Valentinite and Colloform Sphalerite in Epithermal Deposits from Baia Mare Area, Eastern Carpathians
Abstract
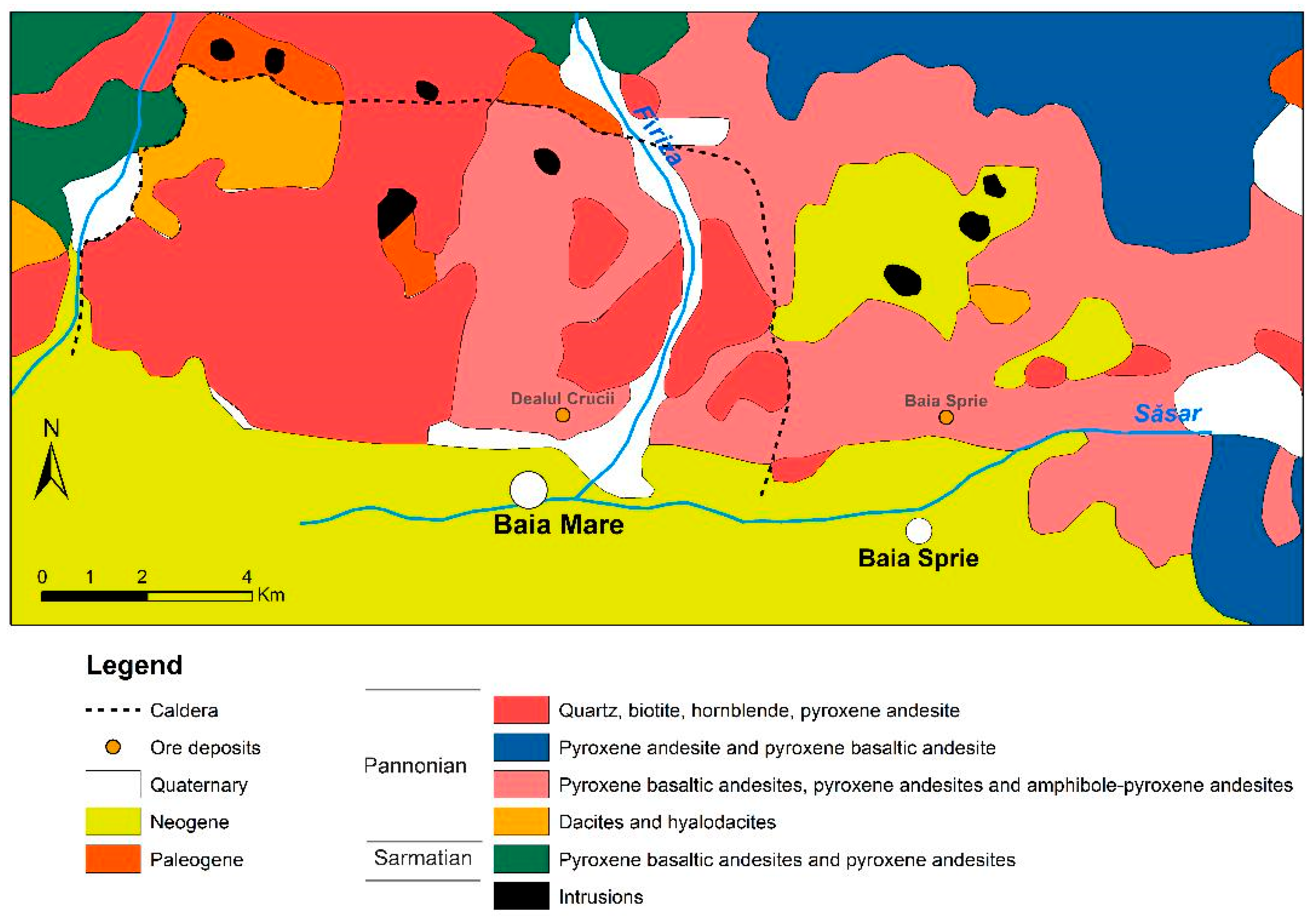
1. Introduction
2. Geological Settings
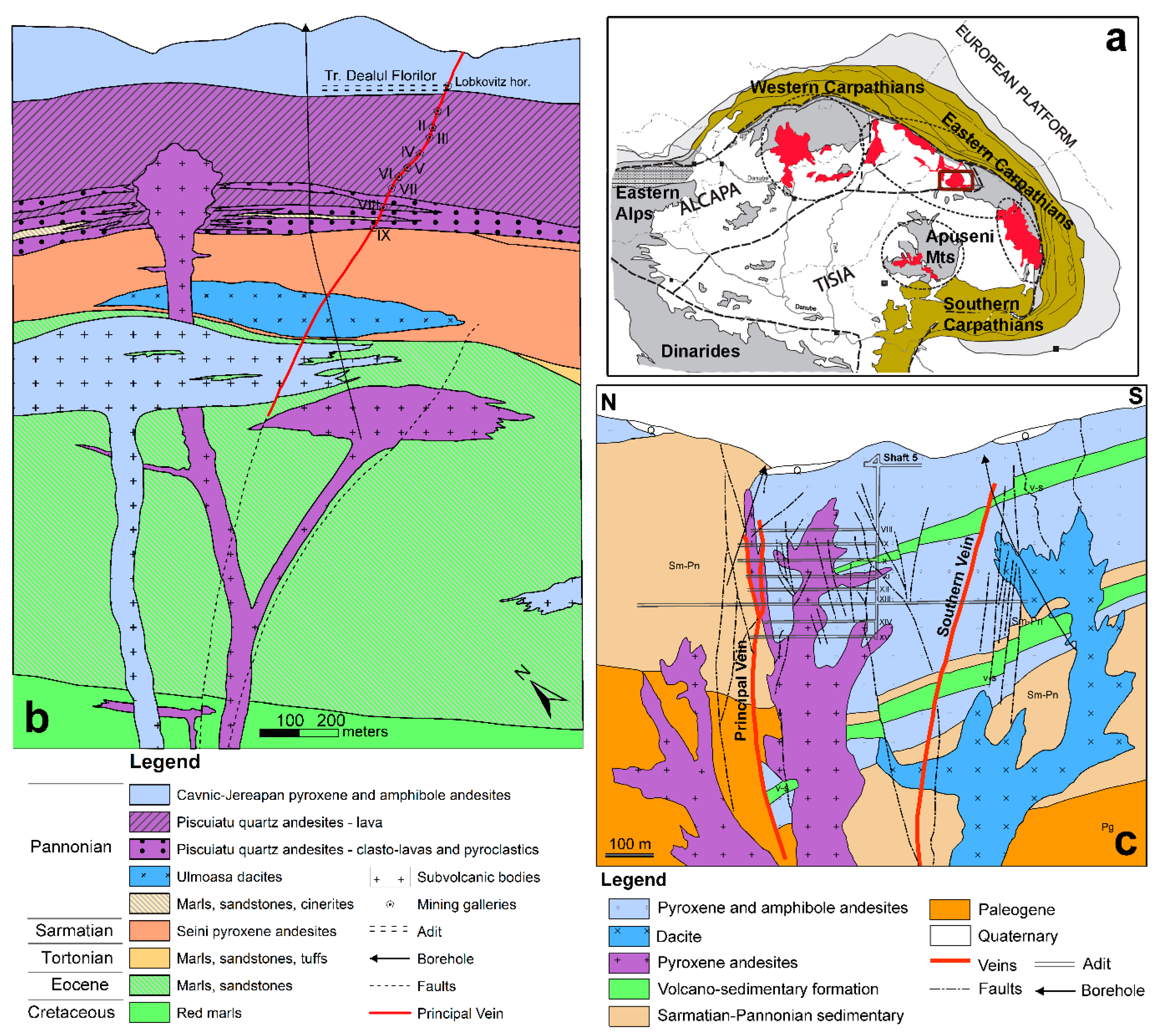
2.1. Dealul Crucii Ore Deposit
- -
- An upper part of golden quartz close to the surface;
- -
- A silver-rich area with many silver minerals, dominated by pyrargyrite, which develops up to the Vth horizon (+40 m).
- -
- The area of gold associated with sulphides between +40 m to −71 m. Here, the silver minerals are rare. Sulphides, especially pyrite, and small quantities of sphalerite, galena, and chalcopyrite with small gold inclusions are predominant.
2.2. Baia Sprie Ore Deposit
3. Methods and Materials
4. Results and Discussions
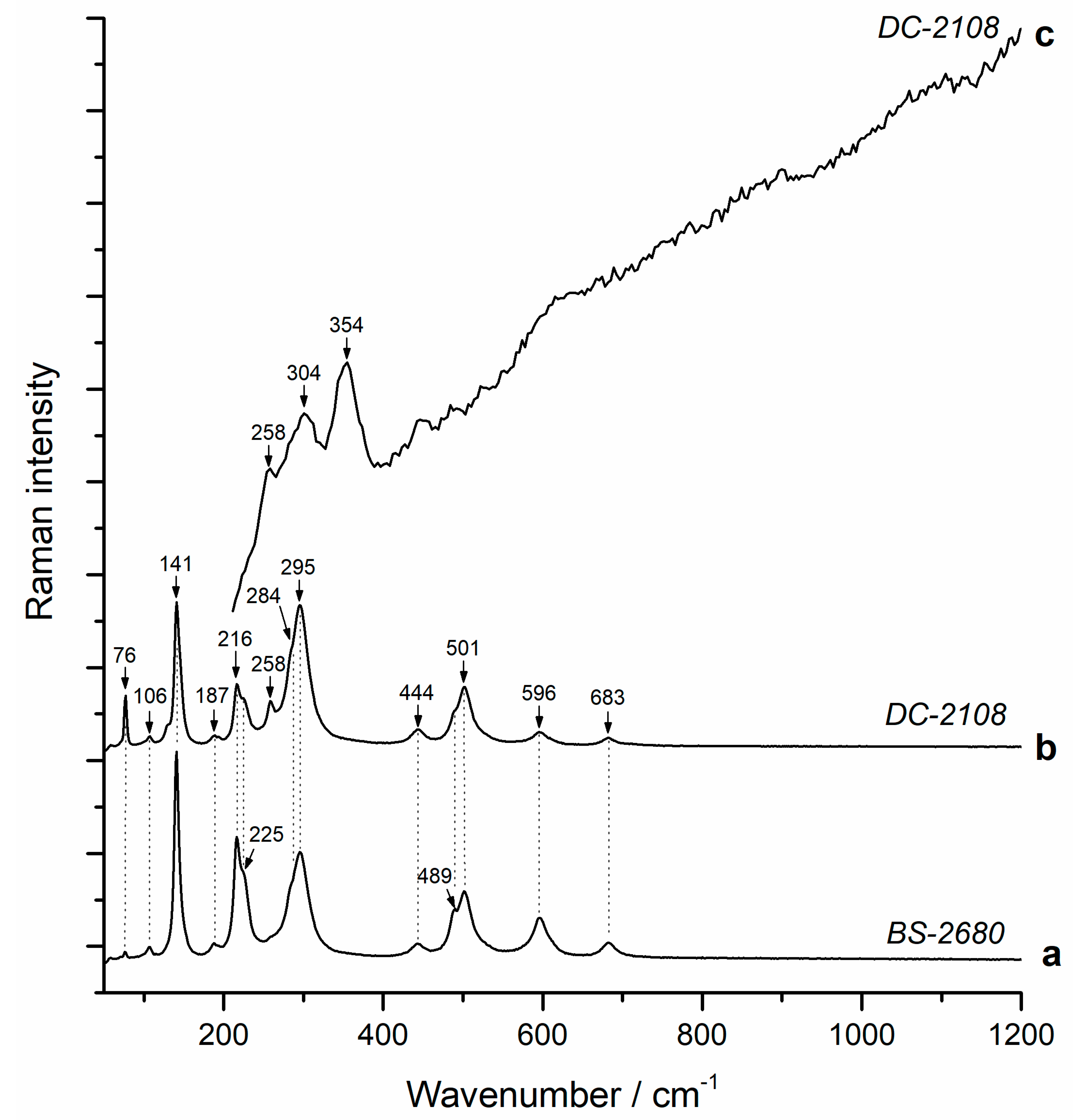
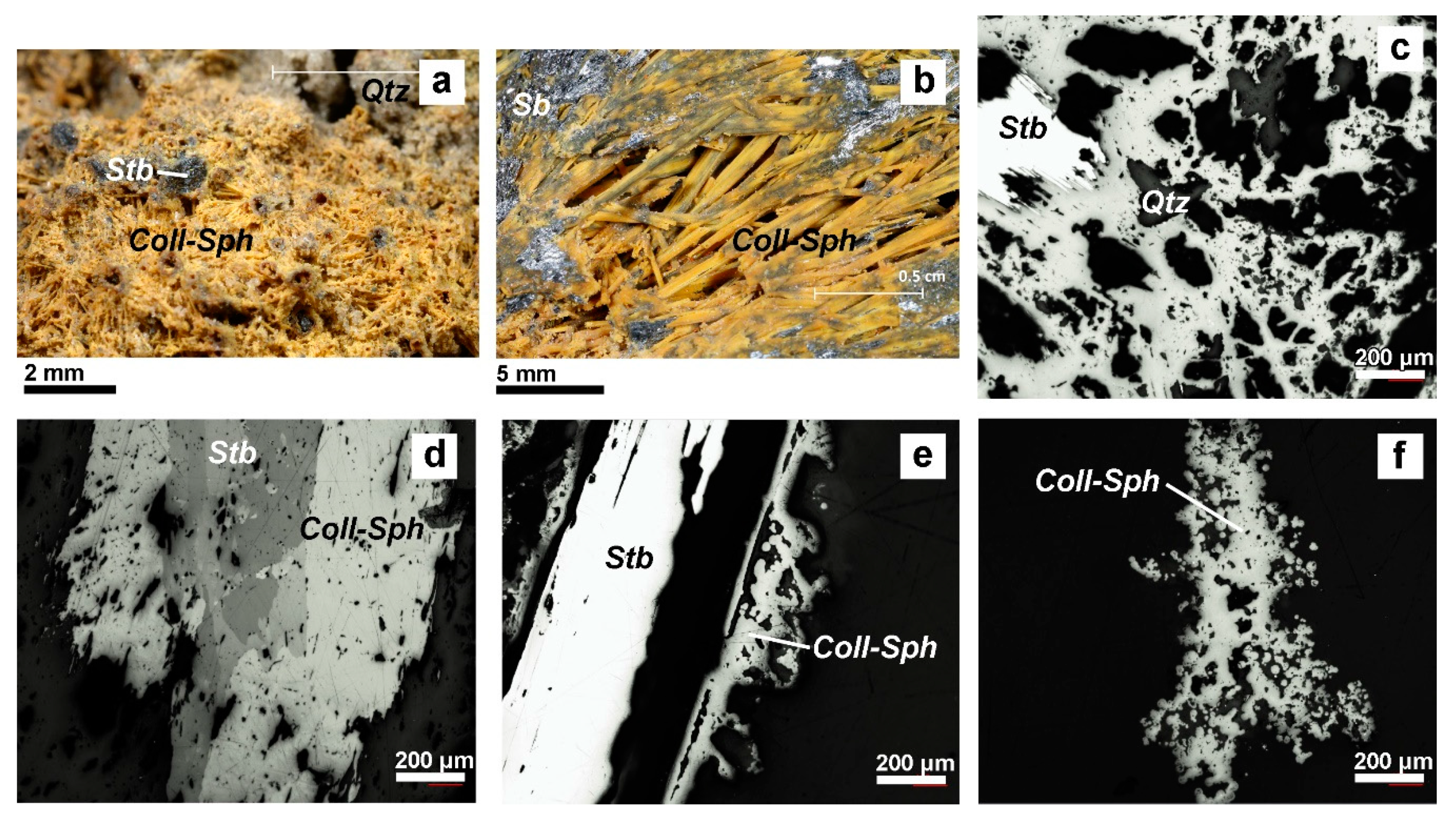
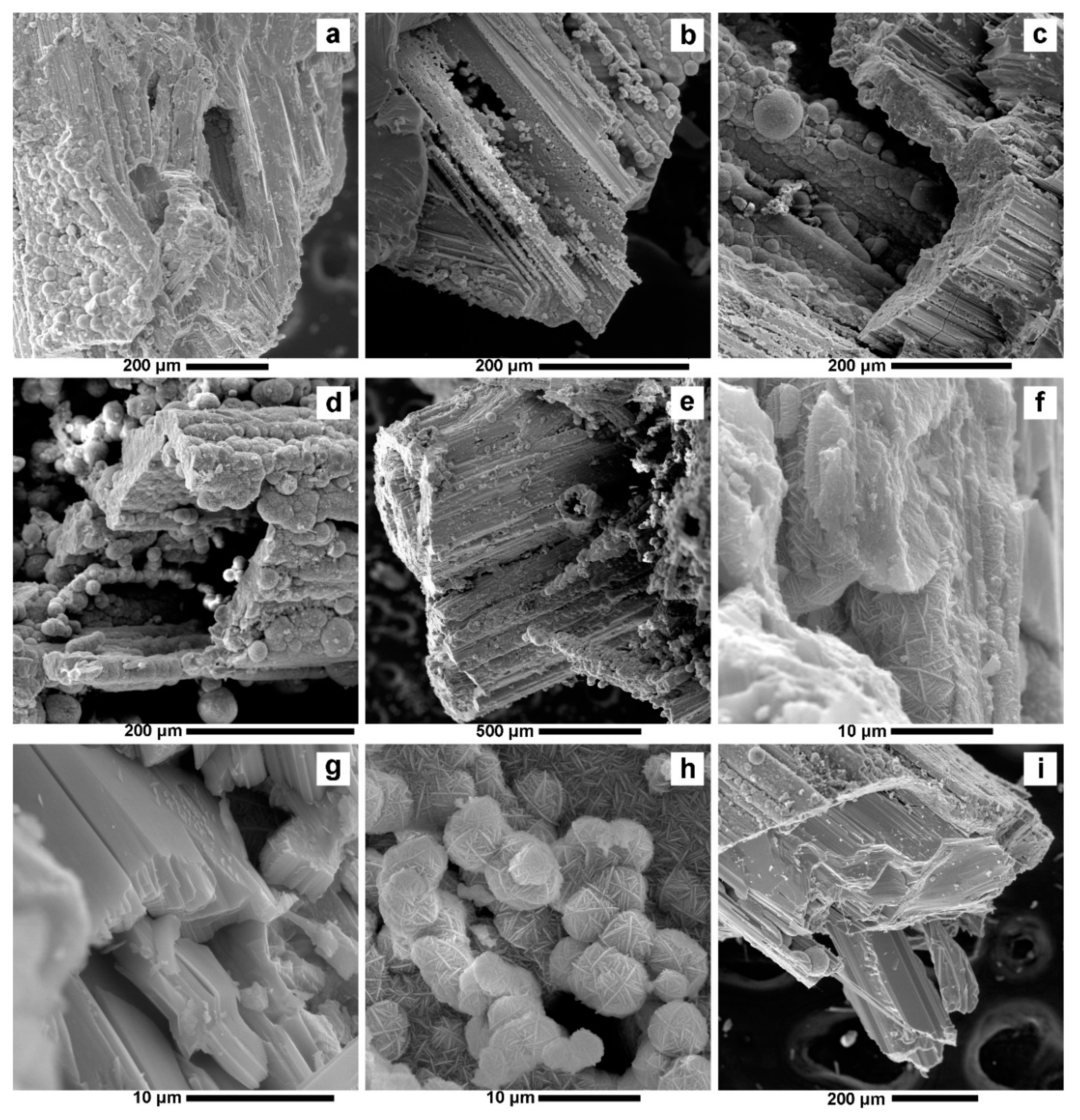
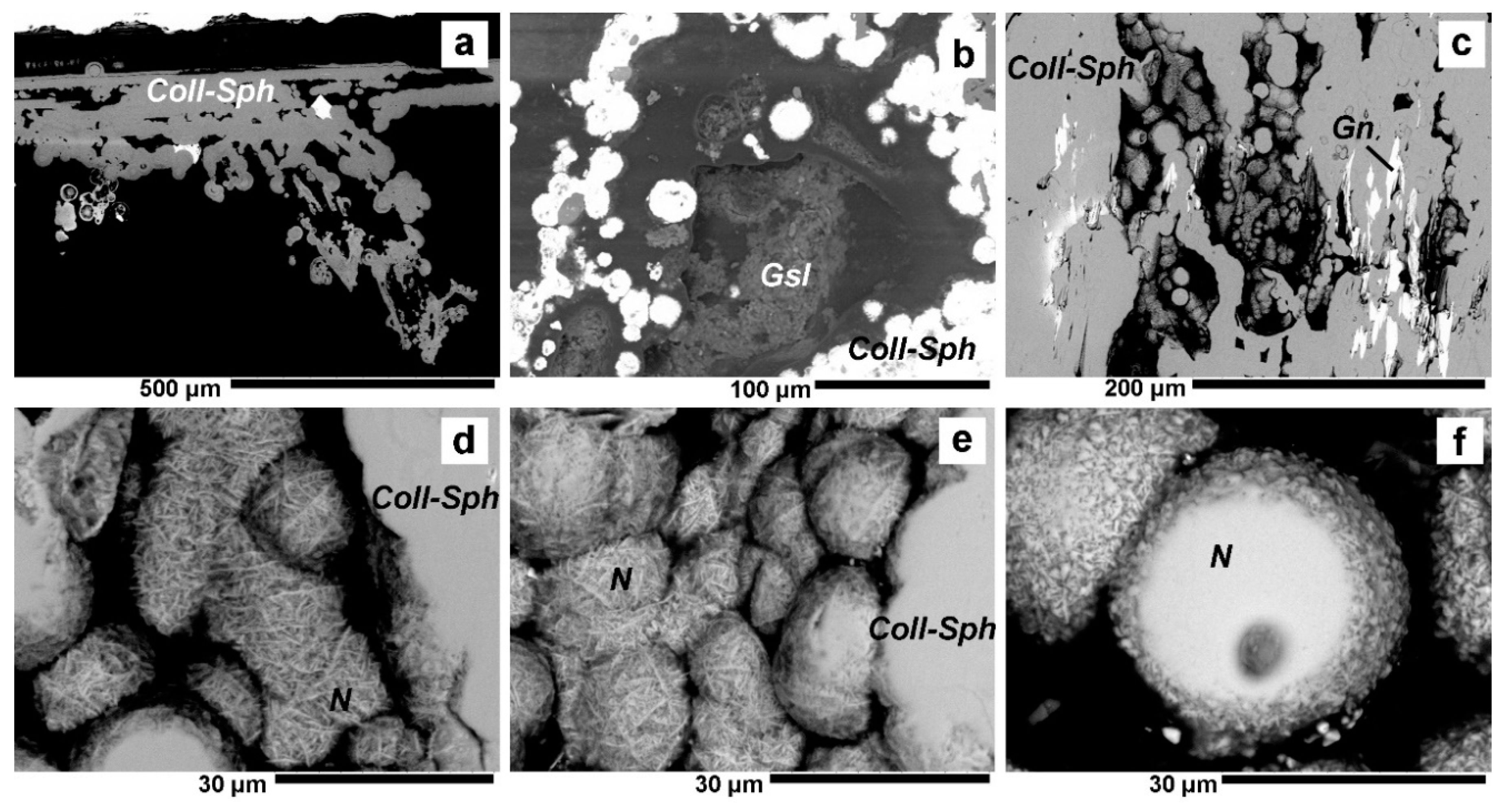
4.1. Valentinite
4.2. Colloform Sphalerite
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iștvan, D.; Halga, S.; Vârșescu, I.; Grancea, L.; Chiuzbăian, C. New data on the upper part of the Baia Sprie deposit, East Carphatians, Romania. An. Științifice ale Univ. Al. I. Cuza Iași Geol. 1996, XLII-supliment, 101–110. [Google Scholar]
- Krenner, J. Andorit uj hazai ezustercz. Mathem es trem. tud. Estesito. 1893, 11, 119–122. [Google Scholar]
- Krenner, J. Egy Felsöbányántatált uj olomerc. Akad. Értek. 1881, 15, 111–118. [Google Scholar]
- Kenngott, A. Mineralogische Notizen, Sitzungsberichte der Akad. Der Wiss. Wien. 1853, 10, 288–299. [Google Scholar]
- Zsivny, V. Klebelsbergit, egy új ásvány Felsöbányáról. Math. t.-t. Ért. 1929, 24, 19–24. [Google Scholar]
- Schroeckinger, J. Dietriechit, ein neuer Alaun aus Ungarn. Verhandl. Geol. Bundesanst. Wien. 1878, 189–191. [Google Scholar]
- Schroeckinger, J. Sphärosiderite von schr hohen Mangangehalte aus ungarn, II. Szikit, ein neues Mangansulphat. Geol. Reichsanst. Wien Verhandl. 1877, 1877, 115–117. [Google Scholar]
- Finalyi, I.; Koch, S. Fülöppite, a new Hungarian Mineral of the plagionite-semseyite group. Min. Mag. 1929, 22, 179–189. [Google Scholar]
- Krenner, J.; Loczka, J. Fizélyit, ein neues ungarisches Silberez. Math. t.-t. Ért. 1923, 40, 18–21. [Google Scholar]
- Svensson, C. The crystal structure of orthorhombic antimony trioxide, Sb2O3. Acta Crystallogr. 1974, B30, 458–461. [Google Scholar] [CrossRef]
- Barrie, C.D.; Boyce, A.J.; Boyle, A.P.; Williams, P.J.; Blake, K.; Wilkinson, J.J.; Lowther, M.; McDermott, P.; Prior, D.J. On the growth of colloform textures: A case study of sphalerite from the Galmoy ore body, Ireland. J. Geol. Soc. Lond. 2009, 166, 563–582. [Google Scholar] [CrossRef]
- Rogers, A.F. A review of the amorphous minerals. Geology 1917, 25, 515–541. [Google Scholar] [CrossRef]
- Roedder, E. The noncolloidal origin of “colloform” textures in sphalerite ores. Econ. Geol. 1968, 63, 45l–471. [Google Scholar] [CrossRef]
- Fowler, A.D.; L’Heureux, I. Self-organized banded sphalerite and branching galena in the Pine Point ore deposit, Northwest Territories. Can. Mineral. 1996, 34, 1211–1222. [Google Scholar]
- Pfaff, K.; Koenig, A.; Wenzel, T.; Ridley, I.; Hildebrandt, H.L.; Leach, L.D.; Markl, G. Trace and minor element variations and sulfur isotopes in crystalline and colloform ZnS: Incorporation mechanisms and implications for their genesis. Chem. Geol. 2011, 286, 118–134. [Google Scholar] [CrossRef]
- Heyl, A.V.; Agnew, A.F.; Lyons, E.J.; Behre, C.H., Jr. The geology of the upper Mississippi Valley zinc lead district. U.S. Ecol. Surv. Prof. Paper. 1959, 309, 310. [Google Scholar]
- McLimans, R.K.; Barnes, H.L.; Ohmoto, H. Sphalerite stratigraphy of the upper Mississippi Valley lead-zinc district, southwes Wtisconsin. Econ. Geol. 1980, 75, 351–361. [Google Scholar] [CrossRef]
- Lasmanis, R. Galena: From Mississippi Valley-Type Deposits. Rocks Miner. 1989, 64, 11–34. [Google Scholar] [CrossRef]
- Pfaff, K.; Hildebrandt, L.H.; Leach, D.L.; Jacob, D.E.; Markl, G. Formation of the Wiesloch Mississippi Valley-type Zn-Pb-Ag deposit in the extensional setting of the Upper Rhinegraben, SW Germany. Miner. Depos. 2010, 45, 647–666. [Google Scholar] [CrossRef]
- Henjes-Kunst, E.; Raith, G.J.; Boyce, J.A. Micro-scale sulfur isotope and chemical variations in sphalerite from the Bleiberg Pb-Zn deposit, Eastern Alps, Austria. Ore Geol. Rev. 2017, 90, 52–62. [Google Scholar] [CrossRef]
- Csontos, L. Tertiary tectonic evolution of the intra-Carpathian area: A review. Acta Vulcanol. 1995, 7, 1–13. [Google Scholar]
- Neubauer, F.; Lips, A.; Kouzmanov, K.; Lexa, J.; Ivășcanu, P. Subduction, slab detachmentand mineralization: The Neogene in the Apuseni Mountains and Carpathians. Ore Geol. Rev. 2005, 27, 13–44. [Google Scholar] [CrossRef]
- Seghedi, I.; Downes, H.; Szakács, A.; Mason, P.; Thirwall, M.T.; Roșu, E.; Pécskay, Z.; Márton, E.; Panaiotu, C. Neogene–Quaternary magmatism and geodynamics in the Carpathian–Pannonian region: A synthesis. Lithos 2004, 72, 117–146. [Google Scholar] [CrossRef]
- Seghedi, I.; Downes, H. Geochemistry and tectonic development of Cenozoic magmatism in the Carpathian–Pannonian region. Gondwana Res. 2011, 20, 655–672. [Google Scholar] [CrossRef]
- Nedelcu, L.; Bălașa, E.; Roșu, N.; Bordea, R. Consideratii Noi asupra zăcământului Dealul Crucii pe baza rezultatelor unor foraje recente. Comptes Rendus des Séances, (1967-1968), 2. Zăcăminte. 1970, Tomes LV, 47–66. [Google Scholar]
- Buzatu, A.; Damian, G.; Dill, G.H.; Buzgar, N.; Apopei, I.A. Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania)—New bismuth minerals occurrence. Ore Geol. Rev. 2015, 65, 132–147. [Google Scholar] [CrossRef]
- Giuşcă, D.; Barcoş, M.; Lang, B.; Stan, N. Neogene volcanism and metallogenesis in the Gutâi Mountains. In Guidebook 12, Symposium on Volcanism and Metallogenesis; Geological Institute of Romania: Bucharest, Romania, 1973; 50p. [Google Scholar]
- Szakács, A.; Pécskay, Z.; Silye, L.; Balogh, K.; Vlad, D.; Fülöp, A. On the age of the Dej Tuff, Transylvanian Basin (Romania). Geol. Carpathica. 2012, 63, 139–148. [Google Scholar] [CrossRef]
- Borcoş, M.; Lang, B.; Peltz, S.; Stan, N. Volcanisme neogene des Monts Gutâi. Geol. Geogr. Geoph. Ser. Geologie. 1973, 17, 1. [Google Scholar]
- Edelstein, O.; Bernard, A.; Kovacs, M.; Crihan, M.; Pecskay, Z. Preliminary date regarding the K-Ar ages of some eruptive rocks from Baia Mare Neogene volcanic zone. Rev. Roum. de Geol. 1992, 36, 45–60. [Google Scholar]
- Damian, F.; Damian, G.; Constantina, C. The Subvulcanic Magmatic Rocks from the Nistru Zone (Gutii Mountains). Carpathian J. Earth Environ. Sci. 2009, 4, 101–122. [Google Scholar]
- Popescu, C.G. A geodinamic Model regarding the Neogene Volcanism and the associated Metallogenetic District (The East Carpathians). An. Univ. Bucureşti Serie Geologie 1994, XLIII, 19–26. [Google Scholar]
- Lang, B.; Edelstein, O.; Steinitz, O.; Kovacs, M.; Halga, S. Ar–Ar dating of adularia—A tool in understanding genetic relations between volcanism and mineralization: Baia Mare area (Gutîi Mountains), Northwestern Romania. Econ. Geol. 1994, 89, 174–180. [Google Scholar] [CrossRef]
- Damian, G.; Damian, F.; Iștvan, D. The Neogene metallogenesis of the central western area of the Baia Mare District (Gutâi Mts – East Carpathians). In the Volum Abstract a Final GEODE-ABCD Workshop, Geodynamics and Ore Deposit Evolution of the Alpine-Balkan- Carpathian-Dinaride Province, Seggauberg; University of Salzburg: Salzburg, Austria, 2003; p. 26. [Google Scholar]
- Popescu, C.G.; Ștefănoiu, A. Specific features and perspectives of Baia Sprie deposit. Revista Minelor. 1972, 23, 326–329. (In Romanian) [Google Scholar]
- Borcoș, M.; Vlad, S.; Udubașa, G.; Găbudeanu, B. Qualitative and quantitative metallogenetic analysis of the ore genetic units in Romania. Rom. J. Miner. Depos. 1998, 78, 1–160. [Google Scholar]
- Popescu, C.G. Metalogenie aplicată şi prognoza geologică partea a II-a; Universităţii Bucureşti: Bucharest, Romania, 1986; p. 210. (In Romanian) [Google Scholar]
- Damian, F. The mineralogical characteristics and the zoning of the hydrothermal types alteration from Nistru ore deposit, Baia Mare Metallogenetic district. Studia Univ. Babeş-Bolyai Geol. 2003, XLVIII, 101–112. [Google Scholar] [CrossRef]
- Manilici, V.; Giușcă, D.; Stiopol, V. Study of Baia Sprie ore deposit. Mem. Com. Geol. Inst. Geol. Bucur. 1965, VII, 1–95. (In Romanian) [Google Scholar]
- Ghitulescu, T.P. Distribution de la mineralizations dans les gissements d’âge tertiaires de Transilvanie. Bul. Soc. Rom. de Geol. 1932, II, 56–97. [Google Scholar]
- Udubaşa, G.; Ilinca, G.; Marincea, Ş.; Săbău, G.; Rădan, S. Minerals in Romania: The State of the Art 1991. Rom. J. Mineral. 1992, 75, 1–51. [Google Scholar]
- Măldărascu, I.; Popescu, C.G. Physical and structural control of hydrothermaldeposits formation from the eastern part of Baia Mare metallogenetic district. Stud. Cercet. Geol. Geofiz. Geogr. 1981, 26, 241–248. (In Romanian) [Google Scholar]
- Borcoș, M.; Lang, B.; Boștinescu, S.; Gheorghita, I. Neogene hydrothermal ore deposits in the volcanic Gutâi Mountains. III. Dealul Crucii–Băiu district. A. Herja, Baia Sprie and uior ore deposits. Rev. Roum. Géol. Geophys. Ser. Géol. 1975, 19, 21–35. [Google Scholar]
- Marias, F. Metallogeny of the Baia Mare Mining District—An Approach on the Cavnic Hydrothermal System; Cornelius Ed.: Glendale Heights, IL, USA, 2005; p. 378. (In Romanian) [Google Scholar]
- Popescu, C.G.; Neacșu, A. Metalogenie aplicată și prognoză geologică; Ed. Univ. București: Bucharest, Romania, 2009; p. 209. (In Romanian) [Google Scholar]
- Damian, G.; Nedelcu, L.; Iștvan, D. Hydrothermal ore deposits at Baia Sprie and Turt, in Excursion guides, Ghe. Udubasa ed., Mineral and mineral occurrences in the Baia Mare mining district. Rom. J. Mineral. 1995, 77, 45–63. [Google Scholar]
- Stanciu, C. Hydrothermal transformation processes in Herja and Baia Sprie ore deposits—Gutîi Mountains. Inst. Geol. Stud. Teh. si Econ. Seria I. 1973, 9, 73–94. (In Romanian) [Google Scholar]
- Damian, G.; Damian, F.; Cook, J.N.; Ciobanu, L.C. Ag-sulphosalts in upper parts of the Baia Mare district (Romania): Microanalyses and implications for the deposit zonality. In Proceedings of the Proceedings and abstracts of the 6th International Symposium on Mineralogy, Cluj-Napoca, Romania, 18–21 September 2003; pp. 37–39. [Google Scholar]
- Petrulian, N.; Steclaci, L. Studiul mineralogic și geochimic al Filonului Nou de la Baia Sprie. Stud. Cerc. Geologie. 1971, XVI, 294–320. [Google Scholar]
- Superceanu, C. Contribuții la paragenezele scheelitului și wolframitului din zăcământul de minereuri complexe de la Baia Sprie. Rev. Min. 1957, 8, 399–404. [Google Scholar]
- Buzatu, A.; Damian, G.; Buzgar, N. Raman and infrared studies of weathering products from Baia Sprie ore deposit (Romania). Rom. J. Miner. Depos. 2012, 85, 7–10. [Google Scholar]
- Buzatu, A.; Buzgar, N.; Damian, G.; Vasilache, V.; Apopei, A.I. The determination of the Fe content in natural sphalerites by means of Raman spectroscopy. Vib. Spectrosc. 2013, 68, 220–224. [Google Scholar] [CrossRef]
- Koch, A.I. Valentinit und orientiert weitengewachsene Baryte von Felsobanya, Steinslaz von Deesakna F.K. Ref. N. Jb. Min. 1926, 53, 81–84. [Google Scholar]
- Tokody, L. Felsobanya asvanyai geokemiai Szempontbol. Math. T.-t Ert. 1942, 61, 191–223. [Google Scholar]
- Schaller, W.T. Crystallography of valentinite (Sb2O3) and andorite (?) (2PbS–Ag2S–3Sb2S3) from Oregon. Am. Mineral. 1937, 22, 651–666. [Google Scholar]
- Palache, C.; Berman, H.; Frondel, C. The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana Yale University 1837-1892, Volume I: Elements, Sulfides, Sulfosalts, Oxides; John Wiley and Sons, Inc.: New York, NY, USA, 1944; pp. 547–550. [Google Scholar]
- Mestl, G.; Ruiz, P.; Delmon, B.; Knozinger, H. Sb2O3/Sb2O4 in reducing/oxidizing environments: An in situ Raman spectroscopy study. J. Phys. Chem. 1994, 98, 11276–11282. [Google Scholar] [CrossRef]
- Orman, R.G. Phase Transitions in Antimony Oxides and Related Glasses. Master’s Thesis, University of Warwick, Coventry, UK, 2005. [Google Scholar]
- Geng, A.; Cao, L.; Wan, C.; Ma, Y. High-pressure Raman investigation of the semiconductor antimony oxide. Phys. Status Solidi. C 2011, 8, 1708–1711. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Liu, V.; Willander, M. Hydrothermal Synthesis of Nanoclusters of ZnS Comprised on Nanowires. Nanomaterials 2013, 3, 564–571. [Google Scholar] [CrossRef] [PubMed]
- ASTM. American Society for Testing and Materials: Powder Difraction File, Alphabetical Index (Chemical and Mineral Name; Int. Cent. Difraction Data: Swarthmore, PA, USA, 1987; p. 664. [Google Scholar]
- Kharbish, S. A Raman spectroscopic investigation of Fe-rich sphalerite: Effect of Fe-substitution. Phys. Chem. Miner. 2007, 34, 551–558. [Google Scholar] [CrossRef]
- Osadchii, E.G.; Gorbaty, Y.E. Raman spectra and unit cell parameters of sphalerite solid solutions (FexZn1-xS). Geochim. Cosmochim. Acta. 2010, 74, 568–573. [Google Scholar] [CrossRef]
- Plotinskaya, O.Y.; Prokofiev, V.Y.; Damian, G.; Damian, F.; Lehmann, B. Tthe Cisma Deposit, Baiut District, Eastern Carpathians, Romania: Sphalerite composition and formation conditions. Carpathian J. Earth Environ. Sci. 2012, 7, 265–273. [Google Scholar]
- Mason, B.; Vitalian, C. The mineralogy of the antimony oxides and antimonates. Mineral. Mag. J. Mineral. Soc. 1953, 30, 100–112. [Google Scholar] [CrossRef]
- Zotov, A.V.; Shikina, N.D.; Akinfiev, N.N. Thermodynamic properties of the Sb(III) hydroxide complex Sb(OH)3(aq) at hydrothermal conditions. Geochim. Cosmochim. Acta 2003, 67, 1821–1836. [Google Scholar] [CrossRef]
- Biver, M.; Shotyk, W. Stibiconite (Sb3O6OH), senarmontite (Sb2O3) and valentinite (Sb2O3): Dissolution rates at pH 2–11 and isoelectric points. Geochim. Cosmochim. Acta 2013, 109, 268–279. [Google Scholar] [CrossRef]
- Kullerrud, G. The FeS-ZnS system, a geological thermometer, Narsk. Geol. Tidsskr. 1953, 22, 61–147. [Google Scholar]
- Barton, P.B.; Toulmin, P. Phase relations involving sphalerite in the Fe-Zn-S system. Econ. Geol. 1966, 61, 815–849. [Google Scholar] [CrossRef]
- Boorman, R.S. Subsolidus studies in the ZnS-FeS-FeS2 system. Econ. Geol. 1967, 62, 614–631. [Google Scholar] [CrossRef]
- Chernyshev, L.V.; Anfilogov, V.N. Subsolidus phase relations in the ZnS-FeS-FeS2 system. Econ. Geol. 1968, 63, 841–843. [Google Scholar] [CrossRef]
- Einaudi, T.M. Spalerite-Pyrhotite-Pyrite Equilibria a Revoluation. Econ. Geol. 1968, 63, 832–834. [Google Scholar] [CrossRef]
- Scott, S.D.; Barnes, H.L. Sphalerite Geothermometry and Geobarometry. Econ. Geol. 1971, 66, 653–669. [Google Scholar] [CrossRef]
- Scot, S.D. Experimental calibration of the sphalerite geobarometer. Econ. Geol. 1973, 68, 466–474. [Google Scholar] [CrossRef]
- Hutchinson, N.M.; Scott, S.D. Sphalerite geobarometry in the Cu-Fe-Zn-S system. Econ. Geol. 1981, 76, 143–156. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhong, W.; Deng, Y.; Au, C.; Du, Y.W. Fabrication and Optical Behaviors of Core–Shell ZnS Nanostructures. Nanoscale Res. Lett. 2010, 5, 1124–1127. [Google Scholar] [CrossRef]
- Vicsek, T.M. Deterministic models of fractal and multifractal growth. Physica D 1989, 38, 356–361. [Google Scholar] [CrossRef]
- Witten, T.A., Jr.; Sander, L.M. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Anitas, E.M. Small-Angle Scattering from Weakly Correlated Nanoscale Mass Fractal Aggregates. Nanomaterials 2019, 9, 648. [Google Scholar] [CrossRef]
- Anitas, E.M.; Marcelli, G.; Szakacs, Z.; Todoran, R.; Todoran, D. Structural Properties of Vicsek-like Deterministic Multifractals. Symmetry 2019, 11, 806. [Google Scholar] [CrossRef]



| No | Sample Name | SO3 | Ag2O | PbO | Bi2O3 | CuO | Sb2O3 | FeO | SnO | Formula |
|---|---|---|---|---|---|---|---|---|---|---|
| 47/1 | BS-2680_an1 | 0.0133 | 0 | 0 | 0.0342 | 0 | 96.7938 | 0.0462 | 0.255 | Sb1.993Sn0.006Fe0.003O3 |
| 48/1 | BS-2680_an2 | 0 | 0 | 0 | 0.0348 | 0 | 95.465 | 0 | 0.2559 | Sb1.996Sn0.006O3 |
| 49/1 | BS-2680_an3 | 0 | 0 | 0 | 0 | 0 | 95.0205 | 0.0407 | 0.2758 | Sb1.995Sn0.006Fe0.002O3 |
| 53/1 | DC-2108_an3 | 0 | 0.028 | 0 | 0 | 0 | 93.892 | 0 | 0.2592 | Sb1.996Sn0.006O3 |
| 54/1 | DC-2108_an4 | 0.0032 | 0 | 0.0309 | 0 | 0.009 | 96.8374 | 0 | 0.2669 | Sb1.995Sn0.006O3 |
| 55/1 | DC-2108_an5 | 0.0063 | 0 | 0 | 0 | 0 | 95.4937 | 0 | 0.2599 | Sb1.996Sn0.006O3 |
| 56/1 | DC-2108_an6 | 0 | 0.011 | 0 | 0.0007 | 0 | 93.227 | 0.025 | 0.2654 | Sb1.995Sn0.006Fe0.001O3 |
| Sample Codename | Cu | Fe | Bi | Sb | S | Pb | Zn | Total | Chemical Formula |
|---|---|---|---|---|---|---|---|---|---|
| 2018_an1 | 0 | 0.0179 | 0.0329 | 1.5717 | 32.6581 | 0.7478 | 63.66 | 98.6879 | Zn0.97Sb0.01S1.01 |
| 2018_an2 | 0 | 0.0188 | 0 | 1.8936 | 31.9448 | 0.9229 | 63.1952 | 97.9753 | Zn0.97Sb0.02S1 |
| 2018_an3 | 0 | 0 | 0.0028 | 2.149 | 32.9386 | 1.1718 | 62.7512 | 99.0134 | Zn0.95Sb0.02Pb0.01S1.02 |
| 2018_an4 | 0.0344 | 0.0613 | 0.0046 | 1.8271 | 31.7961 | 0.811 | 62.7965 | 97.331 | Zn0.97Sb0.02S1.01 |
| 2018_an5 | 0 | 0.0198 | 0 | 1.9453 | 31.8728 | 0.9271 | 62.6001 | 97.3651 | Zn0.97Sb0.02S1.01 |
| 2018_an6 | 0 | 0.0418 | 0.0176 | 1.8369 | 31.6313 | 0.7952 | 63.0341 | 97.3569 | Zn0.98Sb0.02S1 |
| 2018_an7 | 0.0127 | 0.0167 | 0 | 1.8427 | 31.9095 | 0.8546 | 62.601 | 97.2372 | Zn0.97Sb0.02S1.01 |
| 2018_an8 | 0 | 0.029 | 0.0183 | 1.5806 | 31.0534 | 1.0354 | 63.8626 | 97.5793 | Zn0.99Sb0.01Pb0.01S0.99 |
| 2018_an9 | 0 | 0.0479 | 0.0344 | 0.7726 | 31.9911 | 0.56 | 65.0537 | 98.4597 | Zn0.99Sb0.01S1 |
| 2018_an10 | 0 | 0.0133 | 0.0226 | 1.4599 | 32.7883 | 1.0876 | 64.3684 | 99.7401 | Zn0.97Sb0.01Pb0.01S1.01 |
| 2018_an12 | 0 | 0 | 0.0438 | 1.6283 | 31.5759 | 0.8551 | 63.5192 | 97.6223 | Zn0.98Sb0.01S1 |
| 2018_an13 | 0 | 0.0134 | 0 | 1.3705 | 32.2799 | 0.7191 | 63.9183 | 98.3012 | Zn0.98Sb0.01S1.01 |
| 2018_an14 | 0 | 0.0065 | 0 | 1.7952 | 31.8389 | 0.7561 | 63.5187 | 97.9154 | Zn0.98Sb0.01S1 |
| 2018_an15 | 0 | 0.0057 | 0 | 1.685 | 32.1684 | 0.8822 | 63.7386 | 98.4799 | Zn0.98Sb0.01S1.01 |
| KMZ_an1 | 0 | 0.0115 | 0.01 | 2.5884 | 31.6988 | 0.7254 | 61.8225 | 96.8566 | Zn0.97Sb0.02S1.01 |
| KMZ_an2 | 0 | 0.0142 | 0.0601 | 2.6813 | 32.2032 | 0.6121 | 62.1113 | 97.6822 | Zn0.96Sb0.02S1.01 |
| KMZ_an3 | 0 | 0.0379 | 0.1227 | 2.5596 | 31.3526 | 0.8513 | 60.3013 | 95.2254 | Zn0.96Sb0.02S1.02 |
| KMZ_an4 | 0 | 0.0081 | 0.022 | 1.409 | 32.0244 | 0.8022 | 62.0654 | 96.3311 | Zn0.97Sb0.01S1.02 |
| KMZ_an5 | 0 | 0.013 | 0 | 1.7448 | 31.9473 | 0.8585 | 63.3473 | 97.9109 | Zn0.98Sb0.01S1. |
| KMZ_an5 | 0 | 0.013 | 0 | 1.7448 | 31.9473 | 0.8585 | 63.3473 | 97.9109 | Zn0.98Sb0.01S1 |
| Element | Fe | Bi | Sb | Pb | Zn |
|---|---|---|---|---|---|
| Fe | 1 | ||||
| Bi | 0.254121 | 1 | |||
| Sb | −0.10204 | 0.355393 | 1 | ||
| Pb | −0.26614 | −0.18413 | 0.086813 | 1 | |
| Zn | 0.218825 | −0.32911 | −0.85962 | −0.26285 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, G.; Buzatu, A.; Apopei, I.A.; Szakács, Z.L.; Denuț, I.; Iepure, G.; Bârgăoanu, D. Valentinite and Colloform Sphalerite in Epithermal Deposits from Baia Mare Area, Eastern Carpathians. Minerals 2020, 10, 121. https://doi.org/10.3390/min10020121
Damian G, Buzatu A, Apopei IA, Szakács ZL, Denuț I, Iepure G, Bârgăoanu D. Valentinite and Colloform Sphalerite in Epithermal Deposits from Baia Mare Area, Eastern Carpathians. Minerals. 2020; 10(2):121. https://doi.org/10.3390/min10020121
Chicago/Turabian StyleDamian, Gheorghe, Andrei Buzatu, Ionut Andrei Apopei, Zsolt László Szakács, Ioan Denuț, Gheorghe Iepure, and Daniel Bârgăoanu. 2020. "Valentinite and Colloform Sphalerite in Epithermal Deposits from Baia Mare Area, Eastern Carpathians" Minerals 10, no. 2: 121. https://doi.org/10.3390/min10020121
APA StyleDamian, G., Buzatu, A., Apopei, I. A., Szakács, Z. L., Denuț, I., Iepure, G., & Bârgăoanu, D. (2020). Valentinite and Colloform Sphalerite in Epithermal Deposits from Baia Mare Area, Eastern Carpathians. Minerals, 10(2), 121. https://doi.org/10.3390/min10020121





