Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia
Abstract
1. Introduction
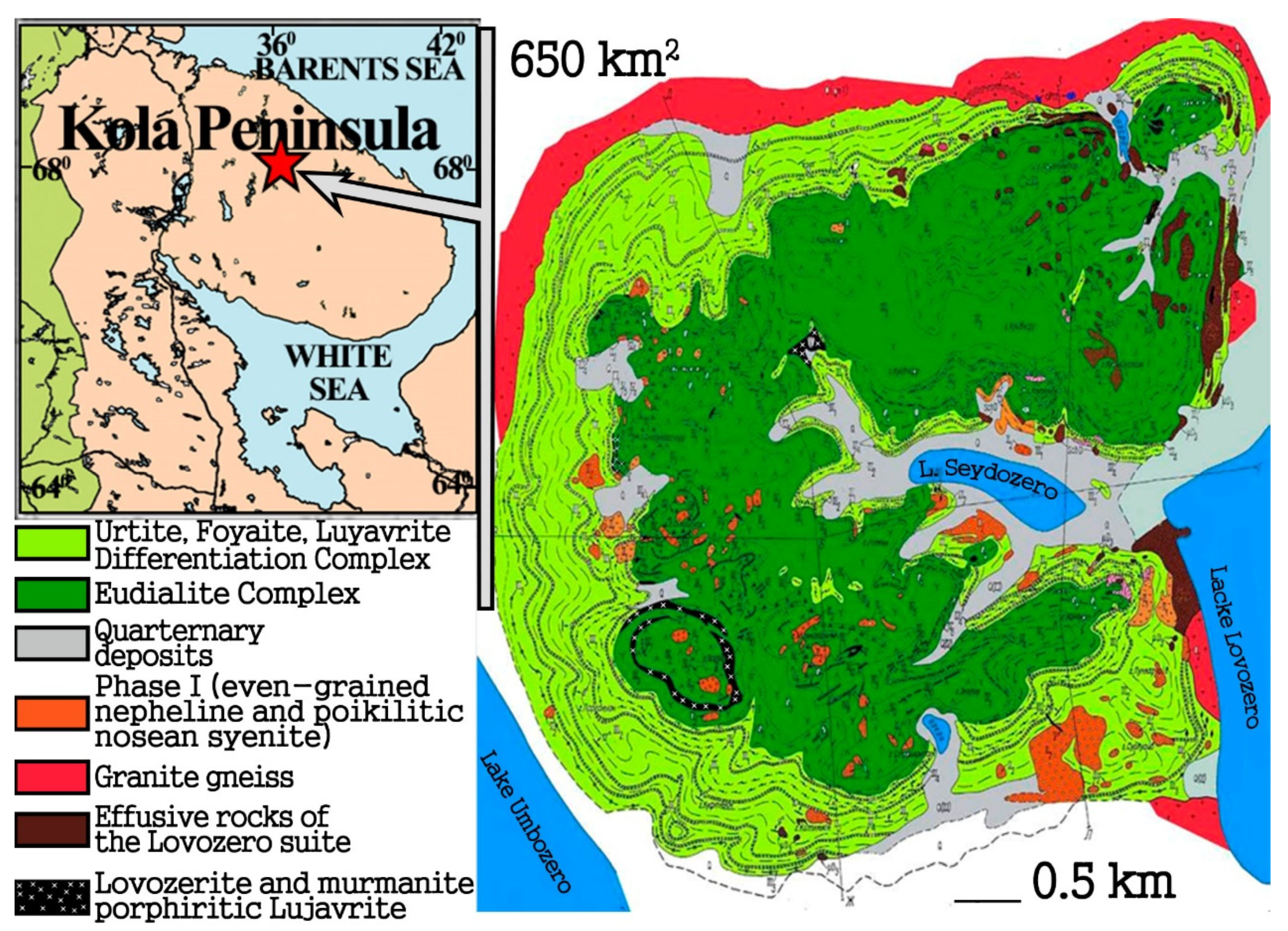
2. Geology
3. Samples and Analytical Method
4. Results
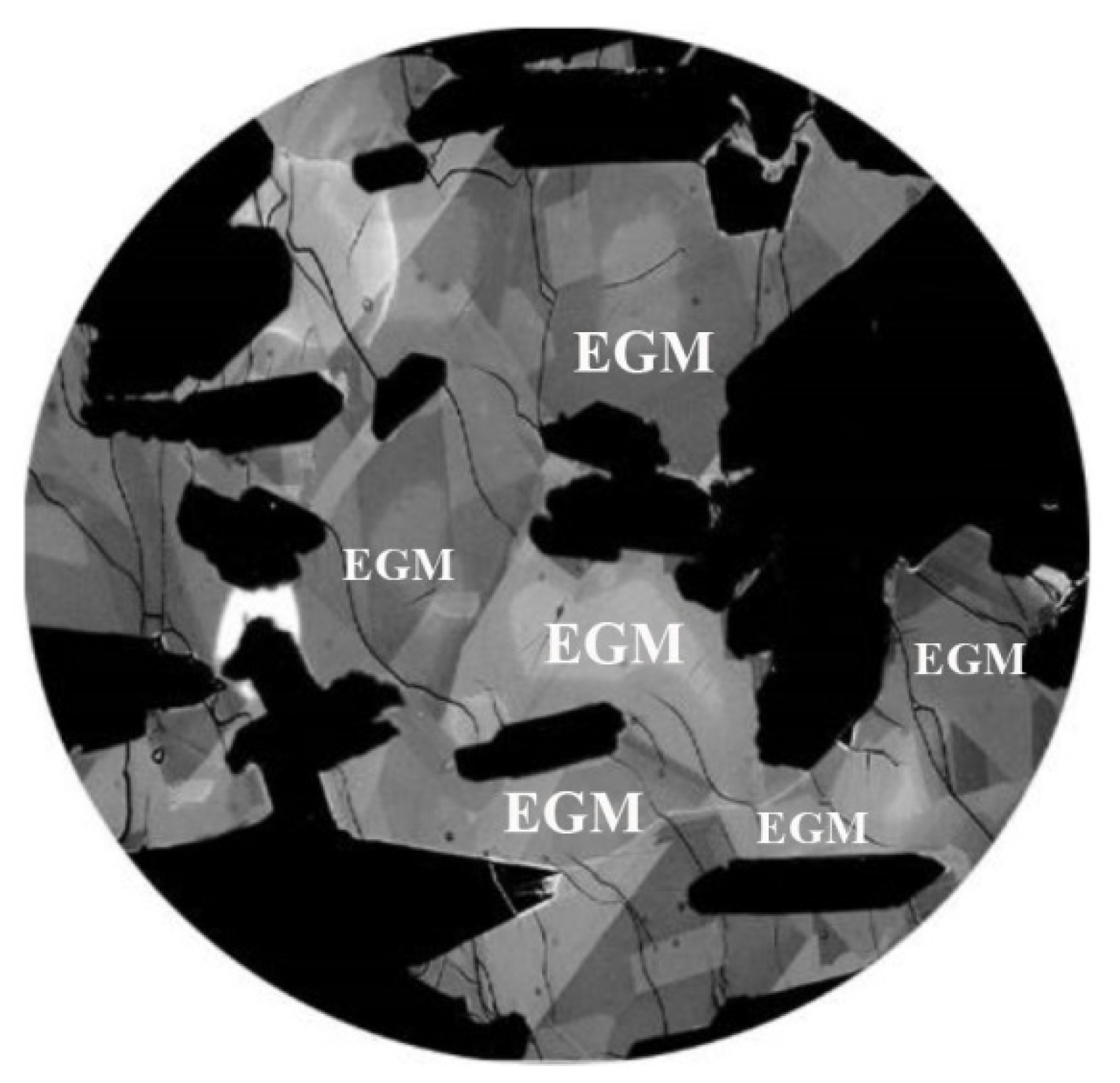
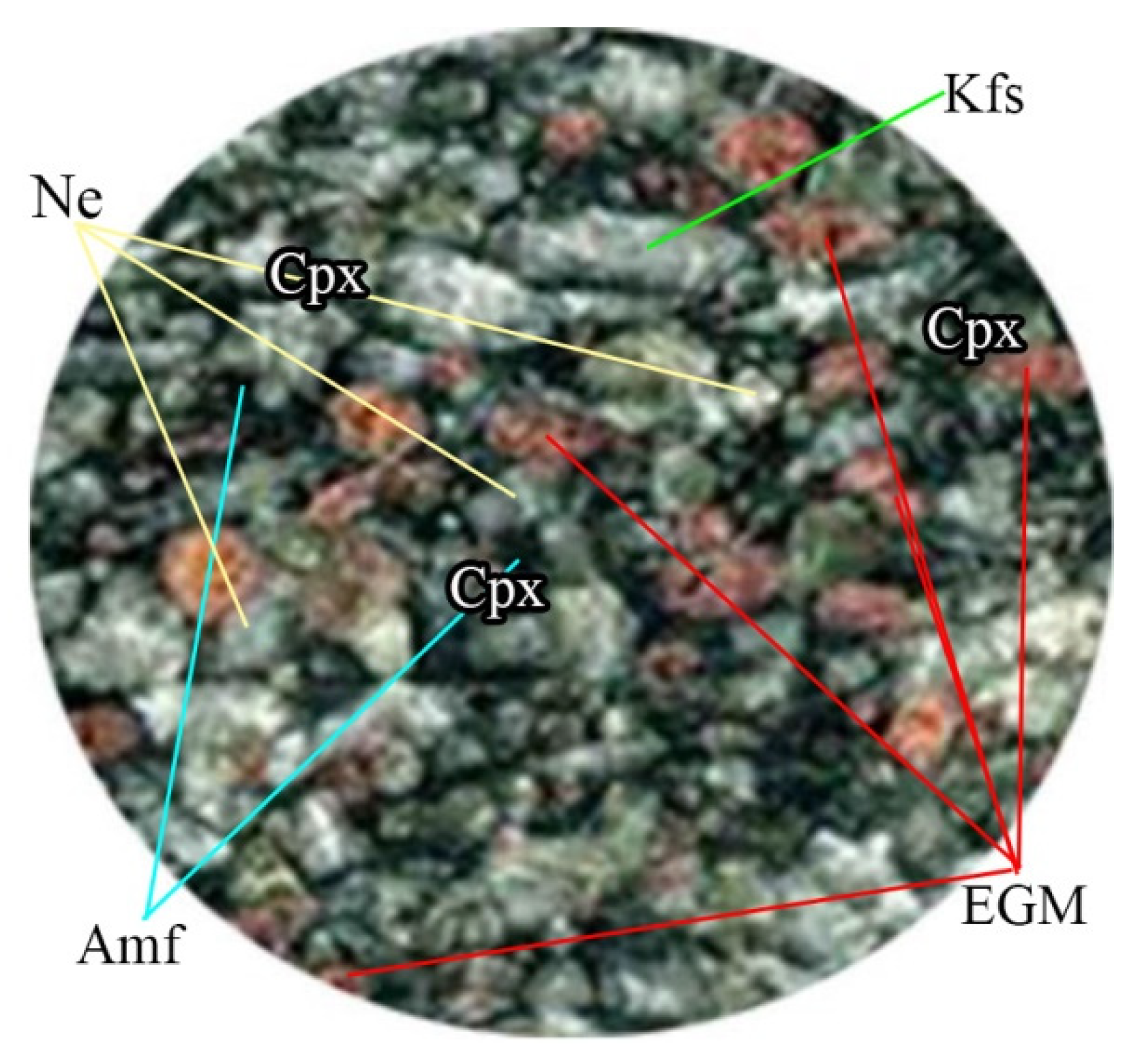
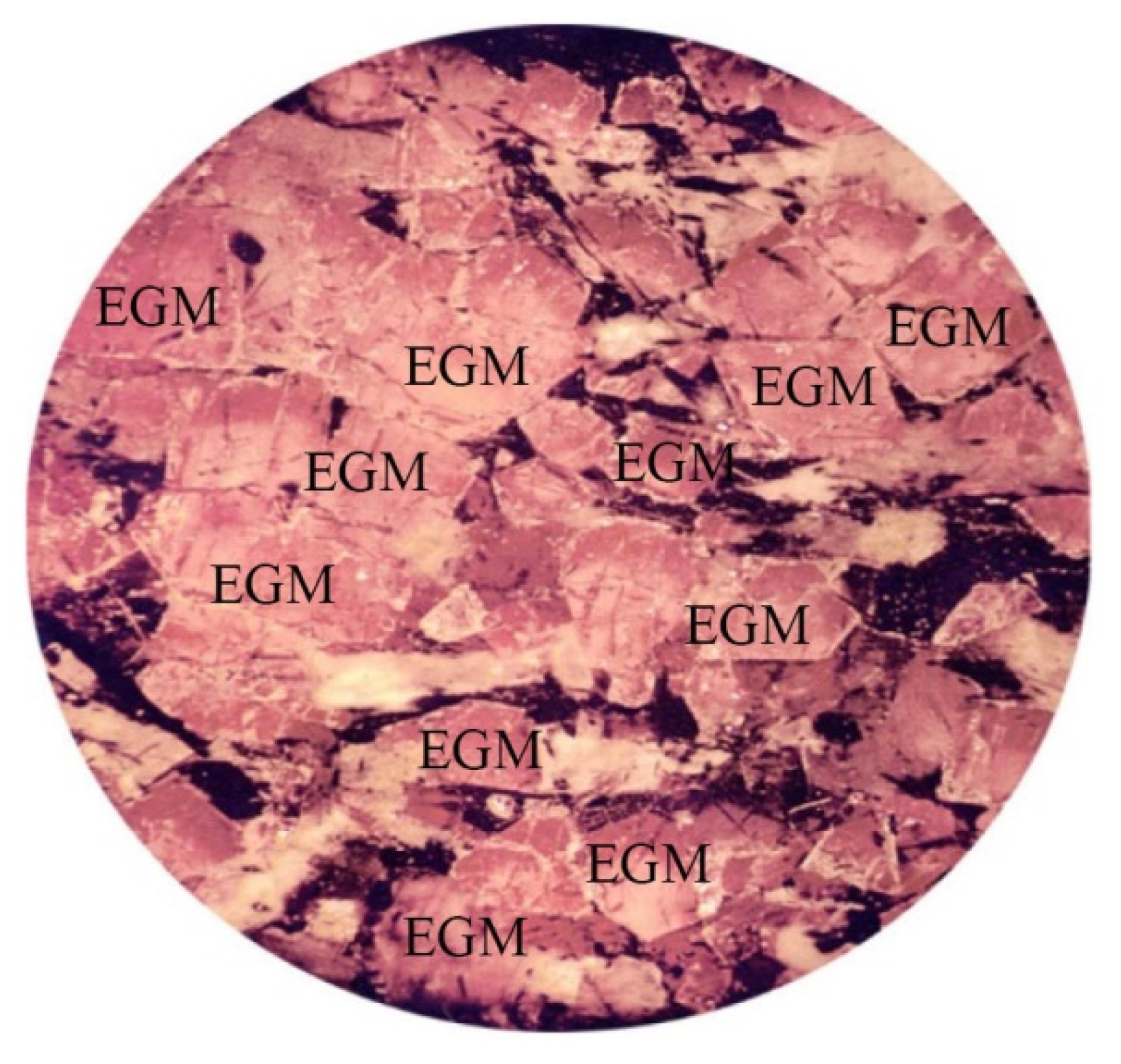
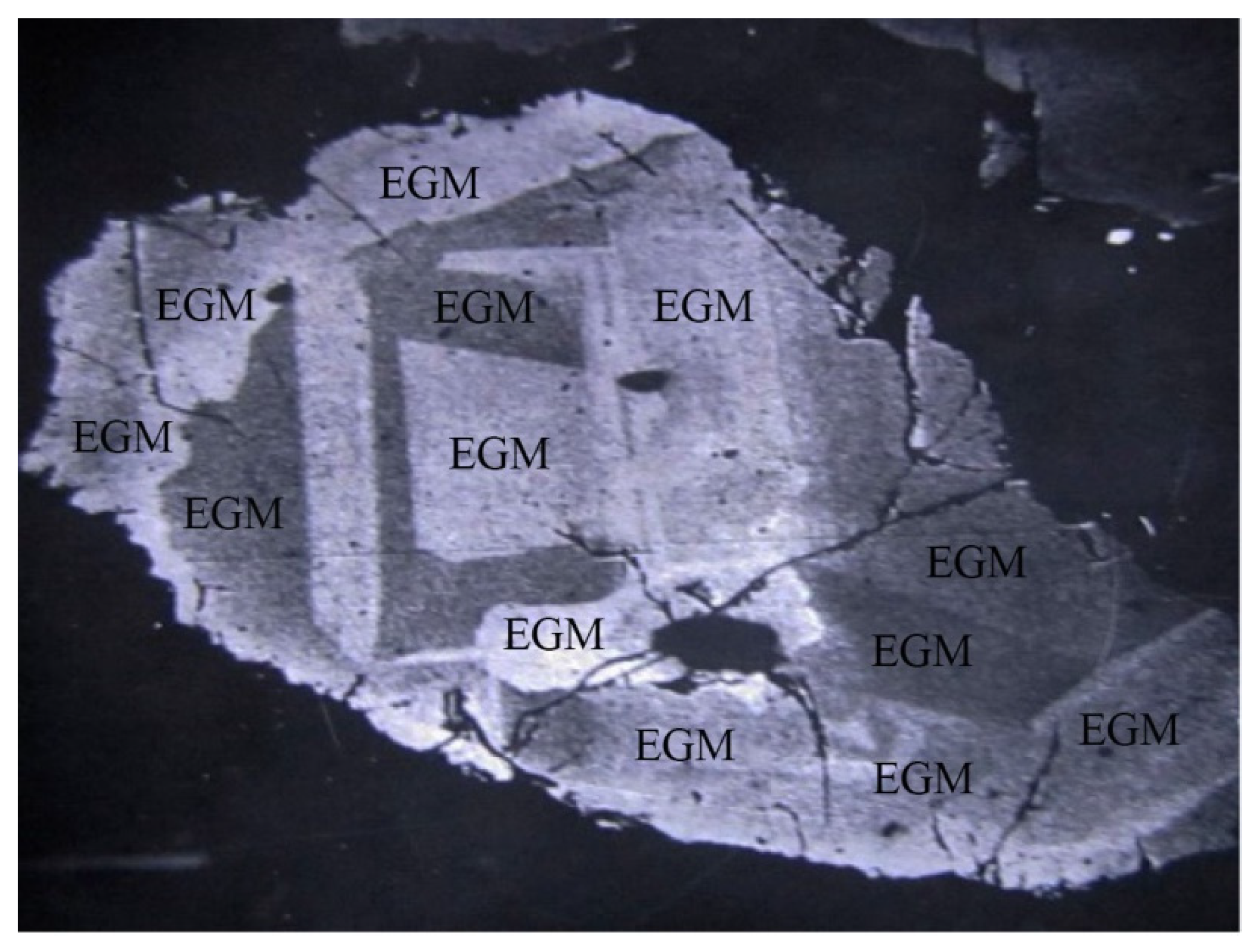
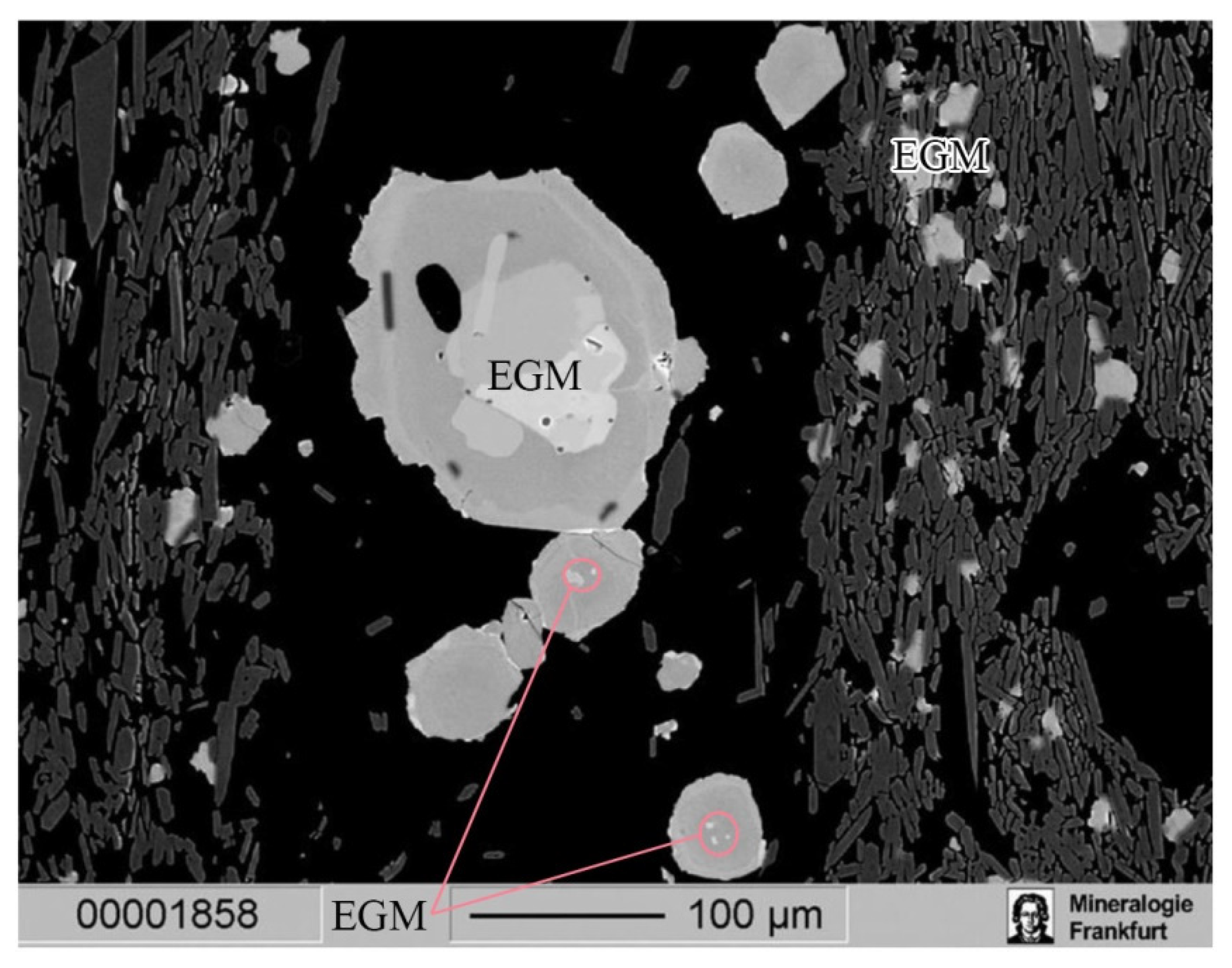
4.1. Petrographic Characteriatics of EGM
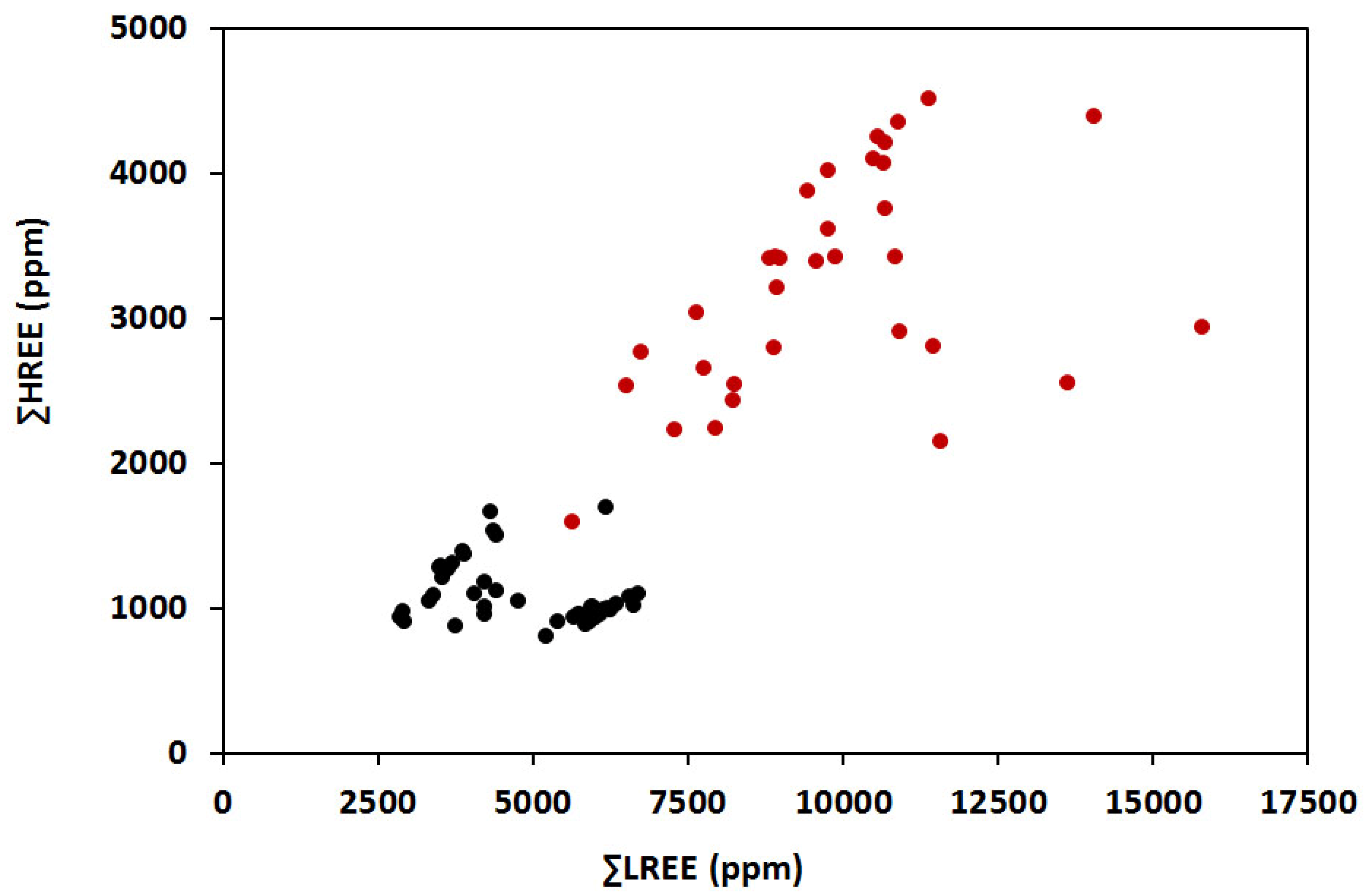
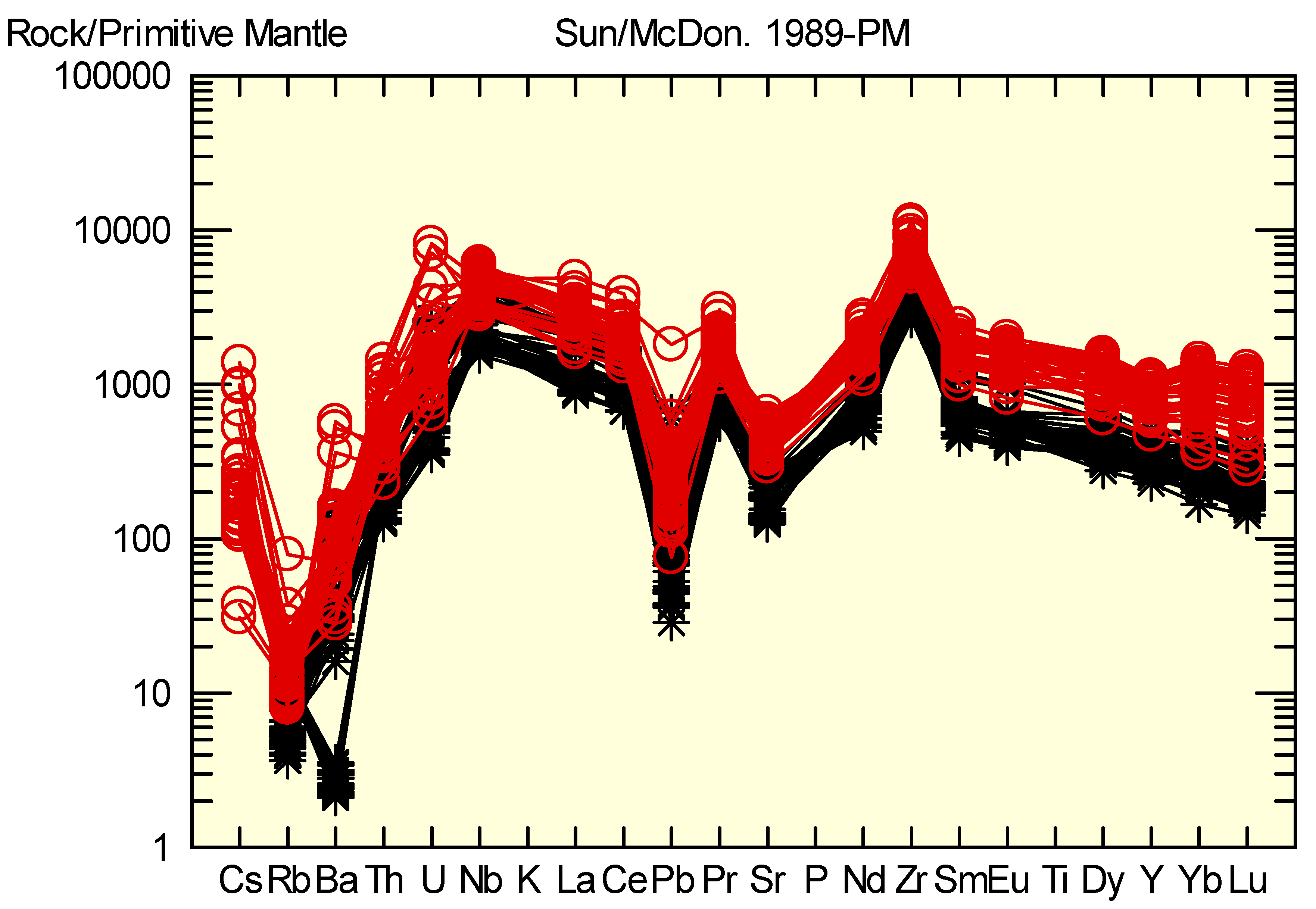
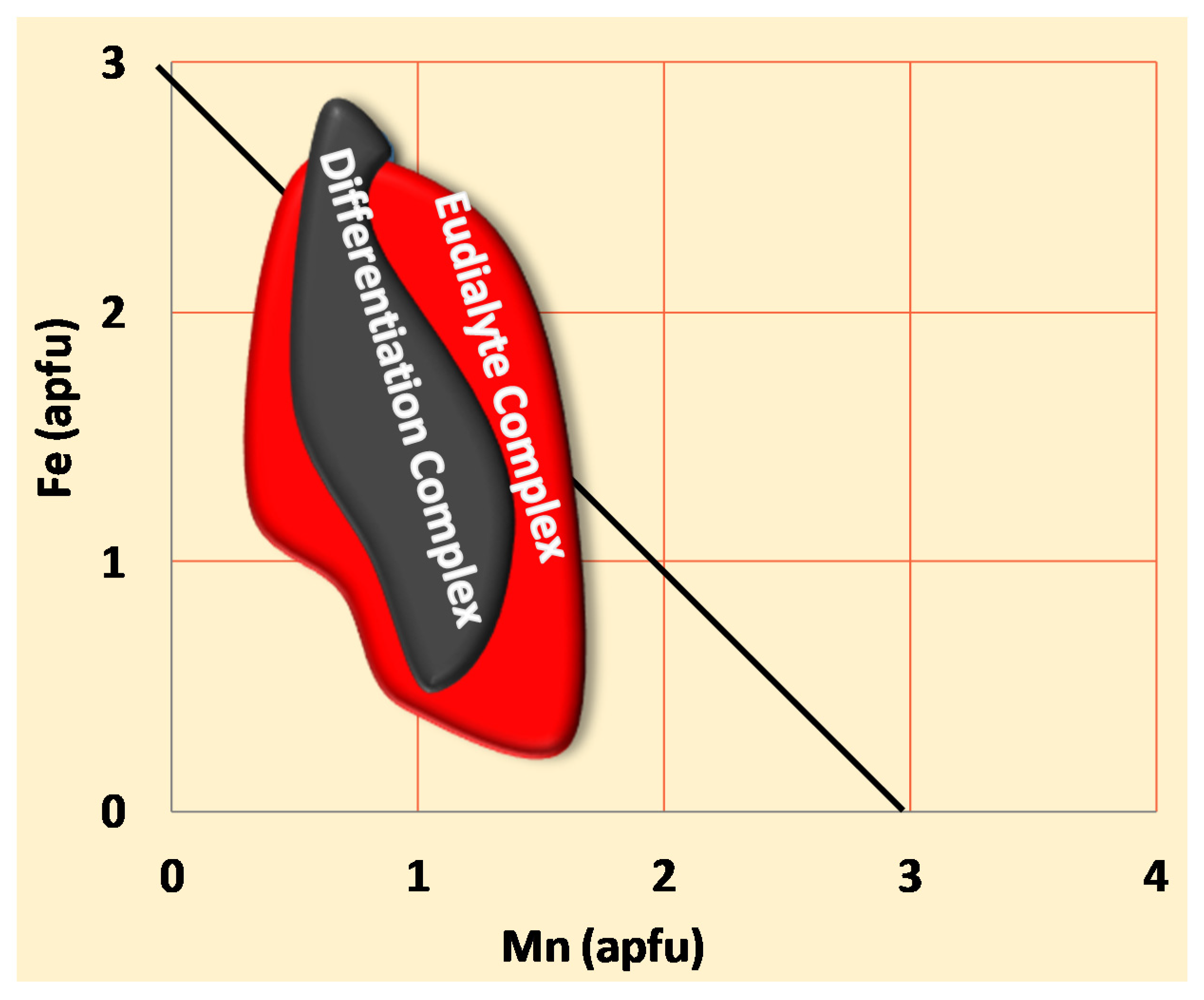
4.2. Compositions of EGM
5. Discussion
5.1. Arrival of EGM on Liquidus
5.2. Genesis of the Lovozero EGM Ore
5.3. Consequences for Layered Agpaitic Complexes
6. Conclusions
- Eudialyte (EGM) of the Lovozero complex is enriched in HREE. EGM of the ore-bearing Phase III of the complex reaches an average of 1.3 wt. % total REEs (max 1.8), and a Ce/Yb = 8.88. In addition, EGM-ores are strongly enriched with Zr and many other HFSE elements commodities.
- The petrography shows that the time of crystallization of EGM relative to liquidus paragenesis changes upward and as the Lovozero complex crystallizes. The interstitial, anhedral EGM crystals in all of Phase II indicate that the bulk magma was not yet saturated in components needed for nucleation of EGM. Only after crystallization, about 85% of the volume of the initial magma was saturated and nucleation of EGM was reached and EGM changed the role to become a cumulus phase.
- Saturation of the bulk magma that leads to EGM nucleation is a prerequisite for eudialyte ores. The ores formed as a result of the suspension and upward transportation of very small crystals and subsequent amalgamation growth below the roof of the magma chamber. The process compares to that suggested for the Khibina apatite-group mineral deposits [26].
- EGM of the neighboring Khibiny complex is anhedral, interstitial, and unlikely to form eudialyte ore deposits, despite a bulk concentration of 531 ppm Zr.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A.; Brown, D.A. The Geochemistry of the Lovozero Alkaline Massif. Part 1. Geology and Petrology; Part 2. Geochemistry; Australian National University Press: Canberra, Australia, 1968; pp. 224–369. [Google Scholar]
- Sørensen, H. Rhythmic igneous layering in peralkaline intrusions. An essay review on Ilímaussaq (Greenland) and Lovozero (Kola, USSR). Lithos 1968, 2, 261–283. [Google Scholar] [CrossRef]
- Sørensen, H. Agpaitic nepheline syenites: A potential source of rare elements. Appl. Geochem. 1992, 7, 417–427. [Google Scholar] [CrossRef]
- Sørensen, H. The agpaitic rocks—An overview. Mineral. Mag. 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Kramm, U.; Kogarko, L.N. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Lahaye, Y.; Brey, G.P. Plume-related mantle source of super-large rare metal deposits from the Lovozero and Khibiny massifs on the Kola Peninsula, Eastern part of Baltic Shield: Sr, Nd and Hf isotope systematics. Miner. Pet. 2010, 98, 197–208. [Google Scholar] [CrossRef]
- Zartman, R.E.; Kogarko, L.N. Lead isotopic evidence for interaction between plume and lower crust during emplacement of peralkaline Lovozero rocks and related rare-metal deposits, East Fennoscandia, Kola Peninsula, Russia. Contrib. Mineral. Petrol. 2017, 172, 1–14. [Google Scholar] [CrossRef]
- Marks, M.; Vennemann, T.; Siebel, W.; Markl, G. Nd-, O-, and H-isotopic evidence for complex, closed-system fluid evolution of the peralkaline Ili’maussaq intrusion, South Greenland. Geochim. Cosmochim. Acta 2004, 68, 3379–3395. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Kogarko, L.N. Alkaline magmatism and enriched mantle reservoirs: Mechanisms, time, and depth of formation. Geochem. Int. 2006, 44, 3–10. [Google Scholar] [CrossRef]
- Piotrowski, J.M.; Edgar, A.D. Melting relations of undersaturated alkaline rocks from South Greenland. Medd. Grønland 1970, 181, 62. [Google Scholar]
- Kogarko, L.N.; Burnham, C.; Shettle, D. Water regime in alkaline magmas. Geochem. Int. 1977, 14, 1–8. [Google Scholar]
- Kogarko, L.N. Ore-forming potential of alkaline magmas. Lithos 1990, 26, 165–175. [Google Scholar] [CrossRef]
- Vlasov, K.A.; Kuzmenko, M.Z.; Eskova, E.M. The Lovozero Alkaline Massif; Izdatel’stvo Akademii Nauk: Moscow, Russia, 1959; 624p. (In Russian) [Google Scholar]
- Borst, A.; Friis, H.; Nielsen, T.; Waight, T. Bulk and Mush Melt Evolution in Agpaitic Intrusions: Insights from compositional zoning in Eudialyte, Ilímaussaq Complex, South Greenland. J. Petrol. 2018, 59, 589–612. [Google Scholar] [CrossRef]
- Cawthorn, R.G. Layering Intrusions. In Developments in Petrology; Elsevier Science: Amsterdam, The Netherlands; Lausanne, Switzerland; New York, NY, USA; Oxford, UK; Tokyo, Japan, 1996; Volume 15, 530p, ISBN 9780080535401. [Google Scholar]
- Kogarko, L.N.; Williams, C.T.; Woolley, A.R. Chemical evolution and petrogenetic implications of loparite in the layered, agpaitic Lovozero Complex, Kola Peninsula, Russia. Mineral. Petrol. 2002, 74, 1–24. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef]
- Arzamastsev, A.A. Unique Palaeozoic Intrusions of the Kola Peninsula. Kola Science Centre Press House; Kola Peninsula Science Centre Press House: Apatity, Russia, 1994; 78p, (In Russian and English). [Google Scholar]
- Kogarko, L.N.; Kononova, V.A.; Orlova, M.P.; Woolley, A.R. Alkaline Rocks and Carbonatites of the World, Part 2; Chapman and Hall: London, UK, 1995; 226p, ISBN 0412614403. [Google Scholar]
- International Mineralogical Association. The Official IMA-CNMNC List of Mineral Names. The New IMA List of Minerals—A Work in Progress—Updated: November 2020. Available online: http://cnmnc.main.jp/IMA_Master_List_%282020-11%29.pdf (accessed on 16 November 2020).
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Saint Petersburg, Russia, 1972; 296p. (In Russian) [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, J.; Kampf, A.; Pekov, I. The nomenclature of eudialyte-group minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N. Sergevanite, Na15(Ca3Mn3)(Na2Fe)Zr3Si26O72(OH)3·H2O, a new eudialyte-group mineral from the Lovozero alkaline massif, Kola Peninsula. Can. Mineral. 2020, 58, 421–436. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Kogarko, L.N. Chemical Composition and Petrogenetic Implications of Apatite in the Khibiny Apatite-Nepheline Deposits (Kola Peninsula). Minerals 2018, 8, 532. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Bea, F.; Glaznev, V.N.; Arzamastseva, L.V.; Montero, P. Kola alkaline province in the Paleozoic: Evaluation of primary mantle magma composition and magma generation conditions. Russ. J. Earth Sci. 2001, 3, 1–32. [Google Scholar] [CrossRef]
- Lindhuber, M.J.; Marks, M.A.W.; Bons, P.D.; Wenzel, T.; Markl, G. Crystal mat-formation as an igneous-layering forming process: Textural and geochemical evidence from the “lower layered” nepheline syenite sequence of the Ilímaussaq complex, South Greenland. Lithos 2015, 224, 295–309. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. The Ilimaussaq Alkaline Complex, South Greenland. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2015; pp. 649–691. [Google Scholar] [CrossRef]
- Lauder, W. Mat formation and crystal settling in magma. Nature 1964, 202, 1100–1101. [Google Scholar] [CrossRef]
- Hunt, E.J.; Finch, A.A.; Donaldson, C.H. Layering in peralkaline magmas, Ilfmaussaq Complex, S Greenland. Lithos 2017, 268, 1–15. [Google Scholar] [CrossRef]
- Irvine, T.N.; Andersen, J.C.Ø.; Brooks, C.K. Included blocks (and blocks within blocks) in the Skaergaard Intrusion: Geological relations and the origins of rhythmic modally graded layers. Geol. Soc. Am. Bull. 1998, 110, 1398–1447. [Google Scholar] [CrossRef]

| Name | CNMMN/CNMNC Approved Formula |
|---|---|
| Barytolamprophyllite | (BaK)Ti2Na3Ti(Si2O7)2O2(OH)2 |
| Belovite-(Ce) | NaCeSr3(PO4)3F |
| Cerite-(Ce) | (Ce,La,Ca)9(Mg,Fe3+)(SiO4)3(SiO3OH)4(OH)3 |
| Lomonosovite | Na6Na2Ti2Na2Ti2(Si2O7)2(PO4)2O4 |
| Loparite-(Ce) | (Na,Ce,Sr)(Ce,Th)(Ti,Nb)2O6 |
| Lorenzenite | Na2Ti2O3(Si2O6) |
| Mosandrite-(Ce) | (Ca3REE)[(H2O)2Ca0.5☐0.5]Ti(Si2O7)2(OH)2(H2O)2 |
| Murmanite | Na2Ti2Na2Ti2(Si2O7)2O4(H2O)4 |
| Nenadkevichite | (Na, ☐)8Nb4(Si4O12)2(O,OH)4·8H2O |
| Nordite-(Ce) | Na3SrCeZnSi6O17 |
| Steenstrupine-(Ce) | Na14Ce6Mn2+2Fe3+2Zr(PO4)7Si12O36(OH)2·3H2O |
| Vitusite-(Ce) | Na3Ce(PO4)2 |
| Vuonnemite | Na6Na2Nb2Na3Ti(Si2O7)2(PO4)2O2(OF) |
| Sample | 382 | 383 | 385 | 389 | 390 | 392 | 393 | 399 | 401 | 402 |
|---|---|---|---|---|---|---|---|---|---|---|
| Nb2O5 | 1.09 | 1.14 | 0.72 | 1.77 | 0.79 | 1.06 | 0.67 | 0.48 | 0.48 | 0.88 |
| TiO2 | 0.33 | 0.37 | 0.62 | 1.33 | 0.41 | 0.38 | 0.41 | 0.48 | 0.48 | 0.48 |
| ZrO2 | 12.33 | 12.81 | 15.90 | 9.90 | 13.62 | 12.81 | 14.41 | 14.55 | 13.65 | 13.59 |
| HfO2 | 0.20 | 0.23 | 0.35 | 0.14 | 0.28 | 0.32 | 0.34 | 0.20 | 0.25 | 0.28 |
| ThO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.10 | 0.10 |
| UO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.16 | 0.10 | 0.10 |
| SiO2 | 49.40 | 49.99 | 48.44 | 49.62 | 48.27 | 48.83 | 48.72 | 49.35 | 48.98 | 48.54 |
| Al2O3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 0.22 | 0.16 |
| Y2O3 | 0.21 | 0.25 | 0.52 | 0.22 | 0.56 | 0.63 | 0.57 | 0.69 | 0.68 | 0.35 |
| La2O3 | 0.58 | 0.50 | 0.29 | 0.50 | 0.39 | 0.44 | 0.37 | 0.19 | 0.24 | 0.37 |
| Ce2O3 | 1.26 | 1.24 | 0.63 | 1.23 | 0.81 | 0.93 | 0.78 | 0.66 | 0.63 | 0.74 |
| Pr2O3 | 0.28 | 0.17 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Nd2O3 | 0.54 | 0.52 | 0.29 | 0.47 | 0.40 | 0.48 | 0.47 | 0.39 | 0.26 | 0.35 |
| CaO | 7.96 | 8.14 | 4.76 | 8.81 | 7.50 | 7.48 | 6.70 | 6.38 | 7.07 | 7.14 |
| MgO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.05 | 0.05 |
| MnO | 3.09 | 2.92 | 2.42 | 3.03 | 1.82 | 2.27 | 1.70 | 2.93 | 2.76 | 3.27 |
| FeO | 1.68 | 1.57 | 3.09 | 0.80 | 4.34 | 4.02 | 4.75 | 1.22 | 0.95 | 1.10 |
| SrO | 1.86 | 1.66 | 0.98 | 3.02 | 0.92 | 1.12 | 0.77 | 1.36 | 1.56 | 1.93 |
| BaO | 0.52 | 0.35 | 0.07 | 0.69 | 0.04 | 0.14 | 0.05 | 0.29 | 0.28 | 0.24 |
| Na2O | 16.71 | 16.70 | 16.97 | 14.39 | 15.96 | 16.05 | 15.84 | 15.70 | 14.59 | 15.67 |
| SO3 | 0.54 | 0.37 | 0.27 | 0.43 | 0.15 | 0.16 | 0.22 | 0.18 | 0.20 | 0.24 |
| Cl | 0.54 | 0.50 | 1.12 | 0.50 | 1.25 | 1.36 | 1.24 | 1.14 | 1.15 | 0.76 |
| Total | 99.12 | 99.43 | 97.59 | 97.00 | 97.66 | 98.63 | 98.16 | 96.85 | 94.83 | 96.49 |
| O=Cl | 0.12 | 0.11 | 0.25 | 0.11 | 0.28 | 0.31 | 0.28 | 0.26 | 0.26 | 0.17 |
| TOTAL | 99.00 | 99.32 | 97.34 | 96.89 | 97.38 | 98.32 | 97.88 | 96.59 | 94.57 | 96.32 |
| A.P.F.U. | ||||||||||
| Nb | 0.26 | 0.27 | 0.17 | 0.41 | 0.19 | 0.25 | 0.16 | 0.11 | 0.11 | 0.21 |
| Ti | 0.13 | 0.14 | 0.25 | 0.52 | 0.16 | 0.15 | 0.16 | 0.19 | 0.19 | 0.19 |
| Zr | 3.13 | 3.22 | 4.13 | 2.49 | 3.55 | 3.29 | 3.73 | 3.72 | 3.52 | 3.52 |
| Hf | 0.03 | 0.03 | 0.05 | 0.02 | 0.04 | 0.05 | 0.05 | 0.03 | 0.04 | 0.04 |
| Th | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 |
| U | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 |
| Si | 25.74 | 25.73 | 25.83 | 25.59 | 25.81 | 25.75 | 25.84 | 25.89 | 25.89 | 25.79 |
| Al | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.14 | 0.10 |
| Y | 0.06 | 0.07 | 0.15 | 0.06 | 0.16 | 0.18 | 0.16 | 0.19 | 0.19 | 0.10 |
| La | 0.11 | 0.09 | 0.06 | 0.10 | 0.08 | 0.09 | 0.07 | 0.04 | 0.05 | 0.07 |
| Ce | 0.24 | 0.23 | 0.12 | 0.23 | 0.16 | 0.18 | 0.15 | 0.13 | 0.12 | 0.14 |
| Pr | 0.05 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Nd | 0.10 | 0.10 | 0.06 | 0.09 | 0.08 | 0.09 | 0.09 | 0.07 | 0.05 | 0.07 |
| Ca | 4.44 | 4.49 | 2.72 | 4.87 | 4.30 | 4.23 | 3.81 | 3.59 | 4.00 | 4.06 |
| Mg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.04 | 0.04 |
| Mn | 1.36 | 1.27 | 1.09 | 1.32 | 0.82 | 1.01 | 0.76 | 1.30 | 1.24 | 1.47 |
| Fe | 0.73 | 0.68 | 1.38 | 0.35 | 1.94 | 1.77 | 2.11 | 0.54 | 0.42 | 0.49 |
| Sr | 0.56 | 0.50 | 0.30 | 0.90 | 0.29 | 0.34 | 0.24 | 0.41 | 0.48 | 0.59 |
| Ba | 0.11 | 0.07 | 0.01 | 0.14 | 0.01 | 0.03 | 0.01 | 0.06 | 0.06 | 0.05 |
| Na | 16.88 | 16.67 | 17.54 | 14.39 | 16.55 | 16.41 | 16.29 | 15.97 | 14.95 | 16.14 |
| S | 0.21 | 0.14 | 0.11 | 0.17 | 0.06 | 0.06 | 0.09 | 0.07 | 0.08 | 0.10 |
| Cl | 0.48 | 0.44 | 1.01 | 0.44 | 1.13 | 1.22 | 1.11 | 1.01 | 1.03 | 0.68 |
| Element (ppm) | 01-1 | 01-2 | 01-3 | 02-1 | 02-2 | 02-3 | 1610-03 | 1610-04 | 463-04 | 1610-06 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sc | 102 | 104 | 108 | 117 | 120 | 120 | 119 | 121 | 79 | 111 |
| Ti | 1331 | 1374 | 1257 | 1267 | 1333 | 1276 | 1340 | 1419 | 1207 | 1318 |
| Mn | 8518 | 8704 | 8581 | 7632 | 8662 | 8144 | 8306 | 8657 | 7609 | 7894 |
| Co | 0.16 | 0.17 | 0.15 | 0.2 | 0.23 | 0.18 | 0.18 | 0.2 | – | 0.2 |
| Cu | 0.85 | 1.08 | 1.06 | 1.21 | 1.14 | 1.08 | 1.27 | 1.13 | 0.75 | 1.24 |
| Rb | 6.74 | 7.61 | 7.51 | 7.82 | 8.55 | 7.91 | 8.52 | 8.93 | 2.85 | 7.52 |
| Sr | 2880 | 2872 | 2841 | 2773 | 2815 | 2738 | 2837 | 2937 | 3273 | 2632 |
| Y | 1487 | 1471 | 1483 | 1453 | 1477 | 1404 | 1307 | 1414 | 1045 | 1324 |
| Nb | 2600 | 2469 | 2168 | 2112 | 2177 | 2119 | 2128 | 1988 | 1327 | 2090 |
| Ba | 22 | 20 | 21 | 15 | 16 | 15 | 25 | 17 | 459 | 15 |
| La | 1703 | 1627 | 1536 | 1462 | 1512 | 1450 | 1438 | 1429 | 1178 | 1435 |
| Ce | 3049 | 2943 | 2787 | 2634 | 2782 | 2705 | 2543 | 2612 | 2324 | 2602 |
| Pr | 349 | 337 | 315 | 304 | 315 | 322 | 303 | 306 | 273 | 304 |
| Nd | 1455 | 1437 | 1311 | 1312 | 1315 | 1322 | 1292 | 1321 | 1145 | 1256 |
| Sm | 364 | 347 | 336 | 322 | 331 | 328 | 316 | 336 | 283 | 308 |
| Eu | 104 | 103 | 95 | 92 | 96 | 98 | 94 | 95 | 78 | 88 |
| Gd | 309 | 302 | 274 | 267 | 297 | 288 | 270 | 268 | 249 | 259 |
| Tb | 52 | 50 | 50 | 46 | 47 | 45 | 45 | 43 | 37 | 43 |
| Dy | 270 | 262 | 269 | 253 | 256 | 244 | 241 | 243 | 204 | 238 |
| Ho | 55 | 50 | 52 | 48 | 52 | 50 | 45 | 47 | 39 | 46 |
| Er | 145 | 130 | 134 | 124 | 130 | 132 | 116 | 121 | 95 | 116 |
| Tm | 20 | 19 | 19 | 18 | 19 | 19 | 17 | 18 | 13 | 16 |
| Yb | 131 | 120 | 118 | 116 | 117 | 112 | 109 | 116 | 83 | 108 |
| Lu | 15 | 15 | 14 | 14 | 14 | 14 | 13 | 14 | 11 | 13 |
| ∑REE | 8021 | 7742 | 7310 | 7012 | 7283 | 7128 | 6842 | 6969 | 6010 | 6832 |
| Ce/Yb | 23 | 25 | 24 | 23 | 24 | 24 | 23 | 22 | 28 | 24 |
| Hf | 1075 | 1067 | 1039 | 1058 | 1046 | 1069 | 1112 | 1123 | 789 | 1126 |
| Ta | 287 | 270 | 181 | 184 | 187 | 193 | 217 | 222 | 127 | 188 |
| Pb | 31 | 31 | 26 | 23 | 26 | 24 | 31 | 27 | 5 | 25 |
| Th | 20 | 21 | 18 | 19 | 20 | 19 | 19 | 17 | 28 | 18 |
| U | 25 | 24 | 22 | 23 | 22 | 21 | 22 | 22 | 39 | 22 |
| Sample | 778 | 153 | 290 | 1 | 373 | 304 | 779 | 336 | 335 |
|---|---|---|---|---|---|---|---|---|---|
| Nb2O5 | 0.76 | 0.67 | 0.75 | 0.99 | 0.51 | 0.77 | 0.71 | 0.73 | 0.61 |
| TiO2 | 0.63 | 0.53 | 0.6 | 0.58 | 0.65 | 0.48 | 0.52 | 0.49 | 0.67 |
| ZrO2 | 14.28 | 13.42 | 13.53 | 13.26 | 13.81 | 13.03 | 13.35 | 13.37 | 14.22 |
| HfO2 | 0.00 | 0.27 | 0.29 | 0.41 | 0.00 | 0.27 | 0.26 | 0.22 | 0.00 |
| SiO2 | 49.49 | 50.09 | 49.95 | 48.84 | 50.19 | 49.39 | 49.81 | 50.27 | 50.04 |
| Al2O3 | 0.20 | 0.00 | 0.00 | 0.16 | 0.23 | 0.14 | 0.15 | 0.11 | 0.16 |
| Y2O3 | 0.69 | 0.7 | 0.63 | 0.7 | 0.56 | 0.57 | 0.85 | 0.33 | 0.34 |
| La2O3 | 0.36 | 0.19 | 0.34 | 0.19 | 0.28 | 0.18 | 0.33 | 0.15 | 0.27 |
| Ce2O3 | 0.71 | 0.63 | 0.57 | 0.6 | 0.51 | 0.58 | 0.66 | 0.38 | 0.66 |
| Pr2O3 | 0.00 | 0.15 | 0.15 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nd2O3 | 0.43 | 0.31 | 0.37 | 0.37 | 0.4 | 0.32 | 0.35 | 0.41 | 0.34 |
| Sm2O3 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.17 | 0.00 |
| Gd2O3 | 0.11 | 0.00 | 0.00 | 0.00 | 0.26 | 0.00 | 0.00 | 0.26 | 0.32 |
| CaO | 8.28 | 7.24 | 7.3 | 8.02 | 7.97 | 7.68 | 8.45 | 8.65 | 7.93 |
| MgO | 0.04 | 0.00 | 0.00 | 0.07 | 0.10 | 0.04 | 0.01 | 0.05 | 0.05 |
| MnO | 1.86 | 2.3 | 1.99 | 2.13 | 1.97 | 3.20 | 1.91 | 2.05 | 2.02 |
| FeO | 3.42 | 3.15 | 3.89 | 3.13 | 4.92 | 4.34 | 3.24 | 5.27 | 4.76 |
| SrO | 0.83 | 1.49 | 1.35 | 2.19 | 0.51 | 0.63 | 0.85 | 0.52 | 0.69 |
| BaO | 0.11 | 0.10 | 0.11 | 0.01 | 0.11 | 0.00 | 0.10 | 0.18 | 0.09 |
| Na2O | 16.24 | 16.98 | 16.18 | 16.24 | 15.41 | 15.82 | 16.14 | 15.22 | 14.92 |
| K2O | 0.28 | 0.3 | 0.38 | 0.25 | 0.29 | 0.21 | 0.28 | 0.22 | 0.29 |
| SO3 | 0.26 | 0.18 | 0.26 | 0.18 | 0.28 | 0.18 | 0.20 | 0.25 | 0.30 |
| Cl | 1.43 | 1.40 | 1.40 | 1.58 | 1.31 | 1.32 | 1.44 | 1.42 | 1.39 |
| TOTAL | 100.64 | 100.1 | 100.04 | 100.05 | 100.27 | 99.15 | 99.73 | 100.72 | 100.07 |
| O=Cl | 0.32 | 0.32 | 0.32 | 0.36 | 0.30 | 0.30 | 0.32 | 0.32 | 0.31 |
| TOTAL | 100.32 | 99.78 | 99.72 | 99.69 | 99.97 | 98.85 | 99.41 | 100.40 | 99.76 |
| A.P.F.U. | |||||||||
| Nb | 0.18 | 0.16 | 0.18 | 0.24 | 0.12 | 0.18 | 0.17 | 0.17 | 0.14 |
| Ti | 0.25 | 0.21 | 0.23 | 0.23 | 0.25 | 0.19 | 0.20 | 0.19 | 0.26 |
| Zr | 3.63 | 3.38 | 3.41 | 3.41 | 3.47 | 3.32 | 3.38 | 3.35 | 3.58 |
| Hf | 0.00 | 0.04 | 0.04 | 0.06 | 0.00 | 0.04 | 0.04 | 0.03 | 0.00 |
| Si | 25.82 | 25.84 | 25.82 | 25.76 | 25.88 | 25.82 | 25.83 | 25.83 | 25.86 |
| Al | 0.12 | 0.00 | 0.00 | 0.10 | 0.14 | 0.09 | 0.09 | 0.07 | 0.10 |
| Y | 0.19 | 0.19 | 0.17 | 0.20 | 0.15 | 0.16 | 0.23 | 0.09 | 0.09 |
| La | 0.07 | 0.04 | 0.06 | 0.04 | 0.05 | 0.03 | 0.06 | 0.03 | 0.05 |
| Ce | 0.14 | 0.12 | 0.11 | 0.12 | 0.10 | 0.11 | 0.13 | 0.07 | 0.12 |
| Pr | 0.00 | 0.03 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nd | 0.08 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 | 0.07 | 0.07 | 0.06 |
| Sm | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 |
| Gd | 0.02 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.04 | 0.05 |
| Ca | 4.63 | 4.00 | 4.04 | 4.53 | 4.40 | 4.30 | 4.70 | 4.76 | 4.39 |
| Mg | 0.03 | 0.00 | 0.00 | 0.06 | 0.08 | 0.03 | 0.01 | 0.04 | 0.04 |
| Mn | 0.82 | 1.01 | 0.87 | 0.95 | 0.86 | 1.42 | 0.84 | 0.89 | 0.88 |
| Fe | 1.49 | 1.22 | 1.51 | 1.24 | 1.91 | 1.71 | 1.41 | 2.26 | 1.85 |
| Sr | 0.25 | 0.45 | 0.40 | 0.67 | 0.15 | 0.19 | 0.25 | 0.15 | 0.21 |
| Ba | 0.02 | 0.02 | 0.02 | 0.00 | 0.02 | 0.00 | 0.02 | 0.04 | 0.02 |
| Na | 16.43 | 16.99 | 16.22 | 16.61 | 15.41 | 16.04 | 16.23 | 15.17 | 14.95 |
| K | 0.19 | 0.20 | 0.25 | 0.17 | 0.19 | 0.14 | 0.19 | 0.14 | 0.19 |
| S | 0.10 | 0.07 | 0.10 | 0.07 | 0.11 | 0.07 | 0.08 | 0.10 | 0.12 |
| Cl | 1.26 | 1.22 | 1.23 | 1.41 | 1.15 | 1.17 | 1.27 | 1.23 | 1.22 |
| Element (ppm) | 1a | 1b | 5b | 9a | 8b | 2a | 3a | 3b | 3c | 4a |
|---|---|---|---|---|---|---|---|---|---|---|
| Sc | 75.21 | 63.06 | 69.18 | 63.08 | 53.95 | 52.56 | 47.5 | 48.11 | 53.65 | 47.76 |
| Ti | 1302 | 1313 | 1257 | 1081 | 1459 | 1158 | 1104 | 1106 | 1237 | 1177 |
| Mn | 11,507 | 11,832 | 12,315 | 13,011 | 18,159 | 19,263 | 15,488 | 15,575 | 16,525 | 20,349 |
| Co | 81.8 | 17.6 | 94.86 | 102.75 | – | – | – | – | – | – |
| Cu | 16.22 | 3.19 | 19.35 | 21.19 | – | – | – | – | – | – |
| Rb | 8.68 | 15.33 | 8.78 | 5.66 | 7.81 | 7.46 | 5.39 | 6.44 | 7.22 | 7.1 |
| Sr | 7004 | 7632 | 6185 | 7118 | 10,434 | 12,501 | 11,673 | 11,971 | 12,443 | 9446 |
| Y | 2924 | 3384 | 2143 | 3288 | 4851 | 3999 | 3916 | 3927 | 4191 | 2867 |
| Nb | 2202 | 2206 | 2177 | 2585 | 3511 | 3715 | 3703 | 3874 | 4051 | 3199 |
| Ba | 197 | 216 | 523 | 488 | 677 | 392 | 639 | 534 | 600 | 980 |
| La | 1188 | 1505 | 1107 | 1373 | 2144 | 1751 | 2079 | 1820 | 2031 | 1687 |
| Ce | 2548 | 3205 | 2320 | 2703 | 4383 | 3538 | 3939 | 3607 | 3946 | 3389 |
| Pr | 367 | 422 | 306 | 340 | 564 | 472 | 497 | 470 | 508 | 436 |
| Nd | 1973 | 1991 | 1460 | 1591 | 2705 | 2366 | 2313 | 2320 | 2460 | 2071 |
| Sm | 653 | 617 | 443 | 502 | 872 | 778 | 744 | 764 | 815 | 639 |
| Eu | 217 | 199 | 138 | 161 | 291 | 269 | 257 | 269 | 278 | 215 |
| Gd | 718 | 607 | 387 | 513 | 913 | 802 | 769 | 799 | 833 | 616 |
| Tb | 137 | 115 | 69 | 100 | 184 | 153 | 150 | 154 | 163 | 112 |
| Dy | 666 | 731 | 442 | 703 | 1 099 | 905 | 895 | 895 | 959 | 635 |
| Ho | 144 | 147 | 86 | 149 | 236 | 187 | 187 | 187 | 199 | 129 |
| Er | 395 | 395 | 226 | 413 | 665 | 506 | 517 | 507 | 542 | 339 |
| Tm | 57 | 56 | 31 | 59 | 97 | 72 | 74 | 72 | 76 | 47 |
| Yb | 382 | 365 | 196 | 388 | 648 | 472 | 487 | 467 | 501 | 308 |
| Lu | 49 | 46 | 24 | 49 | 86 | 60 | 62 | 60 | 64 | 39 |
| ∑REE | 9494 | 10,399 | 7235 | 9045 | 14,885 | 12,331 | 12,971 | 12,391 | 13,376 | 10,661 |
| Ce/Yb | 6.66 | 8.77 | 11.83 | 6.97 | 6.76 | 7.5 | 8.08 | 7.73 | 7.88 | 11.02 |
| Hf | 1809 | 1557 | 1229 | 1291 | 2421 | 1854 | 1809 | 1870 | 2072 | 1785 |
| Ta | 198 | 182 | 145 | 194 | 303 | 371 | 387 | 429 | 423 | 351 |
| Pb | 9.2 | 12.98 | 10.33 | 5.39 | 11.53 | 14.65 | 8.65 | 10.9 | 10.24 | 18.96 |
| Th | 34.13 | 31.65 | 29.11 | 19.47 | 41.06 | 36.88 | 41.57 | 37.61 | 45.42 | 40.66 |
| U | 31.5 | 28.28 | 30.63 | 13.37 | 30.97 | 22.7 | 18.96 | 23.36 | 26.64 | 26.33 |
| Li | 5.84 | 5.31 | 4.6 | 7.54 | – | 3.03 | – | – | – | – |
| Be | 0.78 | 1.21 | 3.48 | 1.27 | 2.69 | 8.97 | 11.43 | 4.43 | 8.35 | 4.94 |
| B | 87.43 | – | – | – | 25.41 | 38.49 | 34.1 | 40.7 | 37.46 | 46.89 |
| V | 1.85 | 1.55 | 2.1 | 1.73 | 2.8 | 1.71 | 1.85 | 2.04 | 2.09 | 2.43 |
| Ni | 4.3 | – | 4.69 | 5.78 | – | – | – | 2.35 | – | – |
| Zn | 11.38 | 22.88 | 18.9 | 18.2 | 23.41 | 54.74 | 22.35 | 21.72 | 24.51 | 58.37 |
| Ga | 41.24 | 15.38 | 52.86 | 44.48 | 12.86 | 12.68 | 18.04 | 14.63 | 16.55 | 12.22 |
| Cs | 1.49 | 1.67 | 1.63 | 1.12 | 1.52 | 1.49 | 0.92 | 1.06 | 1.58 | 4.14 |
| Phase II | Phase III | Phase II | Phase III | ||
|---|---|---|---|---|---|
| Element | ppm | ppm | Element | ppm | ppm |
| Sc | 85.96 | 57.26 | Er | 156 | 483 |
| Ti | 1237 | 1455 | Tm | 22 | 68.95 |
| Co | 4.18 | 74.25 | Yb | 143 | 454 |
| Cu | 1.59 | 14.99 | Lu | 17.11 | 58.64 |
| Rb | 5.5 | 10.61 | ∑REE | 6038 | 13,013 |
| Sr | 3762 | 9828 | Ce/Yb | 15.23 | 8.88 |
| Y | 1505 | 3811 | Hf | 963 | 2076 |
| Nb | 1872 | 3242 | Ta | 179 | 293 |
| Ba | 249 | 891 | Pb | 17.71 | 20.99 |
| La | 1166 | 1980 | Th | 19.4 | 50.91 |
| Ce | 2181 | 4032 | U | 23.64 | 47.63 |
| Pr | 265 | 517 | Li | – | 5.45 |
| Nd | 1161 | 2475 | Be | – | 5.61 |
| Sm | 317 | 762 | B | – | 34.93 |
| Eu | 97.51 | 249 | V | – | 2.63 |
| Gd | 292 | 760 | Ni | – | 3.96 |
| Tb | 51.04 | 145 | Zn | – | 69.38 |
| Dy | 299 | 851 | Ga | – | 21.97 |
| Ho | 58.61 | 177 | Cs | – | 2.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogarko, L.; Nielsen, T.F.D. Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia. Minerals 2020, 10, 1036. https://doi.org/10.3390/min10111036
Kogarko L, Nielsen TFD. Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia. Minerals. 2020; 10(11):1036. https://doi.org/10.3390/min10111036
Chicago/Turabian StyleKogarko, Lia, and Troels F. D. Nielsen. 2020. "Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia" Minerals 10, no. 11: 1036. https://doi.org/10.3390/min10111036
APA StyleKogarko, L., & Nielsen, T. F. D. (2020). Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia. Minerals, 10(11), 1036. https://doi.org/10.3390/min10111036





