A New Reference for the Thermal Equation of State of Iron
Abstract
1. Introduction
2. Methods
2.1. Samples
2.2. High Pressure Laser Heating
2.3. Temperature Measurement
2.4. X-Ray Optics and Diffraction
2.5. Pressure Measurements
2.6. Equation of State and Thermal Model
3. Results and Discussion
3.1. Iron Structure at High-Pressure and High-Temperature
3.2. Room Temperature Equation of State
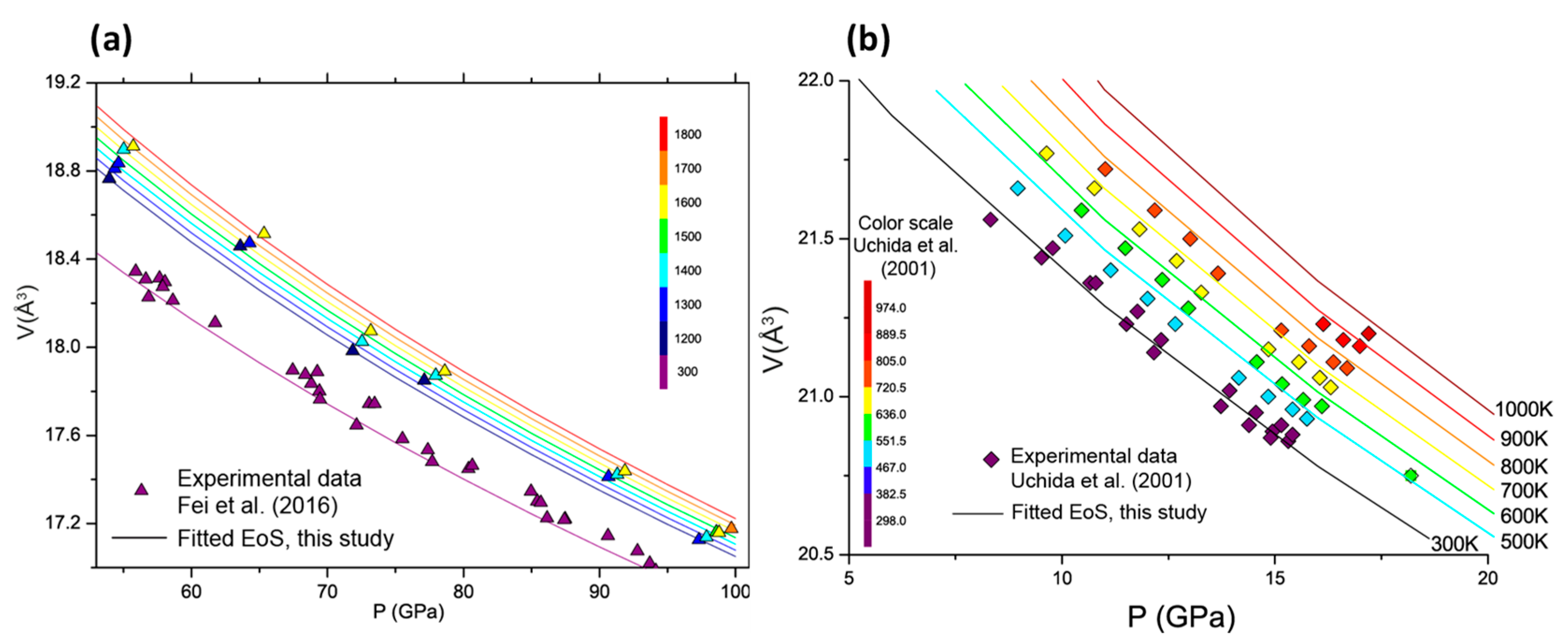
3.3. Thermal Model
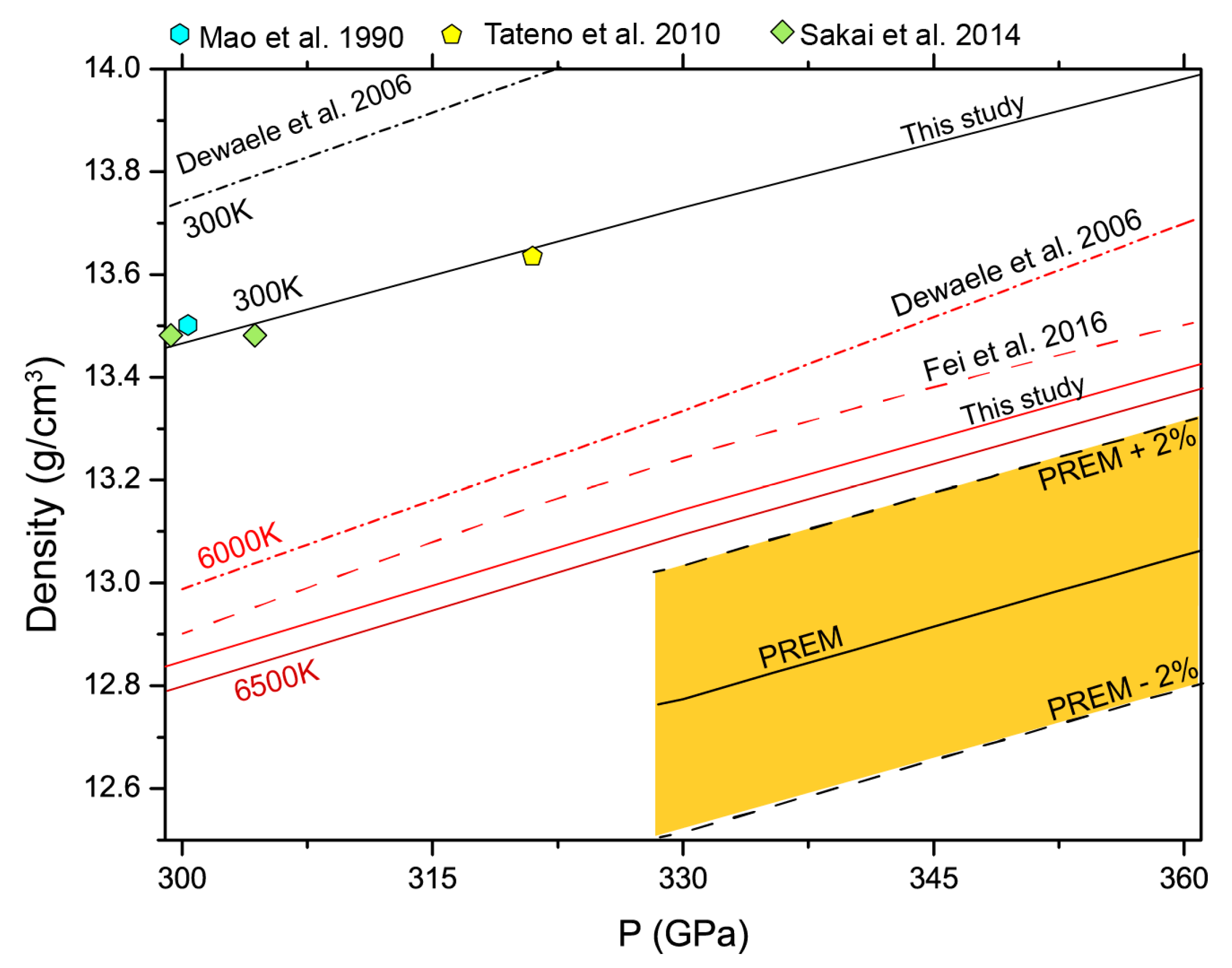
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birch, F. Elasticity and constitution of the Earth’s interior. J. Geophys. Res. 1952, 57, 227–286. [Google Scholar] [CrossRef]
- Anderson, O.L. Mineral physics of iron and of the core. Rev. Geophys. 1995, 33, 429. [Google Scholar] [CrossRef]
- Boehler, R. Temperatures in the Earth’s core from melting-point measurements of iron at high static pressures. Nature 1993, 363, 534–536. [Google Scholar] [CrossRef]
- Ma, Y.; Somayazulu, M.; Shen, G.; Mao, H.; Shu, J.; Hemley, R.J. In situ X-ray diffraction studies of iron to Earth-core conditions. Phys. Earth Planet. Inter. 2004, 143, 455–467. [Google Scholar] [CrossRef]
- Fiquet, G. Sound Velocities in Iron to 110 Gigapascals. Science 2001, 291, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Tateno, S.; Hirose, K.; Ohishi, Y.; Tatsumi, Y. The structure of iron in earth’s inner core. Science 2010, 330, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.S.; Akella, J.; Campbell, A.J.; Mao, H.K.; Hemley, R.J. Phase Diagram of Iron by in Situ X-ray Diffraction: Implications for Earth’s Core. Science 1995, 270, 1473–1475. [Google Scholar] [CrossRef]
- Dubrovinsky, L.S.; Saxena, S.K.; Tutti, F.; Rekhi, S.; LeBehan, T. In Situ X-Ray Study of Thermal Expansion and Phase Transition of Iron at Multimegabar Pressure. Phys. Rev. Lett. 2000, 84, 1720–1723. [Google Scholar] [CrossRef]
- Morard, G.; Boccato, S.; Rosa, A.D.; Anzellini, S.; Miozzi, F.; Henry, L.; Garbarino, G.; Mezouar, M.; Harmand, M.; Guyot, F.; et al. Solving Controversies on the Iron Phase Diagram Under High Pressure. Geophys. Res. Lett. 2018, 45, 11074–11082. [Google Scholar] [CrossRef]
- Poirier, J. Light elements in the Earth’s outer core: A critical review. Phys. Earth Planet. Inter. 1994, 85, 319–337. [Google Scholar] [CrossRef]
- Antonangeli, D.; Siebert, J.; Badro, J.; Farber, D.L.; Fiquet, G.; Morard, G.; Ryerson, F.J. Composition of the Earth’s inner core from high-pressure sound velocity measurements in Fe–Ni–Si alloys. Earth Planet. Sci. Lett. 2010, 295, 292–296. [Google Scholar] [CrossRef]
- Badro, J.; Cote, A.S.; Brodholt, J.P. A seismologically consistent compositional model of Earth’s core. Proc. Natl. Acad. Sci. 2014, 111, 7542–7545. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Allègre, C.J.; Poirier, J.-P.; Humler, E.; Hofmann, A.W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 1995, 134, 515–526. [Google Scholar] [CrossRef]
- Kuwayama, Y.; Hirose, K.; Sata, N.; Ohishi, Y. Phase relations of iron and iron–nickel alloys up to 300 GPa: Implications for composition and structure of the Earth’s inner core. Earth Planet. Sci. Lett. 2008, 273, 379–385. [Google Scholar] [CrossRef]
- Belonoshko, A.B. Equation of state for ∈-iron at high pressures and temperatures. Condens. Matter Phys. 2010, 13, 23605–23615. [Google Scholar] [CrossRef][Green Version]
- Sha, X.; Cohen, R.E. First-principles thermal equation of state and thermoelasticity of hcp Fe at high pressures. Phys. Rev. B 2010, 81, 094105. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Dymshits, A.M.; Litasov, K.D.; Sokolova, T.S. Thermodynamics and Equations of State of Iron to 350 GPa and 6000 K. Sci. Rep. 2017, 7, 41863. [Google Scholar] [CrossRef]
- Funamori, N.; Yagi, T.; Uchida, T. High-pressure and high-temperature in situ x-ray Diffraction study of iron to above 30 Gpa using MA8-type apparatus. Geophys. Res. Lett. 1996, 23, 953–956. [Google Scholar] [CrossRef]
- Uchida, T.; Wang, Y.; Rivers, M.L.; Sutton, S.R. Stability field and thermal equation of state of ε-iron determined by synchrotron X-ray diffraction in a multianvil apparatus. J. Geophys. Res. Solid Earth 2001, 106, 21799–21810. [Google Scholar] [CrossRef]
- Yamazaki, D.; Ito, E.; Yoshino, T.; Yoneda, A.; Guo, X.; Zhang, B.; Sun, W.; Shimojuku, A.; Tsujino, N.; Kunimoto, T.; et al. P-V-T equation of state for ε -iron up to 80 GPa and 1900 K using the Kawai-type high pressure apparatus equipped with sintered diamond anvils. Geophys. Res. Lett. 2012, 39, 2012GL053540. [Google Scholar] [CrossRef]
- Mao, H.K.; Wu, Y.; Chen, L.C.; Shu, J.F.; Jephcoat, A.P. Static compression of iron to 300 GPa and Fe 0.8 Ni 0.2 alloy to 260 GPa: Implications for composition of the core. J. Geophys. Res. 1990, 95, 21737. [Google Scholar] [CrossRef]
- Jephcoat, A.P.; Mao, H.K.; Bell, P.M. Static compression of iron T78 GPa with rare gas solids as pressure-transmitting media. In Elastic Properties and Equations of State; American Geophysical Union: Washington, DC, USA, 1988; Volume 91, pp. 524–531. [Google Scholar]
- Dewaele, A.; Loubeyre, P.; Occelli, F.; Mezouar, M.; Dorogokupets, P.I.; Torrent, M. Quasihydrostatic Equation of State of Iron above 2 Mbar. Phys. Rev. Lett. 2006, 97, 215504. [Google Scholar] [CrossRef]
- Fei, Y.; Murphy, C.; Shibazaki, Y.; Shahar, A.; Huang, H. Thermal equation of state of hcp-iron: Constraint on the density deficit of Earth’s solid inner core. Geophys. Res. Lett. 2016, 43, 6837–6843. [Google Scholar] [CrossRef]
- Sakai, T.; Takahashi, S.; Nishitani, N.; Mashino, I.; Ohtani, E.; Hirao, N. Equation of state of pure iron and Fe0.9Ni0.1 alloy up to 3Mbar. Phys. Earth Planet. Inter. 2014, 228, 114–126. [Google Scholar] [CrossRef]
- Schultz, E.; Mezouar, M.; Crichton, W.; Bauchau, S.; Blattmann, G.; Andrault, D.; Fiquet, G.; Boehler, R.; Rambert, N.; Sitaud, B.; et al. High Pressure–High Temperature Monochromatic X-Ray Diffraction At the Esrf. High Press. Res. 2005, 25, 71–83. [Google Scholar] [CrossRef]
- Mezouar, M.; Crichton, W.A.; Bauchau, S.; Thurel, F.; Witsch, H.; Torrecillas, F.; Blattmann, G.; Marion, P.; Dabin, Y.; Chavanne, J.; et al. Development of a new state-of-the-art beamline optimized for monochromatic single-crystal and powder X-ray diffraction under extreme conditions at the ESRF. J. Synchrotron Radiat. 2005, 12, 659–664. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B.; Alamos, L. Gsas. Rep. lAUR 1994, 1994, 86–748. [Google Scholar]
- Speziale, S.; Zha, C.-S.; Duffy, T.S.; Hemley, R.J.; Mao, H. Quasi-hydrostatic compression of magnesium oxide to 52 GPa: Implications for the pressure-volume-temperature equation of state. J. Geophys. Res. Solid Earth 2001, 106, 515–528. [Google Scholar] [CrossRef]
- Tange, Y.; Nishihara, Y.; Tsuchiya, T. Unified analyses for P-V-T equation of state of MgO: A solution for pressure-scale problems in high P-T experiments. J. Geophys. Res. 2009, 114, B03208. [Google Scholar] [CrossRef]
- Dorogokupets, P.I. P–V–T equations of state of MgO and thermodynamics. Phys. Chem. Miner. 2010, 37, 677–684. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673. [Google Scholar] [CrossRef]
- Datchi, F.; LeToullec, R.; Loubeyre, P. Improved calibration of the SrB4O7:Sm2+optical pressure gauge: Advantages at very high pressures and high temperatures. J. Appl. Phys. 1997, 81, 3333–3339. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Dziewonski, A.M.; Anderson, D.L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 1981, 25, 297–356. [Google Scholar] [CrossRef]
- Alfè, D.; Gillan, M.; Price, G. Composition and temperature of the Earth’s core constrained by combining ab initio calculations and seismic data. Earth Planet. Sci. Lett. 2002, 195, 91–98. [Google Scholar] [CrossRef]

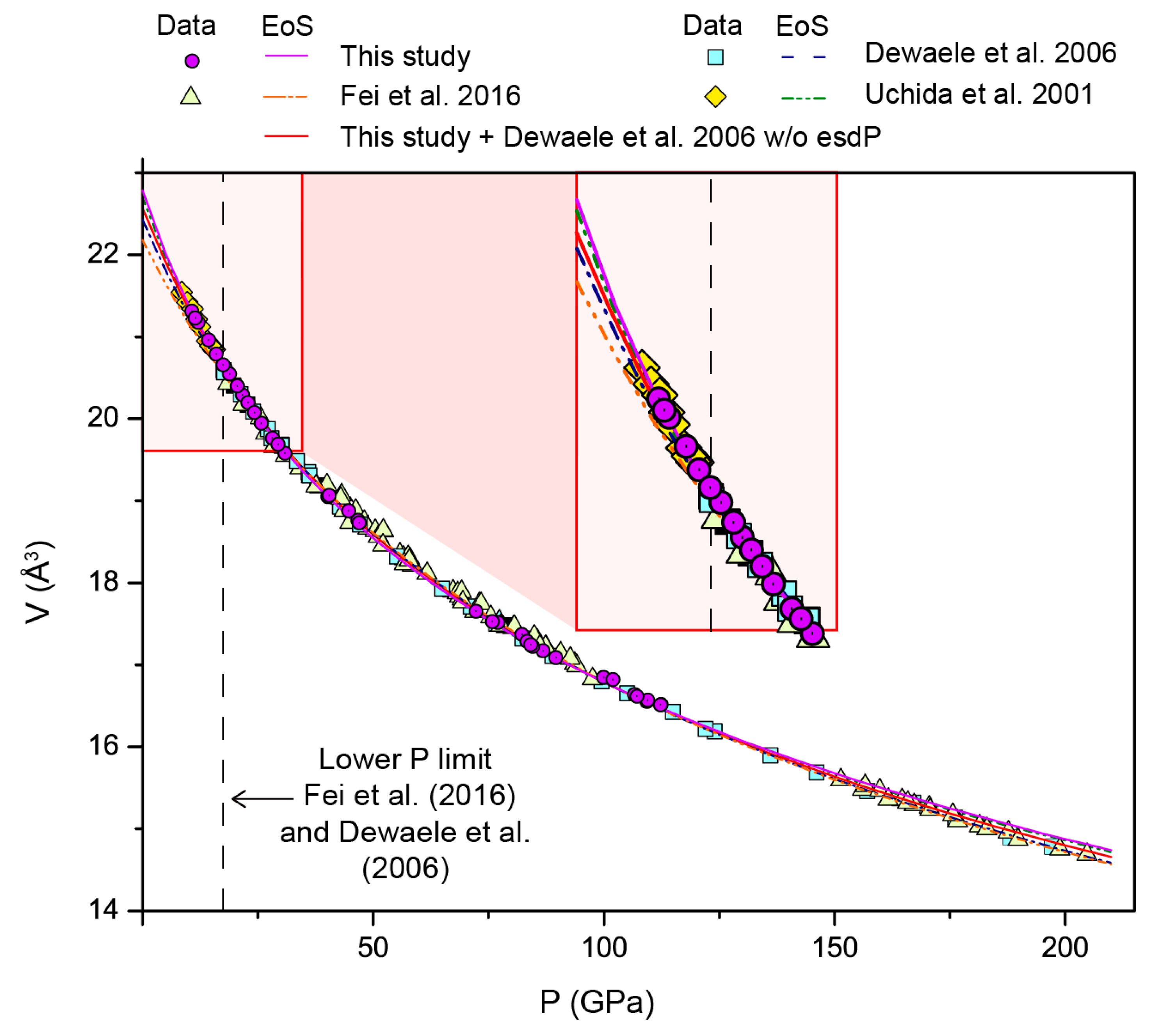
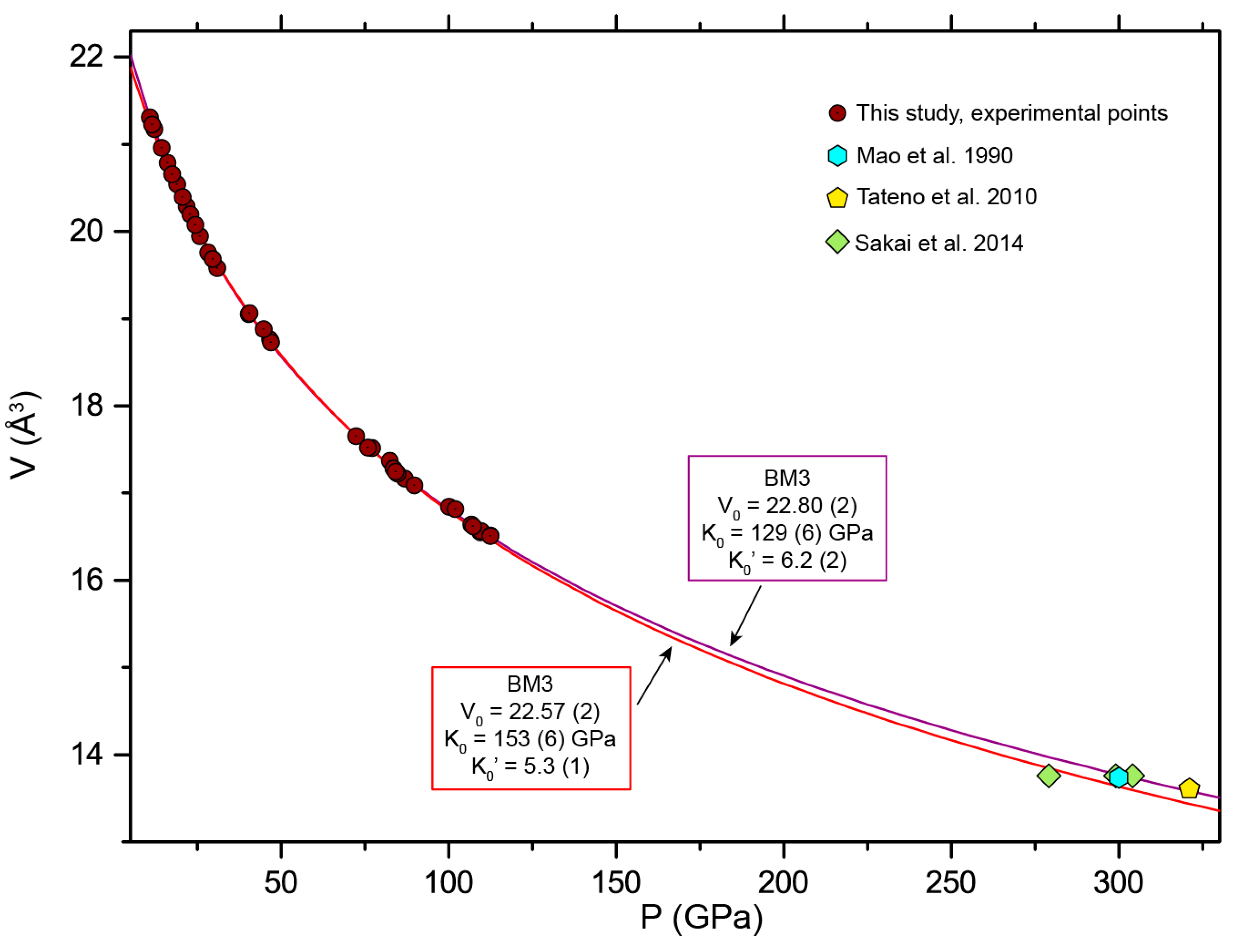
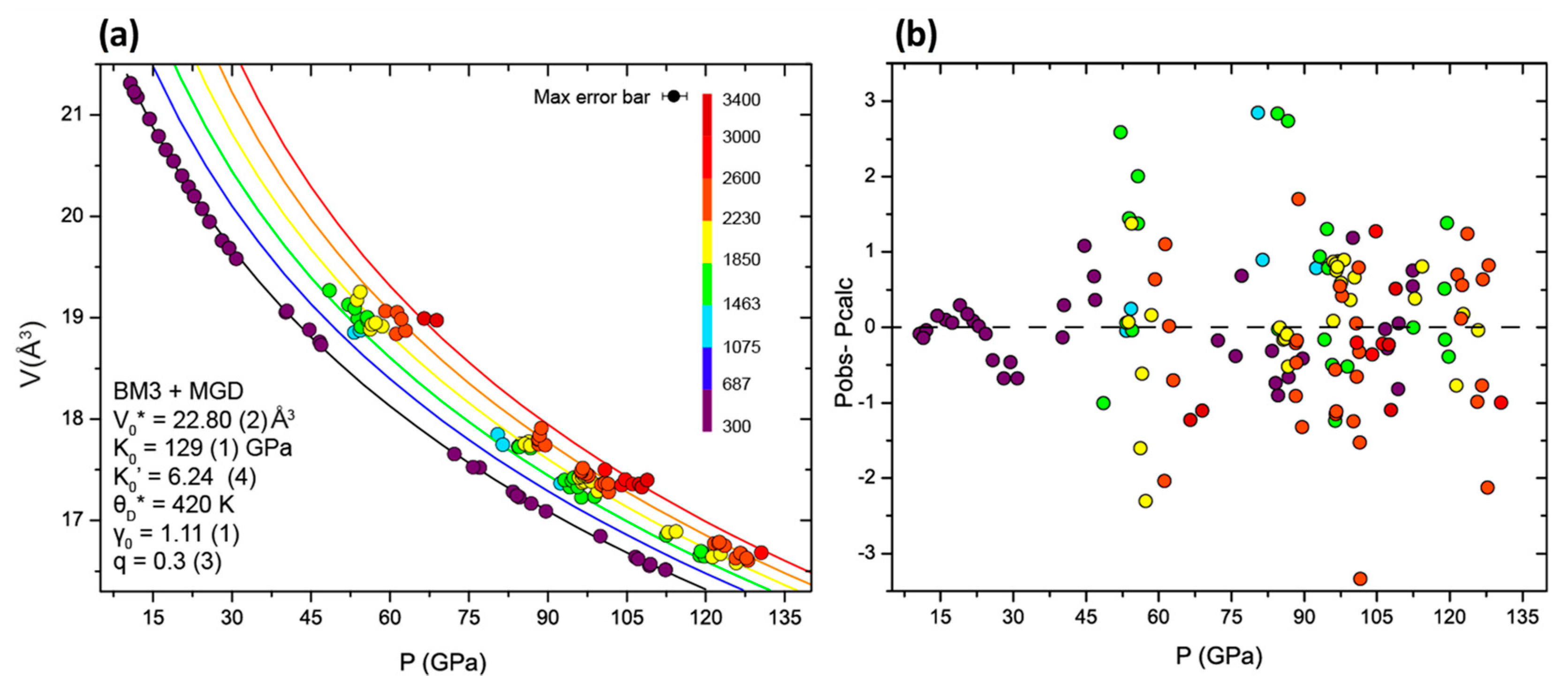
| This Study | This Study + Data from Dewaele et al. 2006 [24] | Fei et al. 2016 [25] | Dewaele et al. 2006 [24] | Uchida et al. 2001 [20] | ||||
|---|---|---|---|---|---|---|---|---|
| with esdP | w/o esdP | |||||||
| Formalism | BM | Vinet | BM | BM | BM | Vinet | BM | |
| V0 (Å3) | 22.80 (2) | 22.81 | 22.75 (1) | 22.57 (1) | 22.18 | 22.47 | 22.43 | 22.7 (3) |
| K0 (GPa) | 129 (6) | 125 (5) | 134 (5) | 153 (3) | 191 (5) | 165 * | 163 (8) | 135 (19) |
| K0’ | 6.2 (2) | 6.5 (2) | 6.1 (2) | 5.3 (1) | 4.5 (1) | 4.97 (4) | 5.4 (2) | 6 (0.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miozzi, F.; Matas, J.; Guignot, N.; Badro, J.; Siebert, J.; Fiquet, G. A New Reference for the Thermal Equation of State of Iron. Minerals 2020, 10, 100. https://doi.org/10.3390/min10020100
Miozzi F, Matas J, Guignot N, Badro J, Siebert J, Fiquet G. A New Reference for the Thermal Equation of State of Iron. Minerals. 2020; 10(2):100. https://doi.org/10.3390/min10020100
Chicago/Turabian StyleMiozzi, Francesca, Jan Matas, Nicolas Guignot, James Badro, Julien Siebert, and Guillaume Fiquet. 2020. "A New Reference for the Thermal Equation of State of Iron" Minerals 10, no. 2: 100. https://doi.org/10.3390/min10020100
APA StyleMiozzi, F., Matas, J., Guignot, N., Badro, J., Siebert, J., & Fiquet, G. (2020). A New Reference for the Thermal Equation of State of Iron. Minerals, 10(2), 100. https://doi.org/10.3390/min10020100





