Grammatikopoulosite, NiVP, a New Phosphide from the Chromitite of the Othrys Ophiolite, Greece
Abstract
:1. Introduction
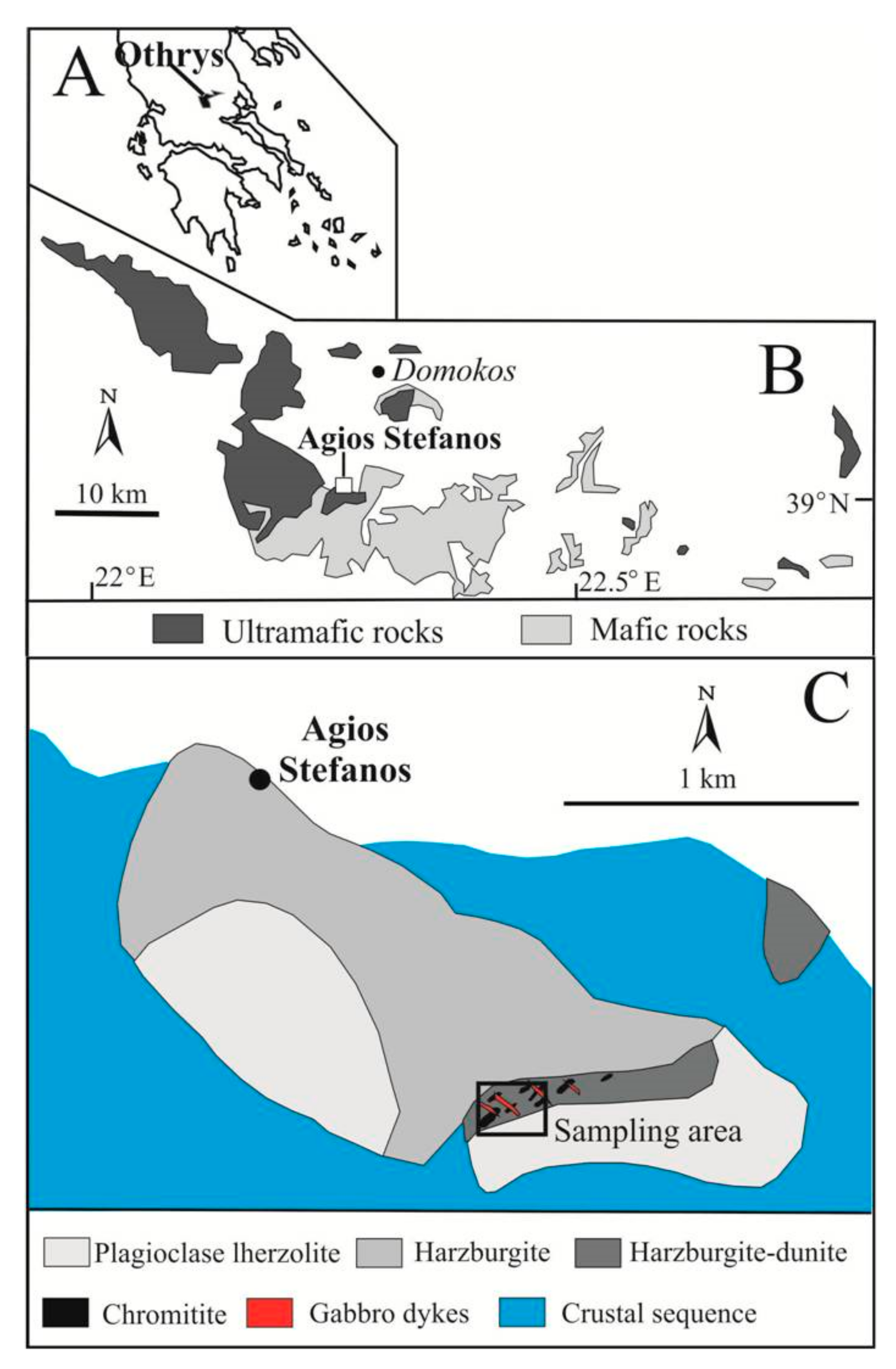
2. Geological Background and Occurrence of Grammatikopoulosite
3. Analytical Methods
4. Physical and Optical Properties
5. Chemical Composition and X-Ray Crystallography
6. Description of the Structure and Relations to Other Species
7. Discussion and Genetical Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaccarini, F.; Bindi, L.; Ifandi, E.; Grammatikopoulos, T.; Stanley, C.; Garuti, G.; Mauro, D. Tsikourasite, Mo3Ni2P1 + x (x < 0.25), a new phosphide from the chromitite of the othrys ophiolite, Greece. Minerals 2019, 9. [Google Scholar]
- Britvin, S.N.; Murashko, M.N.; Vapnik, Y.; Polekhovsky, Y.S.; Krivovichev, S.V. Earth’s phosphides in levant and insights into the source of Archean prebiotic phosphorus. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Zaccarini, F.; Pushkarev, E.; Garuti, G.; Kazakov, I. Platinum-group minerals and other accessory phases in chromite deposits of the Alapaevsk ophiolite, Central Urals, Russia. Minerals 2016, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Sideridis, A.; Zaccarini, F.; Grammatikopoulos, T.; Tsitsanis, P.; Tsikouras, B.; Pushkarev, E.; Garuti, G.; Hatzipanagiotou, K. First occurrences of Ni-phosphides in chromitites from the ophiolite complexes of Alapaevsk, Russia and GerakiniOrmylia, Greece. Ofioliti 2018, 43, 75–84. [Google Scholar]
- Ifandi, E.; Zaccarini, F.; Tsikouras, B.; Grammatikopoulos, T.; Garuti, G.; Karipi, S.; Hatzipanagiotou, K. First occurrences of Ni-V-Co phosphides in chromitite of Agios Stefanos mine, Othrys ophiolite, Greece. Ofioliti 2018, 43, 131–145. [Google Scholar]
- Zaccarini, F.; Ifandi, E.; Tsikouras, B.; Grammatikopoulos, T.; Garuti, G.; Mauro, D.; Bindi, L.; Stanley, C. Occurrences of of new phosphides and sulfide of Ni, Co, V, and Mo from chromitite of the Othrys ophiolite complex (Central Greece). Per. Ital. Mineral. 2019, 88. [Google Scholar] [CrossRef]
- Smith, A.G.; Rassios, A. The evolution of ideas for the origin and emplacement of the western Hellenic ophiolites. Geol. Soc. Am. Spec. Pap. 2003, 373, 337–350. [Google Scholar]
- Hynes, A.J.; Nisbet, E.G.; Smith, G.A.; Welland, M.J.P.; Rex, D.C. Spreading and emplacement ages of some ophiolites in the Othris region (eastern central Greece). Z. Deutsch Geol. Ges. 1972, 123, 455–468. [Google Scholar]
- Smith, A.G.; Hynes, A.J.; Menzies, M.; Nisbet, E.G.; Price, I.; Welland, M.J.; Ferrière, J. The stratigraphy of the Othris Mountains, eastern central Greece: A deformed Mesozoic continental margin sequence. Eclogue Geol. Helv. 1975, 68, 463–481. [Google Scholar]
- Rassios, A.; Smith, A.G. Constraints on the formation and emplacement age of western Greek ophiolites (Vourinos, Pindos, and Othris) inferred from deformation structures in peridotites. In Ophiolites and Oceanic Crust: New Insights from Field Studies and the Ocean Drilling Program; Dilek, Y., Moores, E., Eds.; Geological Society of America: Boulder, CO, USA, 2001; pp. 473–484. [Google Scholar]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Drury, M.R. The Othris Ophiolite, Greece: A snapshot of subduction initiation at a mid-ocean ridge. Lithos 2008, 100, 234–254. [Google Scholar] [CrossRef]
- Barth, M.; Gluhak, T. Geochemistry and tectonic setting of mafic rocks from the Othris Ophiolite, Greece. Contrib. Mineral. Petrol. 2009, 157, 23–40. [Google Scholar] [CrossRef]
- Dijkstra, A.H.; Barth, M.G.; Drury, M.R.; Mason, P.R.D.; Vissers, R.L.M. Diffuse porous melt flow and melt-rock reaction in the mantle lithosphere at a slow-spreading ridge: A structural petrology and LA-ICP-MS study of the Othris Peridotite Massif (Greece). Geochem. Geophys. Geosyst. 2003, 4. [Google Scholar] [CrossRef]
- Magganas, A.; Koutsovitis, P. Composition, melting and evolution of the upper mantle beneath the Jurassic Pindos ocean inferred by ophiolitic ultramafic rocks in East Othris, Greece. Int. J. Earth Sci. 2015, 104, 1185–1207. [Google Scholar] [CrossRef]
- Bortolotti, V.; Chiari, M.; Marcucci, M.; Photiades, A.; Principi, G.; Saccani, E. New geochemical and age data on the ophiolites from the Othrys area (Greece): Implication for the Triassic evolution of the Vardar ocean. Ofioliti 2008, 33, 135–151. [Google Scholar]
- Economou, M.; Dimou, E.; Economou, G.; Migiros, G.; Vacondios, I.; Grivas, E.; Rassios, A.; Dabitzias, S. Chromite deposits of Greece. In Chromites, UNESCO’s IGCP197 Project Metallogeny of Ophiolites; Petrascheck, W., Karamata, S., Eds.; Theophrastus Publ. S.A.: Athens, Greece, 1986; pp. 129–159. [Google Scholar]
- Garuti, G.; Zaccarini, F.; Economou-Eliopoulos, M. Paragenesis and composition of laurite from chromitites of Othrys (Greece): Implications for Os-Ru fractionation in ophiolite upper mantle of the Balkan Peninsula. Mineral. Depos. 1999, 34, 312–319. [Google Scholar] [CrossRef]
- Tsikouras, B.; Ifandi, E.; Karipi, S.; Grammatikopoulos, T.A.; Hatzipanagiotou, K. Investigation of platinum-group minerals (PGM) from Othrys chromitites (Greece) using superpanning concentrates. Minerals 2016, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Bruker. APEX3; Bruker AXS Inc.: Madison, WI, USA, 2016; Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 31 January 2020).
- Bruker. SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 31 January 2020).
- Britvin, S.N.; Rudashevskii, N.S.; Krivovichev, S.V.; Burns, P.C.; Polekhovsky, Y.S. Allabogdanite, (Fe,Ni)2P, a new mineral from the Onello meteorite: The occurrence and crystal structure. Am. Mineral. 2002, 87, 1245–1249. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.J.C. International Tables for Crystallography: Mathematical, Physical, and Chemical Tables; International Union of Crystallography: Chester, UK, 1992; Volume 3. [Google Scholar]
- Ivanov, A.V.; Zolensky, M.E.; Saito, A.; Ohsumi, K.; Yang, S.V.; Kononkova, N.N.; Mikouchi, T. Florenskyite, FeTiP, a new phosphide from the Kaidun meteorite, Locality: Kaidun chondritic meteorite, South Yemen. Am. Mineral. 2000, 85, 1082–1086. [Google Scholar] [CrossRef]
- Zolensky, M.; Gounelle, M.; Mikouchi, T.; Ohsumi, K.; Le, L.; Hagiya, K.; Tachikawa, O. Andreyivanovite: A second new phosphide from the Kaidun meteorite. Am. Mineral. 2008, 93, 1295–1299. [Google Scholar] [CrossRef] [Green Version]
- Fruchart, R.; Roger, A.; Sénateur, J.P. Crystallographic and magnetic properties of solid solutions of the phosphides M2P, M = Cr, Mn, Fe, Co, and N. J. Appl. Phys. 1969, 40, 1250–1257. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 5th ed.; Clarendon Press: Oxford, UK, 1984; p. 1288. [Google Scholar]
- Smith, D.G.W.; Nickel, E.H. A system for codification for unnamed minerals: Report of the subcommittee for unnamed minerals of the IMA commission on new minerals, nomenclature and classification. Can. Mineral. 2007, 45, 983–1055. [Google Scholar] [CrossRef]
- Malvoisin, B.; Chopin, C.; Brunet, F.; Matthieu, E.; Galvez, M.E. Low-temperature Wollastonite formed by carbonate reduction: A marker of serpentinite redox conditions. J. Petrol. 2012, 53, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Etiope, G.; Tsikouras, B.; Kordella, S.; Ifandi, E.; Christodoulou, D.; Papatheodorou, G. Methane flux and origin in the Othrys ophiolite hyperalkaline springs, Greece. Chem. Geol. 2013, 347, 161–174. [Google Scholar] [CrossRef]
- Etiope, G.; Ifandi, E.; Nazzari, M.; Procesi, M.; Tsikouras, B.; Ventura, G.; Steele, A.; Tardini, R.; Szatmari, P. Widespread abiotic methane in chromitites. Sci. Rep. 2018, 8, 8728. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Griffin, W.L.; Huang, J.X.; Gain, S.E.M.; Toledo, V.; Pearson, N.J.; O’Reilly, S.Y. Super-reduced mineral assemblages in “ophiolitic” chromitites and peridotites: The view from Mount Carmel. Eur. J. Mineral. 2017, 29, 557–570. [Google Scholar] [CrossRef]
- Pasek, M.A.; Hammeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natural Acad. Sci. USA 2013, 110, 100089–100094. [Google Scholar] [CrossRef] [Green Version]
- Ballhaus, C.; Wirth, R.; Fonseca, R.O.C.; Blanchard, H.; Pröll, W.; Bragagni, A.; Nagel, T.; Schreiber, A.; Dittrich, S.; Thome, V.; et al. Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes. Geochem. Perspec. Lett. 2017, 5, 42–46. [Google Scholar] [CrossRef]
- Buseck, P.R. Phosphide from meteorites: Barringerite, a new iron-nickel mineral. Science 1969, 165, 169–171. [Google Scholar] [CrossRef]
- Britvin, S.N.; Kolomensky, V.D.; Boldyreva, M.M.; Bogdanova, A.N.; Krester, Y.L.; Boldyreva, O.N.; Rudashevsky, N.S. Nickelphosphide (Ni,Fe)3P—The nickel analogue of schreibersite. Zap. Vserossi. Mineral. Obschch. 1999, 128, 64–72. [Google Scholar]
- Ma, C.; Beckett, J.R.; Rossman, G.R. Monipite, MoNiP, a new phosphide mineral in a Ca-Al-rich inclusion from the Allende meteorite. Am. Mineral. 2014, 99, 198–205. [Google Scholar] [CrossRef]
- Pratesi, G.; Bindi, L.; Moggi-Cecchi, V. Icosahedral coordination of phosphorus in the crystal structure of melliniite, a new phosphide mineral from the Northwest Africa 1054 acapulcoite. Am. Mineral. 2006, 91, 451–454. [Google Scholar] [CrossRef]
- Skala, R.; Cisarova, I. Crystal structure of meteoritic schreibersites: Determination of absolute structure. Phys. Chem. Mineral. 2005, 31, 721–732. [Google Scholar] [CrossRef]






| Sample | P | S | Ni | V | Co | Mo | Fe | Si | Total |
|---|---|---|---|---|---|---|---|---|---|
| VP40-1 | 20.38 | 0.41 | 21.98 | 21.02 | 16.33 | 16.72 | 3.82 | 0.14 | 100.79 |
| VP40-2 | 19.83 | 0.42 | 21.70 | 20.48 | 16.66 | 16.36 | 3.83 | 0.14 | 99.41 |
| VP40-3 | 19.65 | 0.39 | 21.72 | 20.73 | 16.51 | 16.35 | 3.86 | 0.13 | 99.33 |
| VP40-4 | 19.65 | 0.40 | 21.95 | 21.05 | 16.37 | 16.31 | 3.85 | 0.13 | 99.71 |
| VP40-5 | 20.01 | 0.41 | 21.69 | 20.98 | 16.45 | 16.20 | 3.78 | 0.16 | 99.67 |
| average | 19.90 | 0.41 | 21.81 | 20.85 | 16.46 | 16.39 | 3.83 | 0.14 | 99.79 |
| λ nm | R1 | λ nm | R2 |
|---|---|---|---|
| 400 | 47.6 | 400 | 48.8 |
| 420 | 47.9 | 420 | 49.1 |
| 440 | 48.3 | 440 | 49.4 |
| 460 | 48.6 | 470 | 49.9 |
| 470 | 48.8 | 470 | 50.3 |
| 480 | 49.0 | 480 | 50.7 |
| 500 | 49.4 | 500 | 51.5 |
| 520 | 49.9 | 520 | 52.4 |
| 540 | 50.3 | 540 | 53.3 |
| 546 | 50.5 | 546 | 53.5 |
| 560 | 50.9 | 560 | 54.1 |
| 580 | 51.4 | 580 | 54.9 |
| 589 | 51.7 | 589 | 55.2 |
| 600 | 51.9 | 600 | 55.5 |
| 620 | 52.4 | 620 | 56.2 |
| 640 | 53.0 | 640 | 56.8 |
| 650 | 53.2 | 650 | 57.1 |
| 680 | 53.8 | 680 | 58.0 |
| 700 | 54.2 | 700 | 58.6 |
| Atom | Site Occupancy | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|
| M1 | Ni0.57Co0.32Fe0.11 | 0.35709(6) | ¼ | 0.93703(5) | 0.00578(10) |
| M2 | V0.63Mo0.26Co0.11 | 0.47087(6) | ¼ | 0.33109(5) | 0.00595(9) |
| P | P1.00 | 0.23639(12) | ¼ | 0.62449(10) | 0.00547(13) |
| Atoms | Bond Distance |
|---|---|
| M1–P | 2.2453(8) |
| M1–P (×2) | 2.2639(5) |
| M1–P | 2.2728(8) |
| M1–M1 (×2) | 2.6000(6) |
| M1–M2 (×2) | 2.7280(5) |
| M1–M2 (×2) | 2.7487(5) |
| M1–M2 | 2.7677(5) |
| M1–M2 | 2.7696(5) |
| M2–P | 2.4299(8) |
| M2–P (×2) | 2.5008(6) |
| M2–P (×2) | 2.5811(6) |
| M2–M1 (×2) | 2.7280(5) |
| M2–M1 (×2) | 2.7487(5) |
| M2–M1 | 2.7677(5) |
| M2–M1 | 2.7696(5) |
| M2–M2 (×2) | 2.9339(6) |
| Indices | 1 | 2 | ||
|---|---|---|---|---|
| hkl | dobs | Iobs | dcalc | Icalc |
| 101 | 4.43 | 10 | 4.4559 | 14 |
| 002 | - | - | 3.4073 | 5 |
| 102 | 2.950 | 20 | 2.9493 | 19 |
| 111 | 2.785 | 25 | 2.7872 | 24 |
| 201 | 2.699 | 5 | 2.7031 | 5 |
| 112 | 2.273 | 60 | 2.2743 | 65 |
| 210 | 2.269 | 10 | 2.2722 | 8 |
| 201 | 2.230 | 10 | 2.2279 | 9 |
| 211 | 2.157 | 100 | 2.1555 | 100 |
| 103 | 2.118 | 25 | 2.1194 | 27 |
| 013 | 1.915 | 15 | 1.9168 | 14 |
| 301 | 1.888 | 10 | 1.8864 | 12 |
| 113 | 1.824 | 15 | 1.8227 | 20 |
| 020 | 1.784 | 20 | 1.7861 | 21 |
| 004 | 1.702 | 10 | 1.7036 | 10 |
| 302 | 1.700 | 15 | 1.7010 | 22 |
| 213 | 1.608 | 10 | 1.6065 | 7 |
| 114 | 1.489 | 5 | 1.4878 | 6 |
| 303 | 1.482 | 5 | 1.4853 | 6 |
| 400 | 1.470 | 5 | 1.4723 | 6 |
| 123 | 1.367 | 10 | 1.3658 | 7 |
| 322 | 1.233 | 10 | 1.2318 | 9 |
| 314 | 1.211 | 5 | 1.2106 | 6 |
| 215 | 1.170 | 10 | 1.1688 | 9 |
| 511 | 1.102 | 5 | 1.1038 | 6 |
| 513 | 1.005 | 5 | 1.0035 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindi, L.; Zaccarini, F.; Ifandi, E.; Tsikouras, B.; Stanley, C.; Garuti, G.; Mauro, D. Grammatikopoulosite, NiVP, a New Phosphide from the Chromitite of the Othrys Ophiolite, Greece. Minerals 2020, 10, 131. https://doi.org/10.3390/min10020131
Bindi L, Zaccarini F, Ifandi E, Tsikouras B, Stanley C, Garuti G, Mauro D. Grammatikopoulosite, NiVP, a New Phosphide from the Chromitite of the Othrys Ophiolite, Greece. Minerals. 2020; 10(2):131. https://doi.org/10.3390/min10020131
Chicago/Turabian StyleBindi, Luca, Federica Zaccarini, Elena Ifandi, Basilios Tsikouras, Chris Stanley, Giorgio Garuti, and Daniela Mauro. 2020. "Grammatikopoulosite, NiVP, a New Phosphide from the Chromitite of the Othrys Ophiolite, Greece" Minerals 10, no. 2: 131. https://doi.org/10.3390/min10020131
APA StyleBindi, L., Zaccarini, F., Ifandi, E., Tsikouras, B., Stanley, C., Garuti, G., & Mauro, D. (2020). Grammatikopoulosite, NiVP, a New Phosphide from the Chromitite of the Othrys Ophiolite, Greece. Minerals, 10(2), 131. https://doi.org/10.3390/min10020131









