Microbial Diversity Responding to Changes in Depositional Conditions during the Last Glacial and Interglacial Period: NE Ulleung Basin, East Sea (Sea of Japan)
Abstract
1. Introduction
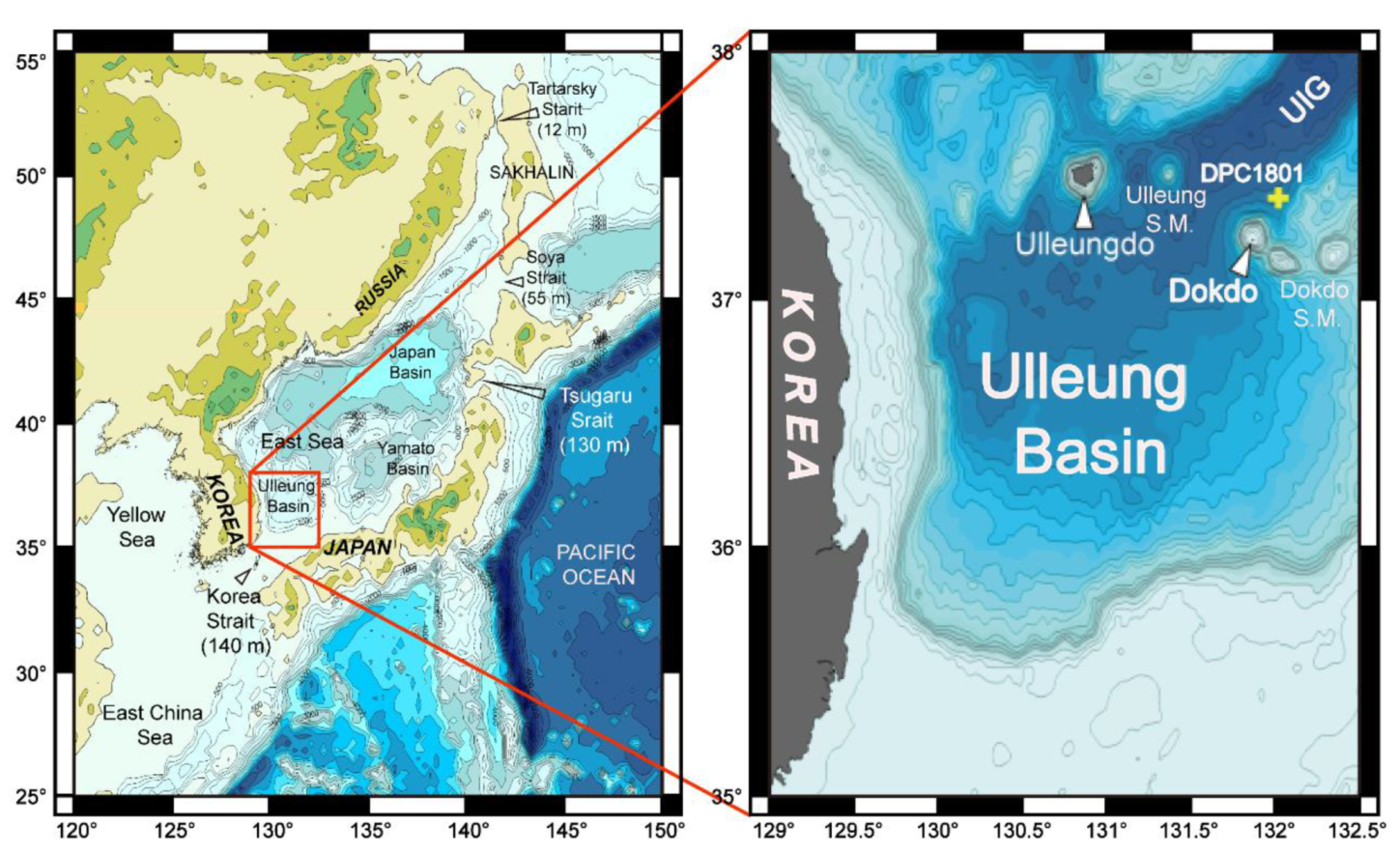
2. Geological Setting
3. Materials and Methods
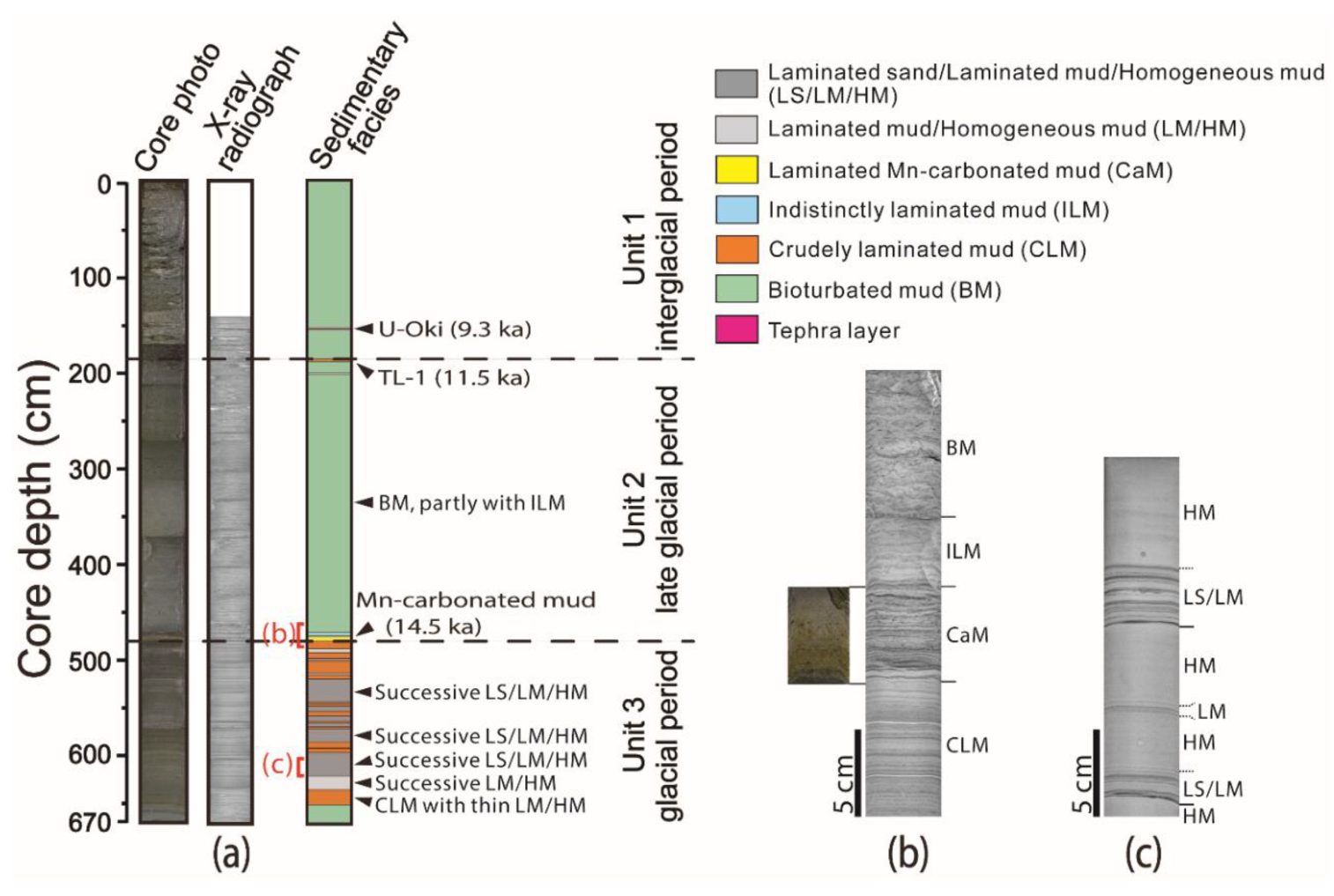
3.1. Core Acquisition and Sedimentary Facies
3.2. X-ray Fluorescence (XRF) and Magnetic Susceptibility (MS)
3.3. X-ray Diffractometer (XRD)
3.4. Transmission Electron Microscope (TEM)
3.5. Extraction of DNA from Sediment Samples
3.6. Next Generation Sequencing (NGS)
3.7. Bioinformatics Analysis
4. Results
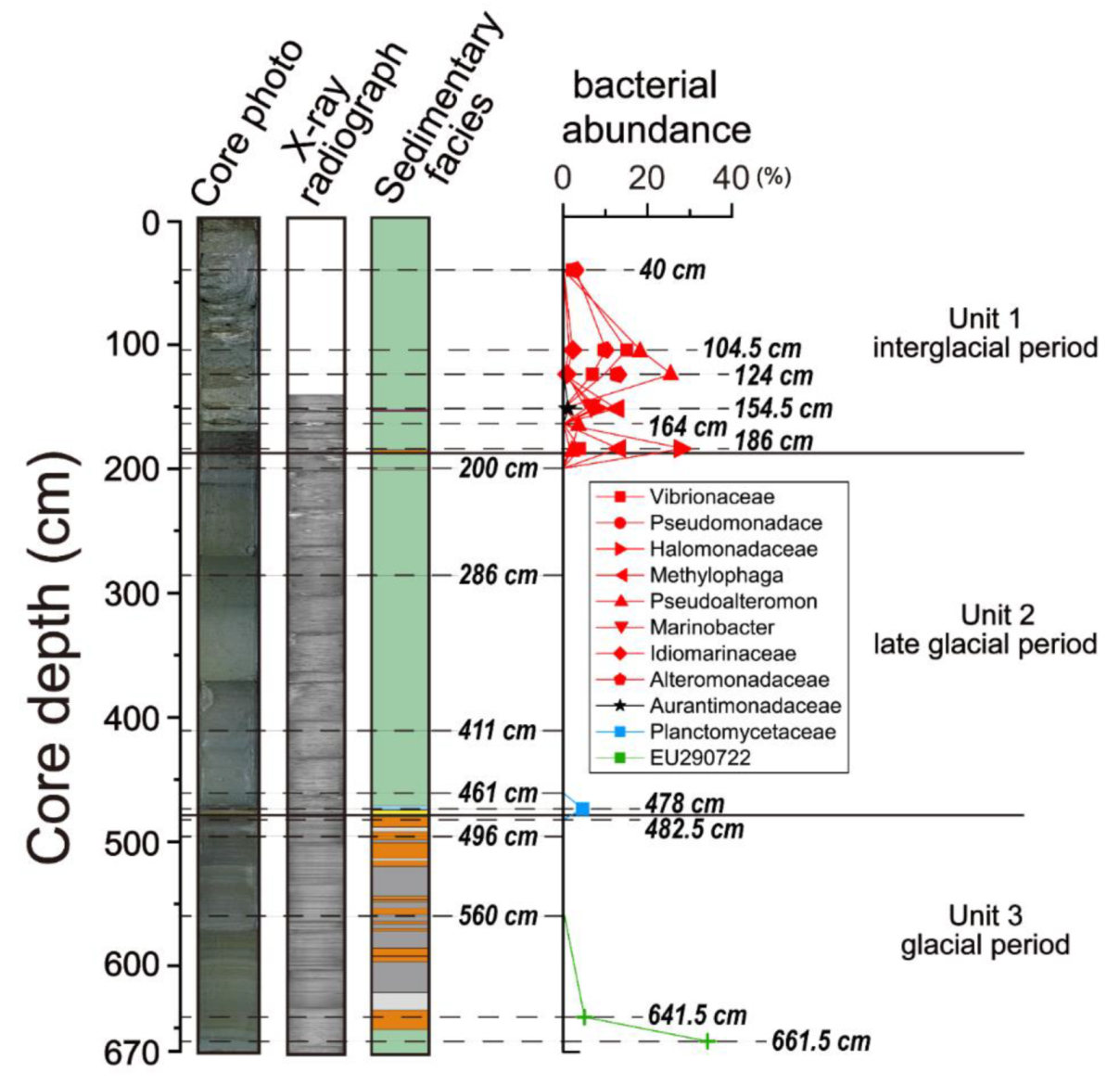
4.1. Sedimentary Facies
4.1.1. Fine-Grained Turbidites
4.1.2. Hemi-Pelagic Sediments
4.1.3. Bottom-Current Deposits
4.1.4. Chemogenic Sediments
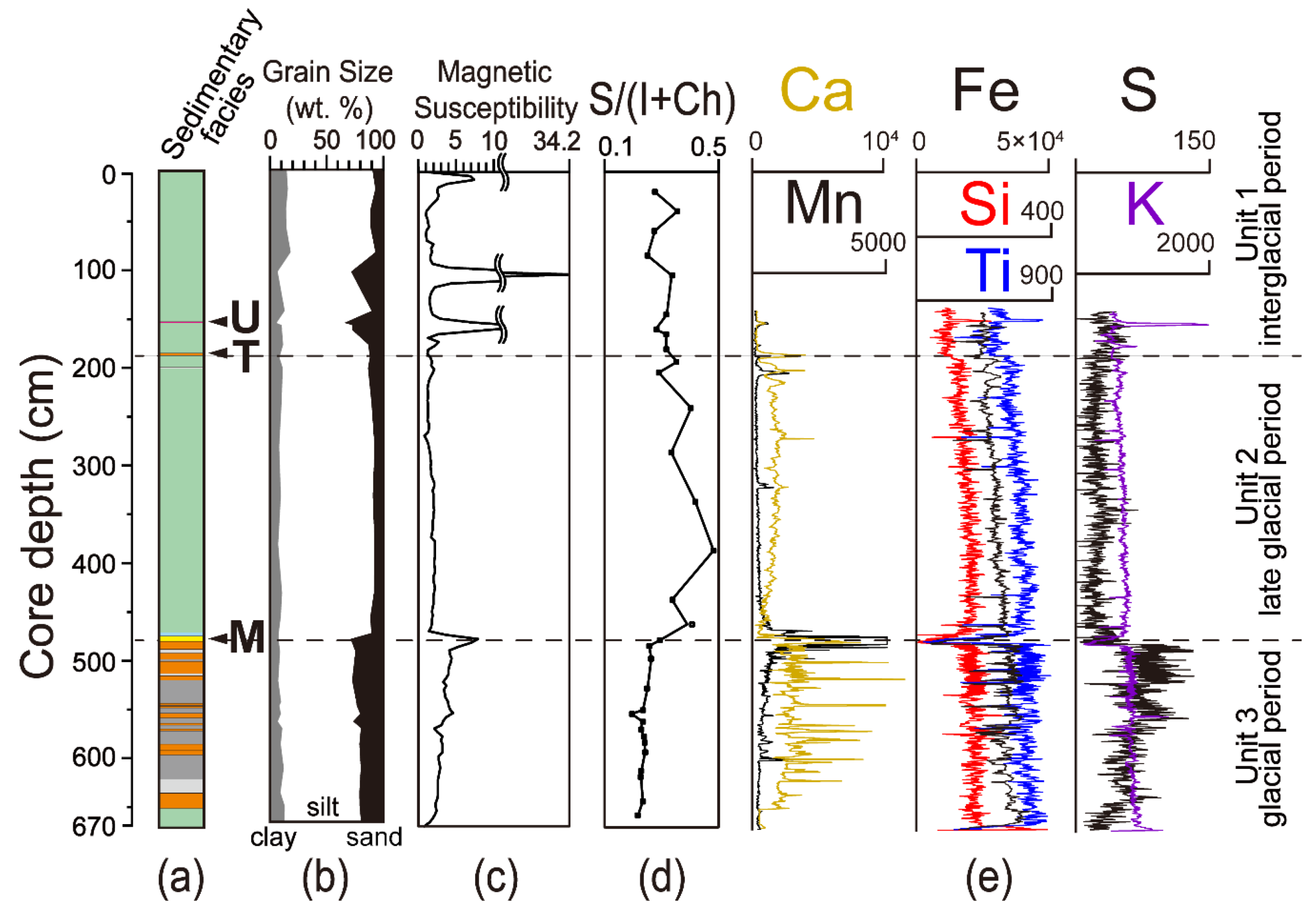
4.2. Grain Size Distribution (GSD), Magnetic Susceptibility (MS) and Distribution of Elemental Composition (XRF)
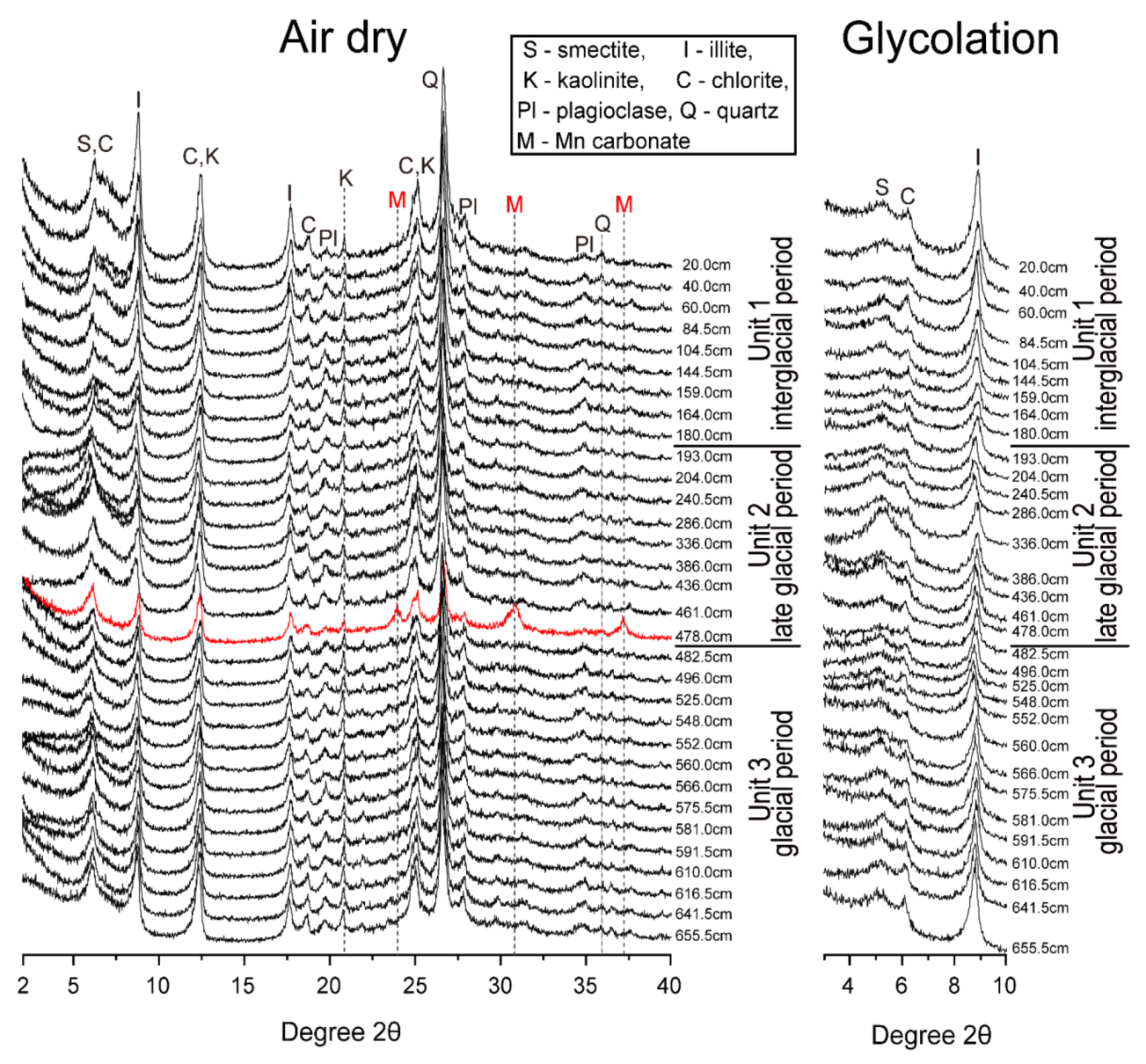
4.3. Mineralogy and Semi-Quantification of Clay Minerals
4.3.1. XRD Patterns
4.3.2. Semi-Quantification of Clay Minerals
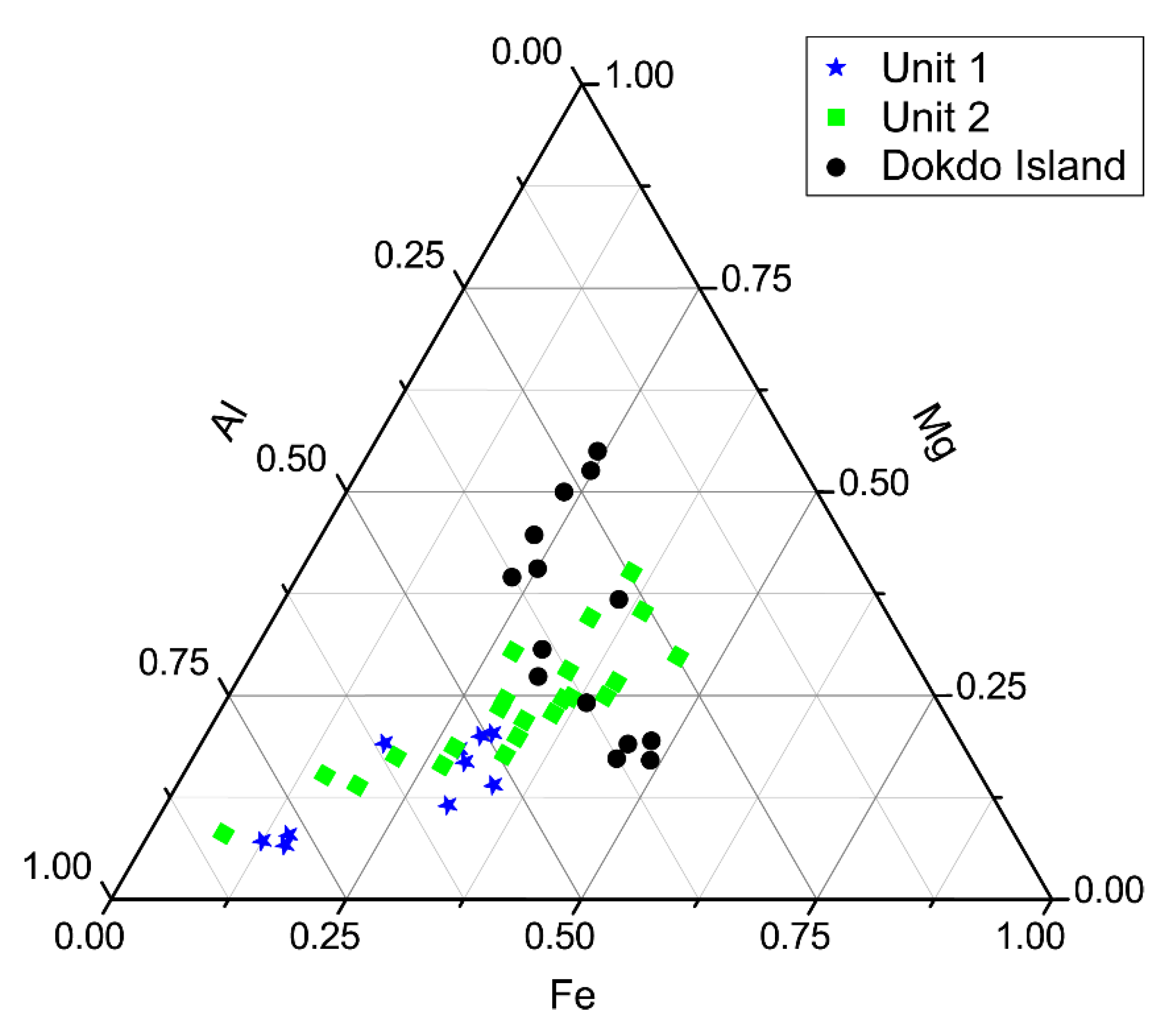
4.4. TEM-EDS
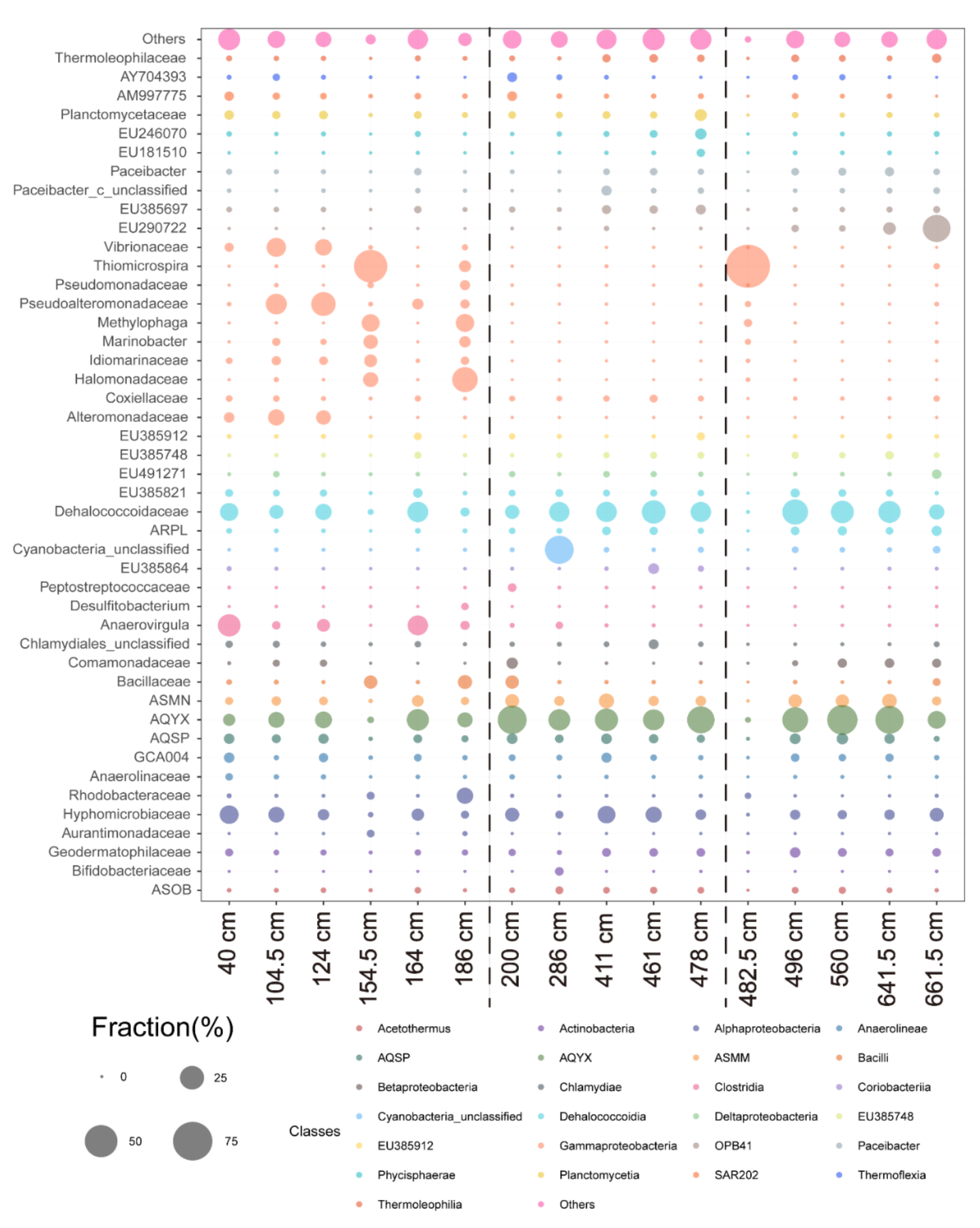
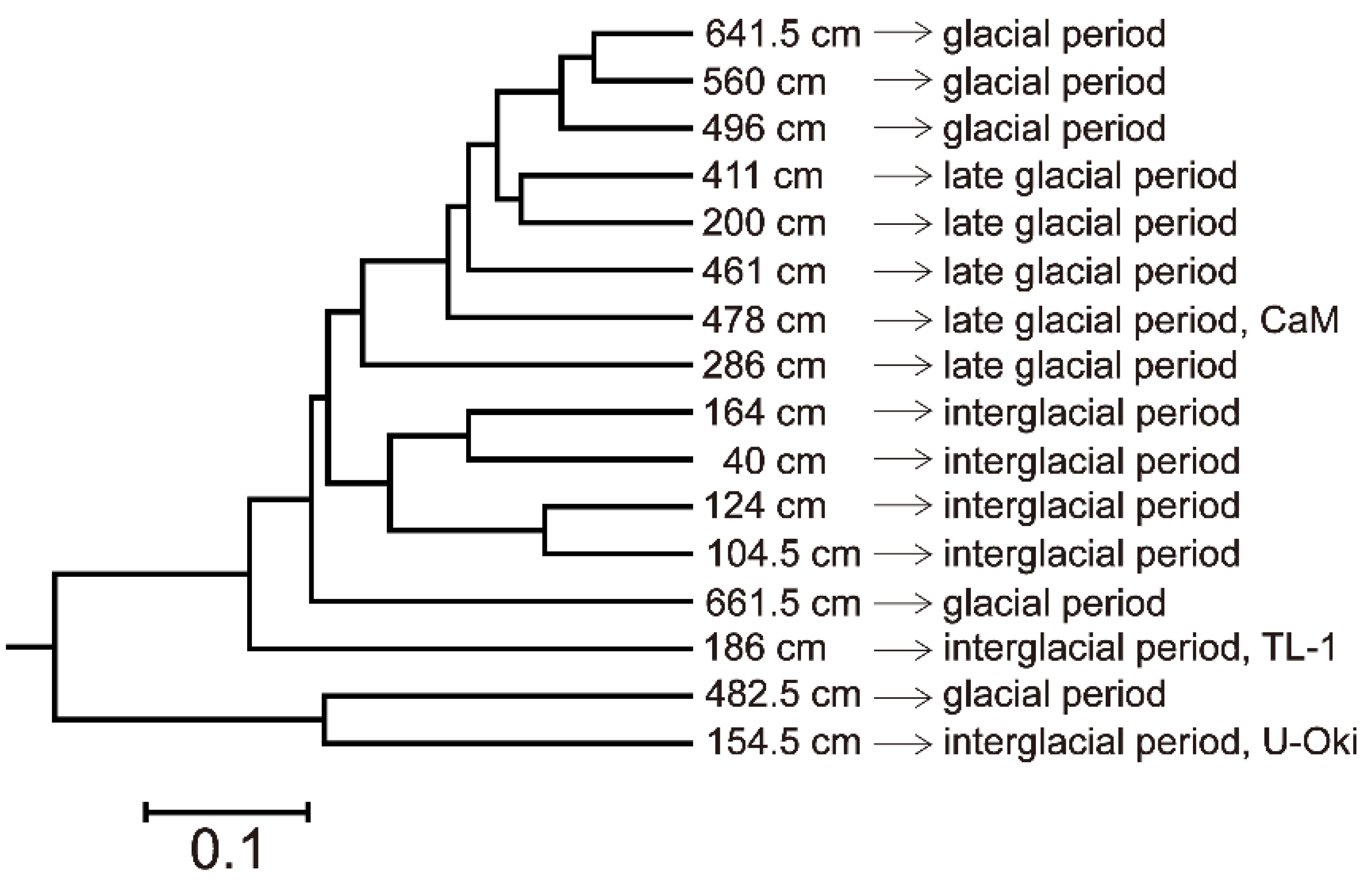
4.5. Microbial Diversity
5. Discussion
5.1. Paleo-Depositional Environments
5.2. Microbial Diversity in Paleo-Depositional Environments
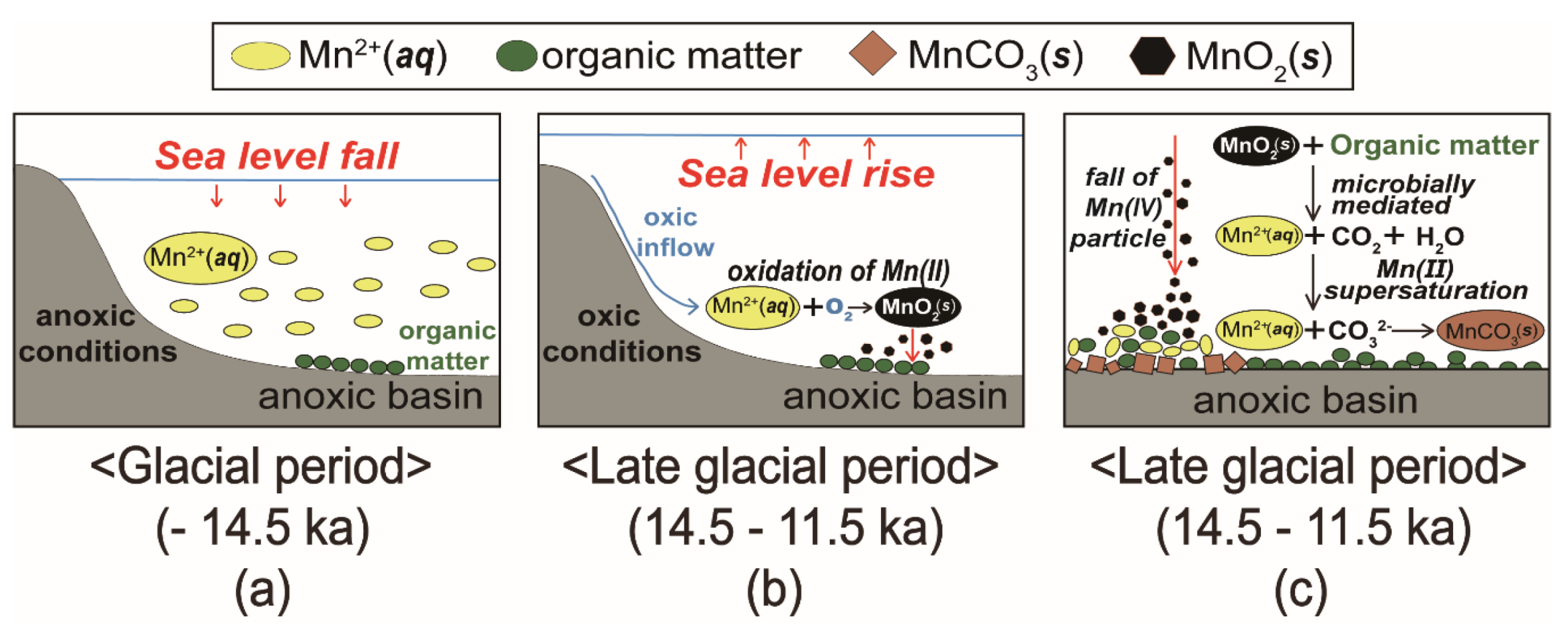
5.3. Formation of Mn-Carbonates
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahk, J.; Chough, S.; Jeong, K.; Han, S. Sedimentary records of paleoenvironmental changes during the last deglaciation in the Ulleung Interplain Gap, East Sea (Sea of Japan). Glob. Planet. Chang. 2001, 28, 241–253. [Google Scholar] [CrossRef]
- Kemp, A.E. Laminated sediments as palaeo-indicators. Geol. Soc. Lond. Spec. Publ. 1996, 116, vii–xii. [Google Scholar] [CrossRef]
- Chun, J.-H.; Cheong, D.; Ikehara, K.; Han, S.-J. Age of the SKP-I and SKP-II tephras from the southern East Sea/Japan Sea: Implications for interstadial events recorded in sediment from marine isotope stages 3 and 4. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 247, 100–114. [Google Scholar] [CrossRef]
- Chamley, H. Clay Mineralogy; Springer: New York, NY, USA, 1989. [Google Scholar]
- Velde, B. Origin and Mineralogy of Clays: Clays and the Environment; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Petschick, R.; Kuhn, G.; Gingele, F. Clay mineral distribution in surface sediments of the South Atlantic: Sources, transport, and relation to oceanography. Mar. Geol. 1996, 130, 203–229. [Google Scholar] [CrossRef]
- Weaver, C.; Pollard, L. The chemistry of clay minerals. Dev. Sedimentol. 1973, 15, 213. [Google Scholar]
- Park, Y.K.; Lee, J.I.; Jung, J.; Hillenbrand, C.-D.; Yoo, K.-C.; Kim, J. Elemental compositions of smectites reveal detailed sediment provenance changes during glacial and interglacial periods: The Southern Drake Passage and Bellingshausen Sea, Antarctica. Minerals 2019, 9, 322. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.; Seabaugh, J.; Newell, S.W.; Eberl, D.D. Role of microbes in the smectite-to-illite reaction. Science 2004, 303, 830–832. [Google Scholar] [CrossRef]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Eberl, D.D.; Dong, H.; Kim, J. The formation of illite from nontronite by mesophilic and thermophilic bacterial reaction. Clays Clay Miner. 2011, 59, 21–33. [Google Scholar] [CrossRef]
- Simon, C.; Daniel, R. Metagenomic analyses: Past and future trends. Appl. Environ. Microbiol. 2011, 77, 1153–1161. [Google Scholar] [CrossRef]
- Cuadros, J.; Michalski, J.R.; Dekov, V.; Bishop, J.; Fiore, S.; Dyar, M.D. Crystal-chemistry of interstratified Mg/Fe-clay minerals from seafloor hydrothermal sites. Chem. Geol. 2013, 360, 142–158. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.; Yang, K.; Park, H.; Elliott, W.C.; Spivack, A.; Koo, T.-H.; Kim, G.; Morono, Y.; Henkel, S. Naturally occurring, microbially induced smectite-to-illite reaction. Geology 2019, 47, 535–539. [Google Scholar] [CrossRef]
- Vorhies, J.S.; Gaines, R.R. Microbial dissolution of clay minerals as a source of iron and silica in marine sediments. Nat. Geosci. 2009, 2, 221. [Google Scholar] [CrossRef]
- Yang, K.; Park, H.; Son, S.-K.; Baik, H.; Park, K.; Kim, J.; Yoon, J.; Park, C.H.; Kim, J. Electron microscopy study on the formation of ferromanganese crusts, western Pacific Magellan Seamounts. Mar. Geol. 2019, 410, 32–41. [Google Scholar] [CrossRef]
- Mande, S.S.; Mohammed, M.H.; Ghosh, T.S. Classification of metagenomic sequences: Methods and challenges. Brief. Bioinform. 2012, 13, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bahk, J.J.; Kim, S.-P.; Park, J.-Y. Physiography and Late Quaternary Sedimentation. In Oceanography of the East Sea (Japan Sea); Springer: New York, NY, USA, 2016; pp. 389–414. [Google Scholar]
- Chough, S.K.; Lee, H.J.; Yoon, S.H. Marine Geology of Korean Seas; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lee, S.H.; Bahk, J.J.; Chough, S.K. Origin of deep-water sediment waves in the Ulleung Interplain Gap, East Sea. Geosci. J. 2003, 7, 65. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, C.H.; Jeong, E.Y.; Hwang, J.S.; Ko, Y.T. Evolution of the Dok Do seamounts, Ulleung Basin, East Sea: Constraints based on the reconstruction of virtual geomagnetic poles using paleomagnetic data. GeoMar. Lett. 2009, 29, 161–169. [Google Scholar] [CrossRef]
- Nitani, H. Beginning of the Kuroshio. In Kuroshio, Physical Aspect of the Japan Current; University of Washington Press: Washington, DC, USA, 1972. [Google Scholar]
- Lie, H.J.; Cho, C.H.; Lee, J.H.; Niiler, P.; Hu, J.H. Separation of the Kuroshio water and its penetration onto the continental shelf west of Kyushu. J. Geophys. Res. Ocean. 1998, 103, 2963–2976. [Google Scholar] [CrossRef]
- Tada, R. Late Quaternary paleoceanography of the Japan Sea. Quat. Res. (Daiyonki-Kenkyu) 1999, 38, 216–222. [Google Scholar] [CrossRef][Green Version]
- Keigwin, L.; Gorbarenko, S. Sea level, surface salinity of the Japan Sea, and the Younger Dryas event in the northwestern Pacific Ocean. Quat. Res. 1992, 37, 346–360. [Google Scholar] [CrossRef]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Morono, Y.; Inagaki, F. Analysis of low-biomass microbial communities in the deep biosphere. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 95, pp. 149–178. [Google Scholar]
- Bykowski, T.; Stevenson, B. Aseptic technique. In Current Protocols in Microbiology; Wiley: Hoboken, NJ, USA, 2008; Volume 11, pp. A.4D.1–A.4D.11. [Google Scholar]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Westcott, S.L.; Schloss, P.D. OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. MSphere 2017, 2, e00073-17. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Yue, J.C.; Clayton, M.K. A similarity measure based on species proportions. Commun. Stat. Theory Methods 2005, 34, 2123–2131. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Bahk, J.; Chough, S.; Han, S. Origins and paleoceanographic significance of laminated muds from the Ulleung Basin, East Sea (Sea of Japan). Mar. Geol. 2000, 162, 459–477. [Google Scholar] [CrossRef]
- Bahk, J.; Lee, S.; Yoo, H.; Back, G.; Chough, S. Late Quaternary sedimentary processes and variations in bottom-current activity in the Ulleung Interplain Gap, East Sea (Korea). Mar. Geol. 2005, 217, 119–142. [Google Scholar] [CrossRef]
- Lee, S.; Bahk, J.; Chough, S.; Back, G.; Yoo, H. Late Quaternary sedimentation in the Ulleung Interplain Gap, East Sea (Korea). Mar. Geol. 2004, 206, 225–248. [Google Scholar] [CrossRef]
- Lee, K.E. Surface water changes recorded in late quaternary marine sediments of the Ulleung Basin, East Sea (Japan Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 247, 18–31. [Google Scholar] [CrossRef]
- Bouma, A.H. Sedimentology of Some Flysch Deposits: A Graphic Approach to Facies Interpretation; Elsevier: Amsterdam, The Netherlands, 1962; Volume 168. [Google Scholar]
- Chough, S.; Lee, G.; Park, B.; Kim, S. Fine structures of turbidite and associated muds in the Ulleung (Tsushima) Basin, East Sea (Sea of Japan). J. Sediment. Res. 1984, 54, 1212–1220. [Google Scholar]
- PIPER, D.W. Turbidite muds and silts on deepsea fans and abyssal plains. In Sedimentation in Submarine Canyons, Fans, and Trenches; Dowden, Hutchinson & Ross: Stroudsburg, PA, USA, 1978. [Google Scholar]
- Stow, D.A.; Faugères, J.-C.; Viana, A.; Gonthier, E. Fossil contourites: A critical review. Sediment. Geol. 1998, 115, 3–31. [Google Scholar] [CrossRef]
- Hansen, M.; Perner, M. A novel hydrogen oxidizer amidst the sulfur-oxidizing Thiomicrospira lineage. ISME J. 2015, 9, 696. [Google Scholar] [CrossRef]
- Brinkhoff, T.; Sievert, S.M.; Kuever, J.; Muyzer, G. Distribution and diversity of sulfur-oxidizingThiomicrospira spp. at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Appl. Environ. Microbiol. 1999, 65, 3843–3849. [Google Scholar] [CrossRef]
- Kuenen, J.; Veldkamp, H. Thiomicrospira pelophila, gen. n., sp. n., a new obligately chemolithotrophic colourless sulfur bacterium. Antonie van Leeuwenhoek 1972, 38, 241–256. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Zhang, Z.; Weng, B.; Huang, F.; Zhu, G.-B.; Zhu, Y.-G. Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. ISME J. 2015, 9, 2059–2067. [Google Scholar]
- Xu, J.-J.; Zhu, X.-L.; Zhang, Q.-Q.; Cheng, Y.-F.; Zhu, Y.-H.; Ji, Z.-Q.; Jin, R.-C. Roles of MnO2 on performance, sludge characteristics and microbial community in anammox system. Sci. Total Environ. 2018, 633, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.E.; Kemp, A.E. Palaeoclimatology and Palaeoceanography from Laminated Sediments; Geological Society of London: London, UK, 1996. [Google Scholar]
- Da Silva, A.-C.; Whalen, M.T.; Hladil, J.; Koptikova, L.; Chen, D.; Spassov, S.; Boulvain, F.; Devleeschouwer, X. Application of magnetic susceptibility as a paleoclimatic proxy on Paleozoic sedimentary rocks and characterization of the magnetic signal–IGCP-580 projects and events. Episodes 2014, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, D.; Yan, D.; Wei, H.; Xiang, L. Evolution from an anoxic to oxic deep ocean during the Ediacaran–Cambrian transition and implications for bioradiation. Chem. Geol. 2012, 306, 129–138. [Google Scholar] [CrossRef]
- Oba, T.; Kato, M.; Kitazato, H.; Koizumi, I.; Omura, A.; Sakai, T.; Takayama, T. Paleoenvironmental changes in the Japan Sea during the last 85,000 years. Paleoceanography 1991, 6, 499–518. [Google Scholar] [CrossRef]
- Yoo, D.G.; Park, S.C. Late Quaternary lowstand wedges on the shelf margin and trough region of the Korea Strait. Sediment. Geol. 1997, 109, 121–133. [Google Scholar] [CrossRef]
- Gorbarenko, S.; Southon, J. Detailed Japan Sea paleoceanography during the last 25 kyr: Constraints from AMS dating and δ18O of planktonic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 156, 177–193. [Google Scholar] [CrossRef]
- Hyun, S.; Bahk, J.J.; Suk, B.-C.; Park, B.-K. Alternative modes of Quaternary pelagic biosiliceous and carbonate sedimentation: A perspective from the East Sea (Japan Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 247, 88–99. [Google Scholar] [CrossRef]
- Chun, J.-H.; Han, S.-J.; Cheong, D.-K. Tephrostratigraphy in the Ulleung Basin, East Sea: Late Pleistocene to Holocene. Geosci. J. 1997, 1, 154–166. [Google Scholar] [CrossRef]
- Lim, D.; Xu, Z.; Choi, J.; Kim, S.; Kim, E.; Kang, S.; Jung, H. Paleoceanographic changes in the Ulleung Basin, East (Japan) Sea, during the last 20,000 years: Evidence from variations in element composition of core sediments. Prog. Oceanogr. 2011, 88, 101–115. [Google Scholar] [CrossRef]
- León, M.J.; Martínez-Checa, F.; Ventosa, A.; Sánchez-Porro, C. Idiomarina aquatica sp. nov, a moderately halophilic bacterium isolated from salterns. Int. J. Syst. Evol. Microbiol. 2015, 65, 4595–4600. [Google Scholar] [CrossRef]
- Brettar, I.; Christen, R.; Höfle, M.G. Idiomarina baltica sp. nov., a marine bacterium with a high optimum growth temperature isolated from surface water of the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 2003, 53, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Jean, W.D.; Leu, T.-Y.; Lee, C.-Y.; Chu, T.-J.; Lin, S.Y.; Shieh, W.Y. Pseudidiomarina marina sp. nov. and Pseudidiomarina tainanensis sp. nov. and reclassification of Idiomarina homiensis and Idiomarina salinarum as Pseudidiomarina homiensis comb. nov. and Pseudidiomarina salinarum comb. nov., respectively. Int. J. Syst. Evol. Microbiol. 2009, 59, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Essén, S.A.; Johnsson, A.; Bylund, D.; Pedersen, K.; Lundström, U.S. Siderophore production by Pseudomonas stutzeri under aerobic and anaerobic conditions. Appl. Environ. Microbiol. 2007, 73, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, X.; Zhang, L.; Dai, J.; Wang, Y.; Tang, Y.; Li, J.; Sun, T.; Fang, C. Pseudomonas xinjiangensis sp. nov., a moderately thermotolerant bacterium isolated from desert sand. Int. J. Syst. Evol. Microbiol. 2009, 59, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Mulet, M.; Rodríguez, A.C.; David, Z.; Lalucat, J.; García-Valdés, E. Pseudomonas aestusnigri sp. nov., isolated from crude oil-contaminated intertidal sand samples after the Prestige oil spill. Syst. Appl. Microbiol. 2014, 37, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, Y.; Wang, K.; Zhang, X.; Zhang, C.; Zhang, S.; Fu, X.; Jiang, J. Pseudomonas zhaodongensis sp. nov., isolated from saline and alkaline soils. Int. J. Syst. Evol. Microbiol. 2015, 65, 1022–1030. [Google Scholar] [CrossRef]
- Pascual, J.; Lucena, T.; Ruvira, M.A.; Giordano, A.; Gambacorta, A.; Garay, E.; Arahal, D.R.; Pujalte, M.J.; Macián, M.C. Pseudomonas litoralis sp. nov, isolated from Mediterranean seawater. Int. J. Syst. Evol. Microbiol. 2012, 62, 438–444. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Zhang, G.I.; Kang, S.-H.; Kim, H.J.; Cho, B.C. Pseudomonas pelagia sp. nov., isolated from a culture of the Antarctic green alga Pyramimonas gelidicola. Int. J. Syst. Evol. Microbiol. 2009, 59, 3019–3024. [Google Scholar] [CrossRef]
- Neufeld, J.D.; Boden, R.; Moussard, H.; Schäfer, H.; Murrell, J.C. Substrate-specific clades of active marine methylotrophs associated with a phytoplankton bloom in a temperate coastal environment. Appl. Environ. Microbiol. 2008, 74, 7321–7328. [Google Scholar] [CrossRef]
- Jetten, M.S.; Wagner, M.; Fuerst, J.; Van Loosdrecht, M.; Kuenen, G.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Neumann, S.; Wessels, H.J.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Kartal, B.; Jetten, M.S.; Van Niftrik, L. Isolation and characterization of a prokaryotic cell organelle from the anammox bacterium K uenenia stuttgartiensis. Mol. Microbiol. 2014, 94, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Sternbeck, J.; Sohlenius, G. Authigenic sulfide and carbonate mineral formation in Holocene sediments of the Baltic Sea. Chem. Geol. 1997, 135, 55–73. [Google Scholar] [CrossRef]
- Scholz, F.; McManus, J.; Sommer, S. The manganese and iron shuttle in a modern euxinic basin and implications for molybdenum cycling at euxinic ocean margins. Chem. Geol. 2013, 355, 56–68. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Kim, S.-H.; Mok, J.-S.; Cho, H.; Lee, T.; Vandieken, V.; Thamdrup, B. Manganese and iron reduction dominate organic carbon oxidation in surface sediments of the deep Ulleung Basin, East Sea. Biogeosciences 2017, 14, 941–958. [Google Scholar] [CrossRef]
- Dubois, M.; Lopez, M.; Orberger, B.; Rodriguez, C.; Boussafir, M.; Dreux, G.; Rodrigues, S.; Pambo, F. The Mn-carbonate rich black shales of the Bangombe Plateau, Francevillian basin, Gabon. In Mineral Resources in a Sustainable World; Springer: New York, NY, USA, 2015; pp. 1905–1908. [Google Scholar]
- Strous, M.; Pelletier, E.; Mangenot, S.; Rattei, T.; Lehner, A.; Taylor, M.W.; Horn, M.; Daims, H.; Bartol-Mavel, D.; Wincker, P. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 2006, 440, 790. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Kim, C.H.; Park, C.H.; Yang, K.; Lee, S.H.; Lee, I.S.; Kwack, Y.J.; Kwak, J.W.; Jung, J.; Kim, J. Microbial Diversity Responding to Changes in Depositional Conditions during the Last Glacial and Interglacial Period: NE Ulleung Basin, East Sea (Sea of Japan). Minerals 2020, 10, 208. https://doi.org/10.3390/min10030208
Lee KH, Kim CH, Park CH, Yang K, Lee SH, Lee IS, Kwack YJ, Kwak JW, Jung J, Kim J. Microbial Diversity Responding to Changes in Depositional Conditions during the Last Glacial and Interglacial Period: NE Ulleung Basin, East Sea (Sea of Japan). Minerals. 2020; 10(3):208. https://doi.org/10.3390/min10030208
Chicago/Turabian StyleLee, Kee Hwan, Chang Hwan Kim, Chan Hong Park, Kiho Yang, Sang Hoon Lee, In Soo Lee, You Jin Kwack, Jae Woo Kwak, Jaewoo Jung, and Jinwook Kim. 2020. "Microbial Diversity Responding to Changes in Depositional Conditions during the Last Glacial and Interglacial Period: NE Ulleung Basin, East Sea (Sea of Japan)" Minerals 10, no. 3: 208. https://doi.org/10.3390/min10030208
APA StyleLee, K. H., Kim, C. H., Park, C. H., Yang, K., Lee, S. H., Lee, I. S., Kwack, Y. J., Kwak, J. W., Jung, J., & Kim, J. (2020). Microbial Diversity Responding to Changes in Depositional Conditions during the Last Glacial and Interglacial Period: NE Ulleung Basin, East Sea (Sea of Japan). Minerals, 10(3), 208. https://doi.org/10.3390/min10030208





