Mineralogical Constraints on the Potassic and Sodic-Calcic Hydrothermal Alteration and Vein-Type Mineralization of the Maronia Porphyry Cu-Mo ± Re ± Au Deposit in NE Greece
Abstract
1. Introduction
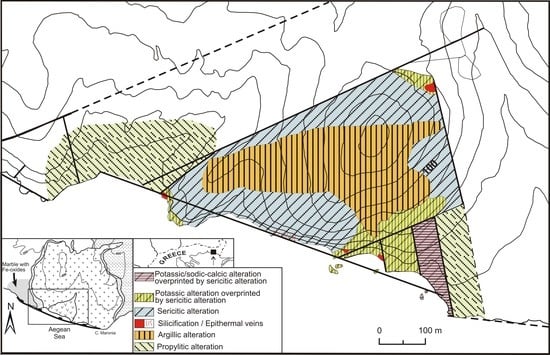
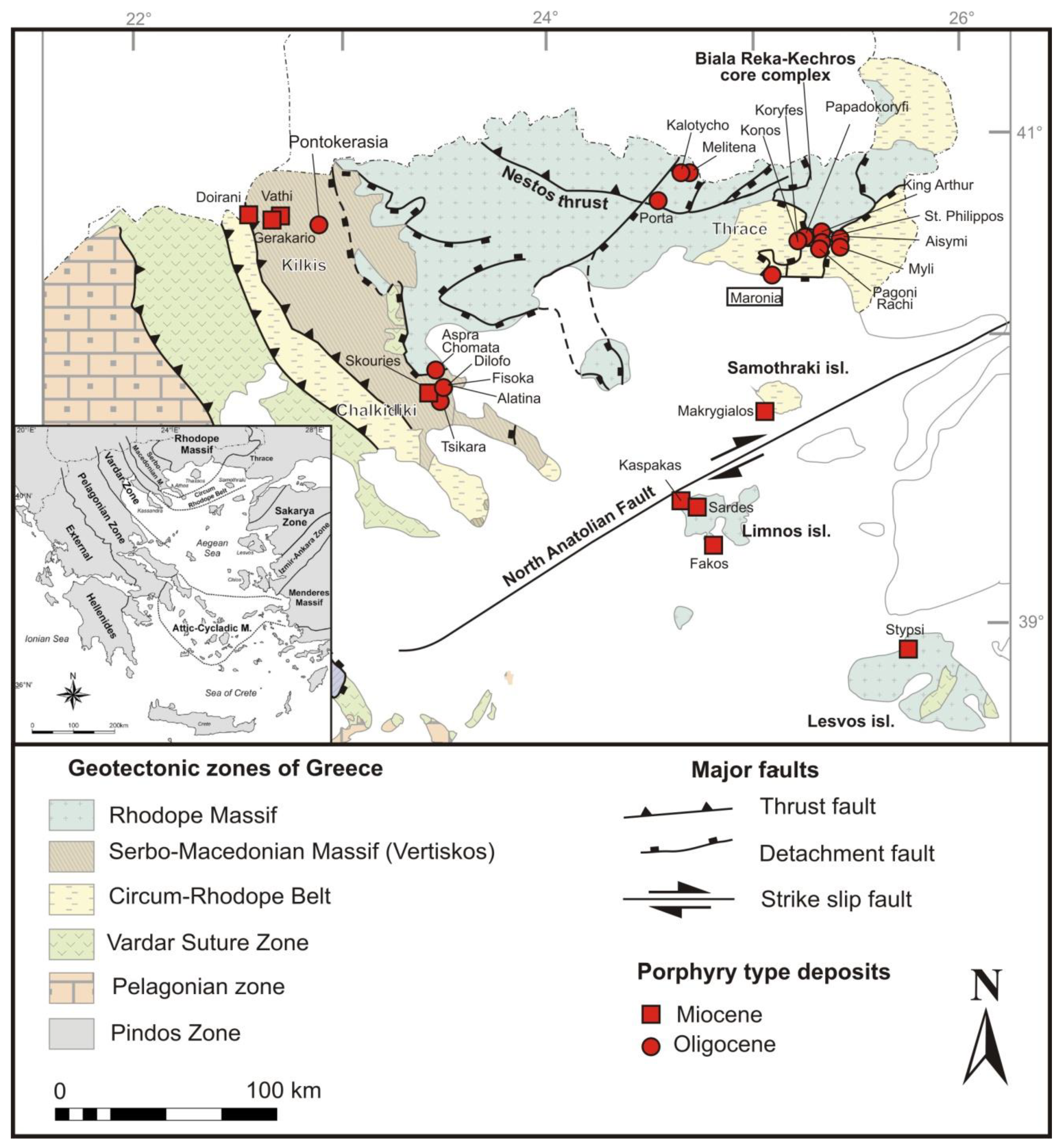
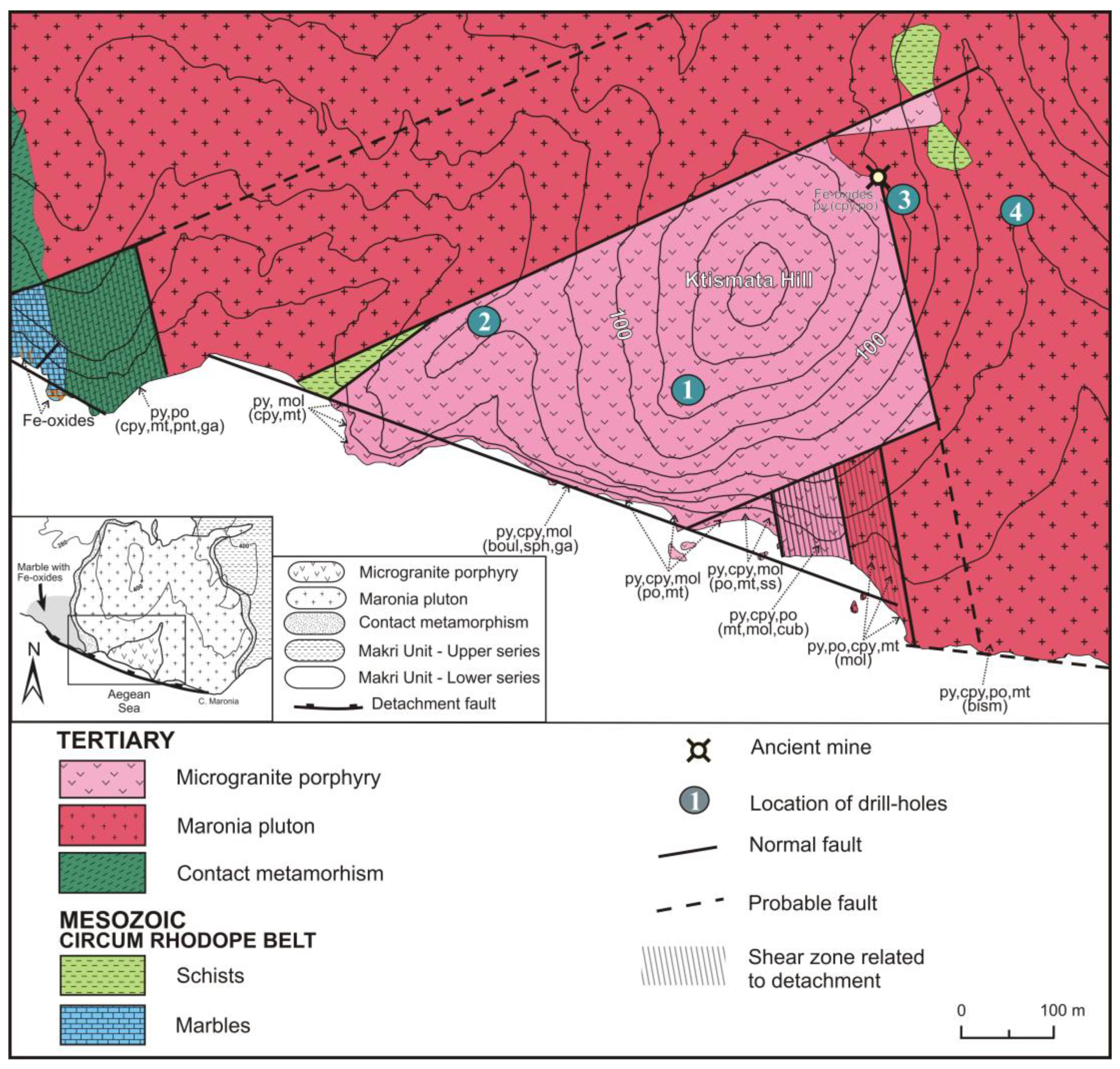
2. Geological Setting
3. Sampling and Analytical Methods
4. Results
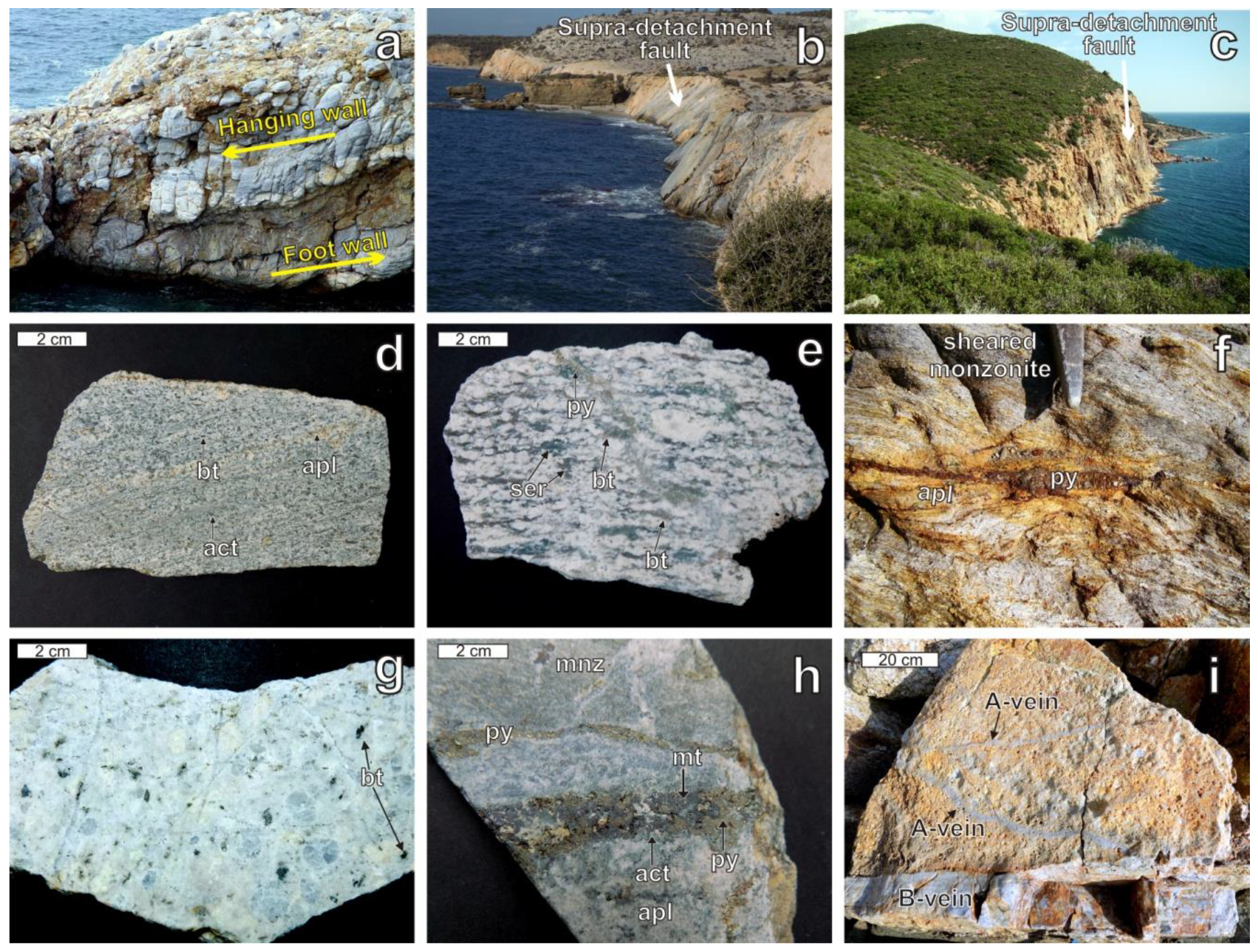
4.1. Structural Control on the Mineralization
4.2. Potassic and Sodic-Calcic Hydrothermal Alteration
4.3. Vein Types
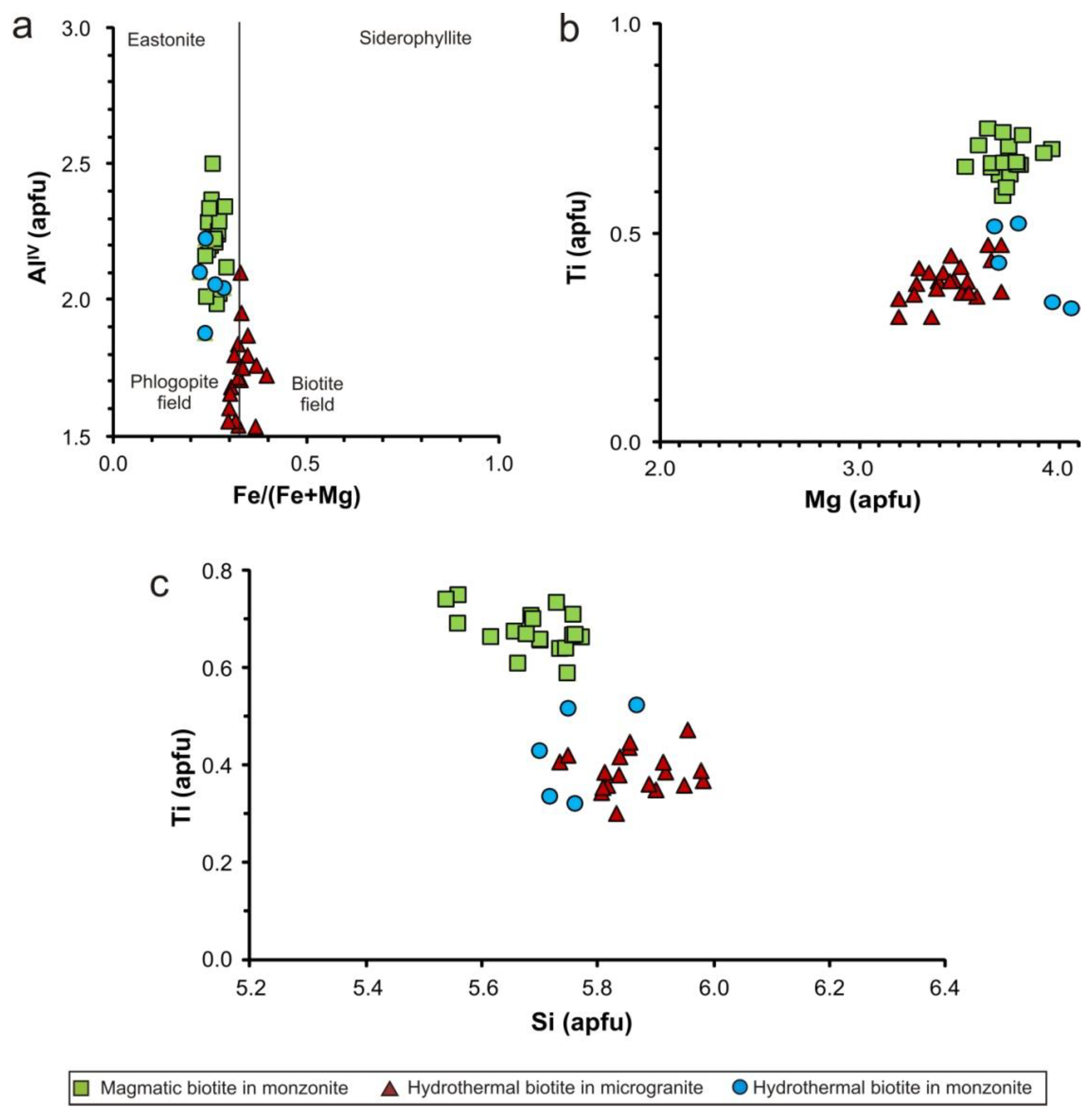
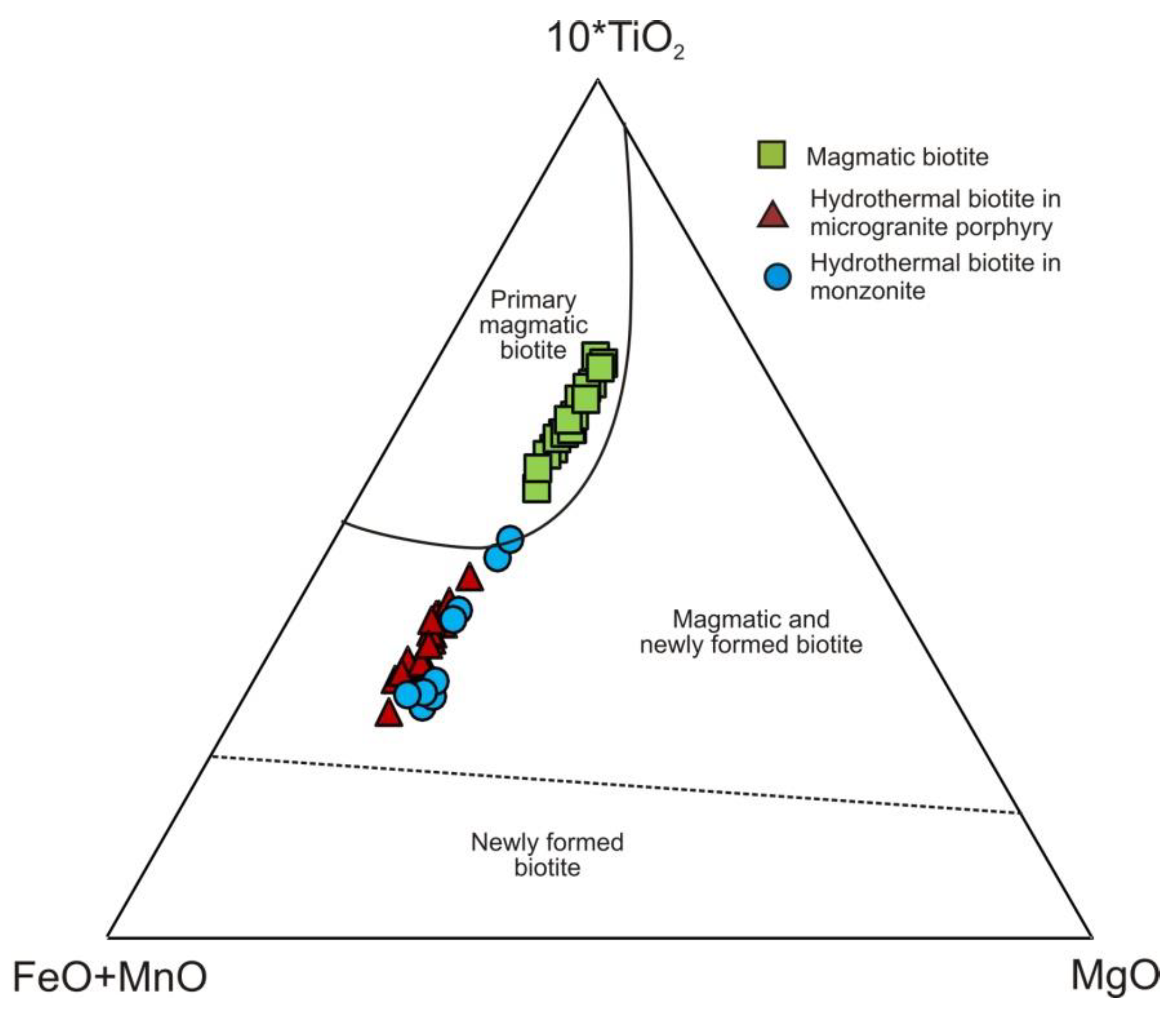
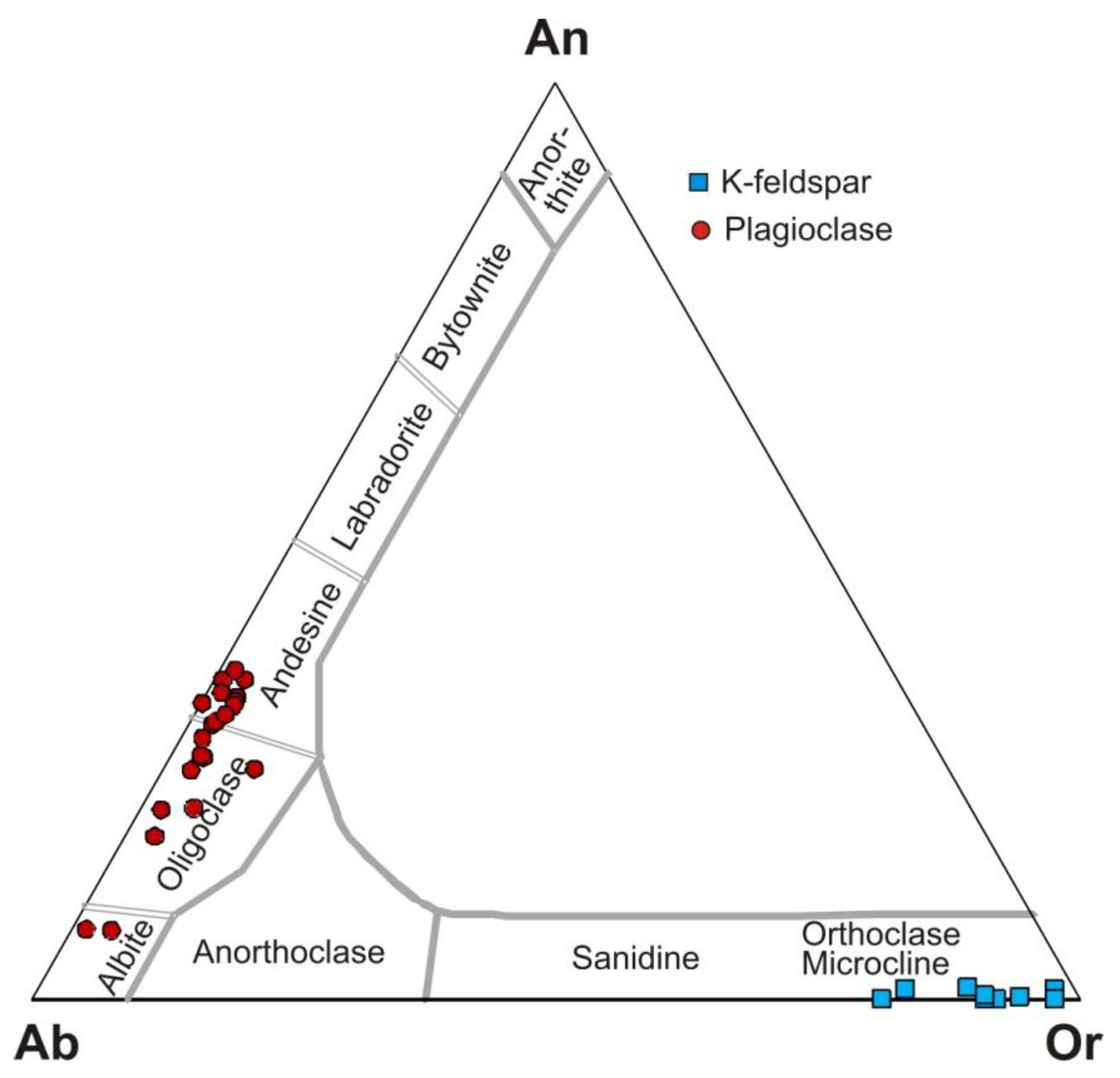
4.4. Mineral Composition and Chemistry of Potassic and Sodic-Calcic Alteration
5. Discussion
5.1. Structural Control of Permeability and Fluid Flow
5.2. Hydrothermal Alteration
5.3. Formation Conditions of Potassic and Potassic/Sodic-Calcic Alteration
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seedorff, E.; Dilles, J.H.; Proffett, J.M., Jr.; Einaudi, M.T.; Zurcher, L.; Stavast, W.J.A.; Johnson, D.A.; Barton, M.D. Porphyry deposits: Characteristics and origin of hypogene features. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Economic Geology Publishing Company: Littleton, CO, USA, 2005; pp. 251–298. [Google Scholar]
- Seedorff, E.; Barton, M.D.; Stavast, W.J.; Maher, D.J. Root zones of porphyry systems: Extending the porphyry model to depth. Econ. Geol. 2008, 103, 939–956. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Lowell, J.D.; Guilbert, J.M. Lateral and vertical alteration-mineralization zoning in porphyry ore deposits. Econ. Geol. 1970, 65, 373–408. [Google Scholar] [CrossRef]
- Beane, R.E.; Titley, S.R. Porphyry copper deposits. Part II. Hydrothermal alteration and mineralization. In Economic Geology 75th Anniversary Volume (1905–1980); Skinner, B.J., Ed.; Economic Geology Publishing Company: Littleton, CO, USA, 1981; pp. 235–269. [Google Scholar]
- Sinclair, W.D. Porphyry deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication: London, UK, 2007; pp. 223–243. [Google Scholar]
- Kesler, S.E.; Chryssoulis, S.L.; Simon, G. Gold in porphyry copper deposits: Its abundance and fate. Ore Geol. Rev. 2002, 21, 103–124. [Google Scholar] [CrossRef]
- Melfos, V.; Voudouris, P. Cenozoic metallogeny of Greece and potential for precious, critical and rare metals exploration. Ore Geol. Rev. 2017, 89, 1030–1057. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Spry, P.G.; Baker, T.; Melfos, V.; Klemd, R.; Haase, K.; Repstock, A.; Djiba, A.; Bismayer, U.; et al. Porphyry and epithermal deposits in Greece: An overview, new discoveries, and mineralogical constraints on their genesis. Ore Geol. Rev. 2019, 107, 654–691. [Google Scholar] [CrossRef]
- Voudouris, P.; Mavrogonatos, C.; Melfos, V.; Spry, P.G.; Magganas, A.; Alfieris, D.; Soukis, K.; Tarantola, A.; Periferakis, A.; Kołodziejczyk, J.; et al. The geology and mineralogy of the Stypsi porphyry Cu-Mo-Au-Re prospect, Lesvos Island, Aegean Sea, Greece. Ore Geol. Rev. 2019, 112, 103023. [Google Scholar] [CrossRef]
- Melfos, V.; Vavelidis, M.; Christofides, G.; Seidel, E. Origin and evolution of the Tertiary Maronia porphyry copper-molybdenum deposit, Thrace, Greece. Miner. Depos. 2002, 37, 648–668. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bindi, L.; Moritz, R.; Ortelli, M.; Kartal, T. Extremely Re-Rich molybdenite from porphyry Cu-Mo-Au prospects in northeastern Greece: Mode of occurrence, causes of enrichment, and implications for gold exploration. Minerals 2013, 3, 165–191. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Kartal, T.; Schleicher, H.; Moritz, R.; Ortelli, M. The Pagoni Rachi/Kirki Cu-Mo-Re-Au-Ag-Te deposit, northern Greece: Mineralogical and fluid inclusion constraints on the evolution of a telescoped porphyry-epithermal system. Can. Mineral. 2013, 51, 411–442. [Google Scholar] [CrossRef]
- Voudouris, P.; Siron, C.R.; Márton, I.; Melfos, V.; Baker, T.; Spry, P.G. Eocene to Miocene hydrothermal deposits of northern Greece and Bulgaria: Relationships between tectonic-magmatic activity, alteration, and gold mineralization—A preface. Soc. Econ. Geol. Guide Ser. 2016, 54, 1–15. [Google Scholar]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Baker, T. Cenozoic porphyry-epithermal and other intrusion-related deposits in northeastern Greece: Geological, mineralogical and geochemical constraints. Soc. Econ. Geol. Guide Ser. 2016, 54, 43–82. [Google Scholar]
- Voudouris, P.C.; Melfos, V.; Baker, T.; Spry, P.G. Diverse styles of Oligocene-Miocene magmatic-hydrothermal deposits in northeastern Greece: Relationships between tectonic-, magmatic activity, alteration and Au-Ag mineralization. Soc. Econ. Geol. Guide Ser. 2016, 54, 83–112. [Google Scholar]
- Mavrogonatos, C.; Voudouris, P.; Berndt, J.; Klemme, S.; Zaccarini, F.; Spry, P.G.; Melfos, V.; Tarantola, Á.; Keith, M.; Klemd, R.; et al. Trace elements in magnetite from the Pagoni Rachi Porphyry prospect, NE Greece: Implications for ore genesis and exploration. Minerals 2019, 9, 725. [Google Scholar] [CrossRef]
- Moshefi, P.; Hosseinzadeh, M.R.; Moayyed, M.; Lentz, D.R. Comparative study of mineral chemistry of four biotite types as geochemical indicators of mineralized and barren intrusions in the Sungun Porphyry Cu-Mo deposit, northwestern Iran. Ore Geol. Rev. 2018, 97, 1–20. [Google Scholar] [CrossRef]
- Runyon, S.E.; Nickerson, P.A.; Seedorff, E.; Barton, M.D.; Mazdab, F.K.; Lecumberri-Sánchez, P.; Steele-MacInnis, M. Sodic-calcic family of alteration in Porphyry systems of Arizona And Adjacent New Mexico. Econ. Geol. 2019, 114, 745–770. [Google Scholar] [CrossRef]
- Menant, A.; Jolivet, L.; Tuduri, J.; Loiselet, C.; Bertrand, G.; Guillou-Frottier, L. 3D subduction dynamics: A first-order parameter of the transition from copper-to gold-rich deposits in the eastern Mediterranean region. Ore Geol. Rev. 2018, 94, 118–135. [Google Scholar] [CrossRef]
- van Hinsbergen, D.J.J.; Hafkenscheid, E.; Spakman, W.; Meulenkamp, J.E.; Wortel, R. Nappe stacking resulting from subduction of oceanic and continental lithosphere below Greece. Geology 2005, 33, 325–328. [Google Scholar] [CrossRef]
- Jolivet, L.; Faccenna, C.; Huet, B.; Labrousse, L.; Le Pourhiet, L.; Lacombe, O.; Lecomte, E.; Burov, E.; Denèle, Y.; Brun, J.-P.; et al. Aegean tectonics: Strain localization, slab tearing and trench retreat. Tectonophysics 2013, 597, 1–33. [Google Scholar] [CrossRef]
- Richards, J.P. Tectonic, magmatic, and metallogenic evolution of the Tethyan orogen: From subduction to collision. Ore Geol. Rev. 2015, 70, 323–345. [Google Scholar] [CrossRef]
- Menant, A.; Jolivet, L.; Vrielynck, B. Kinematic reconstructions and magmatic evolution illuminating crustal and mantle dynamics of the eastern Mediterranean region since the late Cretaceous. Tectonophysics 2016, 675, 103–140. [Google Scholar] [CrossRef]
- Blundell, D.; Arndt, N.; Cobbold, P.R.; Heinrich, C. 9: Processes of tectonism, magmatism and mineralization: Lessons from Europe. Ore Geol. Rev. 2005, 27, 333–349. [Google Scholar] [CrossRef]
- Kaiser-Rohrmeier, M.K.; Von Quadt, A.; Driesner, T.; Heinrich, C.A.; Handler, R.; Ovtcharova, M.; Ivanov, Z.; Petrov, P.; Sarov, S.; Peytcheva, I. Post-orogenic extension and hydrothermal ore formation: High-precision geochronology of the central rhodopian metamorphic core complex (Bulgaria-Greece). Econ. Geol. 2013, 108, 691–718. [Google Scholar] [CrossRef]
- Sánchez, M.G.; McClay, K.R.; King, A.R.; Wijbrams, J.R. Cenozoic crustal extension and its relationship to porphyry Cu-Au-(Mo) and epithermal Au-(Ag) mineralization in the Biga peninsula, northwestern Turkey. In Tectonics and Metallogeny of the Tethyan Orogenic Belt; Richards, J.P., Ed.; Society of Economic Geologists Special Publication: Littleton, CO, USA, 2016; Volume 19, pp. 113–156. [Google Scholar]
- Meinhold, G.; Kostopoulos, D.K. The circum-rhodope belt, northern Greece: Age, provenance, and tectonic setting. Tectonophysics 2013, 595, 55–68. [Google Scholar] [CrossRef]
- Bonev, N.; Marchev, P.; Moritz, R.; Collings, D. Jurassic subduction zone tectonics of the Rhodope Massif in the Thrace region (NE Greece) as revealed by new U-Pb and 40Ar/39Ar geochronology of the Evros ophiolite and high-grade basement rocks. Gondwana Res. 2015, 27, 760–775. [Google Scholar] [CrossRef]
- Papadopoulou, L.; Christofides, G.; Bröcker, M.; Koroneos, Á.; Soldatos, T.; Eleftheriadis, G. Petrology, geochemistry and isotopic characteristics of the shoshonitic plutonic rocks from Maronia area, west Thrace, Greece. Bull. Geol. Soc. Greece 2001, 34, 967–976. [Google Scholar] [CrossRef][Green Version]
- Papadopoulou, L.; Christofides, G.; Koroneos, Á.; Bröcker, M.; Soldatos, T.; Eleftheriadis, G. Evolution and origin of the Maronia pluton, Thrace, Greece. Bull. Geol. Soc. Greece 2004, 36, 568–577. [Google Scholar] [CrossRef]
- Perkins, R.J.; Cooper, F.J.; Condon, D.J.; Tattitch, B.; Naden, J. Post-collisional Cenozoic extension in the northern Aegean: The high-K to shoshonitic intrusive rocks of the Maronia Magmatic Corridor, northeastern Greece. Lithosphere 2018, 10, 582–601. [Google Scholar] [CrossRef]
- Kyriakopoulos, K. A Geochronological, Geochemical and Mineralogical Study of Some Tertiary Plutonic Rocks of the Rhodope Massif and Their Isotopic Characteristics. Ph.D. Thesis, University of Athens, Athens, Greece, 1987. (In Greek). [Google Scholar]
- Del Moro, A.; Innocenti, F.; Kyriakopoulos, C.; Manetti, P.; Papadopoulos, P. Tertiary granitoids from Thrace (Northern Greece): Sr isotopic and petrochemical data. Neues Jahrb. Mineral. Abh. 1988, 159, 113–135. [Google Scholar]
- Biggazzi, G.; Del Moro, A.; Innocenti, F.; Kyriakopoulos, K.; Manetti, P.; Papadopoulos, P.; Norelli, P.; Magganas, A. The magmatic intrusive complex of Petrota, West Thrace: Age and geodynamic significance. Geol. Rhodopica 1989, 1, 290–297. [Google Scholar]
- Mposkos, E.D.; Kostopoulos, D.K. Diamond, former coesite and supersilicic garnet in metasedimentary rocks from the Greek Rhodope: A new ultrahigh-pressure metamorphic province established. Earth Planet. Sci. Lett. 2001, 192, 497–506. [Google Scholar] [CrossRef]
- Carten, R.B. Sodium-calcium metasomatism; chemical, temporal, and spatial relationships at the Yerington, Nevada, porphyry copper deposit. Econ. Geol. 1986, 81, 1495–1519. [Google Scholar] [CrossRef]
- Dilles, J.H.; Einaudi, M.T. Wall-rock alteration and hydrothermal flow paths about the Ann-Mason porphyry copper deposit, Nevada; a 6-km vertical reconstruction. Econ. Geol. 1992, 87, 1963–2001. [Google Scholar] [CrossRef]
- Koděra, P.; Lexa, J.; Fallick, A.E. Formation of the Vysoká-Zlatno Cu-Au skarn-porphyry deposit, Slovakia. Miner. Depos. 2010, 45, 817–843. [Google Scholar] [CrossRef]
- Lang, X.; Tang, J.; Li, Z.; Huang, Y.; Ding, F.; Yang, H.; Xie, F.; Zhang, L.; Wang, Q.; Zhou, Y. U-Pb and Re-Os geochronological evidence for the Jurassic porphyry metallogenic event of the Xiongcun district in the Gangdese porphyry copper belt, southern Tibet, PRC. J. Asian Earth Sci. 2014, 79, 608–622. [Google Scholar] [CrossRef]
- Melfos, V.; Vavelidis, M.; Christofides, G. Cu-Pb-(Sb + As) sulphosalts from the porphyry type Cu-Mo (±Au) mineralization in Maronia area, Thrace county, Greece. In Terranes of Serbia: The Formation of the Geologic Framework of Serbia and the Adjacent Regions; Knezevic, V., Krstic, B., Eds.; Univeristy of Belgarde: Belgrade, Serbia, 1996; pp. 305–310. [Google Scholar]
- Papadopoulou, L. Mineral Phase Equilibria, Crystallization Conditions and Evolution of the Maronia Pluton, Thrace, Greece. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2003. (In Greek). [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals, 2nd ed.; Longman: London, UK, 1992; p. 696. [Google Scholar]
- Jacobs, D.C.; Parry, W.T. Geochemistry of biotite in the Santa Rita porphyry copper deposit, New Mexico. Econ. Geol. 1979, 74, 860–887. [Google Scholar] [CrossRef]
- Selby, D.; Nesbitt, B.E. Chemical composition of biotite from the Casino porphyry Cu-Au-Mo mineralization, Yukon, Canada: Evaluation of magmatic and hydrothermal fluid chemistry. Chem. Geol. 2000, 171, 77–93. [Google Scholar] [CrossRef]
- Nachit, H.; Ibhi, A.; Abia, E.H.; Ohoud, M.B. Discrimination between primary magmatic biotites, reequilibrated biotites and neoformed biotites. Comptes Rendus Geosci. 2005, 337, 1415–1420. [Google Scholar] [CrossRef]
- Boomeri, M.; Nakashima, K.; Lentz, D.R. The Miduk porphyry Cu deposit, Kerman, Iran: A geochemical analysis of the potassic zone including halogen element systematics related to Cu mineralization processes. J. Geochem. Explor. 2009, 103, 17–29. [Google Scholar] [CrossRef]
- Rabbia, O.M.; Hernández, L.B.; French, D.H.; King, R.W.; Ayers, J.C. The El Teniente porphyry Cu-Mo deposit from a hydrothermal rutile perspective. Miner. Depos. 2009, 44, 849. [Google Scholar] [CrossRef]
- Rabbia, O.M.; Hernández, L.B. Mineral chemistry and potential applications of natural-multi-doped hydrothermal rutile from porphyry copper deposits. In Rutile: Properties, Synthesis and Application; Low, I.-M., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 209–228. [Google Scholar]
- Schumacher, J.C. The estimation of the proportion of ferric iron in the electron-microprobe analysis of amphiboles. Can. Mineral. 1997, 35, 238–246. [Google Scholar]
- Weis, P.; Driesner, T. The interplay of non-static permeability and fluid flow as a possible pre-requisite for supercritical geothermal resources. Energy Procedia 2013, 40, 102–106. [Google Scholar] [CrossRef]
- Weis, P.; Driesner, T.; Coumou, D.; Geiger, S. Hydrothermal, multiphase convection of H2O-NaCl fluids from ambient to magmatic temperatures: A new numerical scheme and benchmarks for code comparison. Geofluids 2014, 14, 347–371. [Google Scholar] [CrossRef]
- Weis, P. The dynamic interplay between saline fluid flow and rock permeability in magmatic-hydrothermal systems. Geofluids 2015, 15, 350–371. [Google Scholar] [CrossRef]
- Fossen, H.; Cavalcante, G.C.G. Shear zones—A review. Earth Sci. Rev. 2017, 171, 434–455. [Google Scholar] [CrossRef]
- Reynolds, S.J.; Lister, G.S. Structural aspects of fluid-rock interactions in detachment zones. Geology 1987, 15, 362–366. [Google Scholar] [CrossRef]
- Richards, J.P. Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol. Rev. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Halley, S.; Dilles, J.H.; Tosdal, R.M. Footprints: Hydrothermal alteration and geochemical dispersion around porphyry copper deposits. Soc. Econ. Geol. Newsl. 2015, 100, 12–17. [Google Scholar]
- Dilles, J.H. Petrology of the Yerington Batholith, Nevada: Evidence for evolution of porphyry copper ore fluids. Econ. Geol. 1987, 82, 1750–1789. [Google Scholar] [CrossRef]
- Reynolds, T.J.; Beane, R.E. Evolution of hydrothermal fluid characteristics at the Santa Rita, New Mexico, porphyry copper deposit. Econ. Geol. 1985, 80, 1328–1347. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system: Far Southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Klemm, L.M.; Pettke, T.; Heinrich, C.A.; Campos, E. Hydrothermal evolution of the El Teniente deposit, Chile: Porphyry Cu-Mo ore deposition from low-salinity magmatic fluids. Econ. Geol. 2007, 102, 1021–1045. [Google Scholar] [CrossRef]
- Hendry, D.A.F.; Chivas, A.R.; Long, J.V.P.; Reed, S.J.B. Chemical differences between minerals from mineralizing and barren intrusions from some North American porphyry copper deposits. Contrib. Mineral. Petrol. 1985, 89, 317–329. [Google Scholar] [CrossRef]
- Bowman, J.R.; Parry, W.T.; Kropp, W.P.; Kruer, S.A. Chemical and isotopic evolution of hydrothermal solutions at Bingham, Utah. Econ. Geol. 1987, 82, 395–428. [Google Scholar] [CrossRef]
- Voudouris, P. Mineralogical, Geochemical and Fluid Inclusion Studies on Epithermal Vein Type Gold/Silver Mineralizations at Kassiteres/Sapes, (NE-Greece). Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1993. [Google Scholar]
- Voudouris, P. Mineral composition of mafic minerals and ore deposition from the Kassiteres (Sappes) and Pagoni Rachi (Kirki) porphyry Cu-Mo prospects/W.Thrace. Bull. Geol. Soc. Greece 2001, 34, 1005–1013. (In Greek) [Google Scholar]
- Mavrogonatos, C.; Voudouris, P.; Spry, P.; Melfos, V.; Klemme, S.; Berndt, J.; Periferakis, A. Biotite chemistry from porphyry-style mineralization in Western Thrace, Greece. In Proceedings of the 8th Geochemistry Symposium, Antalya, Turkey, 2–6 May 2018; p. 193. [Google Scholar]
- Moore, W.J.; Czamanske, G.K. Compositions of biotites from unaltered and altered monzonitic rocks in the Bingham Mining District, Utah. Econ. Geol. 1973, 68, 269–274. [Google Scholar] [CrossRef]
- Taylor, R.P. Comparison of biotite geochemistry of Bakircay, Turkey, and Los Pelambres, Chile, porphyry copper systems. Inst. Min. Metall. Trans. 1983, 92, B16–B22. [Google Scholar]
- Brimhall, G.H.; Crecar, D.A. Ore fluids: Magmatic to supergene. In Thermodynamic Modeling of Geologic Materials. Minerals, Fluids, and Melts; Carmichael, I.S.E., Eugster, H., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1987; Volume 17, pp. 235–321. [Google Scholar]
- Chivas, A.R. Geochemical evidence for magmatic fluids in porphyry copper mineralization. Contrib. Mineral. Petrol. 1982, 78, 389–403. [Google Scholar] [CrossRef]
- Czamanske, G.K.; Force, E.R.; Moore, W.J. Some geologic and potential resource aspects of rutile in porphyry copper deposits. Econ. Geol. 1981, 76, 2240–2246. [Google Scholar] [CrossRef]
- Li, J.L.; Gao, J.; Klemd, R.; John, T.; Su, W. Fluid-mediated metal transport in subduction zones and its link to arc-related giant ore deposits: Constraints from a sulfide-bearing HP vein in lawsonite eclogite (Tianshan, China). Geochim. Cosmochim. Acta 2013, 120, 326–362. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and its applications in earth sciences. Earth Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Ford, J.H. A chemical study of alteration at the Panguna porphyry copper deposit, Bougainville, Papua New Guinea. Econ. Geol. 1978, 73, 703–720. [Google Scholar] [CrossRef]
- Cannell, J.; Cooke, D.R.; Walshe, J.L.; Stein, H. Geology, mineralization, alteration, and structural evolution of the El Teniente porphyry Cu-Mo deposit. Econ. Geol. 2005, 100, 979–1003. [Google Scholar] [CrossRef]
- Celis, A. Titanite as an Indicator Mineral for Alkalic Cu-Au Porphyry Deposits in South Central British Columbia. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2015. [Google Scholar]
- Arancibia, O.N.; Clark, A.H. Early magnetite-amphibole-plagioclase alteration-mineralization in the Island copper porphyry copper-gold-molybdenum deposit, British Columbia. Econ. Geol. 1996, 91, 402–438. [Google Scholar] [CrossRef]
- Battles, D.A.; Barton, M.D. Arc-related sodic hydrothermal alteration in the western United State. Geology 1995, 23, 913–916. [Google Scholar] [CrossRef]
- Perring, C.S.; Pollard, P.J.; Dong, G.; Nunn, A.J.; Blake, K.L. The lightning creek sill complex, Cloncurry district, northwest Queensland: A source of fluids for Fe oxide Cu-Au mineralization and sodic-calcic alteration. Econ. Geol. 2000, 95, 1067–1089. [Google Scholar] [CrossRef]
- Chelle-Michou, C.; Chiaradia, M.; Ovtcharova, M.; Ulianov, A.; Wotzlaw, J.F. Zircon petrochronology reveals the temporal link between porphyry systems and the magmatic evolution of their hidden plutonic roots (the Eocene Coroccohuayco deposit, Peru). Lithos 2014, 198, 129–140. [Google Scholar] [CrossRef]
- Proffett, J.M. Geology of the Bajo de la Alumbrera porphyry copper-gold deposit, Argentina. Econ. Geol. 2003, 98, 1535–1574. [Google Scholar] [CrossRef]
- Hendry, D.A.F.; Gunow, A.J.; Smith, R.P.; Reed, S.J.B.; Long, J.V.P. Chemical differences between minerals from mineralizing and barren intrusions associated with molybdenum mineralization at Climax, Colorado. Mineral. Petrol. 1988, 39, 251–263. [Google Scholar] [CrossRef]
- Lerchbaumer, L.; Audétat, A. High Cu concentrations in vapor-type fluid inclusions: An artifact? Geochim. Cosmochim. Acta 2012, 88, 255–274. [Google Scholar] [CrossRef]
- Whitney, J.A.; Hemley, J.J.; Simon, F.O. The concentration of iron in chloride solutions equilibrated with synthetic granitic compositions: The sulfur-free system. Econ. Geol. 1985, 80, 444–460. [Google Scholar] [CrossRef]
- Burnham, C.W. Magmas and hydrothermal fluids. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1997; pp. 63–123. [Google Scholar]
- Seo, J.H.; Guillong, M.; Heinrich, C.A. Separation of molybdenum and copper in porphyry deposits: The roles of sulfur, redox, and pH in ore mineral deposition at Bingham Canyon. Econ. Geol. 2012, 107, 333–356. [Google Scholar] [CrossRef]

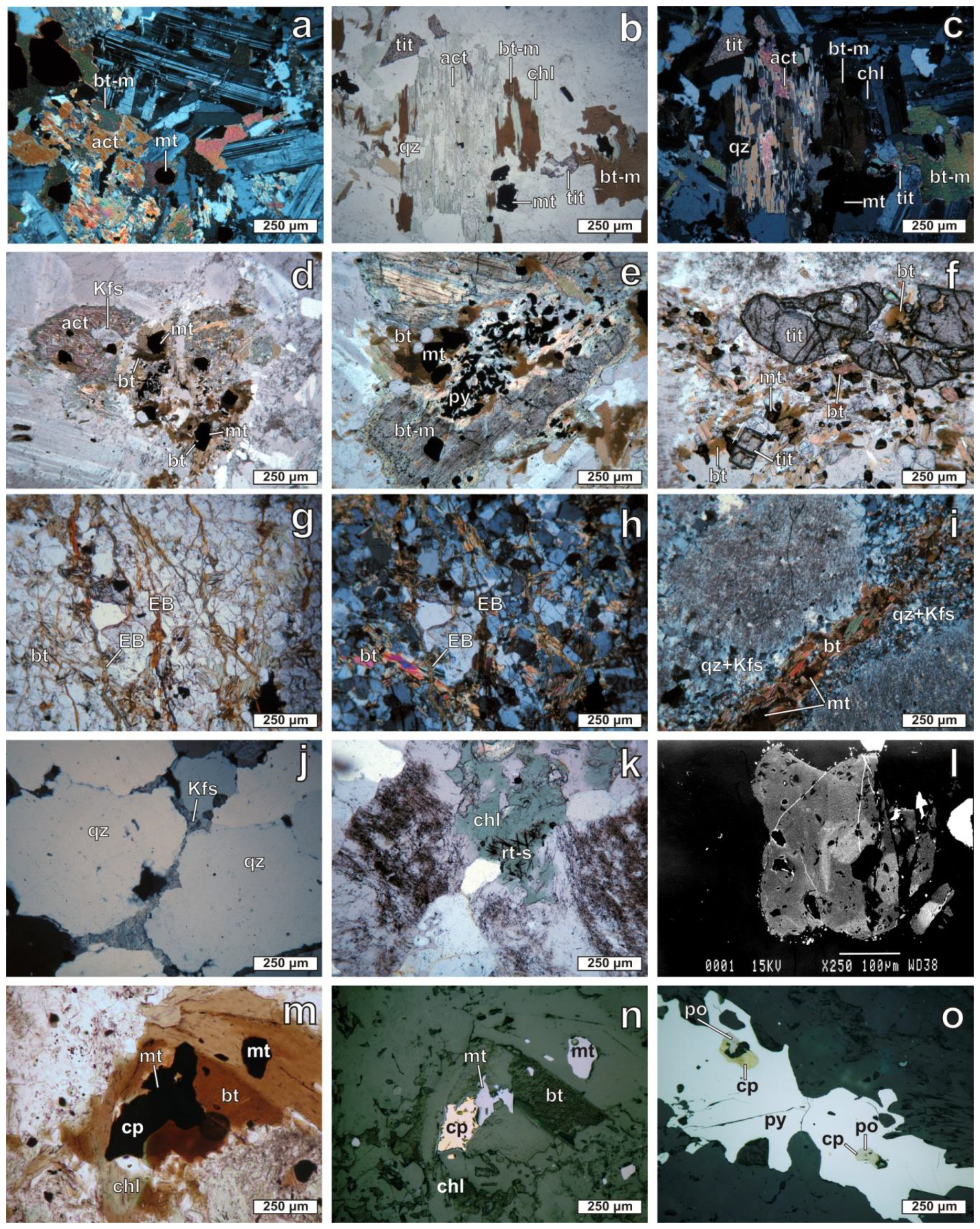
| Associated Vein Types | Alteration Type | Host Rock | Texture | Vein Assemblage | Sulfide Assemblage |
|---|---|---|---|---|---|
| M-type | Sodic-calcic | Monzonite | <5 cm wide | act + pl+ mt + tit ± cal ± ep | py |
| EB-type | Potassic | Monzonite and microgranite porphyry | 1–5 mm wide, uninterrupted sinuous veins | bt + mt | - |
| A-type | Potassic | Microgranite porphyry | 1–3 cm wide, uninterrupted sinuous veins-stockworks | bt + Kfs + mt + qz + rt ± ap | cpy +po + py |
| B-type | Potassic | Microgranite porphyry | 1–3 cm wide, continuous straight veins-stockworks | bt + Kfs + mt + qz | cpy +mol + py |
| D-type | Sericitic | Microgranite porphyry | <10 cm wide, continuous straight veins | qz + ser | py |
| Epithermal-type | Advanced argillic | Microgranite porphyry | 1–5 cm wide, continuous straight veins | qz + pyr | py + sph + ga + ss |
| Biotite Type | Magmatic Biotite | Hydrothermal Biotite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | MA 8b | MA 11 | MR 1 | MR 8b | ||||||||
| 8 | 9 | 14 | 15 | 1 | 10 | 15 | 22 | 3 | 5 | 1 | 10 | |
| SiO2 | 38.96 | 39.19 | 38.85 | 36.27 | 38.61 | 39.47 | 38.54 | 39.73 | 38.74 | 38.19 | 39.04 | 40.17 |
| TiO2 | 5.25 | 5.90 | 6.10 | 6.80 | 3.02 | 2.64 | 3.25 | 4.22 | 3.00 | 3.83 | 4.45 | 4.66 |
| Al2O3 | 14.52 | 14.83 | 14.40 | 15.31 | 13.84 | 14.14 | 14.22 | 13.33 | 14.64 | 14.70 | 13.78 | 14.74 |
| Cr2O3 | 0.44 | 0.86 | 0.04 | 0.04 | bdl | 0.16 | 0.24 | bdl | 0.24 | 0.35 | 1.07 | 0.37 |
| FeO* | 9.69 | 9.82 | 10.68 | 11.47 | 16.62 | 14.43 | 13.72 | 12.22 | 9.99 | 11.74 | 10.60 | 8.88 |
| MnO | 0.39 | 0.14 | 0.07 | 0.21 | 0.18 | 0.59 | 0.37 | 0.48 | 0.61 | 0.54 | bdl | 0.28 |
| MgO | 17.25 | 16.85 | 16.84 | 15.49 | 14.03 | 15.32 | 14.22 | 15.60 | 18.22 | 16.45 | 17.31 | 17.53 |
| CaO | 0.18 | 0.01 | 0.03 | 0.13 | bdl | bdl | bdl | 0.13 | 0.08 | 0.25 | 0.46 | bdl |
| Na2O | 0.39 | 0.32 | 0.06 | 0.37 | 0.43 | 0.23 | 0.02 | bdl | 0.26 | 0.20 | 0.36 | 0.20 |
| K2O | 9.47 | 8.73 | 9.47 | 9.01 | 9.58 | 9.56 | 10.06 | 9.52 | 9.09 | 9.76 | 9.30 | 9.29 |
| F | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Cl | 0.01 | bdl | 0.05 | 0.23 | 0.11 | 0.02 | 0.10 | 0.30 | bdl | 0.19 | 0.09 | bdl |
| O = F, Cl | 0.00 | 0.00 | 0.01 | 0.05 | 0.02 | 0.00 | 0.02 | 0.07 | 0.00 | 0.04 | 0.02 | 0.00 |
| Total initial | 96.55 | 96.65 | 96.59 | 95.33 | 96.42 | 96.56 | 94.74 | 95.53 | 94.87 | 96.20 | 96.46 | 96.12 |
| Fe2O3 calc | 7.56 | 6.97 | 6.90 | 9.13 | 12.25 | 10.50 | 11.37 | 7.37 | 6.12 | 6.65 | 7.49 | 6.76 |
| FeO calc | 2.89 | 3.55 | 4.47 | 3.25 | 5.60 | 4.98 | 3.49 | 5.59 | 4.48 | 5.76 | 3.86 | 2.80 |
| H2O calc | 2.39 | 2.31 | 2.16 | 2.20 | 3.01 | 3.07 | 3.02 | 2.71 | 2.91 | 2.67 | 2.49 | 2.64 |
| Total calc | 99.70 | 99.66 | 99.43 | 98.39 | 100.63 | 100.68 | 98.87 | 98.91 | 98.15 | 99.50 | 99.68 | 99.44 |
| Chemical formulae calculated on the basis of 22 (O, OH, Cl) anions | ||||||||||||
| Si | 5.746 | 5.755 | 5.759 | 5.557 | 5.805 | 5.831 | 5.835 | 5.954 | 5.715 | 5.698 | 5.747 | 5.865 |
| AlIV | 2.289 | 2.215 | 2.214 | 2.346 | 1.724 | 1.872 | 1.798 | 1.556 | 2.228 | 2.046 | 2.060 | 2.105 |
| Fe3+ | 0.000 | 0.030 | 0.026 | 0.097 | 0.470 | 0.298 | 0.366 | 0.490 | 0.056 | 0.256 | 0.193 | 0.029 |
| Z | 8.034 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 |
| AlVI | 0.235 | 0.352 | 0.302 | 0.420 | 0.728 | 0.590 | 0.739 | 0.797 | 0.316 | 0.538 | 0.331 | 0.431 |
| Mg | 3.713 | 3.657 | 3.717 | 3.638 | 3.192 | 3.357 | 3.281 | 3.640 | 3.962 | 3.692 | 3.672 | 3.791 |
| Fe2+ | 0.839 | 0.770 | 1.067 | 1.048 | 1.384 | 1.168 | 1.293 | 0.829 | 0.743 | 0.873 | 0.707 | 0.743 |
| Fe3+ | 0.356 | 0.406 | 0.231 | 0.324 | 0.235 | 0.317 | 0.078 | 0.213 | 0.432 | 0.337 | 0.405 | 0.312 |
| Ti | 0.591 | 0.669 | 0.670 | 0.752 | 0.344 | 0.301 | 0.380 | 0.473 | 0.337 | 0.431 | 0.518 | 0.525 |
| Cr | 0.052 | 0.100 | 0.004 | 0.005 | 0.000 | 0.019 | 0.029 | 0.000 | 0.000 | 0.041 | 0.124 | 0.043 |
| Mn | 0.048 | 0.017 | 0.008 | 0.027 | 0.023 | 0.074 | 0.048 | 0.061 | 0.077 | 0.068 | 0.000 | 0.034 |
| Y | 5.834 | 5.971 | 6.000 | 6.214 | 5.906 | 5.826 | 5.848 | 6.013 | 5.867 | 5.979 | 5.756 | 5.880 |
| K | 1.831 | 1.680 | 1.778 | 1.663 | 1.758 | 1.805 | 1.887 | 1.693 | 1.762 | 1.813 | 1.826 | 1.755 |
| Na | 0.111 | 0.090 | 0.016 | 0.110 | 0.125 | 0.066 | 0.005 | 0.000 | 0.073 | 0.059 | 0.102 | 0.055 |
| Ca | 0.028 | 0.001 | 0.004 | 0.021 | 0.000 | 0.000 | 0.000 | 0.021 | 0.013 | 0.040 | 0.073 | 0.000 |
| X | 1.970 | 1.772 | 1.799 | 1.793 | 1.883 | 1.871 | 1.893 | 1.714 | 1.848 | 1.912 | 2.002 | 1.811 |
| Cl | 0.002 | 0.000 | 0.012 | 0.061 | 0.029 | 0.005 | 0.026 | 0.077 | 0.000 | 0.049 | 0.022 | 0.000 |
| OH | 2.356 | 2.267 | 2.488 | 2.252 | 3.023 | 3.029 | 3.050 | 2.709 | 2.897 | 2.730 | 2.381 | 2.579 |
| O2- | 1.642 | 1.733 | 1.500 | 1.687 | 0.948 | 0.966 | 0.924 | 1.214 | 1.103 | 1.221 | 1.597 | 1.421 |
| Mg/(Mg + Fet) | 0.76 | 0.75 | 0.74 | 0.71 | 0.60 | 0.65 | 0.65 | 0.70 | 0.76 | 0.72 | 0.74 | 0.78 |
| Fet/(Mg + Fet) | 0.24 | 0.25 | 0.26 | 0.29 | 0.40 | 0.35 | 0.35 | 0.30 | 0.24 | 0.28 | 0.26 | 0.22 |
| Sample | MA 8b | MA 8b | MA 8b | MA 11 | MA 11 | MA 11 |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| SiO2 | 62.44 | 62.81 | 63.04 | 64.83 | 64.85 | 62.65 |
| TiO2 | bdl | bdl | bdl | bdl | bdl | bdl |
| Al2O3 | 18.79 | 18.87 | 18.45 | 18.10 | 17.93 | 18.60 |
| FeO* | 0.23 | 0.24 | 0.55 | 0.21 | 0.42 | 0.36 |
| MnO | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO | bdl | bdl | bdl | bdl | bdl | bdl |
| CaO | 0.26 | bdl | bdl | 0.05 | bdl | 0.21 |
| Na2O | 1.11 | 2.10 | 0.90 | 0.65 | 1.02 | 0.23 |
| K2O | 13.84 | 12.53 | 14.61 | 15.91 | 15.17 | 15.42 |
| BaO | 2.85 | 3.13 | 2.50 | bdl | 0.42 | 2.45 |
| Total | 99.52 | 99.68 | 100.05 | 99.75 | 99.81 | 99.92 |
| Chemical formulae calculated on the basis of 8 O anions | ||||||
| Si | 2.944 | 2.947 | 2.957 | 3.002 | 3.003 | 2.950 |
| Ti | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Al | 1.044 | 1.043 | 1.020 | 0.988 | 0.978 | 1.032 |
| Fe3+ | 0.009 | 0.009 | 0.022 | 0.008 | 0.016 | 0.014 |
| Z | 3.997 | 3.999 | 3.998 | 3.998 | 3.998 | 3.997 |
| Mn | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.013 | 0.000 | 0.000 | 0.003 | 0.000 | 0.010 |
| Na | 0.101 | 0.191 | 0.082 | 0.058 | 0.092 | 0.021 |
| K | 0.832 | 0.750 | 0.874 | 0.940 | 0.896 | 0.926 |
| Ba | 0.053 | 0.058 | 0.046 | 0.000 | 0.008 | 0.045 |
| X | 0.999 | 0.998 | 1.002 | 1.001 | 0.996 | 1.002 |
| Or (mol. %) | 89 | 81 | 92 | 94 | 91 | 97 |
| Ab (mol. %) | 10 | 19 | 8 | 6 | 9 | 2 |
| An (mol. %) | 1 | 0 | 0 | 0 | 0 | 1 |
| Sample | Mar 80/7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| SiO2 | 0.26 | 0.92 | 0.68 | 0.14 | 0.75 | 0.84 | 1.29 | 1.11 | 0.30 | 0.11 |
| TiO2 | 96.91 | 93.88 | 94.94 | 94.52 | 94.22 | 94.43 | 92.92 | 92.90 | 96.70 | 98.03 |
| Al2O3 | 0.11 | 0.13 | 0.19 | 0.03 | 0.24 | 0.14 | 0.16 | 0.28 | 0.11 | 0.21 |
| FeO | 1.10 | 1.83 | 1.55 | 1.73 | 1.85 | 1.72 | 1.87 | 1.87 | 0.88 | 0.98 |
| MnO | 0.67 | 0.02 | bdl | bdl | 0.01 | bdl | 0.01 | 0.23 | 0.23 | 0.13 |
| MgO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| CaO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Na2O | bdl | 0.14 | 0.17 | bdl | bdl | bdl | 0.03 | 0.02 | bdl | 0.01 |
| K2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| PbO | 0.10 | 0.05 | bdl | 0.03 | bdl | bdl | 0.16 | bdl | bdl | 0.01 |
| V2O5 | bdl | bdl | bdl | bdl | 0.12 | bdl | 0.29 | 0.21 | bdl | 0.26 |
| SrO | 0.52 | 1.26 | 1.40 | bdl | 1.81 | 2.20 | 2.53 | 3.63 | 1.12 | 0.40 |
| Y2O3 | bdl | bdl | 0.12 | bdl | bdl | 0.02 | bdl | bdl | 0.12 | bdl |
| Nb2O5 | 0.38 | 1.01 | 1.12 | 1.15 | 1.01 | 0.20 | 0.34 | 0.17 | 0.49 | 0.36 |
| WO3 | bdl | bdl | 0.30 | 2.33 | bdl | bdl | bdl | bdl | bdl | bdl |
| Total | 100.05 | 99.24 | 100.47 | 99.93 | 100.01 | 99.55 | 99.6 | 100.42 | 99.95 | 100.50 |
| Chemical formulae calculated on the basis of 2 O anions | ||||||||||
| Si | 0.003 | 0.013 | 0.009 | 0.002 | 0.010 | 0.011 | 0.018 | 0.015 | 0.004 | 0.001 |
| Ti | 0.983 | 0.969 | 0.972 | 0.988 | 0.968 | 0.968 | 0.956 | 0.953 | 0.983 | 0.985 |
| Fe | 0.012 | 0.021 | 0.018 | 0.020 | 0.021 | 0.020 | 0.021 | 0.021 | 0.010 | 0.011 |
| Sr | 0.004 | 0.010 | 0.011 | 0.000 | 0.014 | 0.017 | 0.020 | 0.029 | 0.009 | 0.003 |
| Nb | 0.002 | 0.006 | 0.007 | 0.007 | 0.006 | 0.001 | 0.002 | 0.001 | 0.003 | 0.002 |
| W | 0.000 | 0.000 | 0.001 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Samle | MA 8 | MA 8 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | 4 | 5 | 6 | |
| SiO2 | 54.96 | 55.12 | 55.67 | 54.99 | 55.10 | 55.02 | 55.06 | 55.07 |
| TiO2 | 0.11 | 0.70 | 0.23 | 0.56 | 0.17 | 0.54 | 0.41 | 0.22 |
| Al2O3 | 2.16 | 1.87 | 1.96 | 2.09 | 1.89 | 1.97 | 2.11 | 1.91 |
| FeO* | 6.65 | 6.84 | 6.98 | 6.84 | 6.89 | 7.23 | 6.78 | 7.45 |
| MnO | 0.49 | 0.17 | 0.31 | 0.23 | 0.29 | 0.17 | 0.39 | 0.24 |
| MgO | 19.68 | 19.82 | 19.77 | 19.70 | 19.78 | 19.80 | 19.69 | 19.82 |
| CaO | 12.41 | 13.80 | 12.89 | 13.23 | 13.76 | 12.55 | 12.69 | 13.45 |
| Na2O | 0.48 | 0.19 | 0.31 | 0.42 | 0.28 | 0.25 | 0.22 | 0.35 |
| K2O | bdl | 0.20 | bdl | 0.10 | bdl | 0.11 | bdl | 0.07 |
| Cl | 0.11 | 0.02 | 0.07 | 0.08 | 0.06 | 0.02 | 0.11 | 0.02 |
| Total initial | 97.03 | 98.73 | 98.17 | 98.22 | 98.21 | 97.66 | 97.44 | 98.60 |
| Final wt. % values | ||||||||
| MnO | 0.49 | 0.17 | 0.31 | 0.23 | 0.29 | 0.17 | 0.39 | 0.24 |
| FeO | 5.12 | 6.84 | 5.38 | 6.24 | 6.89 | 6.07 | 5.67 | 5.62 |
| Fe2O3 | 1.70 | 0.00 | 1.78 | 0.67 | 0.00 | 1.29 | 1.24 | 2.04 |
| H2O+ | 2.12 | 2.13 | 2.13 | 2.12 | 2.12 | 2.14 | 2.12 | 2.13 |
| Total calc | 99.32 | 100.86 | 100.48 | 100.41 | 100.33 | 99.93 | 99.68 | 100.93 |
| Chemical formulae calculated on the basis of 23 O anions | ||||||||
| Si | 7.726 | 7.678 | 7.736 | 7.679 | 7.713 | 7.703 | 7.717 | 7.658 |
| Al | 0.274 | 0.307 | 0.264 | 0.321 | 0.287 | 0.297 | 0.283 | 0.313 |
| Ti | 0.000 | 0.015 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.023 |
| Fe3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 |
| T | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 |
| Ti | 0.012 | 0.059 | 0.024 | 0.059 | 0.018 | 0.057 | 0.043 | 0.000 |
| Al | 0.083 | 0.000 | 0.057 | 0.023 | 0.025 | 0.028 | 0.066 | 0.000 |
| Fe3+ | 0.180 | 0.000 | 0.187 | 0.070 | 0.000 | 0.136 | 0.130 | 0.207 |
| Fe2+ | 0.601 | 0.797 | 0.625 | 0.729 | 0.807 | 0.647 | 0.646 | 0.653 |
| Mn2+ | 0.000 | 0.020 | 0.012 | 0.018 | 0.023 | 0.000 | 0.000 | 0.028 |
| Mg | 4.124 | 4.116 | 4.096 | 4.101 | 4.128 | 4.132 | 4.114 | 4.109 |
| C | 5.000 | 4.992 | 5.001 | 5.000 | 5.001 | 5.000 | 4.999 | 4.997 |
| Mn2+ | 0.058 | 0.000 | 0.025 | 0.009 | 0.011 | 0.020 | 0.046 | 0.000 |
| Fe2+ | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.063 | 0.018 | 0.000 |
| Ca | 1.869 | 2.000 | 1.919 | 1.980 | 1.989 | 1.883 | 1.906 | 2.000 |
| Na | 0.072 | 0.000 | 0.056 | 0.011 | 0.000 | 0.034 | 0.030 | 0.000 |
| B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Ca | 0.000 | 0.060 | 0.000 | 0.000 | 0.075 | 0.000 | 0.000 | 0.004 |
| Na | 0.059 | 0.051 | 0.028 | 0.103 | 0.076 | 0.034 | 0.030 | 0.094 |
| K | 0.000 | 0.036 | 0.000 | 0.018 | 0.000 | 0.020 | 0.000 | 0.012 |
| A | 0.059 | 0.147 | 0.028 | 0.121 | 0.151 | 0.054 | 0.030 | 0.110 |
| OH | 1.974 | 1.995 | 1.984 | 1.981 | 1.986 | 1.995 | 1.974 | 1.995 |
| Cl | 0.026 | 0.005 | 0.016 | 0.019 | 0.014 | 0.005 | 0.026 | 0.005 |
| W | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Sum T, C, B, A | 15.059 | 15.139 | 15.029 | 15.121 | 15.152 | 15.054 | 15.029 | 15.107 |
| (Ca + Na)B | 1.99 | 2.06 | 2.00 | 2.00 | 2.06 | 1.94 | 1.96 | 2.00 |
| (Na + K)A | 0.00 | 0.09 | 0.00 | 0.11 | 0.08 | 0.02 | 0.00 | 0.11 |
| Fe/(Fe + Mg) | 0.16 | 0.16 | 0.17 | 0.16 | 0.16 | 0.17 | 0.16 | 0.17 |
| Mg/(Mg + Fe2+) | 0.91 | 0.84 | 0.88 | 0.86 | 0.84 | 0.91 | 0.90 | 0.86 |
| Sample | MA 8 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 | MA 11 |
| 43 | 1 | 2 | 3 | 4 | 13 | 14 | 15 | 16 | |
| SiO2 | 62.01 | 59.20 | 59.48 | 58.63 | 59.10 | 59.78 | 63.84 | 66.78 | 63.15 |
| TiO2 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Al2O3 | 23.60 | 25.08 | 25.14 | 26.04 | 24.84 | 24.79 | 22.43 | 20.61 | 23.18 |
| FeO* | bdl | 0.41 | 0.28 | bdl | 0.87 | 0.22 | 0.16 | bdl | 0.08 |
| MnO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| CaO | 5.22 | 7.23 | 7.22 | 7.48 | 6.94 | 6.66 | 3.74 | 1.61 | 4.36 |
| Na2O | 8.37 | 7.14 | 7.40 | 7.23 | 7.49 | 7.43 | 9.25 | 10.67 | 9.03 |
| K2O | 0.39 | 0.38 | 0.12 | 0.20 | 0.06 | 0.43 | 0.45 | 0.24 | 0.34 |
| BaO | 0.23 | 0.34 | 0.02 | 0.11 | 0.51 | 0.39 | 0.11 | bdl | bdl |
| Total | 99.82 | 99.78 | 99.66 | 99.69 | 99.81 | 99.70 | 99.98 | 99.91 | 100.14 |
| Chemical formulae calculated on the basis of 8 O anions | |||||||||
| Si | 2.759 | 2.729 | 2.662 | 2.628 | 2.654 | 2.680 | 2.824 | 2.932 | 2.791 |
| Al | 1.237 | 1.254 | 1.326 | 1.376 | 1.315 | 1.310 | 1.169 | 1.067 | 1.207 |
| Fe3+ | 0.000 | 0.017 | 0.011 | 0.000 | 0.033 | 0.008 | 0.006 | 0.000 | 0.003 |
| Ti | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Z | 3.997 | 4.001 | 3.999 | 4.004 | 4.002 | 3.998 | 4.000 | 3.998 | 4.001 |
| Mn | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.249 | 0.267 | 0.346 | 0.359 | 0.334 | 0.320 | 0.177 | 0.076 | 0.207 |
| Na | 0.722 | 0.708 | 0.642 | 0.628 | 0.652 | 0.646 | 0.793 | 0.909 | 0.774 |
| K | 0.022 | 0.028 | 0.007 | 0.012 | 0.004 | 0.025 | 0.026 | 0.013 | 0.019 |
| Ba | 0.004 | 0.000 | 0.000 | 0.002 | 0.009 | 0.007 | 0.002 | 0.000 | 0.000 |
| Or (mol. %) | 3 | 3 | 1 | 1 | 1 | 3 | 3 | 1 | 2 |
| Ab (mol. %) | 72 | 62 | 64 | 63 | 65 | 65 | 79 | 91 | 77 |
| An (mol. %) | 25 | 35 | 35 | 36 | 34 | 32 | 18 | 8 | 21 |
| Sample | MA 3a | MA 3a | MA 3a | MA 3a | MA 3a | MA 3a | MA 3a |
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| SiO2 | 30.47 | 30.41 | 30.71 | 30.91 | 30.65 | 30.41 | 30.81 |
| TiO2 | 37.39 | 37.47 | 35.54 | 36.76 | 38.18 | 37.27 | 35.96 |
| Al2O3 | 1.83 | 1.87 | 3.25 | 2.72 | 1.45 | 1.97 | 3.14 |
| FeO* | 0.89 | 0.94 | 1.23 | 1.02 | 1.13 | 1.39 | 1.89 |
| MnO | bdl | 0.18 | 0.31 | bdl | 0.20 | 0.10 | 0.04 |
| MgO | 0.19 | bdl | bdl | bdl | 0.11 | 0.21 | 0.06 |
| CaO | 27.88 | 27.04 | 27.20 | 28.09 | 27.86 | 26.70 | 27.32 |
| Na2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| K2O | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| V2O5 | bdl | 1.21 | 0.82 | bdl | bdl | 0.94 | bdl |
| Total | 98.65 | 99.12 | 99.06 | 99.50 | 99.58 | 98.99 | 99.22 |
| Chemical formulae calculated on the basis of 5 O anions | |||||||
| Si | 1.007 | 0.995 | 1.006 | 1.011 | 1.005 | 0.998 | 1.012 |
| Ti | 0.929 | 0.922 | 0.875 | 0.904 | 0.942 | 0.919 | 0.888 |
| Al | 0.071 | 0.072 | 0.125 | 0.105 | 0.056 | 0.076 | 0.122 |
| Y | 1.000 | 0.994 | 1.001 | 1.009 | 0.998 | 0.996 | 1.010 |
| Fe | 0.025 | 0.026 | 0.034 | 0.028 | 0.031 | 0.038 | 0.052 |
| Mn | 0.000 | 0.005 | 0.009 | 0.000 | 0.006 | 0.003 | 0.001 |
| Mg | 0.009 | 0.000 | 0.000 | 0.000 | 0.005 | 0.010 | 0.003 |
| Ca | 0.987 | 0.948 | 0.954 | 0.984 | 0.979 | 0.938 | 0.961 |
| Na | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| K | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| V | 0.000 | 0.032 | 0.022 | 0.000 | 0.000 | 0.025 | 0.000 |
| X | 1.021 | 1.010 | 1.018 | 1.012 | 1.021 | 1.014 | 1.017 |
| Fe/Al | 0.35 | 0.36 | 0.27 | 0.26 | 0.55 | 0.50 | 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melfos, V.; Voudouris, P.; Melfou, M.; Sánchez, M.G.; Papadopoulou, L.; Filippidis, A.; Spry, P.G.; Schaarschmidt, A.; Klemd, R.; Haase, K.M.; et al. Mineralogical Constraints on the Potassic and Sodic-Calcic Hydrothermal Alteration and Vein-Type Mineralization of the Maronia Porphyry Cu-Mo ± Re ± Au Deposit in NE Greece. Minerals 2020, 10, 182. https://doi.org/10.3390/min10020182
Melfos V, Voudouris P, Melfou M, Sánchez MG, Papadopoulou L, Filippidis A, Spry PG, Schaarschmidt A, Klemd R, Haase KM, et al. Mineralogical Constraints on the Potassic and Sodic-Calcic Hydrothermal Alteration and Vein-Type Mineralization of the Maronia Porphyry Cu-Mo ± Re ± Au Deposit in NE Greece. Minerals. 2020; 10(2):182. https://doi.org/10.3390/min10020182
Chicago/Turabian StyleMelfos, Vasilios, Panagiotis Voudouris, Margarita Melfou, Matías G. Sánchez, Lambrini Papadopoulou, Anestis Filippidis, Paul G. Spry, Anna Schaarschmidt, Reiner Klemd, Karsten M. Haase, and et al. 2020. "Mineralogical Constraints on the Potassic and Sodic-Calcic Hydrothermal Alteration and Vein-Type Mineralization of the Maronia Porphyry Cu-Mo ± Re ± Au Deposit in NE Greece" Minerals 10, no. 2: 182. https://doi.org/10.3390/min10020182
APA StyleMelfos, V., Voudouris, P., Melfou, M., Sánchez, M. G., Papadopoulou, L., Filippidis, A., Spry, P. G., Schaarschmidt, A., Klemd, R., Haase, K. M., Tarantola, A., & Mavrogonatos, C. (2020). Mineralogical Constraints on the Potassic and Sodic-Calcic Hydrothermal Alteration and Vein-Type Mineralization of the Maronia Porphyry Cu-Mo ± Re ± Au Deposit in NE Greece. Minerals, 10(2), 182. https://doi.org/10.3390/min10020182