Abstract
This research was performed to obtain high-value products from clay materials. High-grade nanometric delta-alumina (δ-Al2O3) was obtained from the modification of clay-based minerals, which could be potentially applied in the form of thin film for novel optoelectronic applications. The selective recovery process of alumina from clay materials presents an important advantage regarding the complete removal of other starting constituents such as silica, iron, titanium, alkali, and alkaline earth metals. To accomplish the selective removal of different species, an acid leaching route was used to extract the aluminum, then the iron impurities were eliminated by alkaline precipitation. The solution was acidized to precipitate the aluminum as aluminum chloride hexahydrate. Finally, the aluminum chloride hexahydrate was calcinated to obtain nano-delta-alumina with purity of over 98.5% Al2O3. The dominating crystalline phase was delta–gamma alumina (δ-phase and γ-phase), with a particle size of <140 nm. Then, these nanoparticles (NPs) were prepared as a stable colloidal dispersion to form a mesoporous layer employing the spin-coating technique. Initially, the synthesized alumina was characterized by atomic force microscopy (AFM) and TEM to determine the particle size and its morphology, whereas the colloidal dispersion was analyzed by rheological measurements. Finally, the findings of this investigation made it possible to get thin films with good porosity, which can be used in optoelectronic applications, specifically in perovskite solar cells.
1. Introduction
Bauxite is the world’s primary source of aluminum, and the largest reserves of bauxite are found in Guinea and Australia. In 2018, the annual world production of bauxite amounted to 300 million tons, most of it (74%) produced in Australia (25%), followed by China (23%), Guinea (17%), and Brazil (9%) [1]. After mining, the bauxite is processed into alumina using the Bayer process. Most of the alumina (“metallurgical grade”) is then transformed into aluminum, and the rest of the alumina (“chemical grade”) is used in non-metallurgical applications. Total world alumina production in 2018 was 130.4 million metric tons, from which chemical grade amounted to 8.5 million metric tons. However, due to the depletion of bauxite and increasing demand for alumina, new methods for alumina production have been researched, such as coal fly ash [2].
Clay materials have been evaluated as potential alternative sources of alumina [3], particularly for high-purity alumina (HPA) products. Orbite Technologies Inc. is near the completion of its first HPA commercial production plant in Cap-Chat, Québec [4], and Altech Chemicals Limited started building its HPA plant in February 2019 in Johor, Malaysia [5]. Feedstocks for Orbite´s plant will include red mud from bauxite operations, fly ash, and aluminous clay, among others [6]. Orbite´s aluminous clay deposit is in the Gaspe Region, Québec, Canada [7]. Altech´s plant will produce HPA from kaolin feed shipped from its mine in Meckering, Western Australia [5]. Both Orbite Technologies Inc. and Altech Chemicals Ltd. use acid leaching of their feedstocks with hydrochloric acid to get HPA [6,8].
Aluminous clays and kaolin are being commercialized in Colombia [9] for several industrial applications—mostly for traditional ceramics—and suppliers are currently focusing R&D efforts to add value to these raw materials and to get into more specialized and higher added-value markets. The HPA market attracts special attention, as some Colombian clays contain up to 36 wt % Al2O3, and Colombian washed kaolins can have about 36–38 wt % Al2O3. These alumina grades favorably compare with the 23–26 wt % Al2O3 in the Grande-Vallée aluminous clay deposit (Feytis, 2010) and the 30 wt % Al2O3 in the minus 300 µm fraction in the Meckering deposit [10]. Therefore, a Colombian aluminous clay was selected to study the possibility of using it as an alternative source of alumina production, with special emphasis on the obtention of alumina nanoparticles which can be incorporated into thin films with potential optoelectronic applications.
Clays and kaolins are mainly composed of silicon oxide and aluminum oxide, with iron oxide also present as an impurity which, in some instances, can be present in amounts as high as 4–5 wt % Fe2O3. Obtention of alumina from these materials is usually carried out in aqueous media by a leaching process, which consists of using acid and basic dissolution processes to eliminate undesirable components and obtain high-purity alumina [11]. Several investigations have shown that critical parameters to control the leaching process include acid/base concentration, pH, temperature, time, and molar ratio between acid/base and alumina. It has been shown that high acid concentration favors dissolution of iron and aluminum oxides, whereas, at the same time, silicon oxide remains insoluble [12,13]. Then, removal of dissolved iron from the resulting aluminum–iron solution can be done in an alkaline medium, and an alkaline solution (50 wt %) of sodium hydroxide is used by some researchers for Fe3+ extraction, obtaining dissolved Al3+ in an aqueous medium with low Fe3+ concentration [14,15,16]. In this approach, iron extraction was reached after the chemical reaction between an excess of acid and aluminum chloride, which generated a left-shift of the equilibrium with a resulting higher efficiency in concentration of Al3+ in the solution. This purification stage of the solution with respect to Al3+ comprises Al(OH)3 precipitation, followed by conversion of Al(OH)3 into AlCl3 with the assistance of HCl. The obtained AlCl3 is heated at a temperature sufficient for converting AlCl3 into Al2O3 [17].
One of the controls of the reaction rate of the leaching process and the particle size of the product is pH. It has been found that the reaction progresses at a moderate rate at pH ≤ 7 due to a chelating action that inhibits the grain growth. This inhibition, represented by a Lamer diagram for the synthesis of monodispersed particles, explains the interaction between acidic ions in an aqueous solution, where the acidic ions are strongly adsorbed on the particle surface and, as a result, the crystals remain separated and crystal growth is not promoted. Larger precipitation times favor particle growth since there is a competition between nucleation and particle growth [18,19,20].
On the other hand, to process high-purity alumina, it is necessary to know the physical–chemical properties and structure transformation during calcination. Al2O3 has a transformation of different polymorphic phases: γ → δ → θ → α. The evolution of Al2O3 towards these different phases is due to an atomic reorganization through a crystal lattice (change of oxygen anion positions and cation migration from tetrahedral to octahedral sites); this reorganization depends on the thermal treatment [21]. Thereby, in agreement with the Al2O3 phase diagram, the phases γ-Al2O3 and δ-Al2O3 are formed at temperatures between 700 and 900 °C, θ phase (θ-Al2O3) between 1050 and 1200 °C, and α-Al2O3 around 1400 °C [22,23,24]. In that case, the range of temperatures that are needed is between 700 and 900 °C, given that the phases γ-Al2O3 and δ-Al2O3 are the required phases to guarantee the nano-structure intrinsic to alumina [25].
Once the required material is obtained—in this case, nanometric Al2O3—the stability of its colloidal dispersion is essential for further applications, including thin film formation [26,27]. Therefore, the rheological behavior of colloidal dispersion is another area of study in this work. The properties of colloidal dispersions are intimately linked to the particle size (≤1 µm) and surface area, as well as to the chemical nature of the particle’s surface. In this sense, their interactions are controlled by attractive and repulsive inter-particle forces. The net inter-particle forces are equal to the sum of the attractive van der Waals and the repulsive double-layer forces, called the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory. From this perspective, these interactions can be controlled by manipulation of experimental parameters, such as the solution pH, addition of electrolyte ions, surfactants, polymers, and polyelectrolytes, where the objective is to generate attractive or repulsive forces. In this work, the aim was to first obtain high-purity alumina, and then to formulate a stable colloidal dispersion of the obtained nanoparticles in order to promote a net repulsive inter-particle force of alumina in polar solvent media, using the concept of the electrical double layer [28,29].
Regarding the double-layer forces, there are different mechanisms to generate the electrical double-layer forces, such as ion adsorption, isomorphous ion substitution, differential ion dissolution, and ionization of surface sites [28,30]. The latter process is frequently used in metal oxides, such as alumina and zirconia, where their surfaces involve hydroxy sites (protonation/deprotonation; Equation (1)). The ionization superficially charges particles, promoting a redistribution of the ions in the surrounding solution, with counter-ions preferentially attracted towards and co-ions repelled away from the surface (the combination between counter-ions and co-ions refers to the electrical double layer). Finally, when two similarly charged particles are brought into proximity, their electrical double layers overlap and form a repulsive force. This repulsive force is due to the increased concentration of ions on the surface, which is greater on the surface of the particle than in the bulk aqueous media [28].
Based on what has been previously mentioned, the present work was focused on three different areas of investigation: First, the extraction of high-purity nanoparticles of Al2O3 (98.5 wt %) from aluminous clays by chemical reactions between different reagents, such as hydrochloric acid (HCl) and sodium hydroxide (NaOH). Secondly, the particle size was evaluated and measured, controlling pH and leaching time; the key parameters were: Basic media (NaOH, 50 wt %), acid media (HCl, 15 wt %), holding time (30–60 min), and thermal treatment in a N2 atmosphere in order to control the chloride vapors. The thermal treatment was performed at a 3 °C/min rate until reaching 800 °C over 1 h to mainly obtain δ-Al2O3 phase. Under these conditions, alumina with characteristic peaks of gamma–delta alumina phase (γ-Al2O3, δ-Al2O3) and a particle size of ≤140 nm was obtained. Finally, mesoporous thin films were obtained by preparing nano-Al2O3 stable colloidal nanoparticle (NP) dispersions. It was found that the functionalization of nanoparticles with ammonia, followed by the addition of a slightly polar solvent (butanol or isopropanol) allowed to obtain a stable colloidal dispersion at different concentrations of solids; therefore, preliminary tests were performed between 1 and 10 wt %. It was found that an adequate solid concentration is ≤2 wt %. Then, these colloidal dispersions were used to fabricate mesoporous thin films by the spin-coating technique with a thickness of 60–100 nm and roughness of between 15–20 nm.
2. Materials and Methods
This work is divided into four parts. The first one corresponds to aluminum chloride (AlCl3) extraction from the clay minerals by acid/basic media. The second one is the evaluation of thermal treatment on the alumina phase formation at 800 °C in a nitrogen atmosphere. The third part is the study of the colloidal dispersion of Al2O3 in order to establish the surface chemical equilibrium, and to evaluate the effects of ionization surface sites and the type of solvent in the rheological behavior. The final step is the fabrication of mesoporous thin films from colloidal dispersion of the Al2O3 obtained in the third part by the spin-coating technique; thin-film thickness and roughness were measured. The experimental procedures are described in detail below. Finally, the products obtained from experimental alumina were compared with a reference Aluminum oxide, (Sigma-Aldrich, particle size < 50 nm DLS, 20 wt % in isopropanol, MDL number MFCD00003424).
2.1. Aluminum Chloride Extraction
The Colombian clay material (for more details, see Section 3.1.1) was ground without additives and sieved to the size fraction of 200 mesh (74 µm). Then, the leaching process of clay was carried out into a 500 mL three-neck round flask. This process was thermally controlled using an industrial oil bath. The aluminum chloride extraction was composed of three stages. Initially, acidic leaching assisted by 20 wt % HCl was carried out to extract the quartz phases. Next, the separation of iron ions (Fe3+) from the leaching solution with the addition of 50 wt % NaOH was performed at pH 11. Then, alumina was recovered from the acid leaching liquor with 15 wt % HCl as AlCl3∙6H2O at pH 7. After each stage, the filtration was performed to remove the quartz, iron oxide, and aluminum chloride extraction. The stoichiometric calculations were done by balancing chemical reactions and the molar ratio (Equations (2)–(4)). All of the parameters for each stage are shown in Table 1. It is worth noting that these parameter values were obtained after several tests with different values for temperature, pH, stirring rate, and time.

Table 1.
Parameters for aluminum chloride extraction.
The process of recovery of AlCl3∙6H2O from clay—such as kaolinite/halloysite (Al2O3∙2SiO2∙H2O)—in acidic or basic solutions is a complex process, due to chemical interactions. However, in the present work, the most important component to recover is Al2O3. For this reason and to keep the reaction system simple, only the leaching reactions for this component were studied. The following three reactions (Equations (2)–(4)) correspond to alumina leaching.
Al2O3 + 6HCl → 2AlCl3 + 3H2O
AlCl3 + 3NaOH → Al(OH)3 + 3NaCl
Al(OH)3 + 3HCl + 3H2O → AlCl3∙6H2O
The other compounds present in lower percentages (TiO2, CaO, and ZnO) were dissolved in acid media.
2.2. Thermal Treatment
The obtained compound AlCl3∙6H2O was calcinated in a muffle furnace (Nabertherm LT 15/12/P330, 910 CU.IN, 100–1200 °C, 208 240 VAC) in N2 atmosphere to obtain the γ–δ Al2O3 and to eliminate Cl– ions. The selection of thermal treatment was performed according to the Al2O3 phase diagram [22]. Preliminary thermal treatment tests were performed at 800–900 °C, with a heating rate of 3–5 °C/min, holding time of 60–120 min, and cooling rate of 3–5 °C/min. The best performance was observed at 800 °C, with a heating rate of 3 °C/min, holding time of 60 min, and a cooling rate of 3 °C/min. Equation (5) shows the chemical reactions of AlCl3∙6H2O calcination.
2.3. Alumina Dispersion Process and Mesoporous Thin Film Fabrication
According to the theory, the surface layer of an aluminum (III) ion is a Lewis acid, which can exchange the hydroxyl groups for other coordinating anions [28]. In this sense, the alumina powder was prepared as a stable colloidal nanoparticle (NP) dispersion employing the processes showed in Figure 1. First, the alumina powder was submitted to a physisorption process, which consisted of the addition of the ammonia solution (deprotonation) to charge the alumina surface negatively (functionalized particle). The physisorption process was studied at pHs of 8, 10, and 11. After 24 h of physisorption treatment, the solution was centrifuged and the residual supernatant solution was eliminated. Second, the centrifuged solid was mixed with two polar solvents at different solid concentrations to prepare colloidal dispersions. Slightly polar solvents isopropanol and 1-butanol, as well as solid concentrations between 10 and 20 mg/mL were analyzed. The particle size is essential for a stable colloidal dispersion preparation. To improve the homogeneity of particle size in the colloidal dispersion, a particle size classification was used to eliminate particles over 250 nm using the centrifugation method followed by filtration (Table 2, colloidal dispersion preparation). Furthermore, the alumina dispersion was used to fabricate the mesoporous layer. This was carried out by the spin-coating technique, resulting in a thin film; all of the parameters used are shown in Table 2.
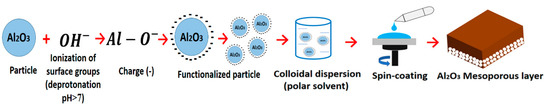
Figure 1.
Alumina dispersion preparation process and spin-coating technique for the manufacture of the mesoporous layer.

Table 2.
Parameters for alumina dispersion preparation and manufacture of mesoporous layer.
2.4. Characterization
The powder was analyzed by different characterization techniques. The chemical composition was studied by X-ray fluorescence (XRF) using an XRF PANanalytical Axios Max (Malvern Panalytical Ltd., Malvern, UK), the crystalline phases after thermal treatments by an X-ray diffraction (XRD; XPert PANanalytical EMPYREAM, Malvern Panalytical Ltd., Malvern, UK) instrument with Co Kα (λ = 1.79 Å) radiation filtered with iron and 2 theta scan from 10° to 90°, and Fourier transform infrared (FT-IR; Shimadzu IRtracer-100, Shimadzu, Kyoto, Japan) in the region 4000–400 cm−1 using an ATR (Attenuated total reflection) accessory for characterizing the types of bonds present in the sample powder; the latter two techniques were used to compare experimental alumina with commercial reference alumina. Rheometer equipment (CSL2 500, TA Instrument, Inc., New Castle, DE, USA) was used to perform the rheological measurements using a concentric cylinder geometry. The particle size was determined by Scanning Electron Microscopy (JSM 5910LV, JEOL, Tokyo, Japan) and Atomic Force Microscopy (MFP-3D infinity AFM, Asylum Research Asylum Research, Oxford Instruments, Goleta, CA, USA). The colloidal dispersion was characterized by transmission electron microscopic analysis (TEM; Tecnai G2 F20 S-Twin TMP field emission, resolution 0.1 nm, accelerating voltage 200 kV, magnification 1,000,000, camera GATAN US 1000XP-P, and detector EDX Oxford Instruments X-MAX, no coating needed).
3. Results and Discussion
3.1. Aluminum Chloride Extraction and Thermal Treatment
3.1.1. Mineral Phases and Chemical Composition of Aluminous Clay
The main mineralogical components in the clay material used for this study are kaolinite/halloysite (Al2O3∙2SiO2∙H2O; >80%) with minor amounts of quartz (SiO2) and gibbsite (Al(OH)3) (see Figure 2). Trace quantities of cristobalite (SiO2) and iron hydroxides were found.
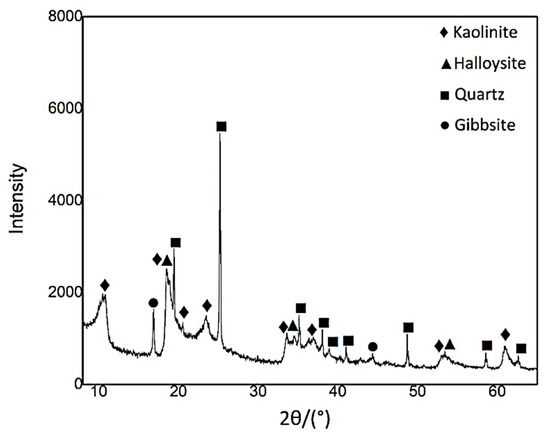
Figure 2.
XRD pattern of aluminous clay.
The chemical composition of aluminous clay was analyzed by XRF analysis, where the major components were silica (SiO2, 47.1%) and alumina (Al2O3, 36.1%), with a lower quantity (≤1%) of other oxides, as it is shown in Table 3.

Table 3.
Chemical composition of aluminous clay used in the study.
3.1.2. Mineral Phase and Chemical Composition of Al2O3
After the aluminum recovery from the kaolinite through the process described in the experimental section, the aluminum chloride obtained was calcined at 800 °C and characterized by scanning electron microscopy (SEM). An agglomerated powder with an average particle size of ≤140 nm was obtained (Figure 3a,b). Additionally, the specific surface area of the nanopowder was 205.04 ± 1.42 m2/g, which was determined using a MALVERN MASTERSIZER 2000 laser diffraction instrument (Malvern Panalytical).
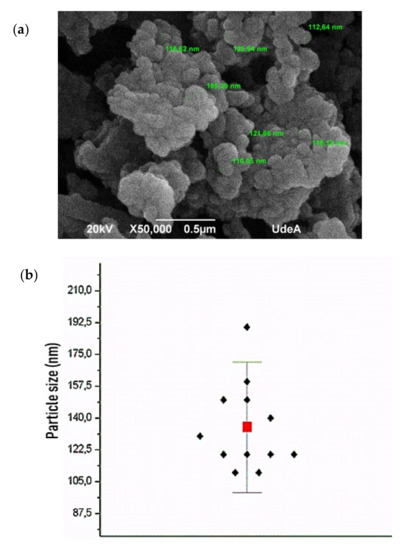
Figure 3.
Obtained Al2O3 nanopowder before colloidal dispersion preparation (a) SEM image and (b) average particle size (red box) and jittered dot plot (black rhombus).
The mineral phases were identified according to XRD patterns with a JCPDS card N° 43-1308 (γ-Al3O3) and JCPDS card N° 46-1131 (δ-Al2O3) (Figure 4b). The formation of δ-Al2O3 was evidenced in the most intense reflections at 80°, 53°, and 43°; we also noticed the formation of the gamma phase in the theta angles 21°, 32.5°, and 45°. However, it was clearly evidenced that our Al2O3 (black line) was less crystalline than the commercial reference Al2O3 dispersion (red line). For this reason, the synthesized Al2O3 presented more γ-Al2O3 phase than the reference Al2O3 [31].
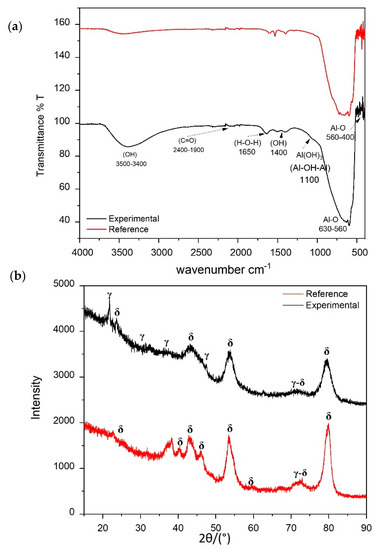
Figure 4.
Comparative (a) FT-IR spectra of the synthesized Al2O3 and a commercial reference (Sigma Aldrich)—transmittance in a.u. (arbitrary unit)—and (b) XRD pattern.
On the other hand, the FT-IR analysis of Al2O3 (Figure 4a) showed bands at 560–400 and 560–630 cm−1, which were attributed to A–O stretching. The weak band at 1100 cm−1 was assigned to Al–OH–Al stretching. The bands at 1400 and 1650 cm−1 correspond to H–O–H stretching, and the bands at 3400–3500 cm−1 to water adsorption. In this case, the only difference between these two types of alumina was related to adsorbed water.
The chemical composition of Al2O3 was obtained by XRF analysis, where the major percentage is alumina (Al2O3, 98.5%), with a low amount of quartz (SiO2, 0.5%) and trace amounts of other compounds (≤1%). These results are summarized in Table 4.

Table 4.
Chemical composition of the synthesized Al2O3. (N.D.: not detected)
3.2. Alumina Dispersion Process and Manufacture of the Mesoporous Layer
3.2.1. Morphological Analysis
In order to remove larger particles, a particle size classification (as discussed in the experimental Section 2.3) was performed. The obtained particles were characterized by transmission electron microscopy (TEM). As shown in Figure 5, the synthesized Al2O3 nanoparticles exhibited particle size below 50 nm and a semi-crystalline structure, formed by non-interconnected grains of semi-spherical alumina nanoparticles. The crystal lattice was calculated according to Bragg’s law λL = dhkl R, where: λ = wavelength, L = camera length (mm), and dhkl = lattice spacing (nm) with the small angle approximation. According to this, the diffraction rings exhibited average diameters of 110.29, 133.73, and 118.16 nm, leading to interplanar spacings of 2.35, 1.94, and 1.38 Å, respectively. From the characteristic lattice planes found in the diffraction pattern for δ-Al2O3, those interplanar spacings can be indexed to the ⟨311⟩, ⟨400⟩, and ⟨440⟩ planes, respectively. Finally, these crystal lattices correspond to the most intense reflections in the XRD spectra in Figure 4b, indicating that alumina composition did not change after the classification and dispersion.
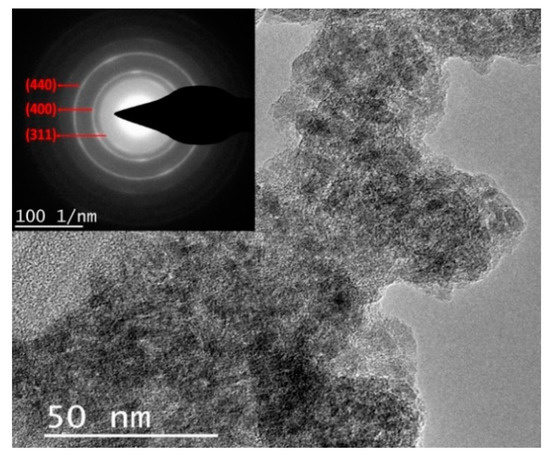
Figure 5.
Morphological analysis by TEM and electron diffraction pattern from the experimental alumina.
3.2.2. Rheological Behavior Measurements
After the physisorption process, an electric layer was formed on the alumina surface due to the excess of hydroxyl groups, leaving the surface negatively charged (δ−). Once the surface-modified nanoparticles get in contact with the polar solvents (isopropanol and 1-butanol), this negative charge (δ−) interacts with the positive charge (δ+) of the protonated chain, and the charge (δ−) of alcohol interacts with the aluminum, forming dipole–dipole interactions and resulting in a stable colloidal dispersion.
Figure 6a shows the viscosity results obtained from the rheological measurements in a room with automatic temperature control (approximately 20 °C). Clear differences were observed in the behaviors of the dispersions with different polar solvents and pHs. In the case of butanol, the viscosity increased with the pH. While there was a different effect for the case of isopropanol, which decreased its viscosity when going from pH = 8 to pH = 10, and then increased at pH = 11. Images of the dispersion in Figure 6b show that butanol-based dispersions (upper) are less stable and tend to flocculate, while the isopropanol-based dispersions (lower) showed better stability. It can be inferred that the decrease of viscosity for isopropanol at pH = 10 and the later increase at pH = 11 is due to the better functionalization at pH = 10, and then the starting of the flocculation at pH = 11.
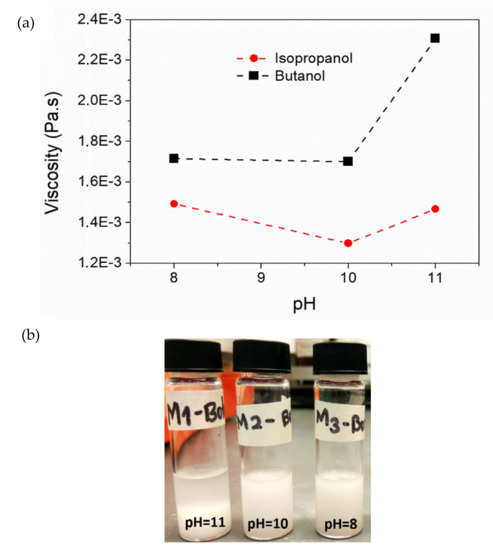
Figure 6.
(a) Influence of polar solvent and pH on the viscosity of the Al2O3 dispersions with a solid concentration of 20 mg/mL; (b) images of dispersions in butanol (left) and isopropanol (right).
3.2.3. Mesoporous Layer
In order to demonstrate the applicability of the obtained dispersions, an Al2O3 thin film was deposited on glass using the colloidal dispersion in isopropanol and pH = 10. Figure 7 shows an AFM image of a film deposited by spin-coating. The mesoporous film shows a good porosity that could potentially serve as a mesoporous scaffold for the fabrication of meso-superstructure perovskite solar cells, as reported elsewhere [32]. However, more studies are needed to demonstrate this application.
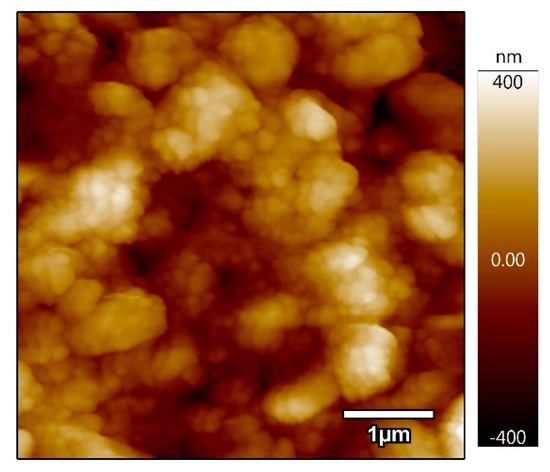
Figure 7.
Atomic force microscopy (AFM) image of the Al2O3 mesoporous thin film.
4. Conclusions
The high-grade nanometric delta-alumina (δ-Al2O3) was obtained from the modification of clay-based minerals by a process that included the selective removal of different species and calcination. First, the clay mineral was leached, the iron impurities were eliminated by alkaline precipitation, and the aluminum was precipitated as aluminum chloride hexahydrate by acidification with HCl. Finally, the aluminum chloride hexahydrate was calcinated to obtain nano-delta-alumina with purity of over 98.5% Al2O3. The dominating crystalline phase was delta–gamma alumina (δ-phase and γ-phase), with a particle size of <140 nm.
A stable colloidal dispersion was developed by understanding and controlling the synthesis of Al2O3 from clay materials and implementing a surface modification to modify the rheological behavior of inter-particle forces. The surface modification by adjusting the pH of the solution and the ionization surfaces on metal oxides can be used to control the electrical layer repulsion between particles and to obtain dispersed conditions. The best pH condition was 10 in isopropanol, resulting in a colloidal dispersion adequate for the manufacture of mesoporous layers. In general, the nano-alumina obtained from a Colombian clay material exhibited the properties required for obtaining thin films with potential optoelectronic applications, particularly as a mesoporous layer in perovskite solar cells. However, further studies are necessary to identify the best application in optoelectronics.
Author Contributions
Conceptualization, F.J. and J.E.L.-R.; methodology, Y.L.B. and J.E.L.-R.; characterization analysis, Y.L.B., D.R., and D.M.Z.; investigation, Y.L.B.; writing—original draft preparation, Y.L.B.; writing—review and editing, F.J., J.E.L.-R., D.R., D.M.Z., and Y.L.B.; supervision, F.J.; project administration, F.J.; funding acquisition, F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Colombia Scientific Program within the framework of the call Ecosistema Científico (Contract No. FP44842-218-2018).
Acknowledgments
Y.L. Botero wants to thank CONICYT PIA program ACM170005 and the Universidad de Antofagasta (Chile) for their support. The authors thank Luis A. Cisternas for helping in the review process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, E.L. Bauxite and Alumina. In USGS—Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2019; pp. 30–31. [Google Scholar]
- Guo, C.; Zou, J.; Ma, S.; Yang, J.; Wang, K. Alumina extraction from coal fly ash via low-temperature potassium bisulfate calcination. Minerals 2019, 9, 585. [Google Scholar] [CrossRef]
- Baba, A.A.; Raji, M.A.; Muhammed, M.O.; Abdulkareem, A.Y.; Olasinde, F.T.; Ayinla, K.I.; Adekola, F.A.; Bale, R.B. Potential of a Nigerian biotite-rich kaolinite ore to industrial alumina by hydrometallurgical process. Metall. Res. Technol. 2019, 116, 222. [Google Scholar] [CrossRef]
- Orbite Technologies Inc. Orbite Provides Corporate Update. Available online: http://www.orbitetech.com/English/investors/news/news-details/2019/Orbite-Provides-Corporate-Update-June-2019/default.aspx (accessed on 9 June 2019).
- Stoneham, A. “White Gold” in a Green Revolution. Aust. Resour. Investig. 2019, 13, 17–18. [Google Scholar]
- Orbite Technologies Inc. The Orbite Process. Available online: http://www.orbitetech.com/English/technology/the-orbite-process/default.aspx (accessed on 6 September 2019).
- Feytis, A. Grande-Vallée Aluminous Clays; Industrial Minerals News. Available online: https://www.indmin.com/Article/2378443/Grande-Valle-aluminous-clays.html (accessed on 6 September 2019).
- Altec Chemicals Limited HPA Processing Technology. Recuperado el 01 de June de 2019. Available online: https://www.altechchemicals.com/hpa-processing-technology (accessed on 6 September 2019).
- Villaquirán-Caicedo, M.A.; De Gutiérrez, R.M.; Gordillo, M.; Gallego, N.C. Synthesis of zeolites from a low-quality Colombian kaolin. Clays Clay Miner. 2016, 64, 75–85. [Google Scholar] [CrossRef]
- Altech Chemicals Limited. Annual Report for the Year Ended 30 June 2018; Altech Chemicals: Subiaco, Australia, 2018; Volume 118. [Google Scholar]
- Nayak, N.; Panda, C.R. Aluminium extraction and leaching characteristics of Talcher Thermal Power Station fly ash with sulphuric acid. Fuel 2010, 89, 53–58. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, S.; Zhu, F.; Tan, X.; Li, W.; Zhang, P.; Miao, S. Digesting high-aluminum coal fly ash with concentrated sulfuric acid at high temperatures. Hydrometallurgy 2018, 180, 41–48. [Google Scholar] [CrossRef]
- Cui, L.; Guo, Y.; Wang, X.; Du, Z.; Cheng, F. Dissolution kinetics of aluminum and iron from coal mining waste by hydrochloric acid. Chin. J. Chem. Eng. 2014, 23, 5–11. [Google Scholar] [CrossRef]
- Boudreault, R.; Fournier, J.; Primeau, D.; Dumont, H.; Samuel, J.F.; Boudreault, J. Methods for Purifying Aluminium Ions. WIPO Patent WO 2014/075173 A1, 2014. [Google Scholar]
- Cui, L.; Cheng, F.; Zhou, J. Preparation of high purity AlCl3·6H2O crystals from coal mining waste based on iron (III) removal using undiluted ionic liquids. Sep. Purif. Tecnol. 2016, 167, 45–54. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Yu, J. Removal of ferric ions from aluminum solutions by solvent extraction, part I: Iron removal. Sep. Purif. Tecnol. 2016, 159, 18–22. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, H.; Yang, X.; Cheng, F. AlCl3.6H2O Recovery from the Acid Leaching Liquor of Coal Gangue by Using Concentrated Hydrochloric Inpouring. Sep. Purif. Tecnol. 2015, 151, 177–183. [Google Scholar] [CrossRef]
- Carrado, K.A.; Decarreau, A.; Petit, S.; Bergaya, F.; Lagaly, G. Chapter 4: Synthetic clay minerals and purification of natural clays. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 115–139. [Google Scholar]
- Otterstedt, J.-E.; Brandreth, D.A. Small Particles Technology; Otterstedt, J.-E., Brandreth, D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Saito, F.; Baron, M.; Dodds, J.; Muramatsu, A. Morphology Control of Materials and Nanoparticles: Chapiter I and II; Waseda, Y., Muramatsu, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Azar, M. Mise en Forme et Frittage des Poudres de Céramique Nanostructurées: Cas d’une Alumine de Transition; L’Institut National des Sciences Appliqées Lyon: Villeurbanne, France, 2009. [Google Scholar]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Technical Paper No. 19; Aluminium Company of America: Pittsburgh, PA, USA, 1987; Volume 19. [Google Scholar]
- Lamouri, S.; Hamidouche, M.; Bouaouadja, N.; Belhouchet, H.; Garnier, V.; Fantozzi, G.; Trelkat, J.F. Control of the γ-alumina to α-alumina phase transformation for an optimized alumina densification. Bol. La Soc. Esp. Ceram. Y Vidr. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Barron, A.R. Aluminum Oxides, Hydroxides, and Hydrated Oxides; OpenStax-CNX module m32521, Version 1.2. Available online: http://cnx.org/content/m32521/1.2/ (accessed on 25 January 2010).
- Gómez Rosales, Z.E. Determinación de la Estabilidad de las Fases Cristalinas de la Alúmina (Al2O3). Master’s Thesis, Universidad Industrial de Santander, Bucaramanga, Colombia, 2006. [Google Scholar]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of halloysite, imogolite, and boron nitride nanotubes in solvent media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Lazzara, G. Colloidal stability of halloysite clay nanotubes. Ceram. Int. 2019, 45, 2858–2865. [Google Scholar] [CrossRef]
- Pashley, R.; Karaman, M. Applied Colloid and Surface Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2004; Volume 143, p. 188. [Google Scholar]
- Pek-Ing, A.; Yee-Kwong, L. Surface chemistry and rheology of Laponite dispersions-Zeta potential, yield stress, ageing, fractal dimension and pyrophosphate. Appl. Clay Sci. 2015, 107, 36–45. [Google Scholar] [CrossRef]
- Johnson, S.B.; Franks, G.V.; Scales, P.J.; Boger, D.V.; Healy, T.W. Surface chemistry—Rheology relationships in concentrated mineral suspensions. Int. J. Miner. Process. 2000, 58, 267–304. [Google Scholar] [CrossRef]
- Feret, F.R.; Roy, D.; Boulanger, C. Determination of alpha and beta alumina in ceramic alumina by X-ray diffraction. Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 1051–1061. [Google Scholar] [CrossRef]
- Ramirez, D.; Schutt, K.; Montoya, J.F.; Mesa, S.; Lim, J.; Snaith, H.J.; Jaramillo, F. Meso-superstructured perovskite solar cells: Revealing the role of the mesoporous layer. J. Phys. Chem. C 2018, 122, 21239–21247. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).