Factors Controlling the Chromium Isotope Compositions in Podiform Chromitites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of the Chromitites
2.2. Chromium Isotope Analysis
2.3. Mineral Chemistry and Whole Rock Analyses
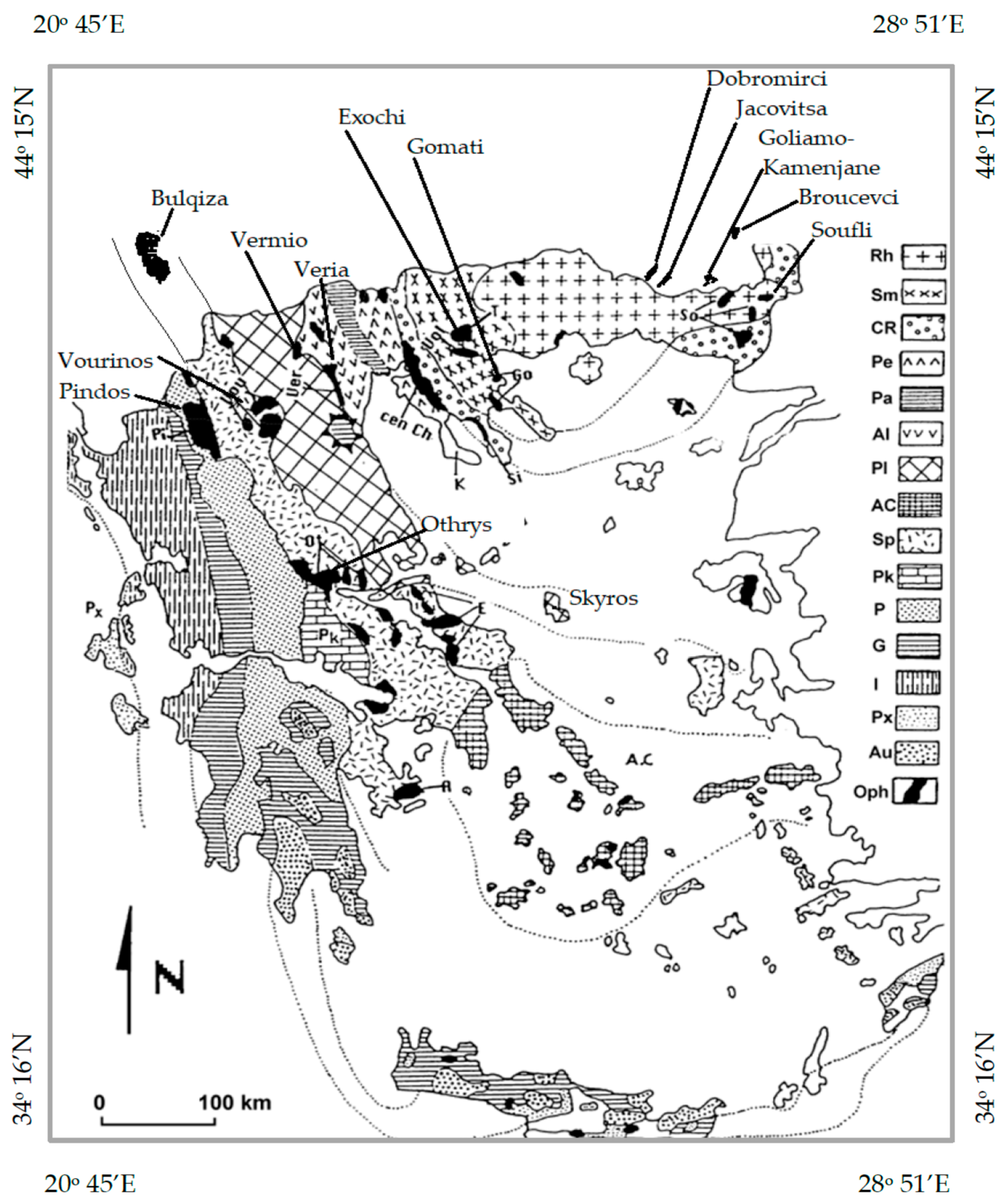
3. A Brief Outline of Characteristics of Ophiolites and Hosted Chromitites
3.1. Ophiolites
3.2. Characteristics of Chromitites
4. Results
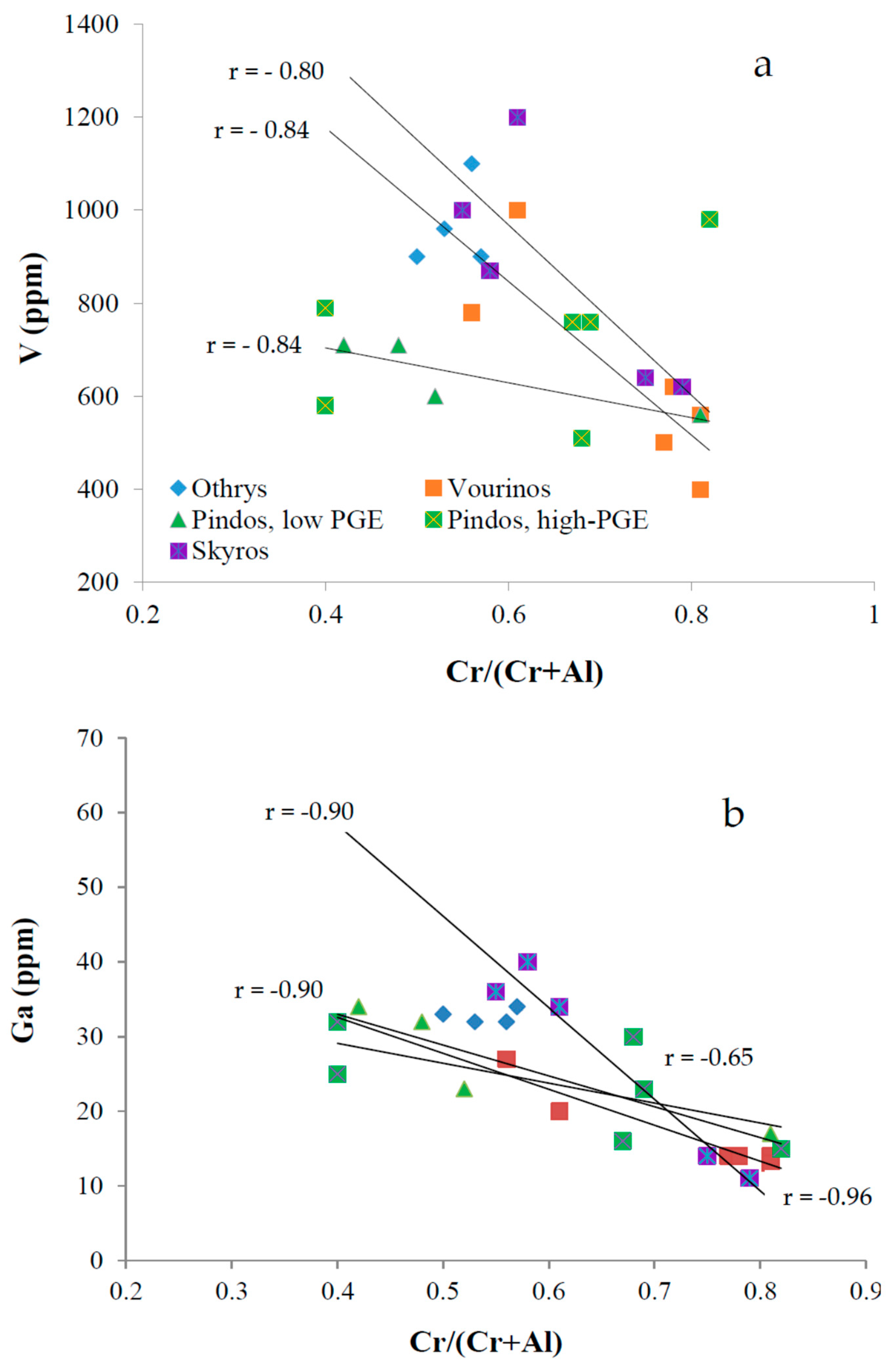
4.1. Distribution of Selected Trace Elements
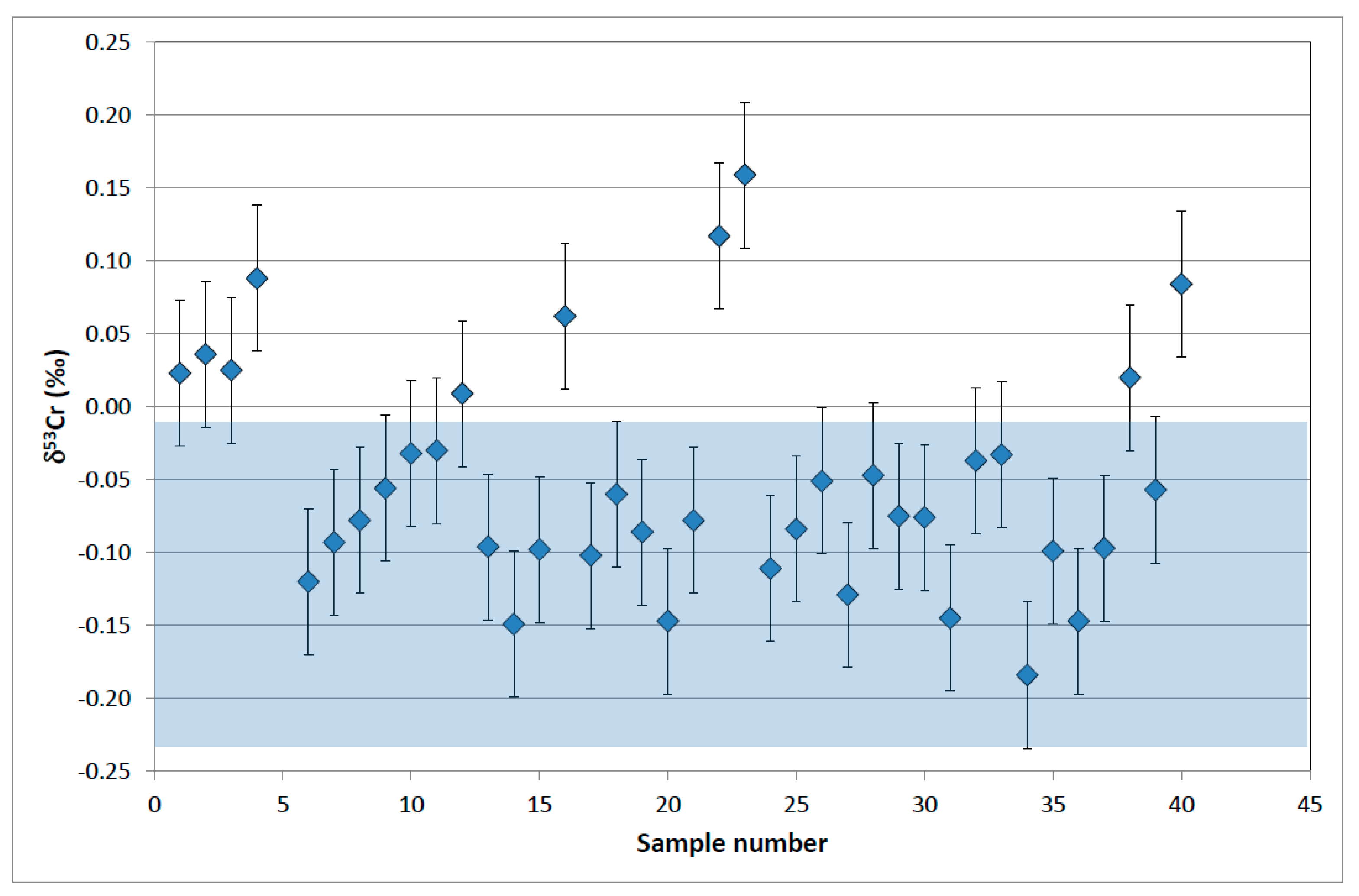
4.2. Chromium Isotope Results
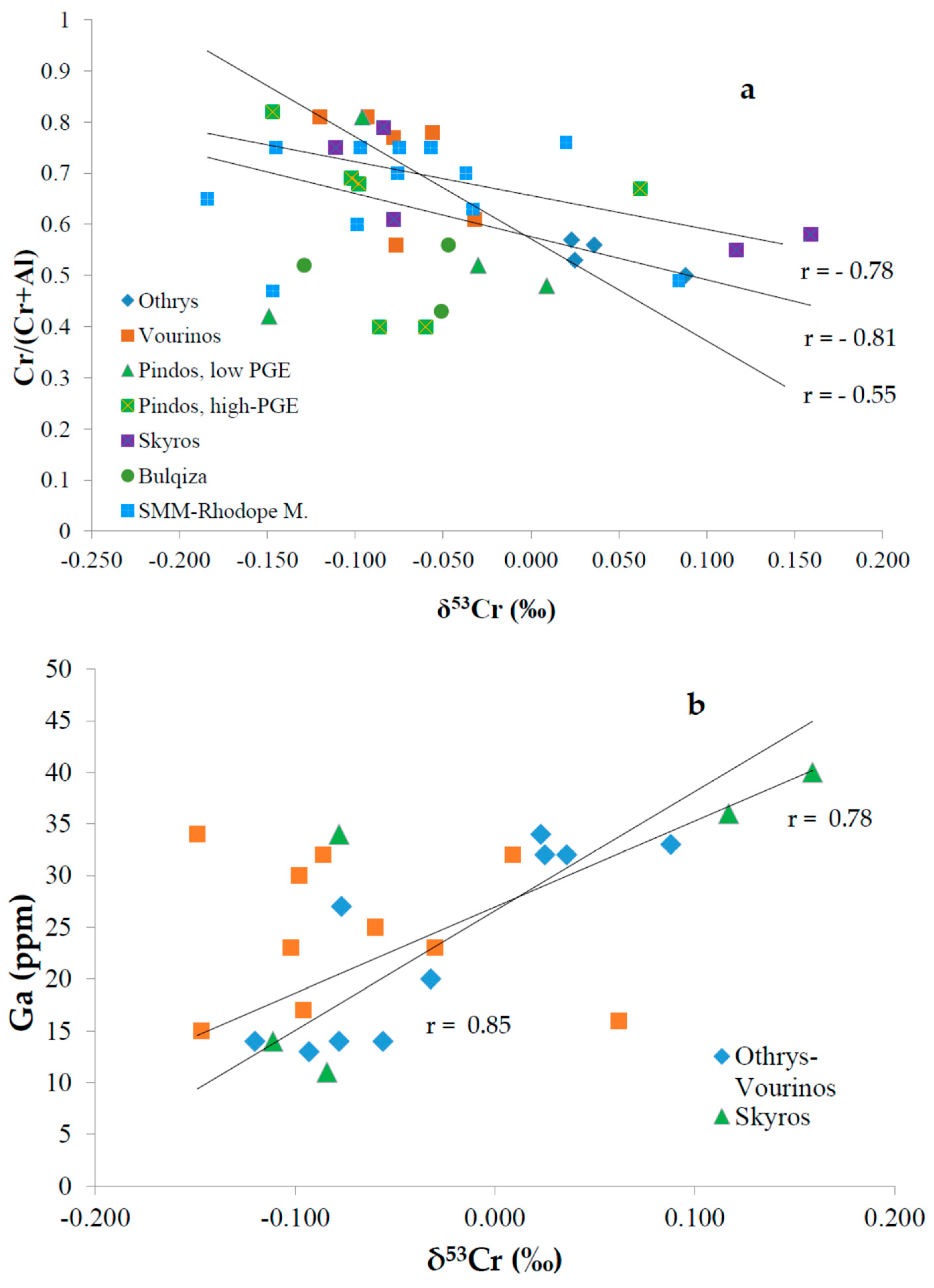
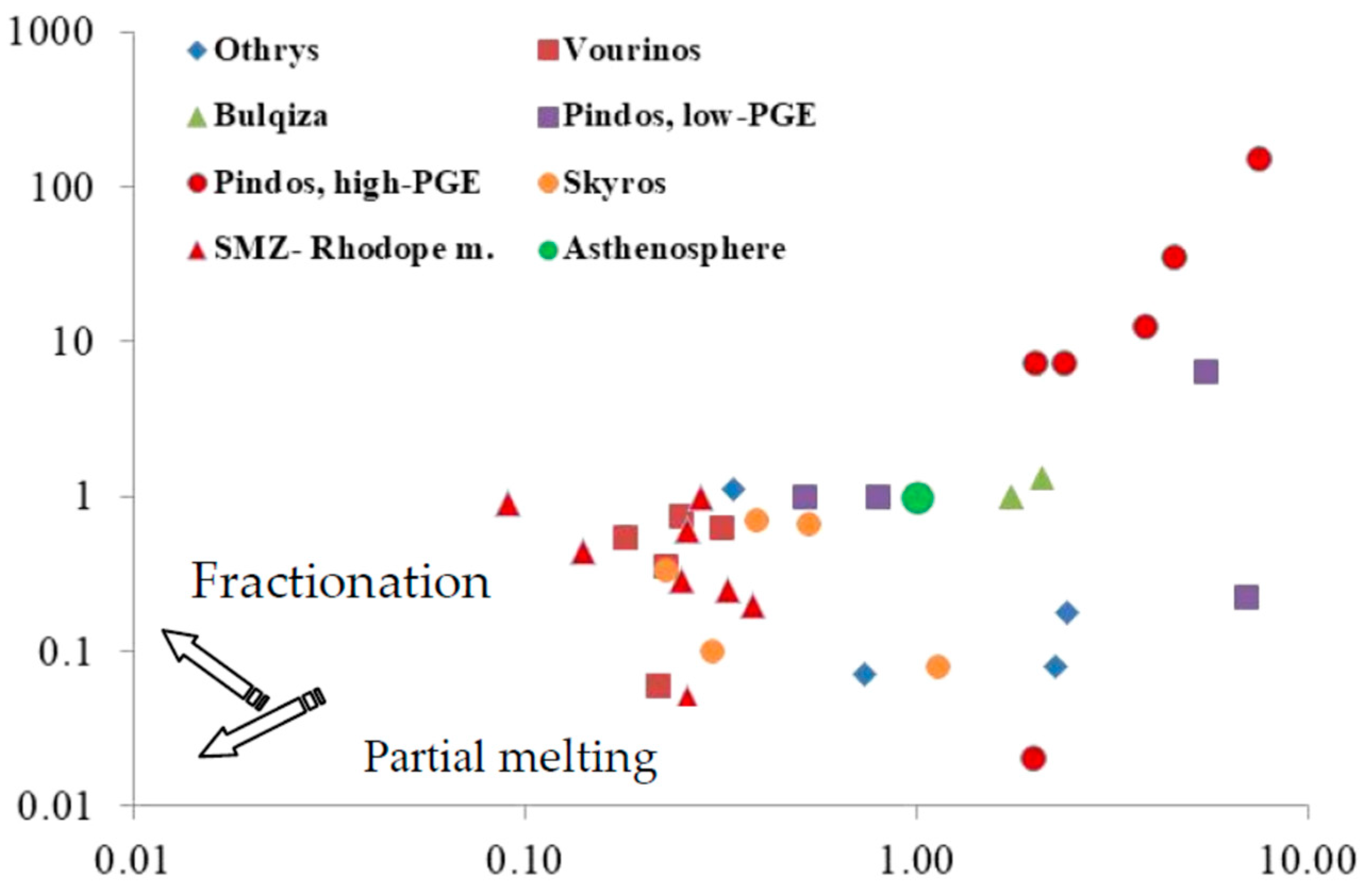
5. Discussion
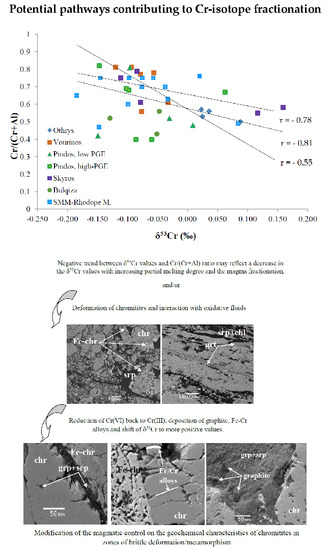
5.1. Potential Pathways Contributing to Chromium Isotope Fractionation
5.1.1. Magmatic Processes
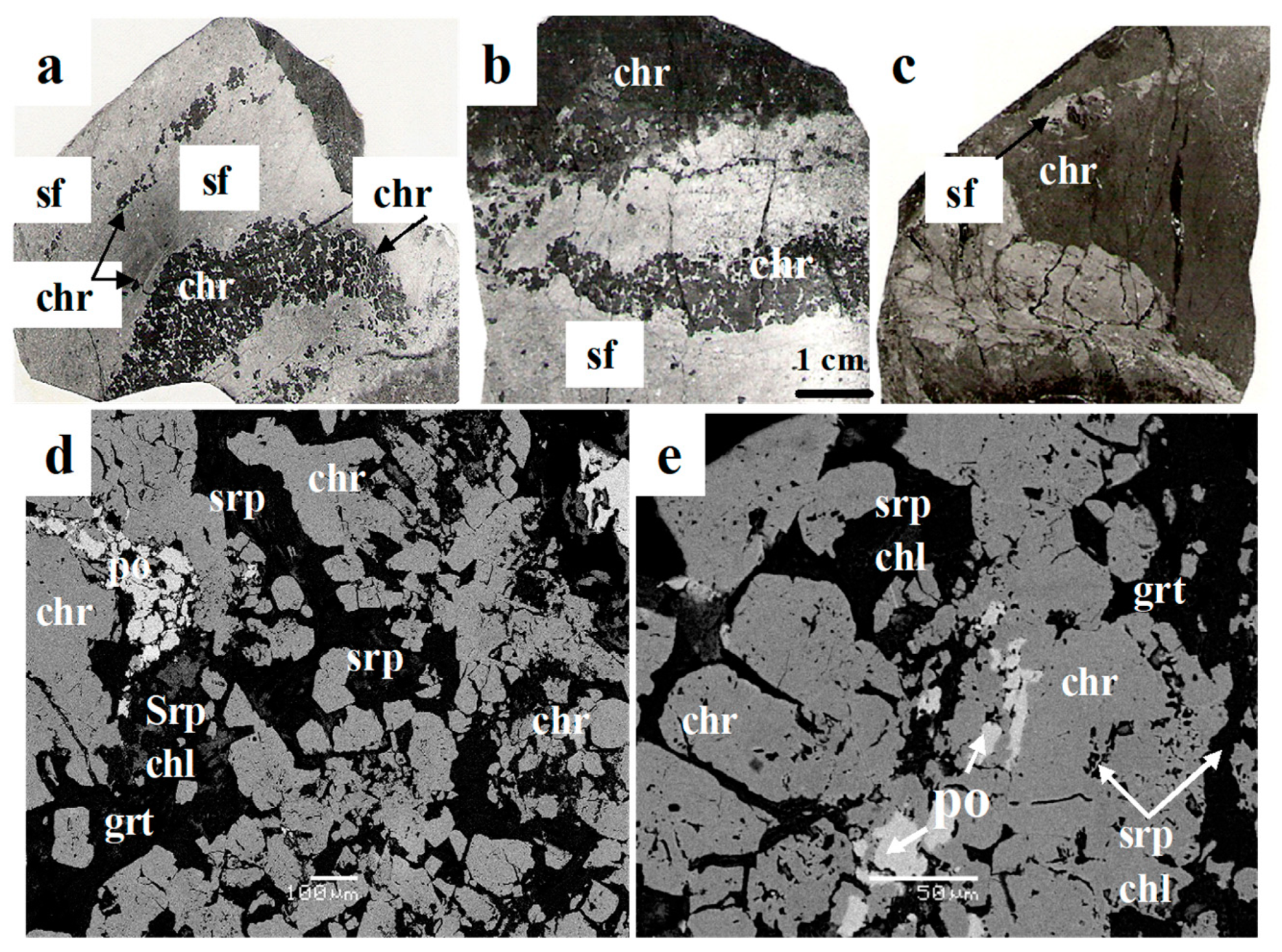
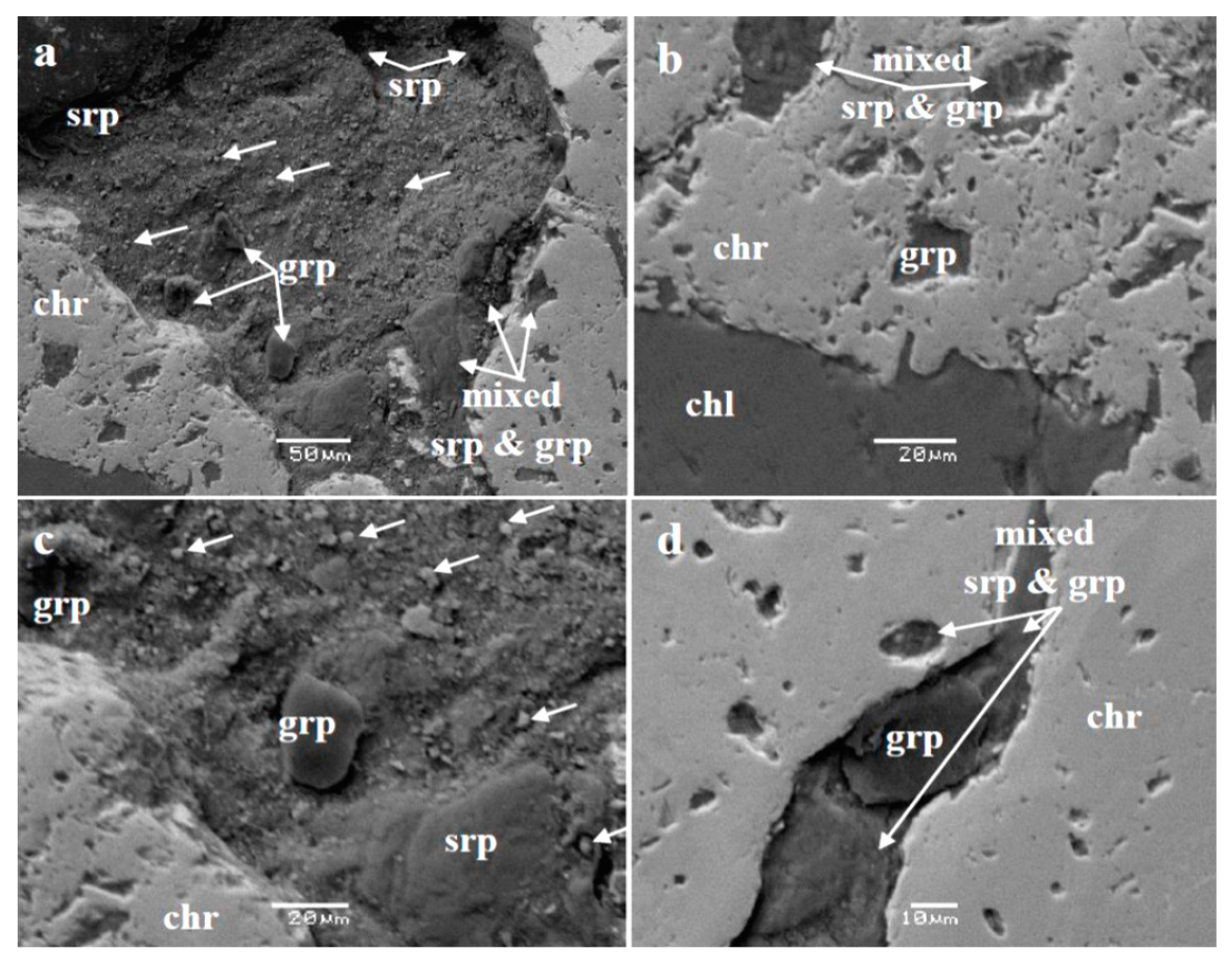
5.1.2. Post-Magmatic Processes
5.2. The Role of Sulphides on the δ53Cr Signatures
5.3. The Potential Role of Abiotic Methane (CH4)
5.4. Weathering of Cr-Bearing Minerals and Environmental Significance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schoenberg, R.; Zink, S.; Staubwasser, M.; Von Blanckenburg, F. The stable Cr isotope inventory of solid Earth reservoirs determined by double spike MC-ICP-MS. Chem. Geol. 2008, 249, 294–306. [Google Scholar] [CrossRef]
- Farkaš, J.; Chrastny, V.; Novak, M.; Cadkova, E.; Pasava, J.; Chakrabarti, R.; Jacobsen, S.B.; Ackerman, L.; Bullen, T.D. Chromium isotope variations (δ53/52Cr) in mantle derived sources and their weathering products: Implications for environmental studies and the evolution of δ53/52Cr in the Earth’s mantle over geologic time. Geochim. Cosmochim. Acta 2013, 123, 74–92. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E. Petrogenesis of the ophiolitic giant chromite deposit of Kempirsai, Kazakhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 10, 1419–1458. [Google Scholar] [CrossRef]
- Arai, S. Conversion of low-pressure chromitites to ultrahigh-pressure chromitites by deep recycling: A good inference. Earth Planet. Sci. Lett. 2013, 379, 81–87. [Google Scholar] [CrossRef]
- Robinson, P.T.; Trumbull, R.B.; Schmitt, A.; Yang, J.S.; Li, J.W.; Zhou, M.F.; Erzinger, J.; Dare, S.; Xiong, F. The origin and significance of crustal minerals in ophiolitic chromitites and peridotites. Gondwana Res. 2015, 27, 486–506. [Google Scholar] [CrossRef]
- Griffin, W.L.; Afonso, J.C.; Belousova, E.A.; Gain, S.E.; Gong, X.H.; González-Jiménez, J.M.; Howell, D.; Huang, J.X.; McGowan, N.; Pearson, N.J.; et al. Mantle recycling: Transition zone metamorphism of Tibetan ophiolitic peridotites and its tectonit implications. J. Petrol. 2016, 57, 655–684. [Google Scholar] [CrossRef]
- Ballhaus, C.; Wirth, R.; Fonseca, R.O.C.; Blanchard, H.; Pröll, W.; Bragagni, A.; Nagel, T.; Schreiber, A.; Dittrich, S.; Thome, V.; et al. Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes. Geochem. Perspect. Lett. 2017, 5, 42–46. [Google Scholar] [CrossRef]
- González-Jiménez, G.M.; Camprubì, A.; Colás, V.; Griffin, W.L.; Proenza, J.A.; O’Reilly, S.Y.; Centeno-García, E.; Garcia-Casco, A.; Belousova, E.; Talavera, C.; et al. The recycling of chromitites in ophiolites from southwestern North America. Lithos 2017, 294–295, 53–72. [Google Scholar] [CrossRef]
- Prichard, H.M.; Neary, C.R.; Potts, P.J. Platinum-group minerals in the Shetland ophiolite complex. In Metallogeny of Basic and Ultrabasic Rocks; Gallagher, M.J., Ixer, R.A., Neary, C.R., Prichard, H.M., Eds.; Institution of Mining and Metallurgy: Edinburgh, UK, 1986; pp. 395–414. [Google Scholar]
- Economou-Eliopoulos, M. Platinum-group element distribution in chromite ores from ophiolite complexes: Implications for their exploration. Ore Geol. Rev. 1996, 11, 363–381. [Google Scholar] [CrossRef]
- Tarkian, M.; Economou-Eliopoulos, M.; Sambanis, G. Platinum-group minerals in chromitites from the Pindos ophiolite complex, Greece. N. J. Mineral. Mon. 1996, 4, 145–160. [Google Scholar]
- Ohnenstetter, M.; Johan, Z.; Cocherie, A.; Fouillac, A.M.; Guerrot, C.; Ohnenstetter, D.; Chaussidon, M.; Rouer, O.; Makovicky, E.; Makovicky, M.; et al. New exploration methods for platinum and rhodium deposits poor in base-metal sulphides-NEXTRIM. Tran. Inst. Min. Metall. Sect. B Appl. Earth Sci. 1999, 108, B119–B150. [Google Scholar]
- Garuti, G.; Zaccarini, F. In situ alteration of platinum-group minerals at low temperature evidence from serpentinized and weathered chromitites of the Vourinos complex (Greece). Can. Mineral. 1997, 35, 611–626. [Google Scholar]
- Proenza, J.A.; Gervilla, F.; Melgarejo, J.C.; Bodinier, J.L. Al- and Cr-rich chromitites from the Mayarí-Baracoa ophiolitic belt (eastern Cuba); consequence of interaction between volatile-rich melts and peridotites in suprasubduction mantle. Econ. Geol. 1999, 94, 547–566. [Google Scholar] [CrossRef]
- Proenza, J.A.; Zaccarini, F.; Escayola, M.; Cábana, C.; Shalamuk, A.; Garuti, G. Composition and textures of chromite and platinum-group minerals in chromitites of the western ophiolitic belt from Córdoba Pampeans Ranges, Argentine. Ore Geol. Rev. 2008, 33, 32–48. [Google Scholar] [CrossRef]
- Uysal, I.; Kapsiotis, A.; Akmaz, R.M.; Saka, S.; Seitz, H.M. The Guleman ophiolitic chromitites (SE Turkey) and their link to a compositionally evolving mantle source during subduction initiation. Ore Geol. Rev. 2018, 93, 98–113. [Google Scholar] [CrossRef]
- Prichard, H.M.; Brough, C. Potential of ophiolite complexes to host PGE deposits. In New Developments in Magmatic Ni-Cu and PGE Deposits; Li, C., Ripley, E.M., Eds.; Geological Publishing House: Beijing, China, 2009; pp. 277–290. [Google Scholar]
- Kapsiotis, A.; Grammatikopoulos, T.A.; Tsikouras, B.; Hatzipanagiotou, K. Platinum-group mineral characterization in concentrates from high-grade PGE Al-rich chromitites of Korydallos area in the Pindos ophiolite complex (NW Greece). Resour. Geol. 2010, 60, 178–191. [Google Scholar] [CrossRef]
- Kapsiotis, A.; Grammatikopoulos, T.; Tsikouras, B.; Hatzipanagiotou, K.; Zaccarini, F.; Garuti, G. Chromian spinel composition and Platinum-group element mineralogy of chromitites from the Milia area, Pindos ophiolite complex, Greece. Can. Mineral. 2009, 47, 1037–1056. [Google Scholar] [CrossRef]
- O’Driscoll, B.; González-Jiménez, J.M. Petrogenesis of the Platinum-group minerals. Rev. Mineral. Geochem. 2016, 81, 489–578. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Qin, L.; Wang, S.J.; Li, S.; Xia, J.; Zhang, Q.; Yang, J. Chromium isotope signature during continental crust subduction recorded in metamorphic rocks. Geochem. Geophys. Geosyst. 2015, 16, 3840–3854. [Google Scholar] [CrossRef]
- Xia, C.; Guodong, L.; Yue, H.; Yuchuan, M. Precipitation Stable Isotope Variability in Tropical Monsoon Climatic Zone of Asia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 042028. [Google Scholar] [CrossRef]
- Xia, J.; Qina, L.; Shena, J.; Carlsonb, R.W.; Ionovd, D.A.; Mockbet, T.D. Chromium isotope heterogeneity in the mantle. Earth Planet Sci. Lett. 2017, 464, 103–115. [Google Scholar] [CrossRef]
- Bonnand, P.; Williams, H.M.; Parkinson, I.J.; Wood, B.J.; Halliday, A.N. Stable chromium isotopic composition of meteorites and metal-silicate experiments: Implications for fractionation during core formation. Earth Planet. Sci. Lett. 2016, 3, 4–7. [Google Scholar] [CrossRef]
- Slejko, F.F.; Petrini, R.; Lutman, A.; Forte, C.; Ghezzi, L. Chromium isotopes tracking the resurgence of hexavalent chromium contamination in a past-contaminated area in the Friuli Venezia Giulia Region, northern Italy. Isot. Environ. Health Stud. 2019, 55, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Chrastny, V.; Cadkova, E.; Naldras, J.; Bullen, T.D.; Tylcer, J.; Szurmanova, Z.; Cron, M.; Prechova, E.; Curik, J.; et al. Common occurrence of a positive δ53Cr shift in Central European waters contaminated by geogenic/industrial chromium relative to source values. Environ. Sci. Technol. 2014, 48, 6089–6096. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Kram, P.; Sebek, O.; Kurik, J.; Andronikov, A.; Veselovsky, F.; Chrastny, V.; Martinkova, E.; Stevanova, M.; Prechova, E.; et al. Temporal changes in Cr fluxes and δ53Cr values in runoff from a small serpentinite catchment (Slavkov Forest, Czech Republic). Chem. Geol. 2017, 472, 22–30. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Frei, R.; Atsarou, C. Application of chromium stable isotopes to the evaluation of Cr (VI) contamination in groundwater and rock leachates from central Euboea and the Assopos basin (Greece). Catena 2014, 122, 216–228. [Google Scholar] [CrossRef]
- Novak, M.; Martinkova, E.; Chrastny, V.; Stepanova, M.; Sebek, O.; Andronikov, A.; Curik, J.; Veselovsky, F.; Prechova, E.; Houskova, M. The fate of Cr(VI) in contaminated aquifers 65 years after the first spillage of plating solutions: A δ53Cr study at four Central European sites. Catena 2017, 158, 371–380. [Google Scholar] [CrossRef]
- Qin, L.; Wang, X. Chromium isotope geochemistry. Rev. Mineral. Geochem. 2017, 82, 379–414. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Eliopoulos, D.G.; Tsoupas, G. On the diversity of the PGE content in chromitites hosted in ophiolites and in porphyry-Cu systems: Controlling factors. Ore Geol. Rev. 2017, 88, 156–173. [Google Scholar] [CrossRef]
- Ellis, A.S.; Johnson, T.M.; Bullen, T.D. Chromium isotopes and the fate of hexavalent chromium in the environment. Science 2002, 295, 2060–2062. [Google Scholar] [CrossRef]
- Trinquier, A.; Elliott, T.; Ulfbeck, D.; Coath, C.; Krot, A.N.; Bizzarro, M. Origin of nucleosynthetic isotope heterogeneity in the solar protoplanetary disk. Science 2009, 324, 295–424. [Google Scholar] [CrossRef]
- Bonnand, P.; Parkinson, I.J.; James, R.H.; Karjalainen, A.M.; Fehr, M.A. Accurate and precise determination of stable Cr isotope compositions in carbonates by double spike MC-ICP-MS. J. Anal. At. Spectrom. 2011, 26, 528–535. [Google Scholar] [CrossRef]
- Moudrakis, D. Introduction to the geology and Macedonia and Trace: Aspects of the geotectonic evolution of the Hellenides. Bull. Geol. Soc. Greece 1992, 30, 31–46. [Google Scholar]
- Jones, G.; Robertson, A. Tectono-stratigraphy and evolution of the Pindos ophiolite and associated units. J. Geol. Soc. 1991, 148, 267–288. [Google Scholar] [CrossRef]
- Rassios, A.; Dilek, Y. Rotational deformation in the Jurassic Mesohellenic Ophiolites, Greece, and its tectonic significance. Lithos 2009, 108, 207–223. [Google Scholar] [CrossRef]
- Rassios, A.H.E.; Moores, E.M. Heterogenous mantle complex, crustal processes, and obduction kinematics in a unified Pindos–Vourinos ophiolitic slab (northern Greece). In Tectonic Development of the Eastern Mediterranean Region; Robertson, A.H.F., Mountrakis, D., Eds.; Geological Society of London, Special Publications: London, UK, 2006; Volume 260, pp. 237–266. [Google Scholar]
- Rassios, A.; Konstantopoulou, G. Emplacement tectonism and the position of chrome ores in the Mega Isoma peridotites, SW Othris, Greece. Bull. Geol. Soc. Greece 1993, 28, 463–474. [Google Scholar]
- Saccani, E.; Photiades, A.; Santato, A.; Zeda, O. New evidence for supra-subduction zone ophiolites in the Vardar Zone from the Vermion Massif (northern Greece): Implication for the tectono-magmatic evolution of the Vardar oceanic basin. Ofioliti 2008, 33, 17–37. [Google Scholar]
- Pearce, J.A.; Lippard, S.J.; Roberts, S. Characteristics and Tectonic Significance of Supra-Subduction Zone Ophiolites. Geol. Soc. Lond. 1984, 16, 77–94. [Google Scholar] [CrossRef]
- Konstantopoulou, G.; Economou-Eliopoulos, M. Distribution of Platinum-group Elements and Gold in the Vourinos Chromitite Ores, Greece. Econ. Geol. 1991, 86, 1672–1682. [Google Scholar] [CrossRef]
- Beccaluva, L.; Coltorti, M.; Saccani, E.; Siena, F. Magma generation and crustal accretion as evidenced by supra-subduction ophiolites of the Albanide-Hellenide Sub Pelagonian zone. Isl. Arc 2005, 14, 551–563. [Google Scholar] [CrossRef]
- Kapsiotis, A.; Rassios, A.; Antonelou, A.; Tzamos, E. Genesis and Multi-episodic Alteration of Zircon-bearing Chromitites from Ayios Stefanos, Othris Massif, Greece: Assessment of an Unconventional Hypothesis on the Origin of Zircon in Ophiolitic Chromitites. Minerals 2016, 6, 124. [Google Scholar] [CrossRef]
- Kapsiotis, A.; Economou-Eliopoulos, M.; Zhenga, H.; Sud, B.X.; Lenaz, D.; Jing, J.J.; Antoneloug, A.; Velicogna, M.; Xia, B. Refractory chromitites recovered from the Eretria mine, East Othris massif (Greece): Implications for metallogeny and deformation of chromitites within the lithospheric mantle portion of a forearc-type ophiolite. Chemie der Erde 2019, 79, 139–152. [Google Scholar] [CrossRef]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Drury, M.R. The Othris Ophiolite, Greece: A snapshot of subduction initiation at a mid-ocean ridge. Lithos 2008, 100, 234–254. [Google Scholar] [CrossRef]
- Economou, M.; Dimou, E.; Economou, G.; Migiros, G.; Vacondios, I.; Grivas, E.; Rassios, A.; Dabitzias, S. Chromite Deposits of Greece: Athens; Theophrastus Publications: Athens, Greece, 1986; pp. 129–159. [Google Scholar]
- Bizimis, M.; Salters, V.J.M.; Bonatti, E. Trace and REE content of clinopyroxenes from supra-subduction zone peridotites. Implications for melting and enrichment processes in island arcs. Chem. Geol. 2000, 165, 67–85. [Google Scholar] [CrossRef]
- Stern, R.J. The anatomy and ontogeny of modern intra-oceanic arc systems. In the Evolving Continents: Understanding Processes of Continental Growth; Kusky, T.M., Zhai, M.G., Xiao, W., Eds.; Geological Society of London: London, UK, 2010; Volume 338, pp. 7–34. [Google Scholar]
- Tsoupas, G.; Economou-Eliopoulos, M. High PGE contents and extremely abundant PGE-minerals hosted in chromitites from the Veria ophiolite complex, northern Greece. Ore Geol. Rev. 2008, 33, 3–19. [Google Scholar] [CrossRef]
- Rogkala, A.; Petrounias, P.; Tsikouras, B.; Hatzipanagiotou, K. New occurrence of pyroxenites in the Veria-Naousa ophiolite (North Greece): Implications on their origin and petrogenetic evolution. Geosciences 2017, 7, 92. [Google Scholar] [CrossRef]
- Economou, M.; Naldrett, A.J. Sulfides associated with podiform bodies of chromite at Tsangli, Eretria, Greece. Mineral. Depos. 1984, 19, 289–297. [Google Scholar] [CrossRef]
- Foose, M.P.; Economou, M.; Panayotou, A. Compositional and mineralogic constraints in the Limassol Forest portion of the Troodos ophiolite complex, Cyprus. Mineral. Depos. 1985, 20, 234–240. [Google Scholar] [CrossRef]
- Etiope, G.; Ifandi, E.; Nazzari, M.; Procesi, M.; Tsikouras, B.; Ventura, G.; Steele, A.; Tardini, R.; Szatmari, P. Widespread abiotic methane in chromitites. Scient. Rep. 2018, 8, 8728. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Vacondios, I. Geochemistry of chromitites and host rocks from the Pindos ophiolite complex, northwestern Greece. Chem. Geol. 1995, 122, 99–108. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Sambanis, G.; Karkanas, P. Trace element distribution in chromitites from the Pindos ophiolite complex, Greece: Implications for the chromite exploration. In Mineral Deposits; Stanley, C.J., Ed.; Balkema: Rotterdam, The Netherlands, 1999; pp. 713–716. [Google Scholar]
- Prichard, H.; Economou-Eliopoulos, M.; Fisher, P.C. Contrasting Platinum-group mineral assemblages from two different podiform chromitite localities in the Pindos ophiolite complex, Greece. Can. Mineral. 2008, 46, 329–341. [Google Scholar] [CrossRef]
- Tarkian, M.; Economou-Eliopoulos, M.; Eliopoulos, D. Platinum-group minerals and tetraauricuprite in ophiolitic rocks of the Skyros Island, Greece. Mineral. Petrol. 1992, 47, 55–66. [Google Scholar] [CrossRef]
- Scarpelis, N.; Economou, M. Genesis and metasomatism of chromite ore from the Gomati area, Chalkidiki, Greece. Ann. Geol. Pays Hell. 1978, 29, 716–728. [Google Scholar]
- Magganas, A.; Economou, M. On the chemical composition of chromite ores from the ophiolitic complex of Soufli, NE Greece. Ofioliti 1988, 13, 15–27. [Google Scholar]
- Zhelyaskova-Panayiotova, M.; Economou-Eliopoulos, M. Platinum-group element and gold concentrations in oxide and sulfide mineralizations from ultramafic rocks of Bulgaria. Ann. Univ. Sofia Geol. Geogr. 1994, 86, 196–218. [Google Scholar]
- Cina, A. Pentlandite Mineralization Related to Albanian Ophiolites. In Proceedings of the XIX CBGA Congress Proceedings, Thessaloniki, Greece, 23–26 September 2010; Volume 100, pp. 317–323. [Google Scholar]
- Tashko, A.; Economou-Eliopoulos, M. An overview of the PGE distribution in the Bulqiza ophiolite complex, Albania. Bull. Geol. Soc. Greece 1994, 32, 193–201. [Google Scholar]
- Karaj, N. Reportition des platinoides chromites et sulphures dans le massif de Bulqiza, Albania. In Incidence sur le Processus Metallogeniques Dans les Ophiolites (These); Universite de Orleans: Orleans, France, 1992; p. 379. [Google Scholar]
- Garuti, G.; Fershtater, G.; Bea, F.; Montero, P.; Pushkarev, E.V.; Zaccarini, F. Platinum-group elements as petrological indicators in mafic-ultramafic complexes of central and southern Urals: Preliminary results. Tectonophysics 1997, 276, 181–194. [Google Scholar] [CrossRef]
- Barnes, S.-L.; Naldrett, A.J.; Gorton, M.P. The origin of the fractionation of the platinum-group elements in terrestrial magmas. Chem. Geol. 1985, 53, 303–323. [Google Scholar] [CrossRef]
- Garuti, G.; Zaccarini, F.; Economou-Eliopoulos, M. Paragenesis and composition of laurite from the chromitites of Othrys (Greece): Implications for Os-Ru fractionation in ophiolitic upper mantle of the Balkan Peninsula. Miner. Depos. 1999, 34, 312–319. [Google Scholar] [CrossRef]
- Colás, V.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; Gervilla, F.; O’Reilly, S.Y.; Pearson, N.J.; Kerestedjian, T.; Proenza, J.A. Fingerprints of metamorphism in chromite: New insights from minor and trace elements. Chem. Geol. 2014, 389, 137–152. [Google Scholar] [CrossRef]
- Pujol-Solà, N.; Proenza, I.A.; Garcia-Casco, A.; González-Jiméne, J.M.; Andreazini, A.; Melgarejo, J.C.; Gervilla, F. An Alternative Scenario on the Origin of Ultra-High Pressure (UHP) and Super-Reduced (SuR) Minerals in Ophiolitic Chromitites: A Case Study from the Mercedita Deposit (Eastern Cuba). Minerals 2018, 8, 433. [Google Scholar] [CrossRef]
- Losi, M.E.; Amrhein, C.; Frankenberger, W.T. Environmental biochemistry of chromium. Rev. Environ. Contam. Toxicol. 1994, 136, 91–121. [Google Scholar] [PubMed]
- Schauble, E.; Rossman, G.R.; Taylor, H.P., Jr. Theoretical estimates of equilibrium chromium-isotope fractionations. Chem. Geol. 2004, 205, 99–114. [Google Scholar] [CrossRef]
- Zhou, M.F.; Robinson, P.T.; Su, B.X.; Gao, J.F.; Li, J.W.; Yang, J.S.; Malpas, J. compositions of chromite, associated minerals, and parental magmas of podiform chromite deposits: The role of slab contamination of asthenospheric melts in suprasubduction zone environments. Gondwana Res. 2014, 26, 262–283. [Google Scholar] [CrossRef]
- Tzamos, E.; Filippidis, A.; Rassios, A.; Grieco, G.; Michailidis, K.; Koroneos, A.; Gamaletsos, P.N. Major and minor element geochemistry of chromite from the Xerolivado–Skoumtsa mine, Southern Vourinos: Implications for chrome ore exploration. J. Geochem. Explor. 2016, 165, 81–93. [Google Scholar] [CrossRef]
- Tzamos, E.; Kapsiotis, A.; Filippidis, A.; Koroneos, A.; Grieco, G.; Rassios, A.E.; Godelitsas, A. Metallogeny of the Chrome Ores of the Xerolivado–Skoumtsa Mine, Vourinos Ophiolite, Greece: Implications on the genesis of IPGE-bearing high-Cr chromitites within a heterogeneously depleted mantle section. Ore Geol. Rev. 2017, 90, 226–242. [Google Scholar] [CrossRef]
- Grieco, G.; Bussolesi, M.; Tzamos, E.; Rassios, A.E.; Kapsiotis, A. Processes of primary and re-equilibration mineralization affecting chromitite ore geochemistry within the Vourinos ultramafic sequence, Vourinos ophiolite (West Macedonia, Greece). Ore Geol. Rev. 2018, 95, 537–551. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Crawford, A.J.; Meffre, S. Factors controlling chemistry of magmatic spinel: An empirical study of associated olivine, Cr-spinel and melt inclusions from primitive rocks. J. Petrol. 2001, 42, 655–671. [Google Scholar] [CrossRef]
- Zaccarini, F.; Proenza, A.J.; Ortega-Gutierrez, F.; Garuti, G. Platinum group minerals in ophiolitic chromitites from Tehuitzingo (Acatlan complex, southern Mexico): Implications for post-magmatic modification. Mineral. Petrol. 2005, 84, 147–168. [Google Scholar] [CrossRef]
- Gervilla, F.; Padrón-Navarta, J.A.; Kerestedjian, T.; Sergeeva, I.; González-Jiménez, J.M.; Fanlo, I. Formation of ferrian chromite in podiform chromitites from the Golyamo Kamenyane serpentinte, Eastern Rhodopes, SE Bulgaria: A two-stage process. Contr. Mineral. Petrol. 2012, 164, 643–657. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Locmelis, M.; Belousova, E.; Griffin, W.L.; Gervilla, F.; Kerestedjian, T.N.; Pearson, N.J.; Sergeeva, I. Genesis and tectonic implications of podiform chromitites in the metamorphosed Ultramafic Massif of Dobromirtsi (Bulgaria). Gondwana Res. 2015, 27, 555–574. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.A.; Kapsiotis, A.; Tsikouras, B.; Hatzipanagiotou, K.; Zaccarini, F.; Garuti, G. Spinel composition, PGE geochemistry and mineralogy of the chromitites from the Vourinos ophiolite complex, northwestern Greece. Can. Mineral. 2011, 49, 1571–1598. [Google Scholar] [CrossRef]
- Mposkos, E.; Krohe, A. Pressure–temperature–deformation paths of closely associated ultra-high-pressure (diamond-bearing) crustal and mantle rocks of the Kimi complex: Implications for the tectonic history of the Rhodope Mountains, northern Greece. Can. J. Earth Sci. 2006, 43, 1755–1776. [Google Scholar] [CrossRef]
- Mposkos, E.; Baziotis, I.; Proyer, A. Pressure–temperature evolution of eclogites from the Kechros complex in the Eastern Rhodope (NE Greece). Int. J. Earth Sci. 2012, 101, 973–996. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. On the origin of the PGE-enrichment in chromitites associated with ophiolite complexes: The case of Skyros Island, Greece. In Digging Deeper Proceedings of the Ninth Biennial SGA Meeting, Dublin 2007; Andrew, C.J., Ed.; Irish Association of Economic Geology: Dublin, Ireland, 2007; Volume 2, pp. 1611–1614. [Google Scholar]
- Ifandi, E.; Zaccarini, Z.; Tsikouras, B.; Grammatikopoulos, T.; Garuti, G.; Karipi, S. First occurrences of Ni-V-Co phosphides in chromitite from the Agios Stefanos Mine, Othrys Ophiolite, Greece. Ofioliti 2018, 43, 131–145. [Google Scholar]
- Tsikouras, B.; Etiope, G.; Ifandi, E.; Kordella, S.; Papatheodorou, G.; Hatzipanagiotou, K. Petrological Implications for the Production of Methane and Hydrogen in Hyperalkaline Springs from the Othrys Ophiolite, Greece. In Proceedings of the 13th International Congress, Chania, Greece, 5–8 September 2013; Bulletin of the Geological Society of Greece: Athens, Greece, 2013; Volume XLVII, pp. 449–457. [Google Scholar]
- Economou-Eliopoulos, M.; Tsoupas, G.; Skounakis, V. Occurrence of Graphite-Like Carbon in Podiform Chromitites of Greece and Its Genetic Significance. Minerals 2019, 9, 152. [Google Scholar] [CrossRef]
- Xu, X.-Z.; Cartigny, P.; Yang, J.-S.; Dilek, Y.; Xiong, F.; Guo, G. Fourier trans-form infrared spectroscopy data and carbon isotope characteristics of the ophiolite-hosted diamonds from the Luobusa ophiolite, Tibet, and Ray-Iz ophiolite, Polar Urals. Lithosphere 2018, 10, 156–169. [Google Scholar] [CrossRef]
- Apollaro, C.; Marini, L.; Critelli, T.; Barca, D.; Bloise, A.; De Rosa, R.; Liberi, F.; Miriello, D. Investigation of rock-to-water release and fate of major, minor, and trace elements in the metabasaltserpentinite shallow aquifer of Mt. Reventino (CZ, Italy) by reaction path modeling. Appl. Geochem. 2011, 26, 1722–1740. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Brozzo, G.; De Rosa, R. Release and fate of Cr(VI) in the ophiolitic aquifers of Italy: The role of Fe(III) as a potential oxidant of Cr(III) supported by reaction path modelling. Sci. Total Environ. 2019, 660, 1459–1471. [Google Scholar] [CrossRef]
- Van der Putten, W.; Ramirez, K.; Poesen, J.; Lenka, L.; Šimek, M.; Anne, W.; Mari, M.; Philippe, L.; Heikki, S.; Andrey, Z.; et al. Opportunities for Soil Sustainability in Europe; European Academies’ Science Advisory Council: Halle, Germany, 2018; 41p. [Google Scholar]
- Vithanage, M.; Kumarathilaka, P.; Oze, C.; Karunatilake, S.; Seneviratne, M.; Hseu, Z.Y.; Gunarathne, V.; Dassanayake, M.; Ok, Y.S.; Rinklebe, J. Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: A critical review. Environ. Int. 2019, 131, 104974. [Google Scholar] [CrossRef]
- Izbicki, J.A.; Ball, J.W.; Bullen, T.D.; Sutley, S.J. Chromium, chromiumisotopes and selected trace elements, western Mojave Desert, USA. Appl. Geochem. 2008, 23, 1325–1352. [Google Scholar] [CrossRef]
- Kazakis, N.; Kantiranis, N.; Kalaitzidou, K.; Kaprara, M.; Mitrakas, M.; Frei, R.; Vargemezis, G.; Tsourlos, P.; Zouboulis, A.; Filippidis, A. Origin of hexavalent chromium in groundwater: The example of the Sarigkiol Basin, Northern Greece. Sci. Total Environ. 2017, 593–594, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Planavsky, N. Cr isotope systematics in the Connecticut River estuary. Chem.Geol. 2019, 506, 29–39. [Google Scholar] [CrossRef]
- Frei, R.; Gaucher, C.; Døssing, L.N.; Sial, A.N. Chromium isotopes in carbonates—A tracer for climate change and for reconstructing the redox state of ancient seawater. Earth Planet. Sci. Lett. 2011, 312, 114–125. [Google Scholar] [CrossRef]
- Frei, R.; Poire, D.; Frei, K.M. Weathering on land and transport of chromium to the ocean in a subtropical region (Misiones, NW Argentina): A chromium stable isotope perspective. Chem. Geol. 2014, 381, 110–124. [Google Scholar] [CrossRef]
- Filippidis, A. Chemical variation of chromite in the central sector of xerolivado chrome mine of Vourinos, Western Macedonia, Greece. Neues Jahrb. Mineral. Mon. 1997, 8, 354–370. [Google Scholar] [CrossRef]
| No | Location | δ53Cr | SEM/EDS | PGE Content (ppb) | Trace Element Content (ppm) | wt % | wt % | wt % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (‰) | Cr≠ | Mg≠ | Os | Ir | Ru | Rh | Pt | Pd | ΣPGE | Pd/Ir | Pt/Pt * | Ni | Co | V | Zn | Mn | Ga | Sc | Fe | Ca | Ti | ||
| Othrys (D), GR | |||||||||||||||||||||||
| 1 | Eretria | 0.023 | 0.57 | 0.63 | 22 | 13 | 67 | 2 | 10 | 1 | 115 | 0.08 | 2.26 | 1600 | 220 | 900 | 340 | 1250 | 34 | 6 | 9.6 | <0.1 | 0.03 |
| 2 | Eretria | 0.036 | 0.56 | 0.66 | 25 | 14 | 25 | 3 | 4 | 1 | 72 | 0.07 | 0.74 | 1400 | 210 | 1100 | 430 | 990 | 32 | 6 | 10 | 0.2 | 0.03 |
| 3 | Domokos | 0.025 | 0.53 | 0.74 | 15 | 11 | 30 | 5 | 24 | 2 | 87 | 0.18 | 2.43 | 1600 | 200 | 960 | 310 | 1300 | 32 | 7 | 9.8 | <0.1 | 0.04 |
| 4 | Domokos | 0.088 | 0.5 | 0.73 | 20 | 10 | 35 | 5 | 8 | 11 | 89 | 1.1 | 0.34 | 1200 | 220 | 900 | 400 | 2500 | 33 | 6 | 9.6 | <0.1 | 0.04 |
| Vourinos (D), GR | |||||||||||||||||||||||
| 5 | Kondro | 0.77 | 0.65 | 26 | 36 | 55 | 17 | 4 | 2 | 140 | 0.06 | 0.22 | 2000 | 240 | 500 | 260 | 1560 | 14 | 6 | 8.1 | <0.1 | 0.04 | |
| 6 | Voidolakos | −0.12 | 0.81 | 0.62 | 14 | 17 | 80 | 12 | 6 | 6 | 135 | 0.35 | 0.23 | 1580 | 210 | 560 | 550 | 1540 | 14 | 6 | 7.9 | <0.1 | 0.04 |
| 7 | Kissavos | −0.093 | 0.81 | 0.66 | 14 | 11 | 49 | 7 | 4 | 7 | 92 | 0.55 | 0.55 | 1900 | 200 | 400 | 300 | 1000 | 13 | 6 | 8.7 | <0.1 | 0.04 |
| 8 | Kissavos | −0.078 | 0.56 | 0.72 | 4 | 3 | 11 | 3 | 3 | 6 | 30 | 0.54 | 0.23 | 1800 | 170 | 780 | 360 | 850 | 27 | 6 | 9.2 | <0.1 | 0.03 |
| 9 | Mikrokleisoura | −0.056 | 0.78 | 0.61 | 30 | 11 | 40 | 16 | 8 | 4 | 109 | 0.63 | 0.26 | 1800 | 180 | 620 | 280 | 950 | 14 | 8 | 7.8 | <0.1 | 0.05 |
| 10 | Mikrokleisoura | −0.032 | 0.61 | 0.52 | 50 | 8 | 42 | 18 | 8 | 6 | 132 | 0.75 | 0.96 | 1000 | 230 | 1000 | 490 | 1760 | 20 | 12 | 15 | 0.5 | 0.1 |
| Pindos, Low PGE | |||||||||||||||||||||||
| 11 | P Dako’s mine | −0.03 | 0.52 | 0.63 | 4 | 6 | 30 | 1 | 4 | 6 | 51 | 1.0 | 0.52 | 1300 | 140 | 600 | 400 | 760 | 23 | 5 | 7.8 | <0.1 | 0.04 |
| 12 | Korydallos | 0.009 | 0.48 | 0.6 | 40 | 20 | 33 | 5 | 25 | 20 | 143 | 1.0 | 0.8 | 1500 | 260 | 710 | 410 | 1200 | 32 | 5 | 9.8 | <0.1 | 0.08 |
| 13 | Kampos Despoti | −0.096 | 0.81 | 0.58 | 30 | 18 | 50 | 3 | 76 | 4 | 181 | 0.22 | 7.01 | 1450 | 290 | 560 | 490 | 1470 | 17 | 5 | 10 | <0.1 | 0.03 |
| 14 | Korydallos | −0.149 | 0.42 | 0.71 | 8 | 3 | 11 | 1 | 75 | 19 | 117 | 6.33 | 5.5 | 1500 | 260 | 710 | 520 | 1300 | 34 | 5.1 | 7.8 | <0.1 | 0.09 |
| Pindos, High-PPGE | |||||||||||||||||||||||
| 15 | Korydallos | −0.098 | 0.68 | 0.57 | 14 | 11 | 57 | 13 | 3460 | 1660 | 5215 | 151 | 7.53 | 1550 | 230 | 510 | 450 | 1200 | 30 | 5.7 | 7.8 | <0.1 | 0.07 |
| 16 | Korydallos | 0.062 | 0.67 | 0.57 | 47 | 49 | 55 | 104 | 3020 | 600 | 3875 | 12.2 | 3.86 | 750 | 270 | 760 | 520 | 1280 | 16 | 6.8 | 9.7 | <0.1 | 0.07 |
| 17 | Korydallos | −0.102 | 0.69 | 0.49 | 62 | 47 | 80 | 112 | 1460 | 337 | 2098 | 7.2 | 2.4 | 720 | 240 | 760 | 620 | 1340 | 23 | 6.7 | 10 | <0.1 | 0.08 |
| 18 | Korydallos | −0.06 | 0.4 | 0.72 | 88 | 74 | 130 | 142 | 1720 | 525 | 2679 | 7.1 | 2.01 | 630 | 240 | 790 | 510 | 1300 | 25 | 6.8 | 9.9 | <0.1 | 0.09 |
| 19 | Korydallos | −0.086 | 0.4 | 0.72 | 45 | 38 | 82 | 78 | 4580 | 1300 | 6123 | 34.2 | 4.6 | 1630 | 240 | 580 | 460 | 1400 | 32 | 5.4 | 7.3 | <0.1 | 0.07 |
| Pindos, High-IPGE | |||||||||||||||||||||||
| 20 | Milia | −0.147 | 0.82 | 0.42 | 150 | 320 | 350 | 82 | 150 | 7 | 1059 | 0.02 | 2.00 | 1600 | 340 | 980 | 1160 | 2000 | 15 | 5 | 12 | 0.1 | 0.01 |
| Skyros, high IPGE | |||||||||||||||||||||||
| 21 | Achladones | −0.078 | 0.61 | 0.64 | 140 | 480 | 1200 | 160 | 280 | 39 | 2300 | 0.08 | 1.13 | 1300 | 250 | 1200 | 540 | 1300 | 34 | 5 | 11 | <0.1 | 0.04 |
| 22 | Achladones | 0.117 | 0.55 | 0.69 | 60 | 40 | 300 | 13 | 23 | 28 | 464 | 0.7 | 0.39 | 1600 | 200 | 1000 | 400 | 1650 | 36 | 5 | 9.4 | <0.1 | 0.04 |
| 23 | Achladones | 0.159 | 0.58 | 0.51 | 13 | 31 | 159 | 7 | 20 | 21 | 251 | 0.67 | 0.53 | 1500 | 240 | 870 | 450 | 1600 | 40 | 5 | 9.6 | <0.1 | 0.04 |
| Skyros, Low PGE | |||||||||||||||||||||||
| 24 | Ag. Ioannis | −0.111 | 0.75 | 0.69 | 30 | 15 | 85 | 6 | 4 | 5 | 145 | 0.33 | 0.23 | 1250 | 220 | 640 | 420 | 2150 | 14 | 6 | 11 | <0.1 | 0.08 |
| 25 | Parapisti | −0.084 | 0.79 | 0.69 | 25 | 20 | 90 | 5 | 3 | 2 | 145 | 0.1 | 0.3 | 1200 | 200 | 620 | 400 | 1900 | 11 | 5 | 10 | <0.1 | 0.05 |
| 26 | Ceruja | −0.051 | 0.43 | 0.69 | 3 | 7 | 18 | 3 | 25 | 7 | 63 | 1 | 1.74 | 2640 | 170 | 610 | 300 | 960 | |||||
| 27 | Ceruja | −0.129 | 0.52 | 0.58 | 3 | 7 | 15 | 2 | 28 | 9 | 64 | 1.3 | 2.1 | 1640 | 160 | 1030 | 420 | 1210 | |||||
| 28 | Ceruja | −0.047 | 0.56 | 0.65 | 3 | 7 | 18 | 3 | 25 | 7 | 63 | 1 | 1.74 | 2150 | 160 | 990 | 340 | 1100 | |||||
| Vermio-Veria | |||||||||||||||||||||||
| 29 | Veria | −0.075 | 0.75 | 0.62 | 70 | 15 | 100 | 9 | 3 | 2 | 199 | 0.13 | 0.23 | 1500 | 200 | 650 | 700 | 2800 | |||||
| 30 | Vermio | −0.076 | 0.7 | 0.65 | 30 | 20 | 55 | 4 | 4 | 31 | 144 | 0.05 | 0.12 | 880 | 170 | 700 | 500 | 1400 | |||||
| 31 | Rhodope massif, GR | −0.145 | 0.75 | 0.66 | 70 | 15 | 100 | 9 | 3 | 2 | 199 | 0.13 | 0.23 | 1770 | 150 | 550 | 300 | 1800 | |||||
| Rhodope massif, Bu | |||||||||||||||||||||||
| 32 | Soufli | −0.037 | 0.7 | 0.62 | 20 | 25 | 85 | 7 | 7 | 5 | 150 | 0.2 | 0.38 | 1260 | 210 | 610 | 360 | 1850 | |||||
| 33 | Tsoutoura | −0.033 | 0.63 | 0.65 | 25 | 6 | 40 | 2 | 3 | 6 | 82 | 1.00 | 0.28 | 1430 | 220 | 670 | 360 | 1270 | |||||
| 34 | Dobromirci | −0.184 | 0.65 | 0.68 | 16 | 32 | 120 | 6 | 4 | 14 | 190 | 0.44 | 0.14 | 520 | 160 | 590 | 230 | 2300 | |||||
| 35 | Goliamo Kamenjane | −0.099 | 0.6 | 0.62 | 3 | 6 | 14 | 3 | 3 | 1 | 30 | 2000 | 780 | 500 | 3700 | 7500 | |||||||
| 36 | Broucevci | −0.147 | 0.47 | 0.72 | 5 | 13 | 23 | 4 | 2 | 12 | 60 | 0.92 | 0.09 | 800 | 180 | 600 | 370 | 2500 | |||||
| 37 | Jacovitsa | −0.097 | 0.75 | 0.61 | 22 | 42 | 53 | 7 | 3 | 2 | 129 | 0.05 | 0.26 | 790 | 240 | 650 | 400 | 3500 | |||||
| SMM, GR | |||||||||||||||||||||||
| 38 | Exochi | 0.02 | 0.76 | 0.62 | 23 | 24 | 73 | 6 | 5 | 7 | 140 | 0.29 | 0.25 | 1240 | 240 | 670 | 400 | 1850 | |||||
| 39 | Exochi | −0.057 | 0.75 | 0.6 | 25 | 10 | 70 | 4 | 4 | 6 | 119 | 0.6 | 0.26 | 1030 | 150 | 1300 | 300 | 1270 | |||||
| 40 | Gomati | 0.084 | 0.49 | 0.67 | 9 | 20 | 60 | 6 | 5 | 4 | 104 | 0.25 | 0.33 | 1050 | 330 | 660 | 350 | 2700 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Economou-Eliopoulos, M.; Frei, R.; Mitsis, I. Factors Controlling the Chromium Isotope Compositions in Podiform Chromitites. Minerals 2020, 10, 10. https://doi.org/10.3390/min10010010
Economou-Eliopoulos M, Frei R, Mitsis I. Factors Controlling the Chromium Isotope Compositions in Podiform Chromitites. Minerals. 2020; 10(1):10. https://doi.org/10.3390/min10010010
Chicago/Turabian StyleEconomou-Eliopoulos, Maria, Robert Frei, and Ioannis Mitsis. 2020. "Factors Controlling the Chromium Isotope Compositions in Podiform Chromitites" Minerals 10, no. 1: 10. https://doi.org/10.3390/min10010010
APA StyleEconomou-Eliopoulos, M., Frei, R., & Mitsis, I. (2020). Factors Controlling the Chromium Isotope Compositions in Podiform Chromitites. Minerals, 10(1), 10. https://doi.org/10.3390/min10010010