Low Temperature Serpentinite Replacement by Carbonates during Seawater Influx in the Newfoundland Margin
Abstract
1. Introduction
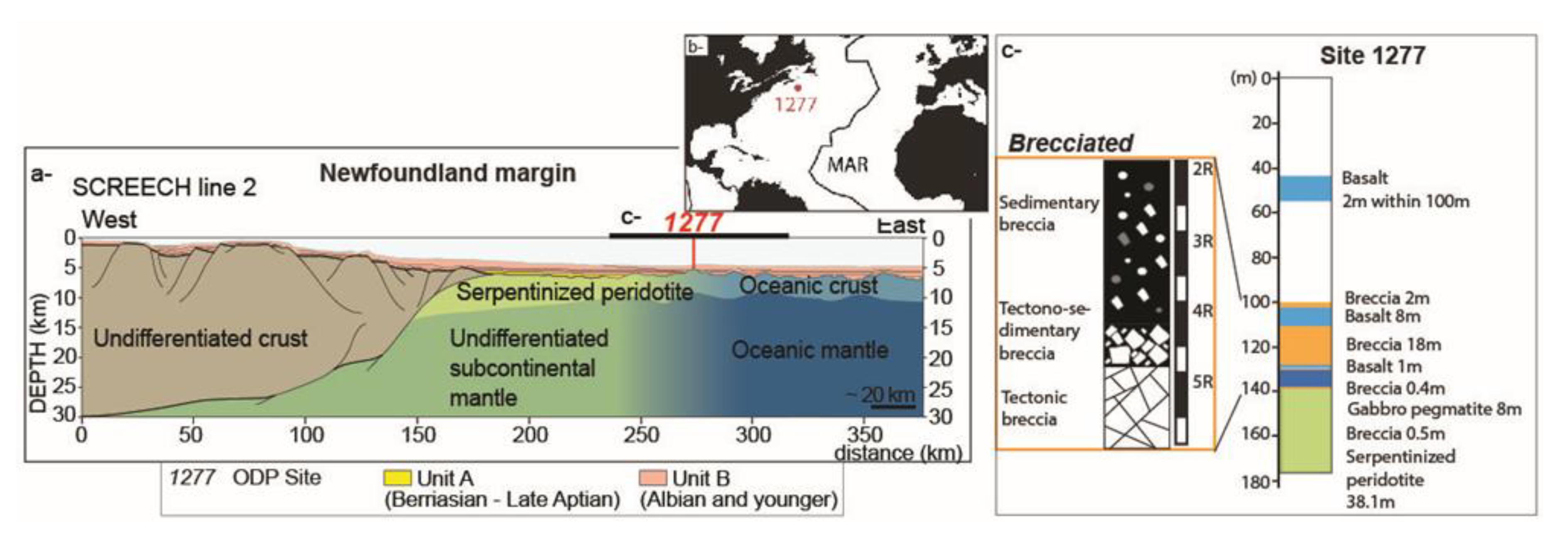
2. Geological Setting
3. Methods
3.1. Microscopy
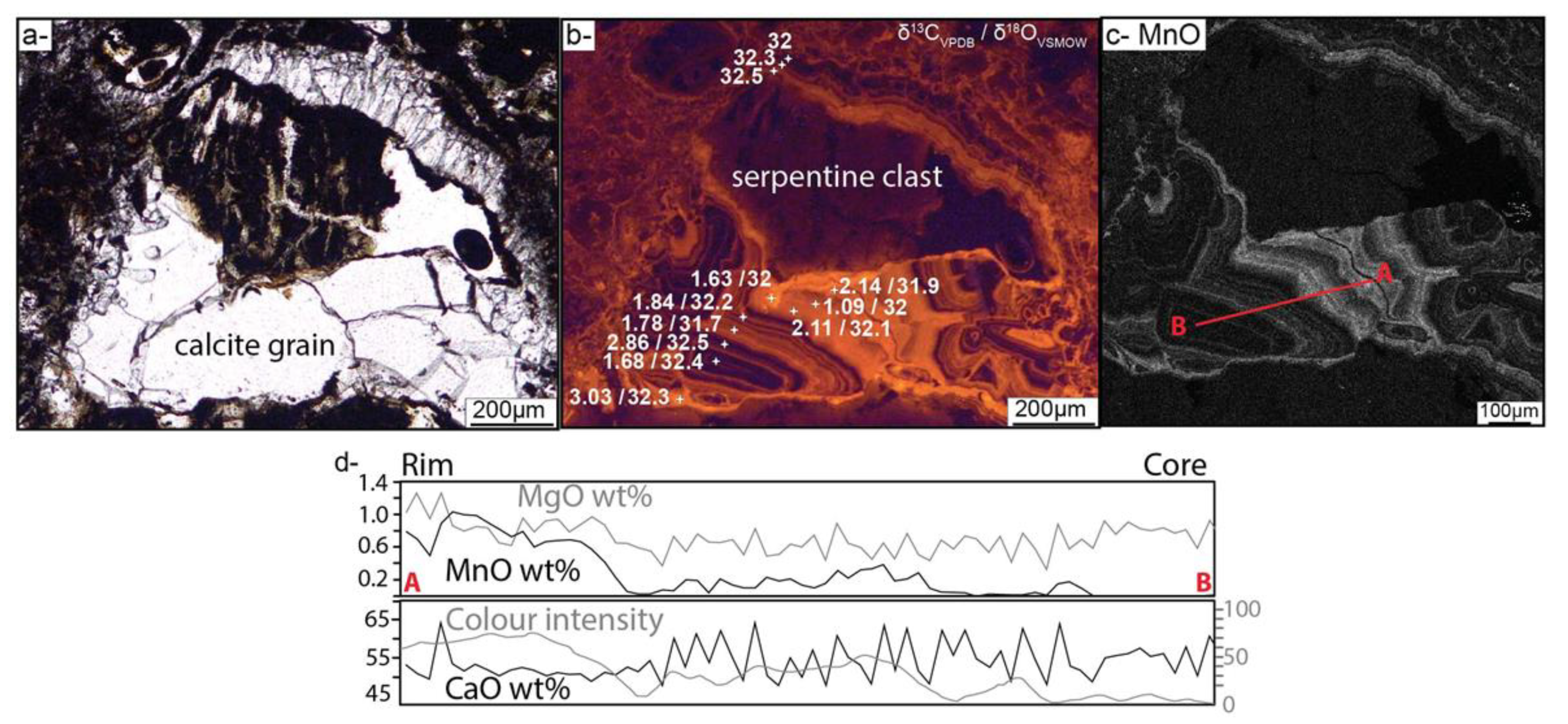
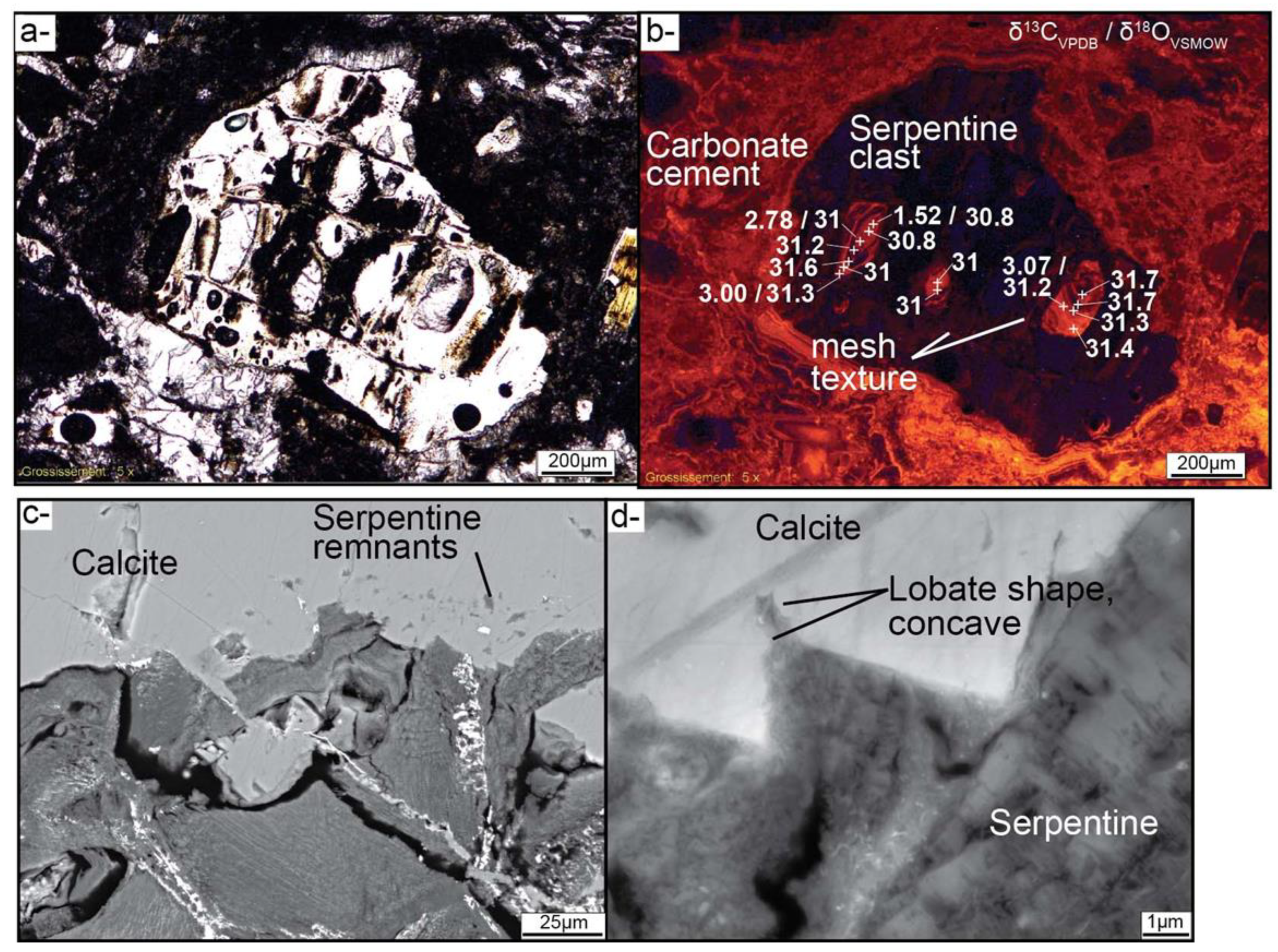
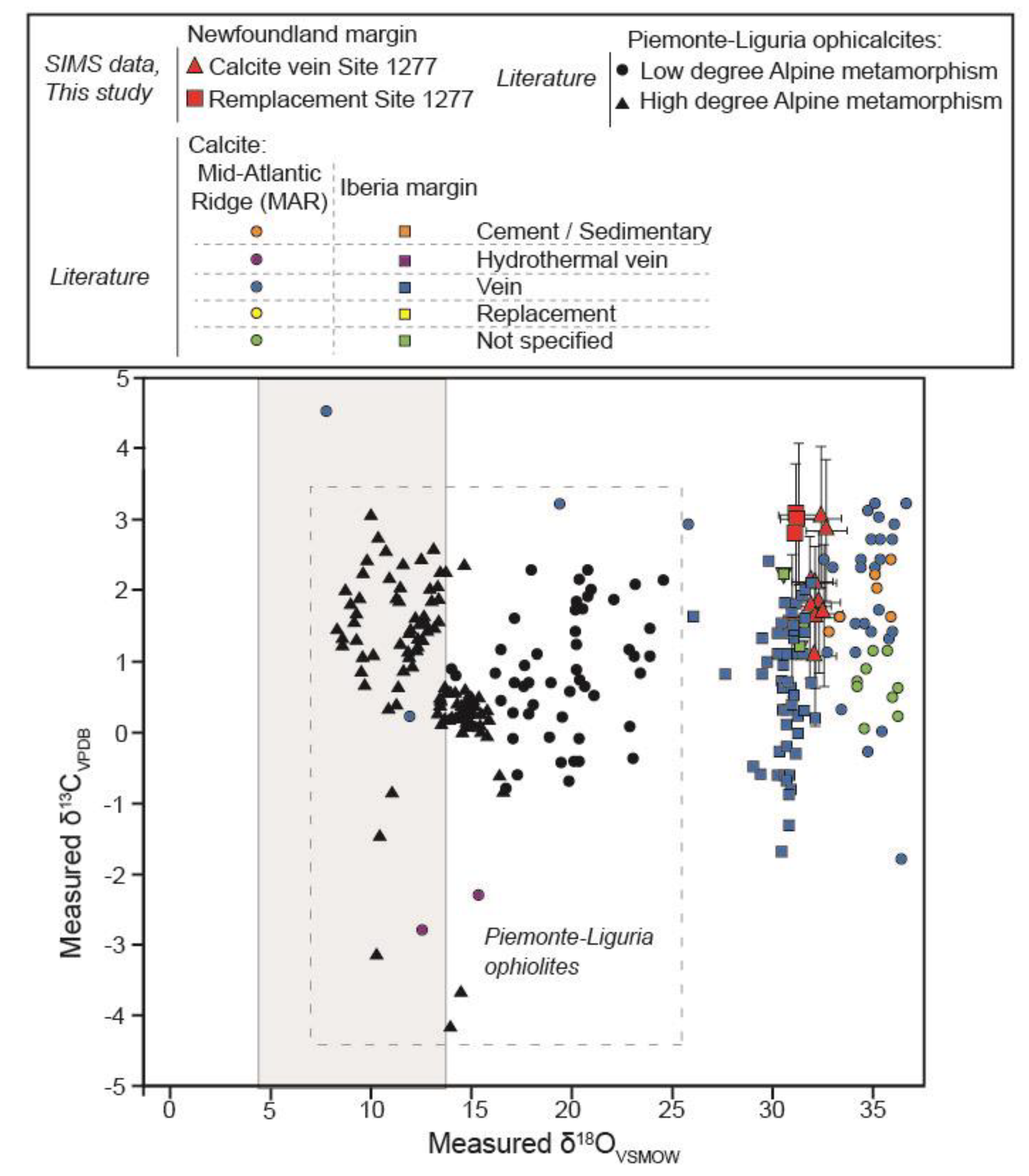
3.2. Secondary Ion Mass Spectrometer (SIMS) Analyses of Calcite
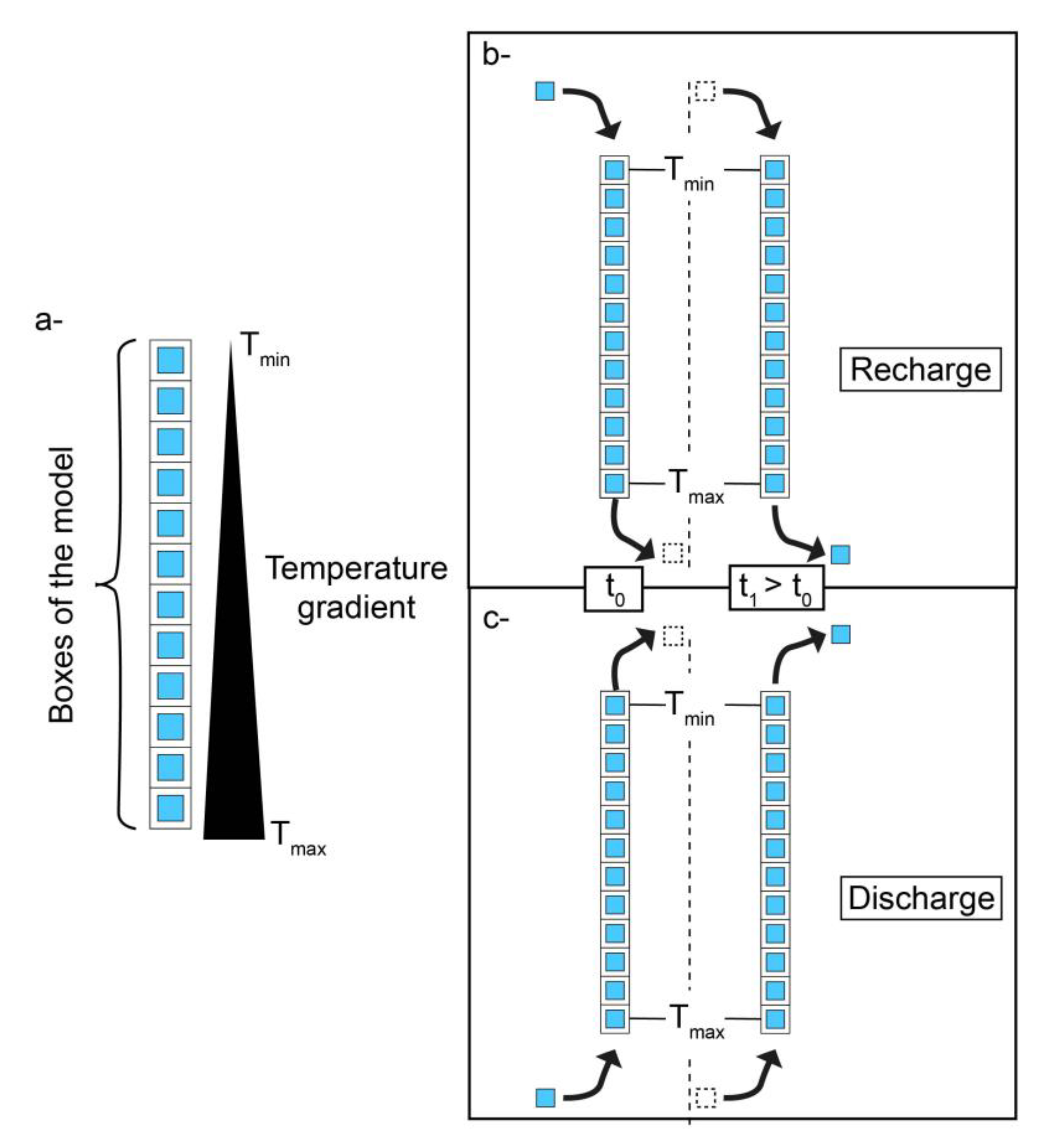
3.3. Thermodynamic Modeling
4. Results
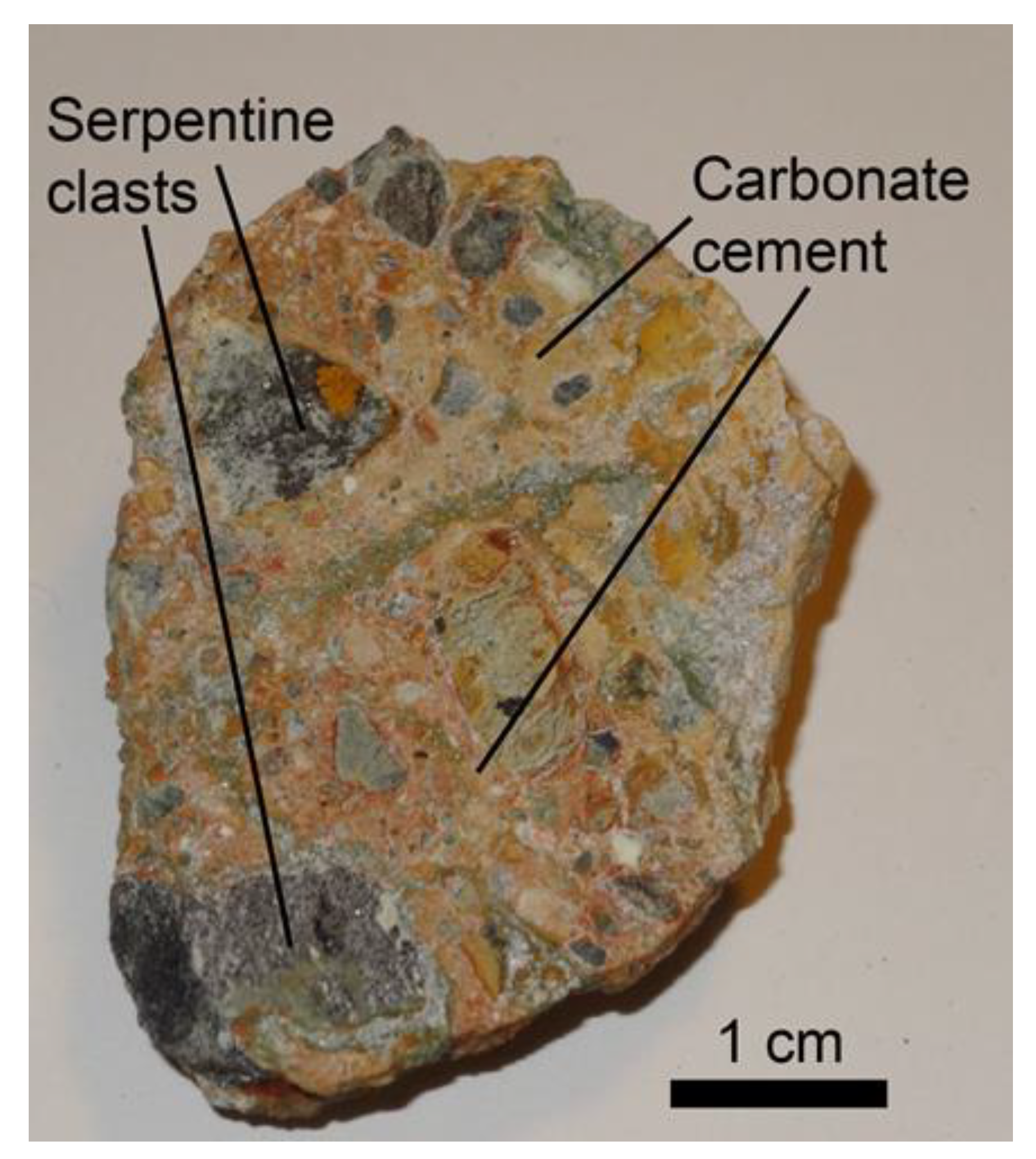
4.1. Brecciation and Carbonate Crystallization
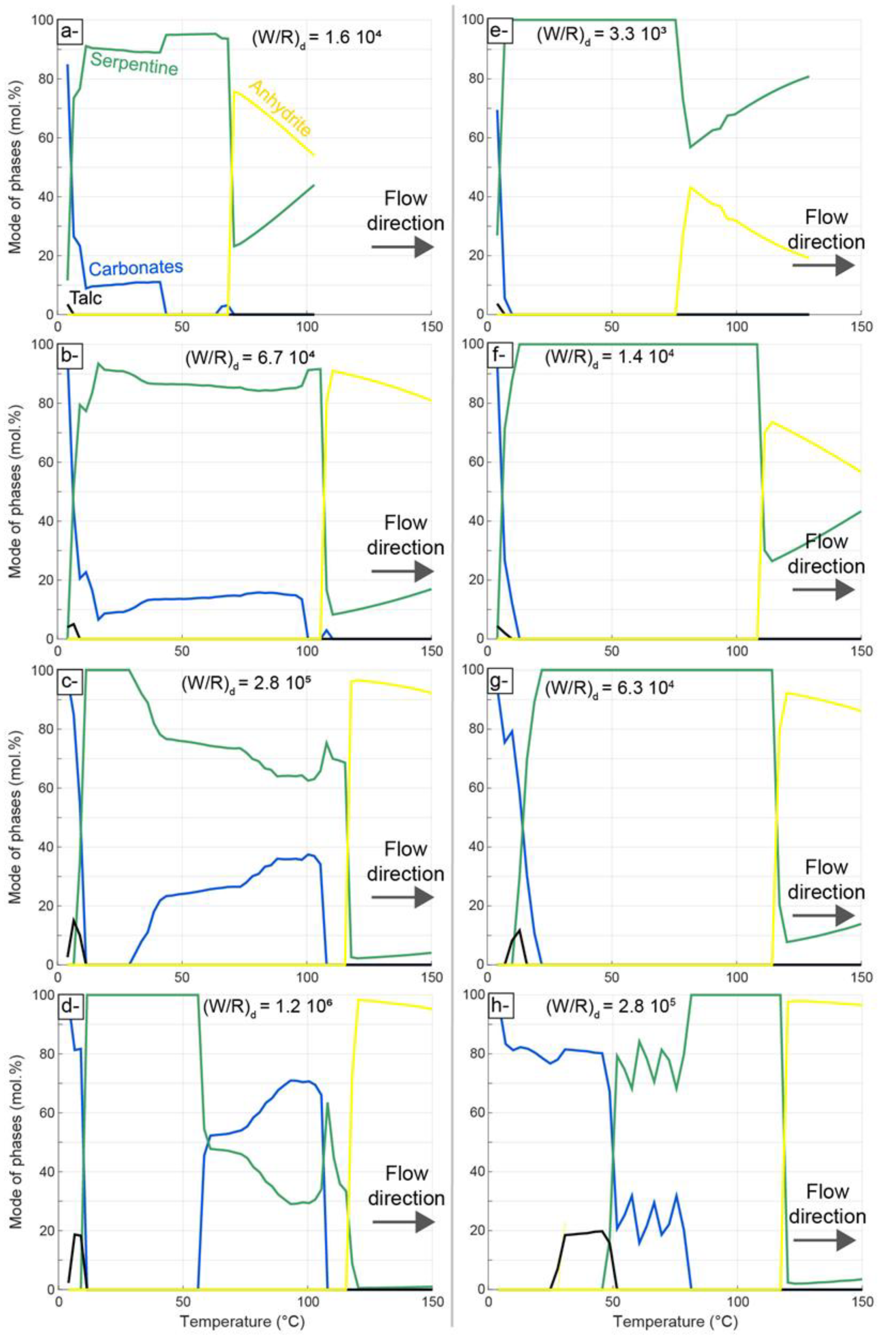
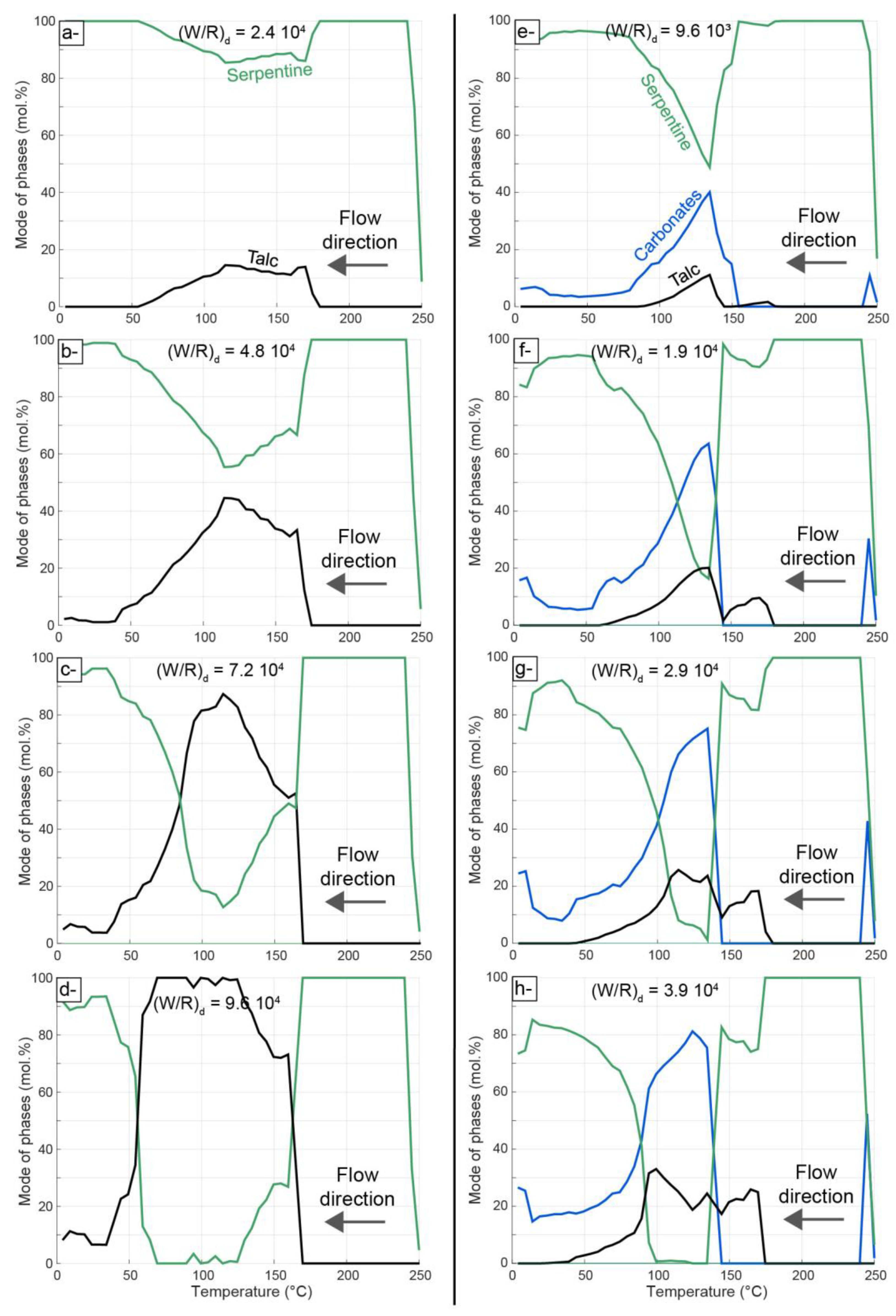
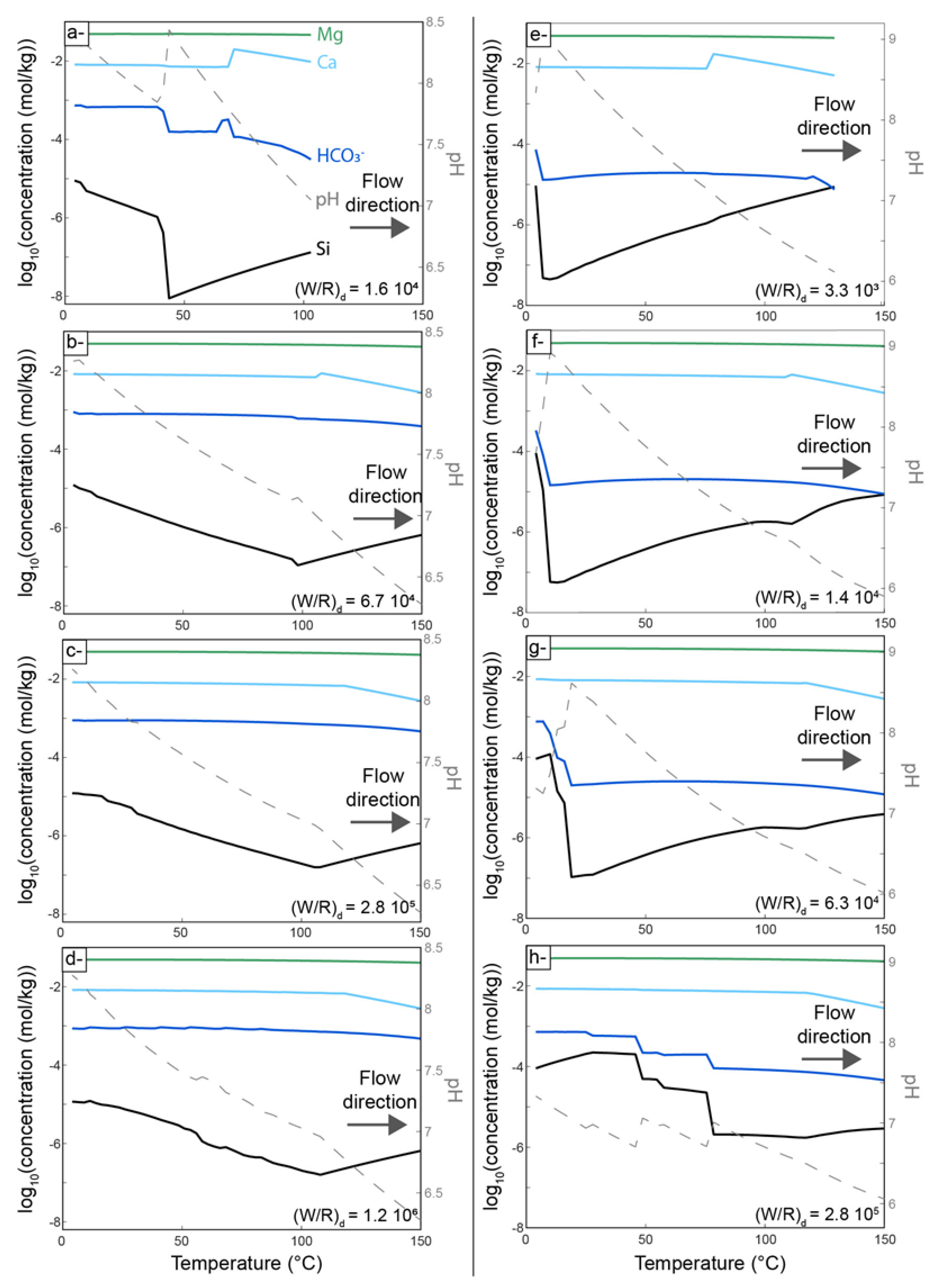
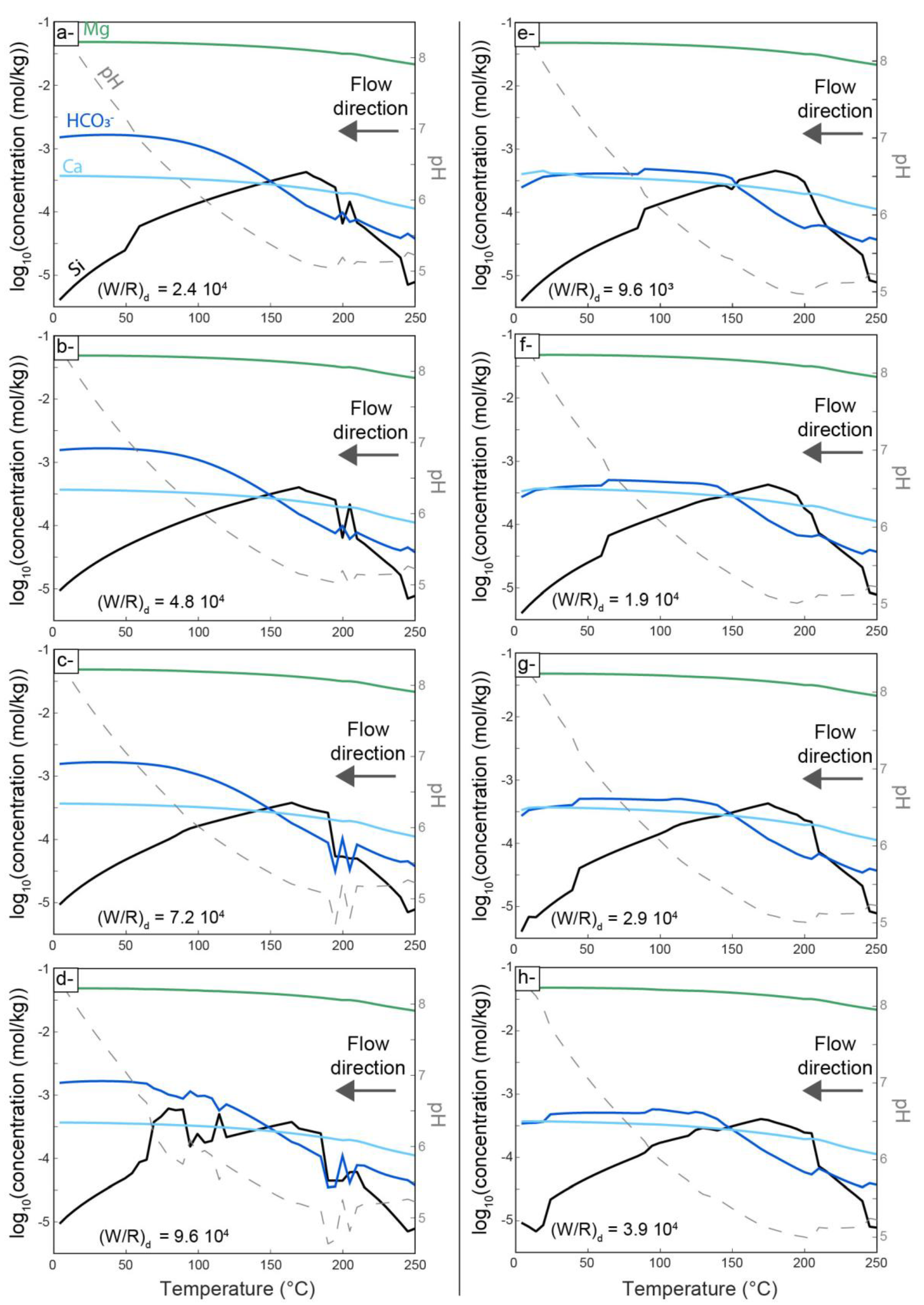
4.2. Thermodynamic Modeling of Carbonation
5. Discussion
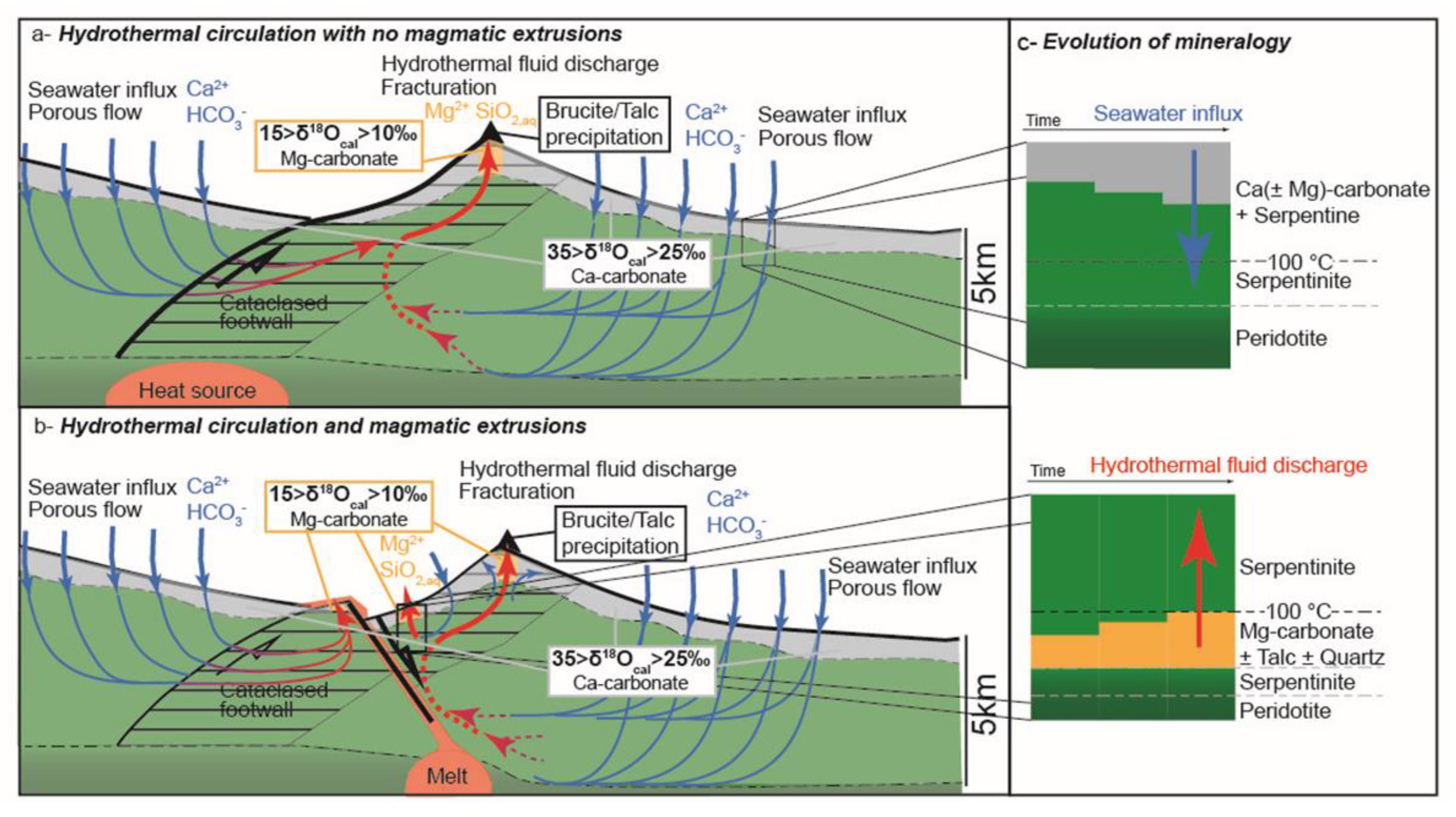
5.1. Serpentine Replacement by Carbonate During Seawater Influx in the NF Margin
5.2. Carbonation in Passive Margins and Slow-Spreading MORs
5.3. Insights from Numerical Modeling
5.4. Implications for Ophicarbonates Formation Preserved in Ophiolites
5.5. Implications for CO2 Mineral Sequestration
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarzenbach, E.M.; Früh-Green, G.L.; Bernasconi, S.M.; Alt, J.C.; Plas, A. Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chem. Geol. 2013, 351, 115–133. [Google Scholar] [CrossRef]
- Morgan, J.K.; Milliken, K.L. Petrography of calcite veins in serpentinized peridotite basement rocks from the Iberia Abyssal Plain, Sites 897 and 899: Kinematic and environmental implications. In Proceedings of the Ocean Drilling Program, Scientific Results; Whitmarsh, R.B., Sawyer, D.S., Klaus, A., Eds.; National Science Foundation: College Station, TX, USA, 1996; Volume 149. [Google Scholar]
- Agrinier, P.; Cornen, G.; Beslier, M.O. Mineralogical and oxygen isotopic features of serpentinites recovered from the ocean/continent transition in the Iberia Abyssal Plain. In Proceedings-Ocean Drilling Program Scientific Results; National Science Foundation: College Station, TX, USA, 1996; pp. 541–552. [Google Scholar]
- Weissert, H.; Bernoulli, D. Oxygen isotope composition of calcite in Alpine ophicarbonates: A hydrothermal of Alpine metamorphic signal? Eclogae Geol. Helv. 1985, 77, 29–43. [Google Scholar]
- Clerc, C.; Boulvais, P.; Lagabrielle, Y.; de Saint Blanquat, M. Ophicalcites from the northern Pyrenean belt: A field, petrographic and stable isotopic study. Int. J. Earth Sci. 2014, 103, 141–163. [Google Scholar] [CrossRef]
- Beinlich, A.; Plümper, O.; Hövelmann, J.; Austrheim, H.; Jamtveit, B. Massive serpentinite carbonation at Linnajavri, N-Norway. Terra Nova 2012, 24, 446–455. [Google Scholar] [CrossRef]
- Surour, A.A.; Arafa, E.H. Ophicarbonates: Calichified serpentinites from Gebel Mohagara, Wadi Ghadir area, eastern desert, Egypt. J. Afr. Earth Sci. 1997, 24, 315–324. [Google Scholar] [CrossRef]
- Lavoie, D.; Cousineau, P.A. Ordovician ophicalcites of southern Quebec Appalachians: A proposed early seafloor tectonosedimentary and hydrothermal origin. J. Sedimentol. Res. 1995, 65, 337–347. [Google Scholar]
- Lemoine, M.; Tricart, P.; Boillot, G. Ultramafic and gabbroic ocean floor of the Ligurian Tethys (Alps, Corsica, Apennines): In search of a genetic model. Geology 1987, 15, 622–625. [Google Scholar] [CrossRef]
- Picazo, S.; Cannat, M.; Delacour, A.; Escartín, J.; Rouméjon, S.; Silantyev, S. Deformation associated with the denudation of mantle-derived rocks at the Mid-Atlantic Ridge 13°–15° N: The role of magmatic injections and hydrothermal alteration. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Cannat, M. Emplacement of mantle rocks in the seafloor at mid-ocean ridges. J. Geophys. Res. Solid Earth 1993, 98, 4163–4172. [Google Scholar] [CrossRef]
- Manatschal, G.; Bernoulli, D. Architecture and tectonic evolution of nonvolcanic margins: Present-day Galicia and ancient Adria. Tectonics 1999, 18, 1099–1119. [Google Scholar] [CrossRef]
- Lafay, R.; Baumgartner, L.P.; Schwartz, S.; Picazo, S.; Montes-Hernandez, G.; Vennemann, T. Petrologic and stable isotopic studies of a fossil hydrothermal system in ultramafic environment (Chenaillet ophicalcites, eastern Alps, France): Processes of carbonate cementation. Lithos 2017, 294, 319–338. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J. In situ carbonation of peridotite for CO2 storage. Proc. Natl. Acad. Sci. USA 2008, 105, 17295–17300. [Google Scholar] [CrossRef]
- Matter, J.; Kelemen, P. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Johannes, W. Experimental investigation of the reaction forsterite + H2O ⇌ serpentine + brucite. Contrib. Mineral. Petrol. 1968, 19, 309–315. [Google Scholar] [CrossRef]
- Mével, C. Serpentinization of abyssal peridotites at mid-ocean ridges. Comptes Rendus Geosci. 2003, 335, 825–852. [Google Scholar] [CrossRef]
- Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Jean-Baptiste, P.; Holm, N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36 14′ N.; MAR). Chem. Geol. 2002, 191, 345–359. [Google Scholar] [CrossRef]
- Barreyre, T.; Escartin, J.; Sohn, R.A.; Cannat, M.; Ballu, V.; Crawford, W. Temporal variability and tidal modulation of hydrothermal exit-fluid temperatures at the Lucky Strike deep-sea vent-field, Mid-Atlantic Ridge. J. Geophys. Res. 2014, 119, 2543–2566. [Google Scholar] [CrossRef]
- Cooper, M.J.; Elderfield, H.; Schultz, A. Diffuse hydrothermal fluids from Lucky Strike hydrothermal vent field: Evidence for a shallow conductively heated system. J. Geophys. Res. 2000, 105, 19369–19375. [Google Scholar] [CrossRef]
- Kelley, D.S.; Karson, J.A.; Früh-Green, G.L.; Yoerger, D.R.; Shank, T.M.; Butterfield, D.A.; Hayes, J.M.; Schrenk, M.O.; Olson, E.J.; Proskurowski, G.; et al. A serpentinite-hosted ecosystem: The Lost City hydrothermal field. Science 2005, 307, 1428–1434. [Google Scholar] [CrossRef]
- Lang, S.Q.; Butterfield, D.A.; Schulte, M.; Kelley, D.S.; Lilley, M.D. Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim. Cosmochim. Acta 2010, 74, 941–952. [Google Scholar] [CrossRef]
- Früh-Green, G.L.; Kelley, D.S.; Bernasconi, S.M.; Karson, J.A.; Ludwig, K.A.; Butterfield, D.A.; Boschi, C.; Proskurowski, G. 30,000 years of hydrothermal activity at the Lost City vent field. Science 2003, 301, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.A.; Kelley, D.S.; Butterfield, D.A.; Nelson, B.K.; Früh-Green, G. Formation and evolution of carbonate chimneys at the Lost City Hydrothermal Field. Geochim. Cosmochim. Acta 2006, 70, 3625–3645. [Google Scholar] [CrossRef]
- Klein, F.; Humphris, S.E.; Guo, W.; Schubotz, F.; Schwarzenbach, E.M.; Orsi, W.D. Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proc. Natl. Acad. Sci. USA 2015, 112, 12036–12041. [Google Scholar] [CrossRef] [PubMed]
- Alt, J.C.; Shanks, W.C. Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: Sulfur geochemistry and reaction modeling. Geochim. Cosmochim. Acta 2003, 67, 641–653. [Google Scholar] [CrossRef]
- Bach, W.; Rosner, M.; Jöns, N.; Rausch, S.; Robinson, L.F.; Paulick, H.; Erzinger, J. Carbonate veins trace seawater circulation during exhumation and uplift of mantle rock: Results from ODP Leg 209. Earth Planet. Sci. Lett. 2011, 311, 242–252. [Google Scholar] [CrossRef]
- Manatschal, G.; Sauter, D.; Karpoff, A.M.; Masini, E.; Mohn, G.; Lagabrielle, Y. The Chenaillet Ophiolite in the French/Italian Alps: An ancient analogue for an oceanic core complex? Lithos 2011, 124, 169–184. [Google Scholar] [CrossRef]
- Schroeder, T.; John, B.E. Strain localization on an oceanic detachment fault system, Atlantis Massif, 30 N.; Mid-Atlantic Ridge. Geochem. Geophys. Geosyst. 2004, 5. [Google Scholar] [CrossRef]
- Bonnemains, D.; Escartín, J.; Mével, C.; Andreani, M.; Verlaguet, A. Pervasive silicification and hanging wall overplating along the 13°20’ N oceanic detachment fault (Mid-Atlantic Ridge). Geochem. Geophys. Geosyst. 2017, 18, 2028–2053. [Google Scholar] [CrossRef]
- Sibson, R.H. Fault rocks and fault mechanisms. J. Geol. Soc. 1977, 133, 191–213. [Google Scholar] [CrossRef]
- Manatschal, G.; Froitzheim, N.; Rubenach, M.; Turrin, B.D. The role of detachment faulting in the formation of an ocean-continent transition: Insights from the Iberia Abyssal Plain: From Wilson, R.L.C.; Whitmarsh, R.B.; Taylor, B.; Froitzheim, N. Non-volcanic rifting of continental margins: A comparaison of evidence from land and sea. Geol. Soc. Lond. Spec. Publ. 2001, 187, 405–428. [Google Scholar]
- Woodcock, N.H.; Mort, K. Classification of fault breccias and related fault rocks. Geol. Mag. 2008, 145, 435–440. [Google Scholar] [CrossRef]
- Agrinier, P.; Mével, C.; Girardeau, J. Hydrothermal alteration of the peridotites cored at the ocean/continent boundary of the Iberian margin: Petrologic and stable isotope evidence. In Proceedings-Ocean Drilling Program Scientific Results; Boillot, G., Winterer, E.L., Eds.; National Science Foundation: College Station, TX, USA, 1988; Volume 103, pp. 225–234. [Google Scholar]
- Milliken, K.L.; Morgan, J.K. Chemical evidence for near seafloor precipitation of calcite in serpentinites (Site 897) and serpentinite breccias (Site 899), Iberia Abyssal Plain. In Proceedings of the Ocean Drilling Program, Scientific Results; Whitmarsh, R.B., Sawyer, D.S., Eds.; National Science Foundation: College Station, TX, USA, 1996; Volume 149. [Google Scholar]
- Skelton, A.D.; Valley, J.W. The relative timing of serpentinisation and mantle exhumation at the ocean–continent transition, Iberia: Constraints from oxygen isotopes. Earth Planet. Sci. Lett. 2000, 178, 327–338. [Google Scholar] [CrossRef]
- Whitmarsh, R.B.; Beslier, M.O.; Wallace, P.J. Proceedings ODP, Initial Reports; Ocean Drilling Program: College Station, TX, USA, 1998; Volume 173. [Google Scholar] [CrossRef]
- Jagoutz, O.; Muntener, O.; Manatschal, G.; Rubatto, D.; Péron-Pinvidic, G.; Turrin, B.D.; Villa, I.M. The rift-to-drift transition in the North Atlantic: A stuttering start of the MORB machine? Geology 2007, 35, 1087–1090. [Google Scholar] [CrossRef]
- Eddy, M.P.; Jagoutz, O.; Ibañez-Mejia, M. Timing of initial seafloor spreading in the Newfoundland-Iberia rift. Geology 2017, 45, 527–530. [Google Scholar] [CrossRef]
- Sutra, E.; Manatschal, G. How does the continental crust thin in a hyperextended rifted margin? Insights from the Iberia margin. Geology 2012, 40, 139–142. [Google Scholar] [CrossRef]
- Machel, H.G.; Mason, R.A.; Mariano, A.N.; Mucci, A. Causes and emission of luminescence in calcite and dolomite. In Luminescence Microscopy and Spectroscopy—Qualitative and Quantitative Applications. SEPM (Society for Sedimentary Geology) Short Course; Barker, C.E., Kopp, O.C., Eds.; SEPM: Tulsa, OK, USA, 1991; Volume 25, pp. 9–25. [Google Scholar]
- Lane, S.J.; Dalton, J.A. Electron microprobe analysis of geological carbonates. Am. Mineral. 1994, 79, 745–749. [Google Scholar]
- Kim, S.T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Wolery, T.J. EQ3/6: A Software Package for Geochemical Modeling of Aqueous Systems: Package Overview and Installation Guide (Version 7.0); Lawrence Livermore National Laboratory: Livermore, CA, USA, 1992; p. 41.
- Klein, F.; Bach, W.; Jöns, N.; McCollom, T.; Moskowitz, B.; Berquó, T. Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15° N on the Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 2009, 73, 6868–6893. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Mildner, D.F.; Gagnon, C.V.; Saar, M.O.; Seyfried, W.E., Jr. Nanoscale constraints on porosity generation and fluid flow during serpentinization. Geology 2016, 44, 103–106. [Google Scholar] [CrossRef]
- Johnson, J.W.; Oelkers, E.H.; Helgeson, H.C. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 C. Comput. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- Malvoisin, B. Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth Planet. Sci. Lett. 2015, 430, 75–85. [Google Scholar] [CrossRef]
- Milési, V.; Guyot, F.; Brunet, F.; Richard, L.; Recham, N.; Benedetti, M.; Dairou, J.; Prinzhofer, A. Formation of CO2, H2 and condensed carbon from siderite dissolution in the 200–300 °C range and at 50 MPa. Geochim. Cosmochim. Acta 2015, 154, 201–211. [Google Scholar] [CrossRef]
- Evans, B.W. Control of the products of serpentinization by the Fe2+ Mg−1 exchange potential of olivine and orthopyroxene. J. Petrol. 2008, 49, 1873–1887. [Google Scholar] [CrossRef]
- Andreani, M.; Munoz, M.; Marcaillou, C.; Delacour, A. μXANES study of iron redox state in serpentine during oceanic serpentinization. Lithos 2013, 178, 70–83. [Google Scholar] [CrossRef]
- Emmanuel, S.; Berkowitz, B. Suppression and stimulation of seafloor hydrothermal convection by exothermic mineral hydration. Earth Planet. Sci. Lett. 2006, 243, 657–668. [Google Scholar] [CrossRef]
- Rudge, J.F.; Kelemen, P.B.; Spiegelman, M. A simple model of reaction-induced cracking applied to serpentinization and carbonation of peridotite. Earth Planet. Sci. Lett. 2010, 291, 215–227. [Google Scholar] [CrossRef]
- Malvoisin, B.; Brantut, N.; Kaczmarek, M.A. Control of serpentinisation rate by reaction-induced cracking. Earth Planet. Sci. Lett. 2017, 476, 143–152. [Google Scholar] [CrossRef]
- Lasaga, A.C. Kinetic Theory in the Earth Sciences; Princeton University Press: Chichester, UK, 2014; Volume 402. [Google Scholar]
- Frost, B.R.; Beard, J.S. On silica activity and serpentinization. J. Petrol. 2007, 48, 1351–1368. [Google Scholar] [CrossRef]
- Saldi, G.D.; Jordan, G.; Schott, J.; Oelkers, E.H. Magnesite growth rates as a function of temperature and saturation state. Geochim. Cosmochim. Acta 2009, 73, 5646–5657. [Google Scholar] [CrossRef]
- Artemyev, D.A.; Zaykov, V.V. The types and genesis of ophicalcites in Lower Devonian olistostromes at cobalt-bearing massive sulfide deposits in the West Magnitogorsk paleoisland arc (South Urals). Russ. Geol. Geophys. 2010, 51, 750–763. [Google Scholar] [CrossRef]
- Boudier, F.; Baronnet, A.; Mainprice, D. Serpentine mineral replacements of natural olivine and their seismic implications: Oceanic lizardite versus subduction-related antigorite. J. Petrol. 2010, 51, 495–512. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hirth, G. Reaction-driven cracking during retrograde metamorphism: Olivine hydration and carbonation. Earth Planet. Sci. Lett. 2012, 345, 81–89. [Google Scholar] [CrossRef]
- Rouméjon, S.; Cannat, M. Serpentinization of mantle-derived peridotites at mid-ocean ridges: Mesh texture development in the context of tectonic exhumation. Geochem. Geophys. Geosyst. 2014, 15, 2354–2379. [Google Scholar] [CrossRef]
- Rumori, C.; Mellini, M.; Viti, C. Oriented, non-topotactic olivine—Serpentine replacement in mesh-textured, serpentinized peridotites. Eur. J. Mineral. 2004, 16, 731–741. [Google Scholar] [CrossRef]
- Viti, C.; Mellini, M. Mesh textures and bastites in the Elba retrograde serpentinites. Eur. J. Mineral. 1998, 10, 1341–1359. [Google Scholar] [CrossRef]
- Andreani, M.; Grauby, O.; Baronnet, A.; Muñoz, M. Occurrence, composition and growth of polyhedral serpentine. Eur. J. Mineral. 2008, 20, 159–171. [Google Scholar] [CrossRef]
- Müntener, O.; Manatschal, G. High degrees of melt extraction recorded by spinel harzburgite of the Newfoundland margin: The role of inheritance and consequences for the evolution of the southern North Atlantic. Earth Planet. Sci. Lett. 2006, 252, 437–452. [Google Scholar] [CrossRef]
- Klinkhammer, G.; Bender, M.; Weiss, R.F. Hydrothermal manganese in the Galapagos Rift. Nature 1977, 269, 319–320. [Google Scholar] [CrossRef]
- Pingitore, N.E.; Eastman, M.P.; Sandidge, M.; Oden, K.; Freiha, B. The coprecipitation of manganese (II) with calcite: An experimental study. Mar. Chem. 1988, 25, 107–120. [Google Scholar] [CrossRef]
- Haberman, D.; Neuser, R.D.; Richter, D.K. Low limit of Mn2+—Activated cathodoluminescence of calcite: State of the art. Sediment. Geol. 1996, 116, 13–24. [Google Scholar] [CrossRef]
- Dromgoole, E.L.; Walter, L.M. Iron and manganese incorporation into calcite: Effects of growth kinetics, temperature and solution chemistry. Chem. Geol. 1990, 81, 311–336. [Google Scholar] [CrossRef]
- Barnaby, R.J.; Rimstidt, J.D. Redox conditions of calcite cementation interpreted from Mn and Fe contents of authigenic calcites. Geol. Soc. Am. Bull. 1989, 101, 795–804. [Google Scholar] [CrossRef]
- Barnes, I.; O’Neil, J.R. The relationship between fluids in some fresh alpine-type ultramafics and possible modern serpentinization, western United States. Geol. Soc. Am. Bull. 1969, 80, 1947–1960. [Google Scholar] [CrossRef]
- Kitano, Y.; Hood, D.W. Calcium carbonate crystal forms formed from sea water by inorganic processes. J. Oceanogr. Soc. Jpn. 1962, 18, 35–39. [Google Scholar] [CrossRef][Green Version]
- Müller, G.; Irion, G.; Förstner, U. Formation and diagenesis of inorganic Ca-Mg carbonates in the lacustrine environment. Naturwissenschaften 1972, 59, 158–164. [Google Scholar] [CrossRef]
- Romanek, C.S.; Jiménez-Lopez, C.; Rodriguez Navarro, A.; Sanchez-Roman, M.; Sahai, N.; Coleman, M. Inorganic synthesis of Fe-Ca-Mg carbonates at low temperature. Geochim. Cosmochim. Acta 2009, 73, 5361–5376. [Google Scholar] [CrossRef]
- Martin, B.; Fyfe, W.S. Some experimental and theoretical observations on the kinetics of hydration reactions with particular reference to serpentinization. Chem. Geol. 1970, 6, 185–202. [Google Scholar] [CrossRef]
- Malvoisin, B.; Brunet, F.; Carlut, J.; Rouméjon, S.; Cannat, M. Serpentinization of oceanic peridotites: 2. Kinetics and processes of San Carlos olivine hydrothermal alteration. J. Geophys. Res. Solid Earth 2012, 117. [Google Scholar] [CrossRef]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Wenner, D.B.; Taylor, H.P. Temperatures of serpentinization of ultramafic rocks based on O18/O16 fractionation between coexisting serpentine and magnetite. Contrib. Mineral. Petrol. 1971, 32, 165–185. [Google Scholar] [CrossRef]
- Baumgartner, L.P.; Valley, J.W. Stable isotope transport and contact metamorphic fluid flow. Rev. Mineral. Geochem. 2001, 43, 415–467. [Google Scholar] [CrossRef]
- Boschi, C.; Dini, A.; Baneschi, I.; Bedini, F.; Perchiazzi, N.; Cavallo, A. Brucite-driven CO2 uptake in serpentinized dunites (Ligurian Ophiolites, Montecastelli, Tuscany). Lithos 2017, 288, 264–281. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and mechanisms of mineral carbonation in peridotite: Natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Früh-Green, G.L.; Weissert, H.; Bernoulli, D. A multiple fluid history recorded in Alpine ophiolites. J. Geol. Soc. 1990, 147, 959–970. [Google Scholar] [CrossRef]
- Pozzorini, D.; Früh-Green, G.L. Stable isotope systematics of the Ventina Ophicarbonate Zone, Bergell contact aureole. Schweiz. Mineral. und Petrol. Mitt. 1996, 76, 549–564. [Google Scholar]
- Abart, R.; Pozzorini, D. Implications of kinetically controlled mineral-fluid exchange on the geometry of stable-isotope fronts. Eur. J. Miner. 2000, 12, 1069–1082. [Google Scholar] [CrossRef]
- Manatschal, G.; Nievergelt, P. A continent-ocean transition recorded in the Err and Platta nappes (Eastern Switzerland). Eclogae Geol. Helv. 1997, 90, 3–28. [Google Scholar]
- Driesner, T. Aspects of petrographical, structural and stable isotope geochemical evolution of ophicarbonates breccias from ocean floor to subduction and uplift: An example from Chatillon, Middle Aosta Valley, Italian Alps. Schweiz. Mineral. und Petrol. Mitt. 1993, 73, 69–84. [Google Scholar]
- Farver, J.R. Oxygen self-diffusion in calcite: Dependence on temperature and water fugacity. Earth Planet. Sci. Lett. 1994, 121, 575–587. [Google Scholar] [CrossRef]
- Labotka, T.C.; Cole, D.R.; Riciputi, L.R. Diffusion of C and O in calcite at 100 MPa. Am. Mineral. 2000, 85, 488–494. [Google Scholar] [CrossRef]
- Rosenbaum, J.M. Stable isotope fractionation between carbon dioxide and calcite at 900 °C. Geochim. Cosmochim. Acta 1994, 58, 3747–3753. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Numerical simulation of CO2 disposal by mineral trapping in deep aquifers. Appl. Geochem. 2004, 19, 917–936. [Google Scholar] [CrossRef]
- Andreani, M.; Luquot, L.; Gouze, P.; Godard, M.; Hoise, E.; Gibert, B. Experimental study of carbon sequestration reactions controlled by the percolation of CO2-rich brine through peridotites. Environ. Sci. Technol. 2009, 43, 1226–1231. [Google Scholar] [CrossRef]
- Boschi, C.; Dini, A.; Dallai, L.; Ruggieri, G.; Gianelli, G. Enhanced CO2-mineral sequestration by cyclic hydraulic fracturing and Si-rich fluid infiltration into serpentinites at Malentrata (Tuscany, Italy). Chem. Geol. 2009, 265, 209–226. [Google Scholar] [CrossRef]
- King, H.E.; Plümper, O.; Putnis, A. Effect of secondary phase formation on the carbonation of olivine. Environ. Sci. Technol. 2010, 44, 6503–6509. [Google Scholar] [CrossRef]
- Paukert, A.N.; Matter, J.M.; Kelemen, P.B.; Shock, E.L.; Havig, J.R. Reaction path modeling of enhanced in situ CO2 mineralization for carbon sequestration in the peridotite of the Samail Ophiolite, Sultanate of Oman. Chem. Geol. 2012, 330, 86–100. [Google Scholar] [CrossRef]
- Klein, F.; McCollom, T.M. From serpentinization to carbonation: New insights from a CO2 injection experiment. Earth Planet. Sci. Lett. 2013, 379, 137–145. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Dipple, G.M. Serpentinite carbonation for CO2 sequestration. Elements 2013, 9, 115–121. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picazo, S.; Malvoisin, B.; Baumgartner, L.; Bouvier, A.-S. Low Temperature Serpentinite Replacement by Carbonates during Seawater Influx in the Newfoundland Margin. Minerals 2020, 10, 184. https://doi.org/10.3390/min10020184
Picazo S, Malvoisin B, Baumgartner L, Bouvier A-S. Low Temperature Serpentinite Replacement by Carbonates during Seawater Influx in the Newfoundland Margin. Minerals. 2020; 10(2):184. https://doi.org/10.3390/min10020184
Chicago/Turabian StylePicazo, Suzanne, Benjamin Malvoisin, Lukas Baumgartner, and Anne-Sophie Bouvier. 2020. "Low Temperature Serpentinite Replacement by Carbonates during Seawater Influx in the Newfoundland Margin" Minerals 10, no. 2: 184. https://doi.org/10.3390/min10020184
APA StylePicazo, S., Malvoisin, B., Baumgartner, L., & Bouvier, A.-S. (2020). Low Temperature Serpentinite Replacement by Carbonates during Seawater Influx in the Newfoundland Margin. Minerals, 10(2), 184. https://doi.org/10.3390/min10020184





