A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater
Abstract
1. Introduction
2. Materials and Methods
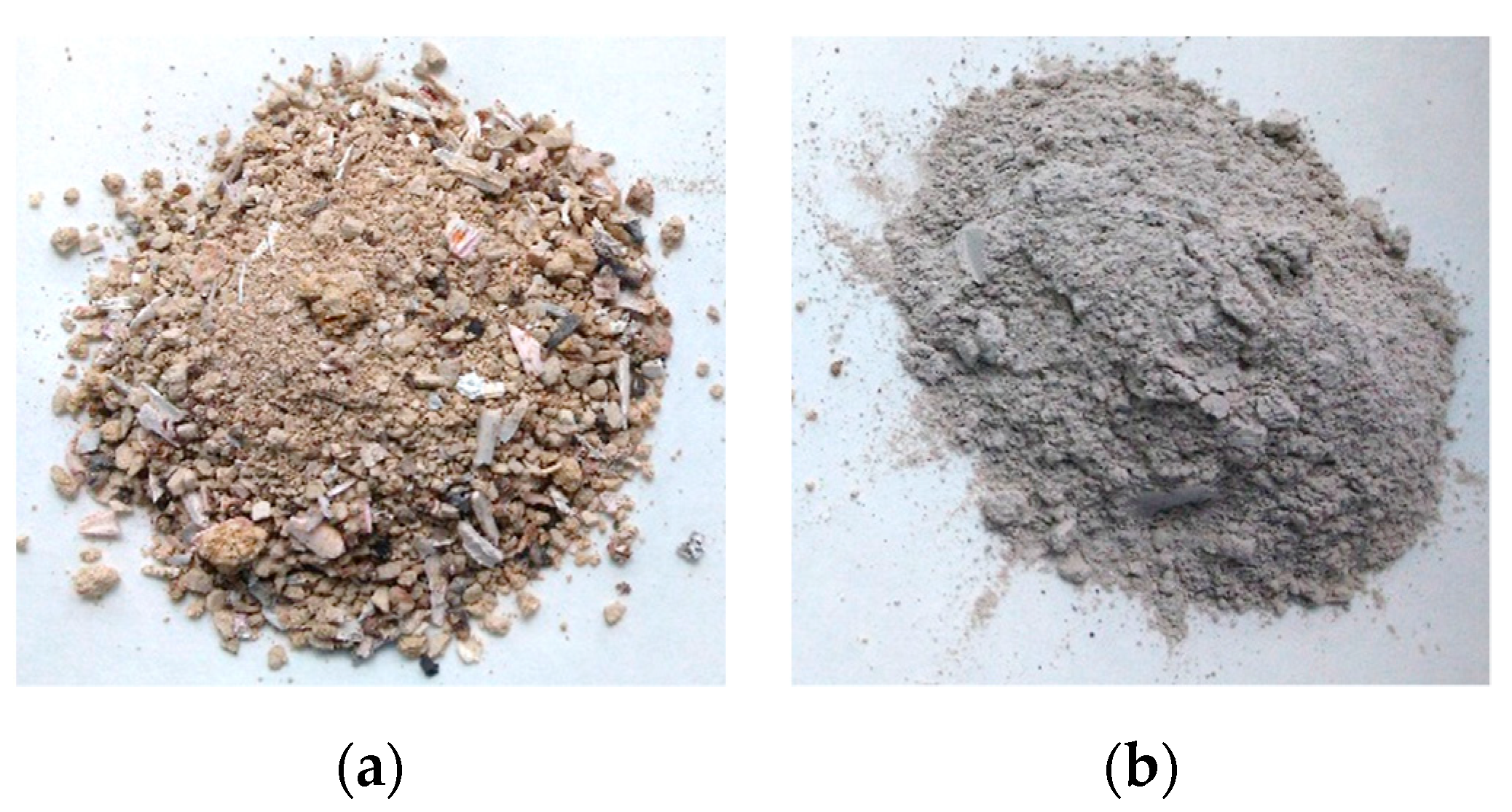
2.1. Adsorbent
2.2. Preparation of Synthetic Wastewater
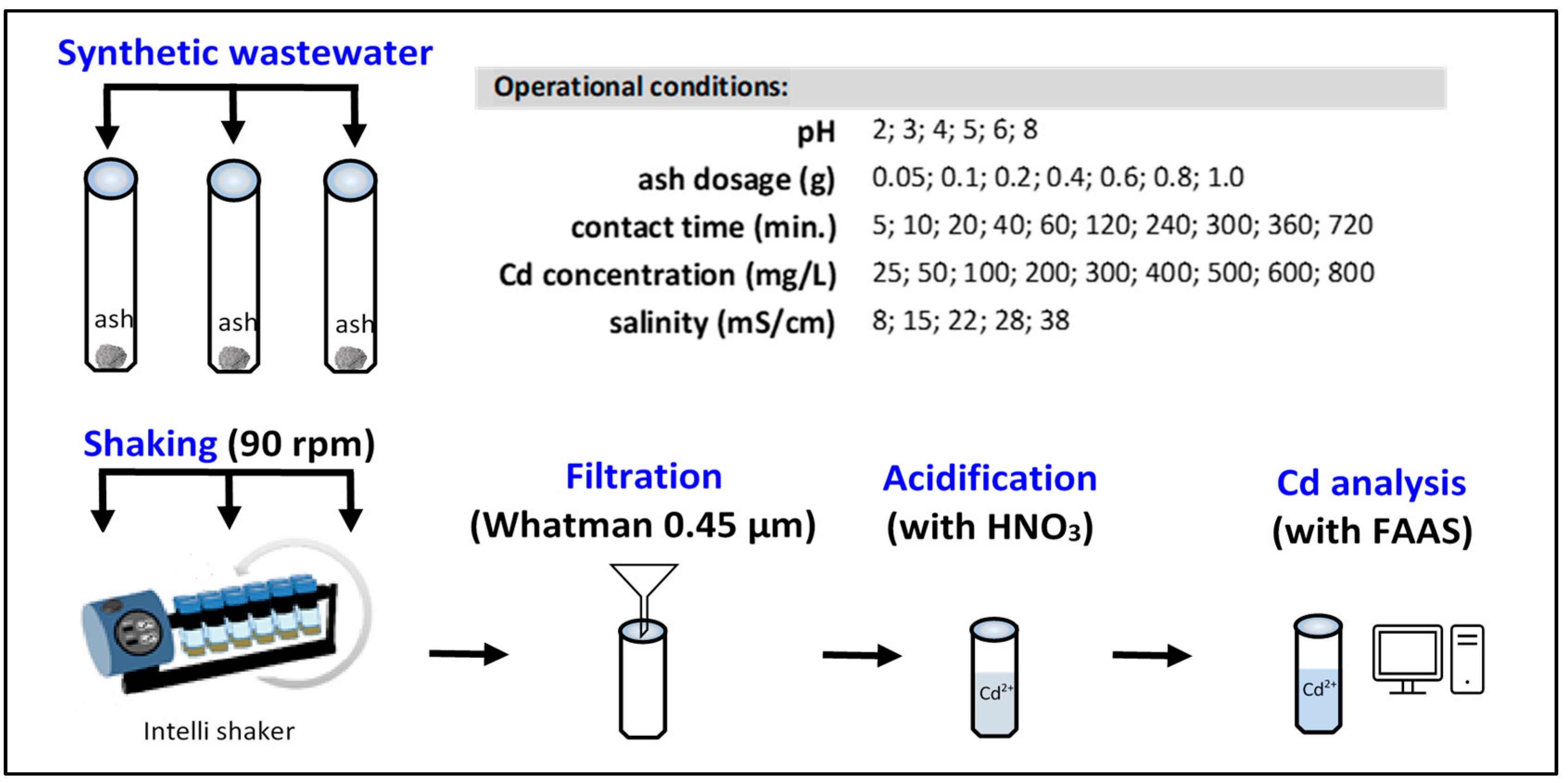
2.3. Batch Adsorption Experiments
2.4. Calculations and Models
2.5. Analytical Methods
2.6. Statistical Analysis and Quality Control
3. Results and Discussion
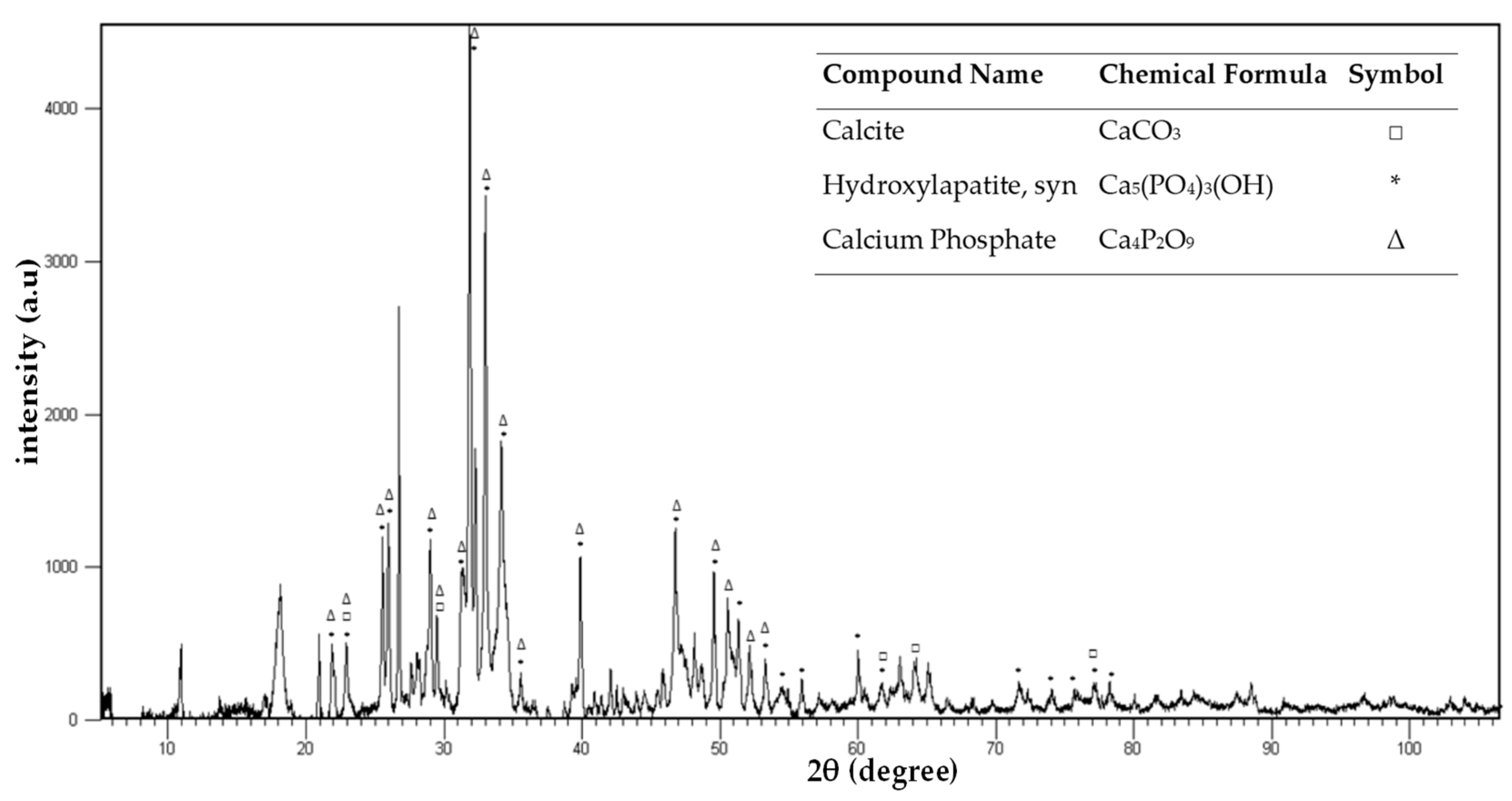
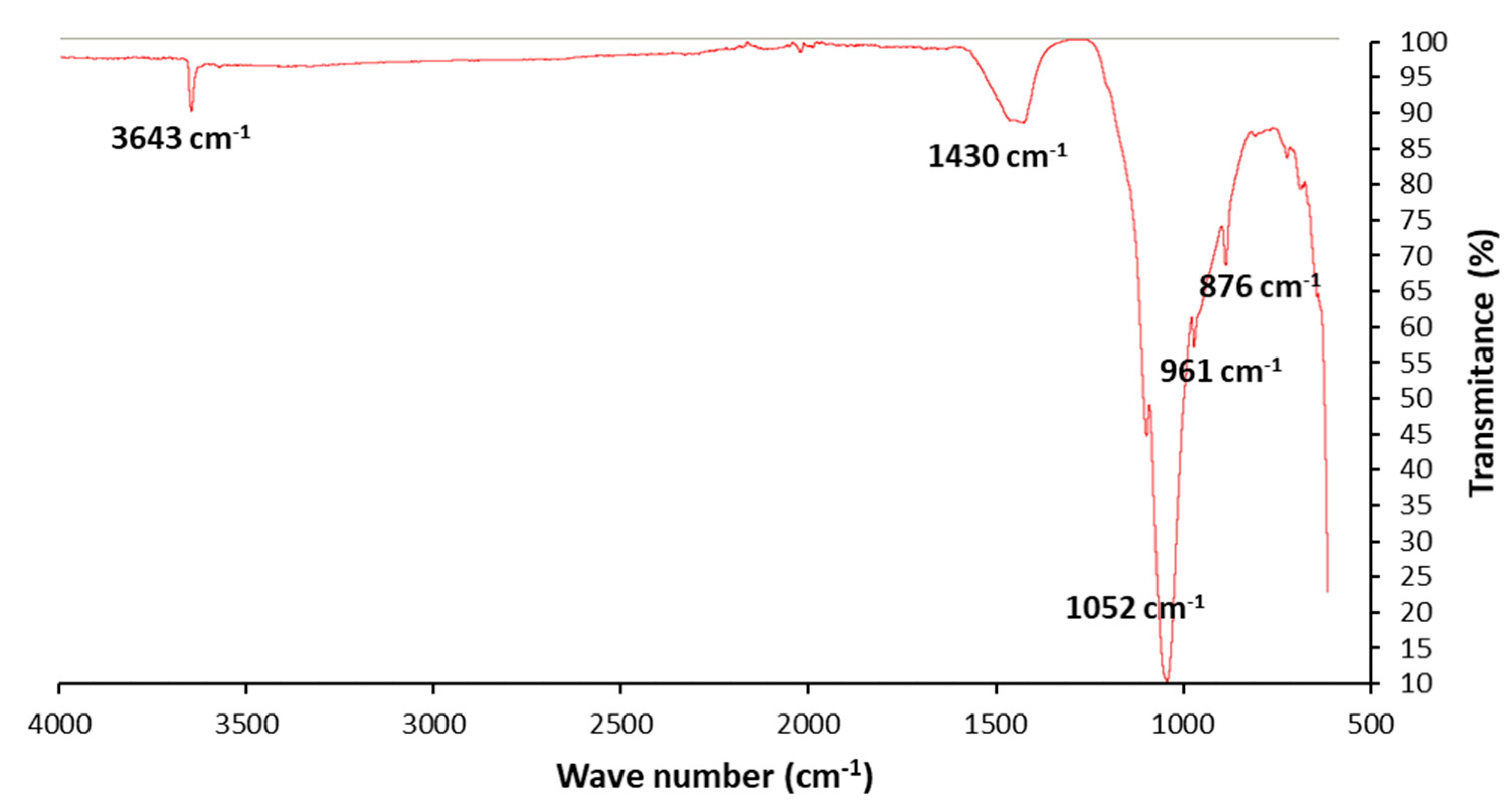
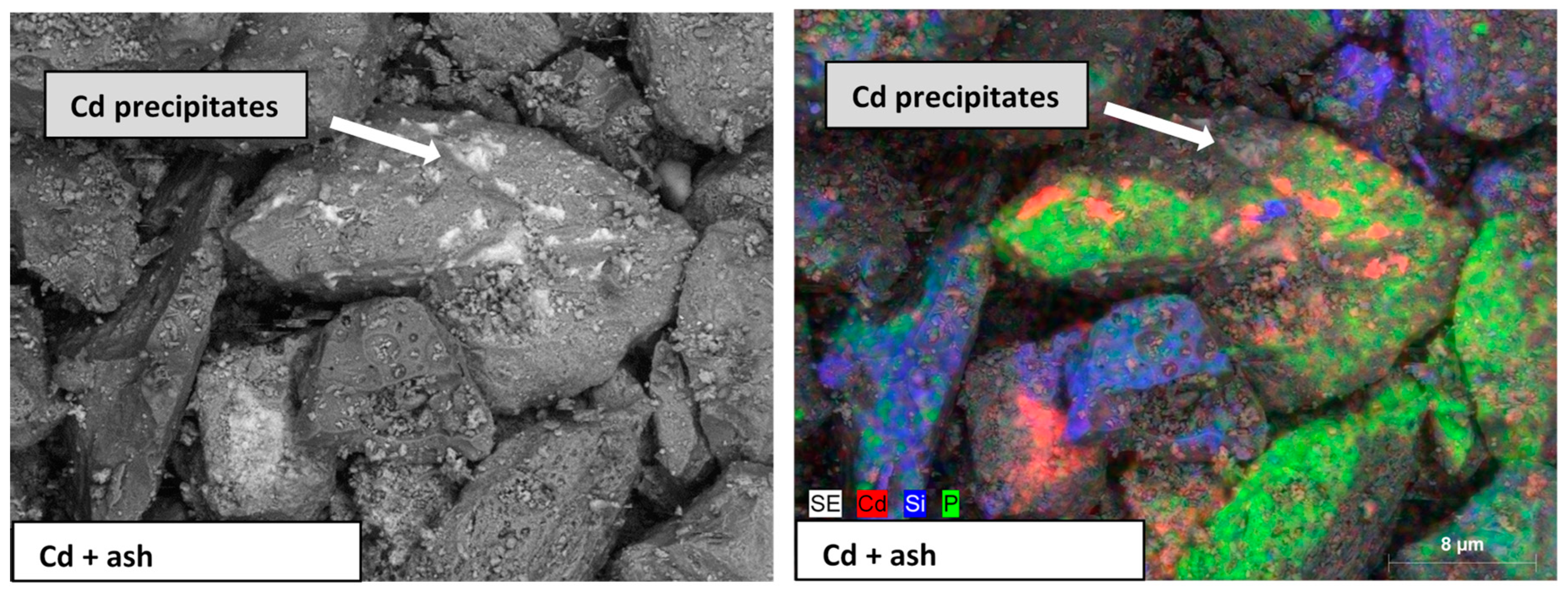
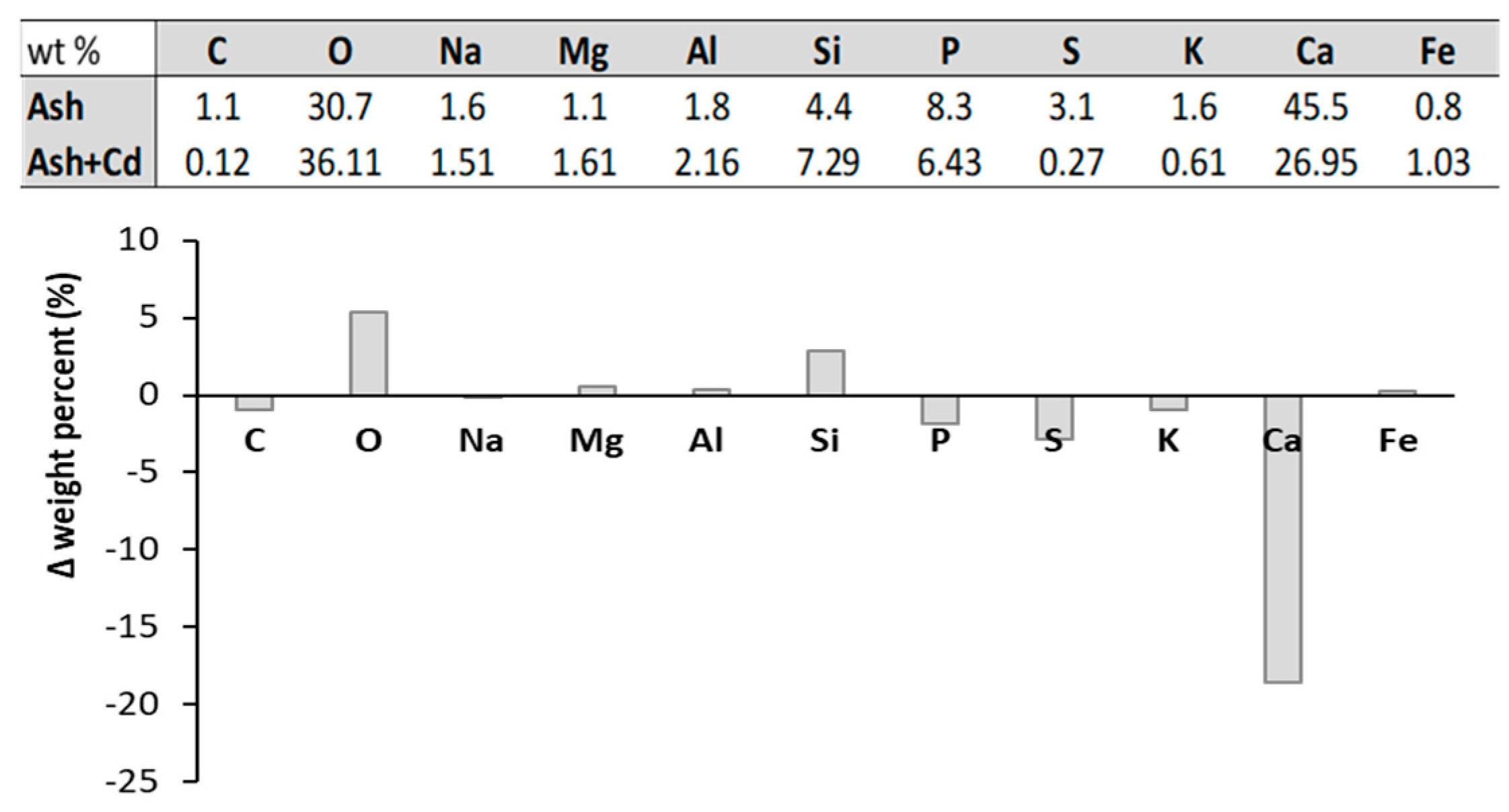
3.1. Ash Characterization
3.2. Effect of Operational Conditions on Cd Sorption onto Poultry Ash
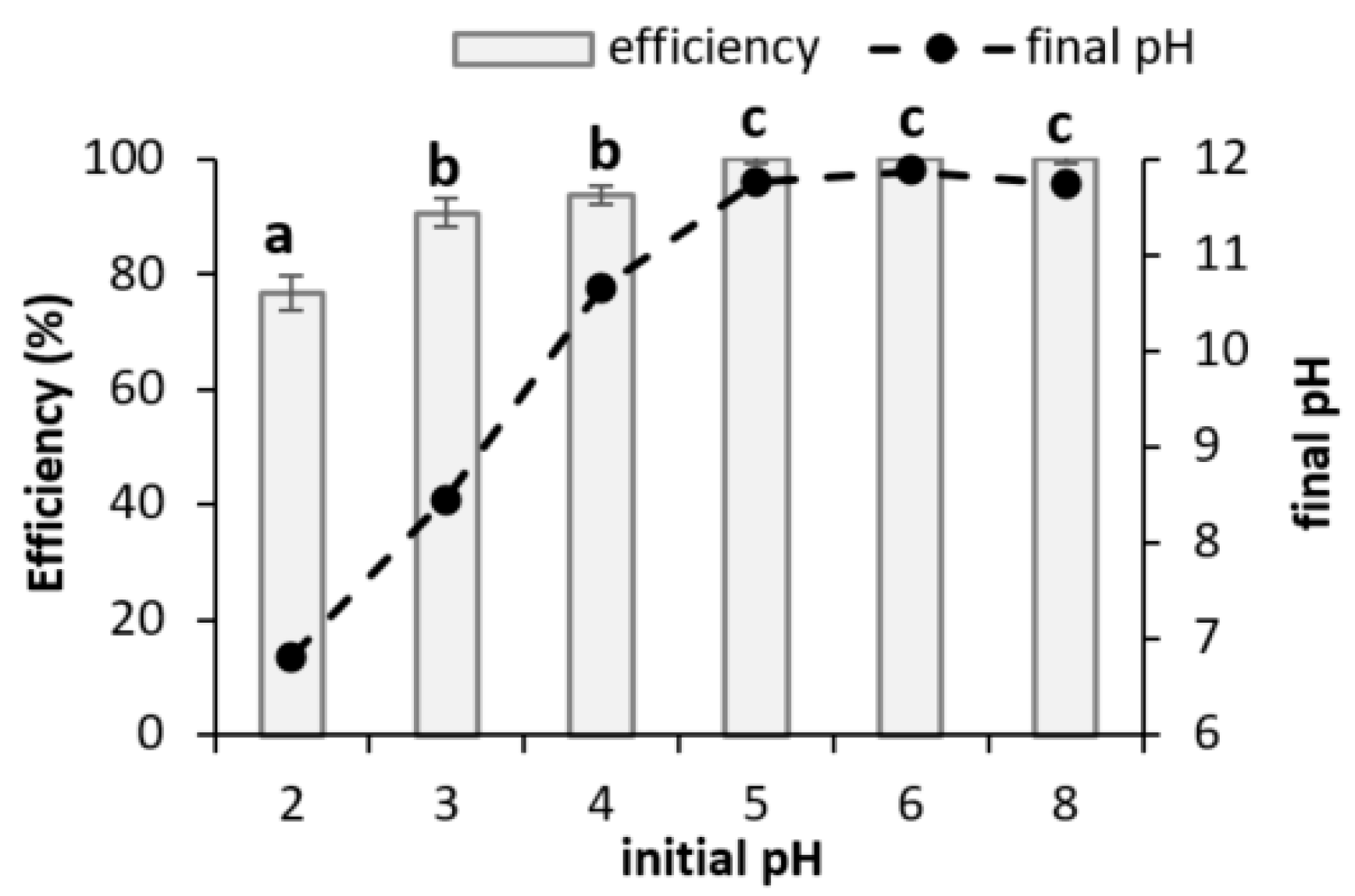
3.2.1. Initial pH in Wastewater
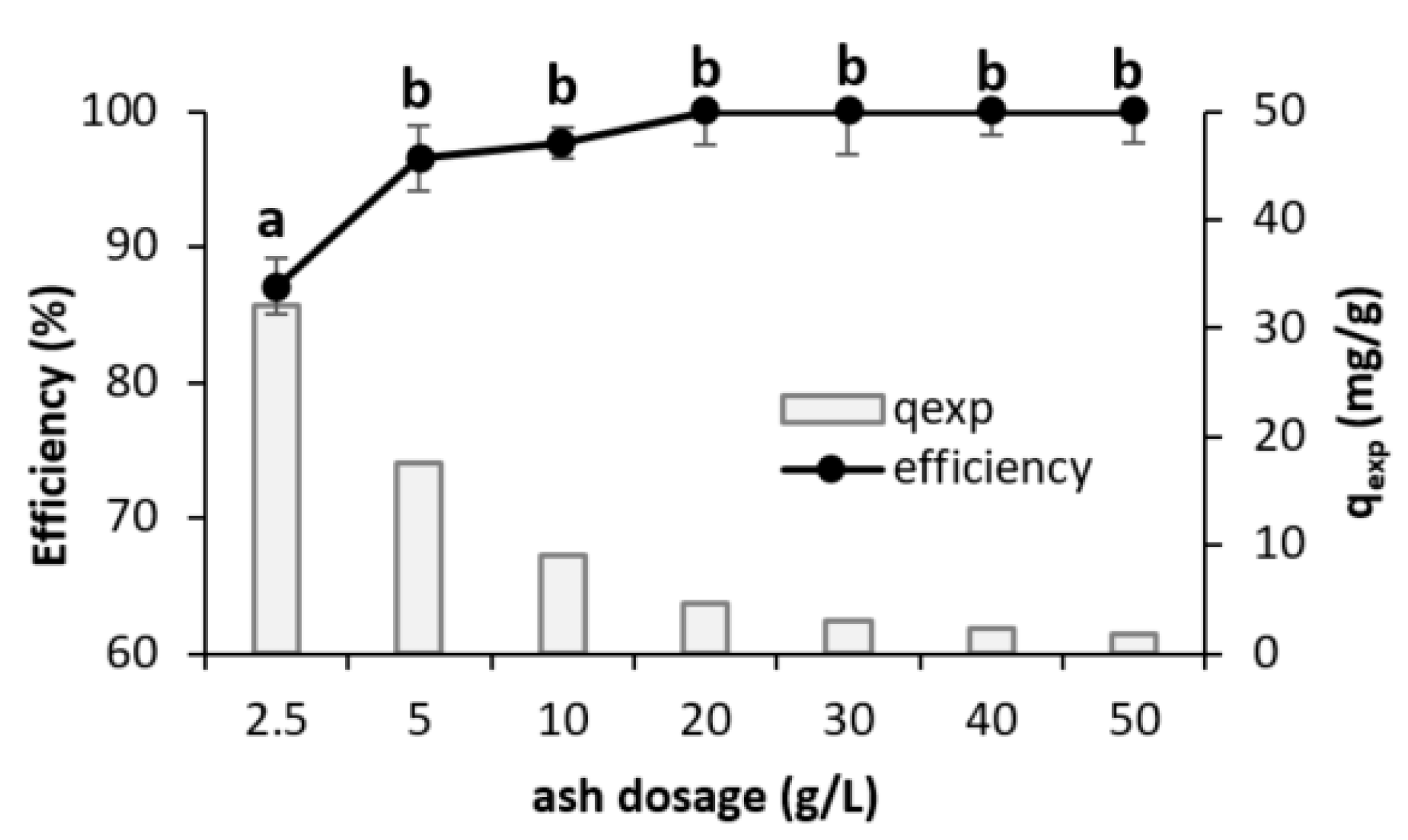
3.2.2. Adsorbent Dosage
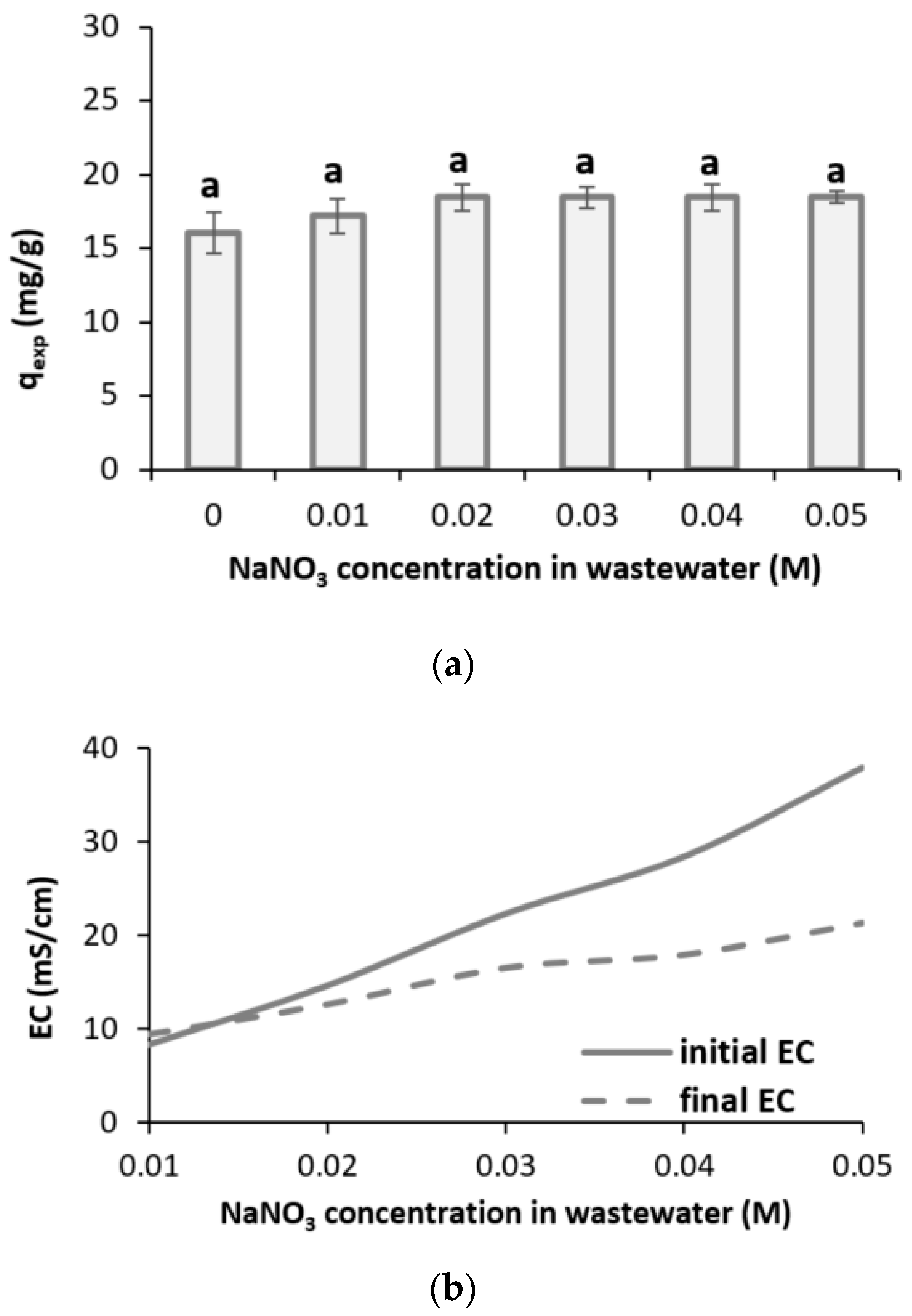
3.2.3. Salinity
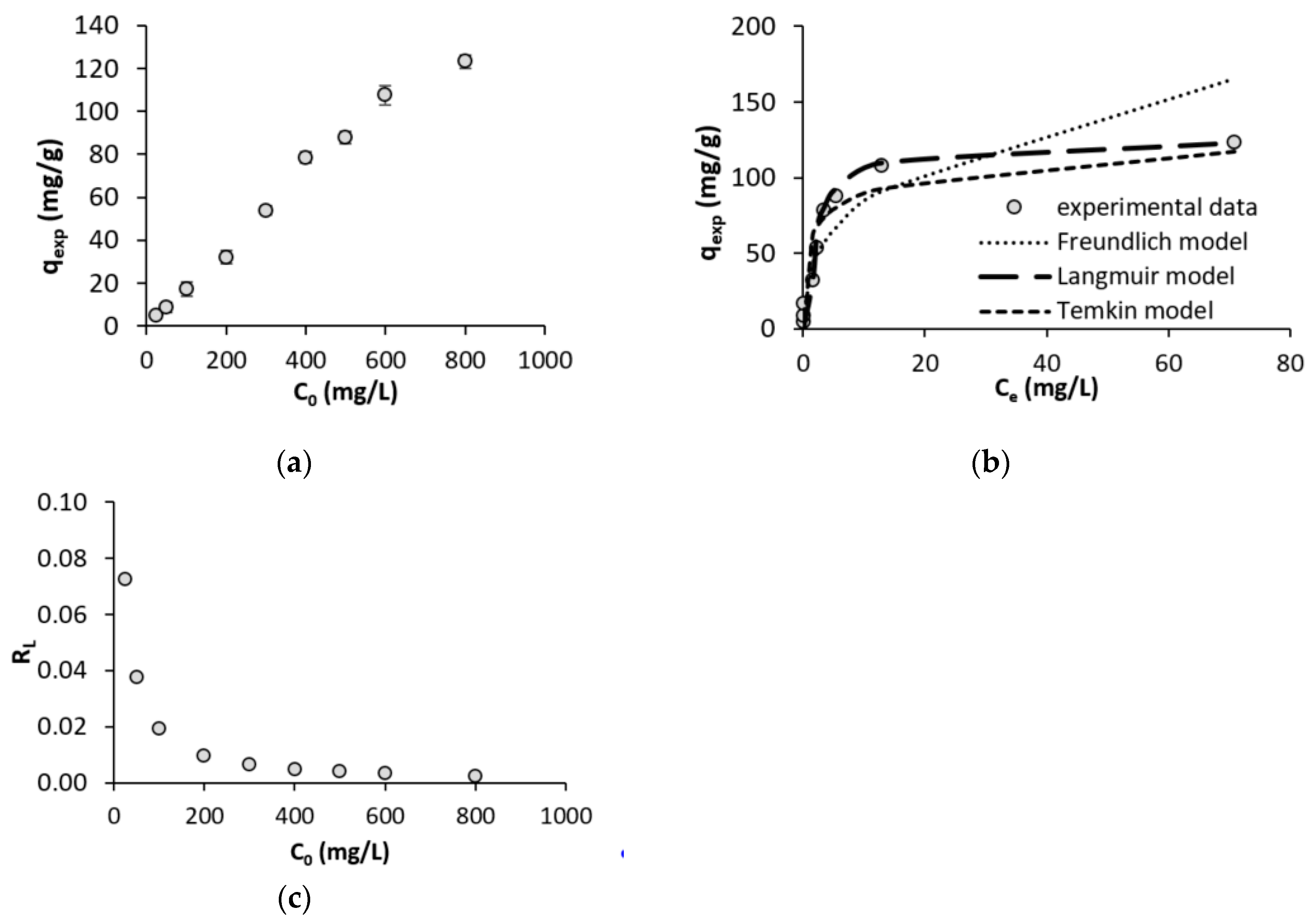
3.3. Initial Cd Concentration and Adsorption Isotherms
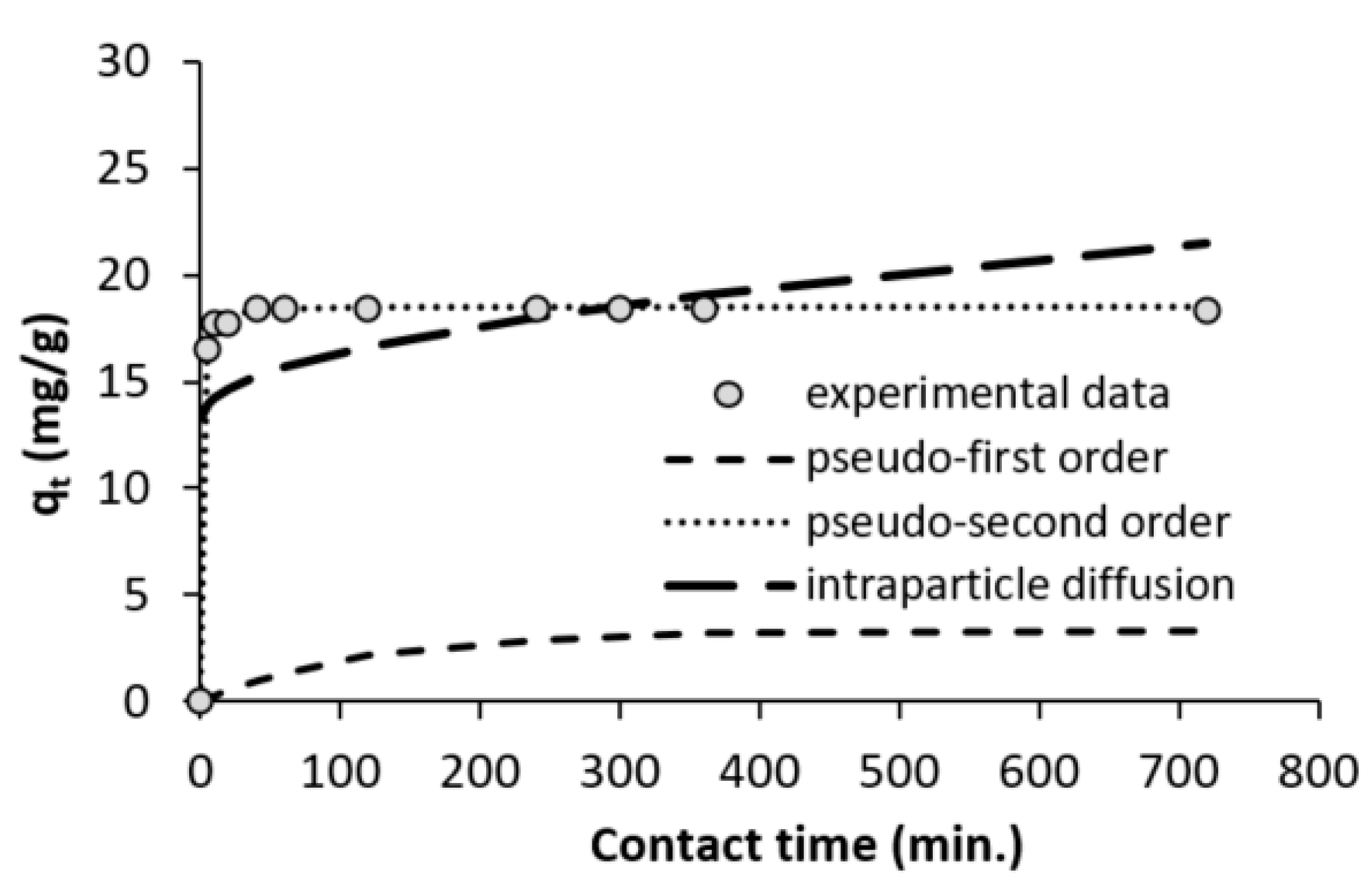
3.4. Contact Time and Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Hussain, A.; Alamzeb, S.; Begum, S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013, 136, 1515–1523. [Google Scholar] [CrossRef]
- Friberg, L.; Elinder, C.; Kjellstrom, T. Cadmium. Environmental Health Criteria 134; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Kumar, R.; Chawla, J.; Kaur, I. Removal of cadmium ion from wastewater by carbon-based nanosorbents: A review. J. Water Health 2015, 13, 18–33. [Google Scholar] [CrossRef]
- Kuchekar, S.R.; Patil, M.P.; Aher, H.R.; Gaikwad, V.B.; Han, S.H. Adsorptive removal of cadmium (II) ion from industrial wastewater by natural adsorbent. J. Mater. Environ. Sci. 2019, 10, 1117–1122. [Google Scholar]
- Vullo, D.L.; Ceretti, H.M.; Daniel, M.A.; Ramirez, S.A.M.; Zalts, A. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour. Technol. 2008, 99, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Manjula, D.M.; Oviyaa Sri, M. Heavy metals removal from industrial wastewater by nano adsorbent prepared from cucumis melopeel activated carbon. J. Nanomed. Res. 2017, 5, 00102. [Google Scholar] [CrossRef]
- Ordinance of the Minister of Environment (OME). Ordinance of the Minister of Environment on the conditions for wastewater discharge to water and ground and on pollutants in water environment. J. Law 2014, 1800, 1–42. (In Polish) [Google Scholar]
- Padmaja, M.; Bhavani, R.; Pamila, R. Adsorption of Cadmium from Aqueous Solutions Using Low cost Materials-A Review. Int. J. Eng. Technol. 2018, 7, 26–29. [Google Scholar] [CrossRef]
- Sukpreabprom, H.; Arqueropanyo, O.A.; Naksata, W.; Sooksamiti, P.; Janhom, S. Single and binary adsorption of Cd (II) and Zn (II) ions from aqueous solutions onto bottom ash. Korean J. Chem. Eng. 2015, 32, 896–902. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 1–38. [Google Scholar] [CrossRef]
- Eurostat. 2019. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190325-1 (accessed on 2 October 2020).
- Reddy, N.; Santosh, M.S. Recovery and Applications of Feather Proteins. In Protein Byproducts: Transformation from Environmental Burden into Value-Added Products; Dhillon, G.S., Ed.; Academic Press: Oxford, UK, 2016; pp. 255–274. [Google Scholar]
- Kwiatkowski, K.; Krzysztoforski, J.; Bajer, K.; Dudyński, M. Bioenergy from feathers gasification–Efficiency and performance analysis. Biomass Bioenergy 2013, 59, 402–411. [Google Scholar] [CrossRef]
- de la Rosa, G.; Reynel-Avila, H.E.; Bonilla-Petriciolet, A.; Cano-Rodriguez, I.; Velasco-Santos, C.; Martínez-Hernández, A.L. Recycling poultry feathers for Pb removal from wastewater: Kinetic and equilibrium studies. Proc. World Acad. Sci. Eng. Technol. 2008, 1, 185–193. [Google Scholar]
- Chen, B.; Yan, L.; Liu, X.; Worral, J.L. Poultry keratin based decolorants for dyeing wastewater treatment. J. Bioresour. Bioproduct. 2016, 1, 30–35. [Google Scholar]
- Nodoushan, M.H.S.; Ehrampoush, M.H. Study of Co (II) adsorption from aqueous solution using protein granules produced from chicken feather. Environ. Health Eng. Manag. J. 2015, 2, 193–197. [Google Scholar]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef]
- Zhang, H.; Carrillo, F.; López-Mesas, M.; Palet, C. Valorization of keratin biofibers for removing heavy metals from aqueous solutions. Text. Res. J. 2019, 89, 1153–1165. [Google Scholar] [CrossRef]
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; Tinôco, I.D.F.F.; Barbosa, R.C.; de Oliveira Resende, M.; Coelho, S.T. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Dudyński, M.; Kwiatkowski, K.; Bajer, K. From feathers to syngas–technologies and devices. Waste Manag. 2012, 32, 685–691. [Google Scholar] [CrossRef]
- Ghiaci, M.; Dorostkar, N.; Gil, A. Chicken bone ash as an efficient metal biosorbent for cadmium, lead, nickel, and zinc from aqueous solutions. Desal. Water Treat. 2014, 52, 3115–3121. [Google Scholar] [CrossRef]
- Asokbunyarat, V.; van Hullebusch, E.D.; Lens, P.N.; Annachhatre, A.P. Coal Bottom Ash as Sorbing Material for Fe (II), Cu (II), Mn (II), and Zn (II) Removal from Aqueous Solutions. Water Air Soil Pollut. 2015, 226, 143. [Google Scholar] [CrossRef]
- Asl, S.M.H.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, H.; Hu, X.; Liu, F.; Wang, L.; Zhao, X.; Ji, P. Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+. J. Hazard. Mater. 2020, 392, 122461. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Zhang, G.; Li, J.; He, Z.; Ji, P.; Zhao, J. Possibility of removing cadmium pollution from the environment using a newly synthesized material coal fly ash. Environ. Sci. Pollut. Res. 2020, 27, 4997–5008. [Google Scholar] [CrossRef]
- Park, J.H.; Eom, J.H.; Lee, S.L.; Hwang, S.W.; Kim, S.H.; Kang, S.W.; Yun, J.-J.; Cho, J.-S.; Lee, Y.-H.; Seo, D.C. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Zhu, T. Mercury removal from coal combustion flue gas by modified fly ash. J. Environ. Sci. 2013, 25, 393–398. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Cho, D.W.; Tsang, D.C.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C.S. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Shin, W.S.; Kang, K.; Kim, Y.K. Adsorption characteristics of multi-metal ions by red mud, zeolite, limestone, and oyster shell. Environ. Eng. Res. 2014, 19, 15–22. [Google Scholar] [CrossRef]
- Lan, T.; Li, P.; Rehman, F.U.; Li, X.; Yang, W.; Guo, S. Efficient adsorption of Cd2+ from aqueous solution using metakaolin geopolymers. Environ. Sci. Pollut. Res. 2019, 26, 33555–33567. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Nasab, S.B.; Moradzadeh, M.; Divband, S.; Koupai, J.A. Cadmium removal from aqueous solution using nano/milli-sized particles of cedar leaf ash. Desalin. Water Treat. 2014, 1–9, 1–11. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, N.; Hu, H.; Huang, J.; Kalkhajeh, Y.K.; Wu, X.; Xu, N.; Fu, X.; Zhan, L. Adsorption of Cd (II) in water by mesoporous ceramic functional nanomaterials. R. Soc. Open Sci. 2019, 6, 182195. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Bordoloi, N.; Goswami, R.; Kumar, M.; Kataki, R. Biosorption of Co (II) from aqueous solution using algal biochar: Kinetics and isotherm studies. Bioresour. Technol. 2017, 244, 1465–1469. [Google Scholar] [CrossRef]
- Popoola, L.T.; Yusuff, A.S.; Adesina, O.A.; Lala, M.A. Brilliant green dye sorption onto snail shell-rice husk: Statistical and error function models as parametric isotherm predictors. J. Environ. Sci. Technol. 2019, 12, 65–80. [Google Scholar] [CrossRef]
- Ismail, K.N.; Hussin, K.; Idris, M.S. Physical, chemical and mineralogical properties of fly ash. J. Nucl. Relat. Technol. 2007, 4, 47–51. [Google Scholar]
- Pitawala, L.M.S.D.; Wijeyawardana, H.M.P.; Nanayakkara, K.G.N. Utilization of coal fly ash in the adsorptive removal of fluoride from contaminated groundwater. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 849, p. 012025. [Google Scholar]
- Fahimi, A.; Bilo, F.; Assi, A.; Dalipi, R.; Federici, S.; Guedes, A.; Fiameni, L. Poultry litter ash characterisation and recovery. Waste Manag. 2020, 111, 10–21. [Google Scholar] [CrossRef]
- Valentim, B.; Flores, D.; Guedes, A.; Guimarães, R.; Shreya, N.; Paul, B.; Ward, C.R. Notes on the occurrence of phosphate mineral relics and spheres (phosphospheres) in coal and biomass fly ash. Inter. J. Coal Geol. 2016, 154, 43–56. [Google Scholar] [CrossRef]
- Tan, W.F.; Wang, L.A.; Huang, C.; Liu, Y.Y.; Green, J.E.; Newport, D.; Green, T. Utilization of municipal solid waste incineration fly ash in lightweight aggregates. J. Cent. South Univ. 2012, 19, 835–841. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Prinsloo, L.C.; Mathebula, C.L.; Swart, H.C.; Coetsee, E.; Doucet, F.J. Surface and bulk characterization of an ultrafine South African coal fly ash with reference to polymer applications. Appl. Surf. Sci. 2014, 317, 73–83. [Google Scholar] [CrossRef]
- Rajamma, R.; Ball, R.J.; Tarelho, L.A.; Allen, G.C.; Labrincha, J.A.; Ferreira, V.M. Characterisation and use of biomass fly ash in cement-based materials. J. Hazard. Mater. 2009, 172, 1049–1060. [Google Scholar] [CrossRef]
- Staroń, P.; Kowalski, Z.; Staroń, A.; Seidlerová, J.; Banach, M. Residues from the thermal conversion of waste from the meat industry as a source of valuable macro-and micronutrients. Waste Manag. 2016, 49, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Agrela, F.; Cabrera, M.; Morales, M.M.; Zamorano, M.; Alshaaer, M. Biomass fly ash and biomass bottom ash. In New Trends in Eco-Efficient and Recycled Concrete; de Brito, J.A., Ed.; Woodhead Publishing: Duxford UK, 2019; pp. 23–58. [Google Scholar]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Olgun, A. Studies on cement and mortar containing low-calcium fly ash, limestone, and dolomitic limestone. Cem. Concr. Com. 2008, 30, 194–201. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Santos, D.B.; Mansur, H.S. A microstructural approach to adherence mechanism of poly (vinyl alcohol) modified cement systems to ceramic tiles. Cem. Concr. Res. 2007, 37, 270–282. [Google Scholar] [CrossRef]
- Sukpreabprom, H.; Arquero, O.A.; Naksata, W.; Sooksamiti, P.; Janhom, S. Isotherm, kinetic and thermodynamic studies on the adsorption of Cd (II) and Zn (II) ions from aqueous solutions onto bottom ash. Inter. J. Environ. Sci. Dev. 2014, 5, 165–170. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, B.; Angove, M. Competitive adsorption behavior of heavy metals on kaolinite. J. Colloid Interface Sci. 2005, 290, 28–38. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J. Activated carbon-fly ash-nanometal oxide composite materials: Preparation, characterization, and tributyltin removal efficiency. J. Chem. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Treatment from abandoned mine landfill leachates. Adsorption technology. J. Mater. Res. Tech. 2019, 8, 2732–2740. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.; Nguyen, T.A.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef]
- Li, W.C.; Law, F.Y.; Chan, Y.H.M. Biosorption studies on copper (II) and cadmium (II) using pretreated rice straw and rice husk. Environ. Sci. Pollut. Res. 2017, 24, 8903–8915. [Google Scholar] [CrossRef]
- Benaissa, H.; Benguella, B. Effect of anions and cations on cadmium sorption kinetics from aqueous solutions by chitin: Experimental studies and modeling. Environ. Pollut. 2004, 130, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Gao, L.Y.; Deng, J.H.; Chen, S.H.; Cai, K.Z. Quantitative contribution of Cd2+ adsorption mechanisms by chicken-manure-derived biochars. Environ. Sci. Pollut. Res. 2018, 25, 28322–28334. [Google Scholar] [CrossRef] [PubMed]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd (II), Co (II), Cu (II), Pb (II), and Zn (II) ions from aqueous solutions via adsorption on fau-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 103895. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xiu, Z.M.; Liu, N.; Bi, H.T.; Lv, C.X. Adsorption of lead and cadmium ions from aqueous solutions by modified oil shale ash. Oil Shale 2012, 29, 268–278. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.M.; Yang, Q.; Zeng, G.M.; Shen, X.X.; Zhang, Y.; Liu, J.J. Adsorption of Cd (II) and Cu (II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J. Hazard. Mater. 2007, 147, 534–539. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, C.K.; Ali, I.; Sharma, M.; Saini, V.K. Removal of cadmium and nickel from wastewater using bagasse fly ash—A sugar industry waste. Water Res. 2003, 37, 4038–4044. [Google Scholar] [CrossRef]
- Singanan, M. Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. Sci. Asia 2011, 37, 115–119. [Google Scholar] [CrossRef]
- Lim, S.F.; Lee, A.Y.W. Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ. Sci. Pollut. Res. 2015, 22, 10144–10158. [Google Scholar] [CrossRef]
- Lundager Madsen, H.E.; Abbona, F.; Barrese, E. Effects of cadmium on crystallization of calcium phosphates. Crystal Res. Tech. J. Exp. Industr. Crystal. 2004, 39, 235–239. [Google Scholar] [CrossRef]
- Xu, Y.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ. Sci. Tech. 1994, 28, 1472–1480. [Google Scholar] [CrossRef] [PubMed]

| Physical Property | Chemical Composition | Contaminants | ||||||
|---|---|---|---|---|---|---|---|---|
| Type * | Unit | Value | Type | Unit | Value | Type | Unit | Value |
| SA | m2/g | 1.19 | P2O5 | wt% | 39.9 | Cd | mg/kg | 0.0 |
| TPV | cm3/g | 0.0023 | CaO | 35.5 | Cr | 1.8 ± 0.5 | ||
| TAP | m2/g | 0.618 | SiO2 | 10.9 | Cu | 2.4 ± 0.09 | ||
| Na2O | 3.5 | Ni | 0.0 | |||||
| MgO | 2.2 | Pb | 0.0 | |||||
| K2O | 2.2 | Zn | 0.3 ± 0.03 | |||||
| Al2O3 | 1.5 | ∑BTEXs | <0.1 | |||||
| Fe2O3 | 0.7 | ∑PAHs | <0.1 | |||||
| ∑PCBs | <1 | |||||||
| Adsorption Model | Parameters | Unit | Value |
|---|---|---|---|
| Freundlich | KF | L/g | 37.2 |
| n | – | 2.85 | |
| R2 | − | 0.8494 | |
| SSE | − | 3.2 | |
| Langmuir | qm | mg/g | 126.6 |
| KL | L/mg | 0.51 | |
| R2 | − | 0.9961 | |
| SSE | − | 0.8 | |
| Temkin | B | mg/L | 14.454 |
| Kt | J/mol | 47.6086 | |
| R2 | − | 0.8798 | |
| SSE | − | 3.8 |
| Adsorbent | Adsorption Conditions | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| Bottom ash | 1 g/L, pH 6, 60 min, 30 °C | 13.7 | [10] |
| Modified coal fly ash | 2 g/L, pH 5, 120 min, 25 °C | 69.3 | [26] |
| Geopolymers | 4 g/L, pH 5, 400 min, 25 °C | 33.98–67.83 | [32] |
| Fly ash from wood pellets | 2 g/L, pH 5, 24 h, 25 °C | 142.9 | [28] |
| Bottom ash from wood pellets | 23.3 | ||
| Coal fly ash | 3 g/L, pH 6, 20 min, 30 °C | 78.14 | [27] |
| Zeolite from coal fly ash | 10 g/L, 90 min, 25 °C | 74.074 | [59] |
| Cedar leaf ash | 10 g/L, 30-45 min, pH 5, 20 °C | 4.33–7.85 | [33] |
| Modified oil shale ash | 5 g/L, pH 7, 15 min, 20 °C | 12.05 | [60] |
| Hydroxylapatite | 2.5 g/L, pH 5, 180 min, 20 °C | 111.1 | [61] |
| Bagasse fly ash | 10 g/L, pH 6, 150 min, 30 °C | 1.24 | [62] |
| Poultry ash | 5 g/L, pH 5, 24 h, 25 °C | 126.6 | this study |
| Kinetic Model | Parameters | Unit | Value |
|---|---|---|---|
| Pseudo-first order | qe | mg/g | 3.33 |
| k2 | 1/min | 0.0124 | |
| R2 | – | 0.8125 | |
| SSE | – | 6.8355 | |
| Pseudo-second order | qe | mg/g | 18.55 |
| k2 | g/mg/min | 0.121 | |
| R2 | – | 1.000 | |
| SSE | – | 0.002 | |
| Intraparticle diffusion | Ci | mg/g | 0.3046 |
| ki | mg/g min1/2 | 13.305 | |
| R2 | – | 0.2166 | |
| SSE | – | 0.1539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusiatin, Z.M.; Kumpiene, J.; Janiszewska, S.; Kasiński, S.; Pecio, M.; Piec, R.; Radziemska, M. A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater. Minerals 2020, 10, 1048. https://doi.org/10.3390/min10121048
Gusiatin ZM, Kumpiene J, Janiszewska S, Kasiński S, Pecio M, Piec R, Radziemska M. A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater. Minerals. 2020; 10(12):1048. https://doi.org/10.3390/min10121048
Chicago/Turabian StyleGusiatin, Zygmunt Mariusz, Jurate Kumpiene, Sylwia Janiszewska, Sławomir Kasiński, Mariusz Pecio, Robert Piec, and Maja Radziemska. 2020. "A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater" Minerals 10, no. 12: 1048. https://doi.org/10.3390/min10121048
APA StyleGusiatin, Z. M., Kumpiene, J., Janiszewska, S., Kasiński, S., Pecio, M., Piec, R., & Radziemska, M. (2020). A Mineral By-Product from Gasification of Poultry Feathers for Removing Cd from Highly Contaminated Synthetic Wastewater. Minerals, 10(12), 1048. https://doi.org/10.3390/min10121048








