Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Characterization
2.3. Testing of the Slag’s Pozzolanic Activity
3. Results
3.1. Chemical Analysis and Determination of the Content of Heavy Metals
3.2. Thermogravimetric and Differential Thermal Analysis (TGA/DTA)
3.3. Textural Properties
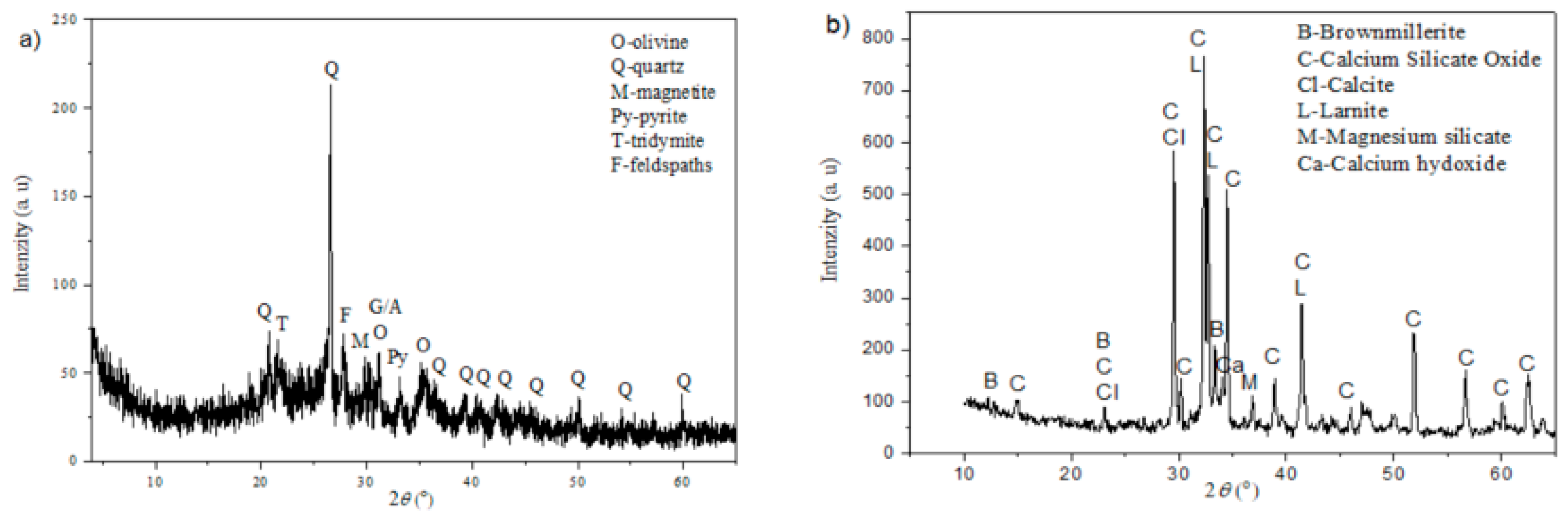
3.4. X-ray Structural Analysis (XRD)
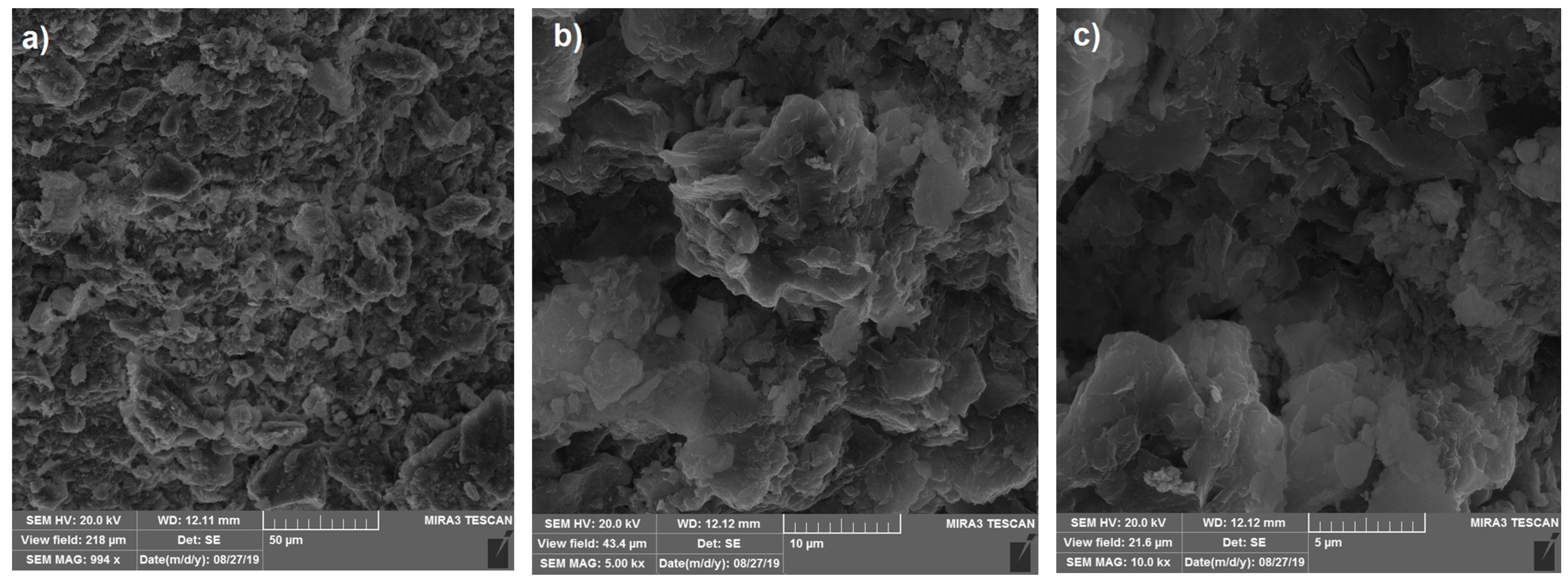
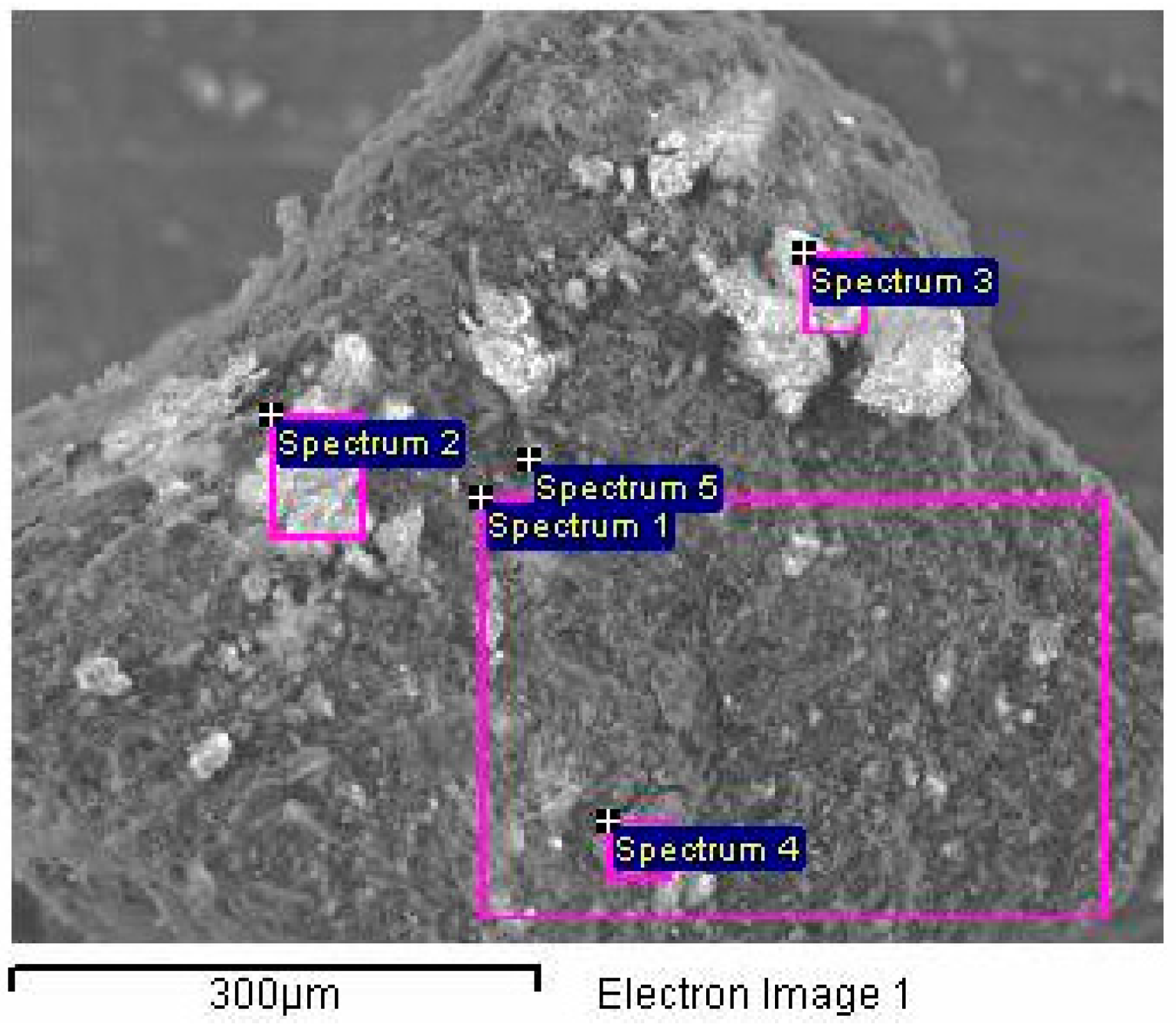
3.5. Scanning Electron Microscopy (SEM) Analysis
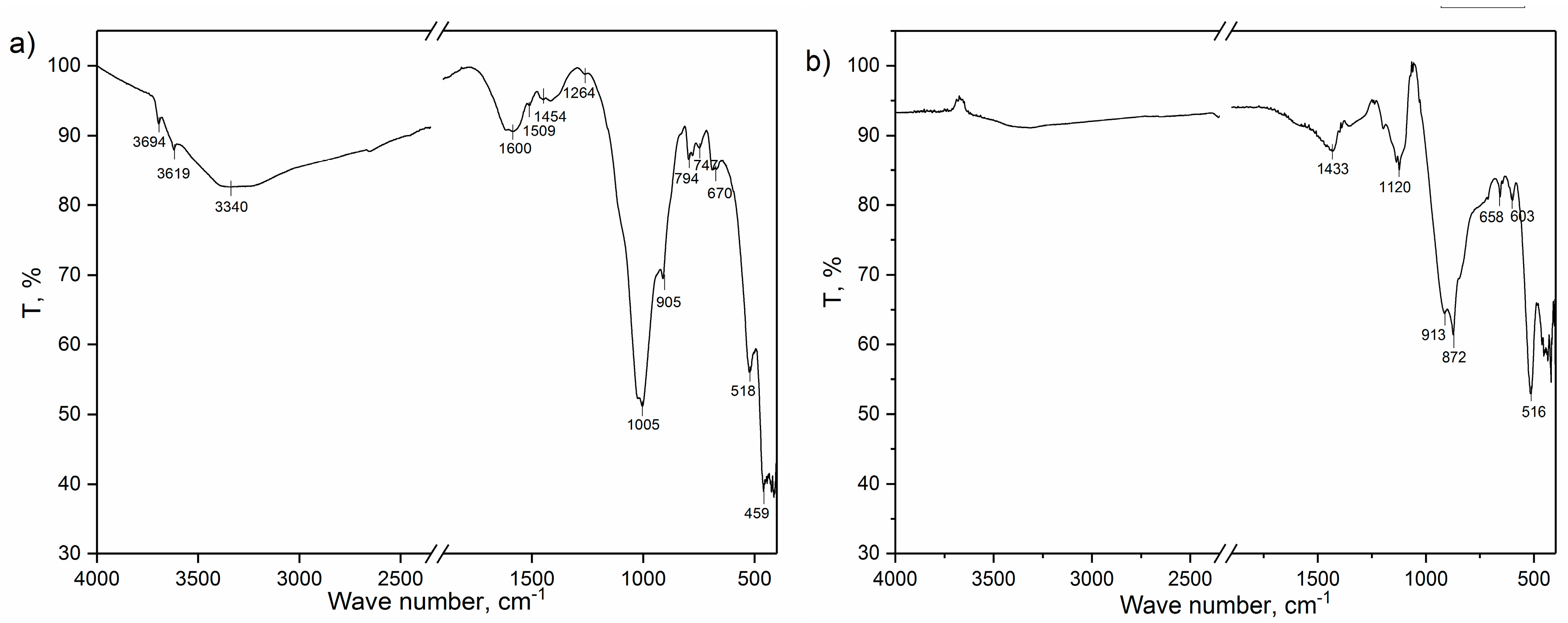
3.6. Infrared Spectroscopy with Fourier Transform (FTIR)
3.7. Testing of the Slag’s Pozzolanic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mining Technology. Available online: https://www.mining-technology.com/features/feature-the-worlds-biggest-coal-reserves-by-country (accessed on 19 May 2020).
- Milovanović, Z. Chapter: 7 Edition: Monographs “Energy and process plants”. In Thermal Power Plants—Theoretical Bases; Faculty of Mechanical Engineering, University of Banja Luka: Banja Luka, Bosnia and Herzegovina, 2005; pp. 385–392. [Google Scholar]
- American Geosciences Institute. Available online: https://www.americangeosciences.org/critical-issues/faq/what-are-the-different-types-of-coal (accessed on 19 May 2020).
- Aydin, S.; Baradan, B. Effect of pumice and fly ash incorporation on high temperature resistance of cement based mortars. Cem. Concr. Res. 2007, 37, 988–995. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/605024/major-lignite-consuming-countries-globally/date (accessed on 8 April 2020).
- Dwivedi, A.; Jain, M. Fly ash—Waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Terzić, A.; Mijatović, N.; Milićević, L.J.; Radojević, Z. Slag from coal combustion process as a secondary raw material for use in building composites. In Contemporary Materials and Constructions with Regulations, Belgrade, Serbia; Jevtić, D., Ed.; Society for Testing and Research of Materials and Structures: Belgrade, Serbia, 2016; pp. 51–60. [Google Scholar]
- Li, R.; Wang, L.; Yang, T.; Raninger, B. Investigation of MSWI fly ash melting characteristic by DSC–DTA. Waste Manag. 2007, 27, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Wey, M.Y.; Hwang, J.H.; Chen, J.C. Stability of heavy metals in bottom ash and fly ash under various incinerating conditions. J. Hazard. Mater. 1998, 57, 145–154. [Google Scholar] [CrossRef]
- Terzić, A.; Pezo, L.; Mijatović, N.; Stojanović, J.; Kragović, M.; Miličić, L.J.; Andrić, L.J. The effect of alternations in mineral additives (zeolite, bentonite, fly ash) on physico-chemical behavior of Portland cement based binders. Constr. Build. Mater. 2018, 180, 199–210. [Google Scholar] [CrossRef]
- Sun, X.; Yi, Y. pH evolution during water washing of incineration bottom ash and its effect on removal of heavy metals. Waste Manag. 2020, 104, 213–219. [Google Scholar] [CrossRef] [PubMed]
- European List of Waste, the Classification of Waste Based on: The European List of Waste (Commission Decision 2000/532/EC—Consolidated Version) and Annex III to Directive 2008/98/EC (Consolidated Version). Available online: http://data.europa.eu/eli/dec/2000/532/2015-06-01 (accessed on 25 September 2020).
- Official Journal of the Republic of Serbia No. 12/95. Available online: http://www.slglasnik.com/ (accessed on 1 February 2017).
- Decree of 20 December 2005—Official Journal of the Republic of Serbia No.113. Available online: http://www.slglasnik.com/ (accessed on 20 December 2005).
- Liu, X.; Ni, C.; Meng, K.; Zhang, L.; Liu, D.; Sun, L. Strengthening mechanism of lightweight cellular concrete filled with fly ash. Constr. Build. Mater. 2020, 251, 118954. [Google Scholar] [CrossRef]
- Wong, G.; Fan, X.; Gan, M.; Ji, Z.; Ye, H.; Zhou, Z.; Wang, Z. Resource utilization of municipal solid waste incineration fly ash in iron ore sintering process: A novel thermal treatment. J. Clean. Prod. 2020, in press. [Google Scholar] [CrossRef]
- Cai, J.; Pan, J.; Li, X.; Tan, J.; Li, J. Electrical resistivity of fly ash and metakaolin based geopolymers. Constr. Build. Mater. 2020, 23420, 117–868. [Google Scholar] [CrossRef]
- Hu, C. Microstructure and mechanical properties of fly ash blended cement pastes. Constr. Build. Mater. 2014, 73, 618–625. [Google Scholar] [CrossRef]
- Zabielska-Adamska, K. Laboratory compaction of fly ash and fly ash with cement additions. J. Hazard. Mater. 2008, 151, 481–489. [Google Scholar] [CrossRef]
- Nadesan, M.; Dinakar, P. Mix design and properties of fly ash waste lightweight aggregates in structural lightweight concrete. Case Stud. Constr. Mater. 2017, 7, 336–347. [Google Scholar] [CrossRef]
- Terzić, A.; Pavlović, L.J.; Radojević, Z.; Pavlović, V.; Mitić, V. Novel Utilization of Fly Ash for High-temperature Mortars: Phase Composititon, Microstructure and Performance Correlation. Int. J. Appl. Ceram. Technol. 2015, 12, 133–146. [Google Scholar] [CrossRef]
- Wang, S.; Baxter, L.; Fonseca, F. Biomass fly ash in concrete: SEM, EDX and ESEM analysis. Fuel 2008, 87, 372–379. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Zajac, M.; Stabler, C.; Lothenbach, B. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]
- Sun, H.; Quian, J.; Yang, Y.; Fan, C.; Yue, Y. Optimization of gypsum and slag contents in blended cement containing slag. Cem. Concr. Compos. 2020, 112, 103674. [Google Scholar] [CrossRef]
- Yoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Characterization of blast furnace slag-blended Portland cement for immobilization of Co. Cem. Concr. Res. 2020, 134, 106089. [Google Scholar] [CrossRef]
- Voinovitch, I.; Debrad-Guedon, J.; Louvrier, J. The Analysis of Silicates; Israel Programfor Scientific Translations: Jerusalem, Israel, 1966. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, Berichte der Bunsengesellschaft für Physikalische Chemie; Hansen, N., Ed.; Academic Press: New York, NY, USA; London, UK, 1967; p. 71. [Google Scholar]
- Dubinin, M.M. Physical adsorption. In Progress in Surface Press, and Membrane Science; Cadenead, D.A., Danielli, J.F., Rosenberg, M.D., Eds.; Academic Press: New York, NY, USA; London, UK, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Dollimore, D.; Heal, G.R. An improved distribution method for the calculation of pore size from adsorption data. J. Appl. Chem. 1964, 14, 109–114. [Google Scholar] [CrossRef]
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Ltd.: Oxford, UK, 1998. [Google Scholar]
- Marinković, S.; Trifunović, P.; Tokalić, R.; Matijašević, S.; Kostić-Pulek, A. DTA/TGA studies of bottom ash from the Nikola Tesla power plant from Serbia for the purpose of its utilization in road construction. In Proceedings of the 36th International Conference of SSCHE, Tatranské Matliare, Slovakia, 25–29 May 2009; pp. 094-1–094-8. [Google Scholar]
- Maroto-Valer, M.; Taulbee, D.; Hower, J. Characterization of differing forms of unburned carbon present in fly ash separated by density gradient centrifugation. Fuel 2001, 80, 759–800. [Google Scholar] [CrossRef]
- Hill, R.; Sarkar, S.; Rathbone, R.; Hower, J. An examination of fly ash carbon and itsinteractions with air entraining agent. Cem. Concr. Res. 1997, 27, 193–204. [Google Scholar] [CrossRef]
- Millán-Corrales, G.; González-López, J.R.; Palomo, A.; Fernandez-Jiménez, A. Replacing fly ash with limestone dust in hybrid cements. Constr. Build. Mater. 2020, 243, 118–169. [Google Scholar] [CrossRef]
- Soriano, I.; Tashima, M.; Bonilla, M.; Payá, J.; Monzó, J.; Borrachero, M. Use of high resolution thermo gravimetric analysis (HRTG) technique in spent FCC catalyst/Portland cement pastes. J. Therm. Anal. Calorim. 2015, 120, 1511–1517. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. Chapter 5 (Thermogravimetric Analysis), A Practical Guide to Micostructural Analysis of Cementitious Materials, 1st ed.; Taylor and Francis Group: Oxford, UK, 2016; p. 36. [Google Scholar]
- Zeng, Q.; Li, K.; Chong, T.; Dangla, P. Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 2012, 27, 560–569. [Google Scholar] [CrossRef]
- Bertier, P.; Schweinar, K.; Stanjek, H.; Ghanizadeh, A.; Clarkson, C.R.; Busch, A.; Kampman, N.; Prinz, D.; Amann-Hildenbrabd, A.; Krooss, B.M.; et al. On the use and abuse of N2 physisorption for the characterization of the pore structure of shales. Clay Miner. Soc. Workshop Lect. 2016, 21, 151–161. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, V.A.; James, P.O.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, W.K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, S.R.; Bajaj, C.H.; Jasra, V.R. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Wu, H.; Xie, H.; He, G.; Guan, Y.; Zhang, Y. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite. Appl. Clay Sci. 2016, 119, 161–169. [Google Scholar] [CrossRef]
- Fesenko, O.; Yatsenko, L.; Brodin, M. Nano materials Imaging Techniques, Surface Studies, and Applications; Springer: Berlin, Germany, 2012; p. 146. [Google Scholar]
- Database of ATR-FT-IR Spectra of Various Materials. Available online: http://lisa.chem.ut.ee/IR_spectra/paint/fillers/quartz/ (accessed on 23 May 2020).
- Correcher, V.; Garcia-Guinea, J.; Bustillo, A.M.; Garcia, R. Study of the thermoluminescence emission of a natural α-cristobalite. Radiat. Eff. Defects Solids 2009, 164, 59–67. [Google Scholar] [CrossRef]
- Bosch-Reig, F.; Gimeno-Adelantado, J.V.; Bosch-Mossi, F.; Doménech-Carbó, A. Quantification of minerals from ATR-FTIR spectra with spectral interferences using the MRC method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 181, 7–12. [Google Scholar] [CrossRef]
- Obradović, M. Research and Comparison of the Influence of Low Rank Coals Characteristics on Their Grindability and Milling Process Parameters. Ph.D. Thesis, Faculty of Mechanical Engineering, University of Belgrade, Belgrade, Serbia, 2015. [Google Scholar]
- Mitrović, D. Origin and Paleoenvironmental Reconstruction of the “Kovin” Coal Deposit—Implications from Petrographic and Geochemical Investigations. Ph.D. Thesis, Faculty of Chemistry, University of Belgrade, Belgrade, Serbia, 2018. [Google Scholar]
- Mayo, D.V.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mane, V.G. Studies of Some Transition Metal Complexes of Schiffõs Bases. Lulu.Com. 2019. Available online: https://books.google.rs/books?hl=en&lr=&id=RVePDwAAQBAJ&oi=fnd&pg=PA19&ots=_Z8TVoH9Ph&sig=U2YqsX98CeXlMr4exwqcajZTtok&redir_esc=y#v=onepage&q&f=false (accessed on 25 September 2020).
- Singh, S.; Menon, S.; Gupta, K.; Jayavel, R. Preferentially oriented single crystal growth of brownmillerite CaFeO2.5 by flux growth technique. Mater. Lett. 2014, 131, 332–335. [Google Scholar] [CrossRef]
- Choudhary, R.; Koppala, S.; Swamiappan, S. Bioactivity studies of calcium magnesium silicate prepared from eggshell wastebysol–gelcombustionsynthesis. J. Asian Ceram. Soc. 2015, 3, 173–177. [Google Scholar] [CrossRef]
| Parameter | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 21.20 | 10.81 | 15.70 | 6.32 | 2.50 | 1.78 | 2.67 | 0.63 | 0.036 | 0.62 | 37.45 |
| CEM I 42.5R | 21.62 | 2.60 | 7.00 | 60.16 | 2.34 | 2.55 | 0.33 | 0.66 | - | 2.68 | |
| Standard | 70 < SiO2 + Fe2O3 + Al2O3 | ≤10.0 | ≤4.0 | ≤3.0 | ≤5.0 | - | ≤5.0 | ≤11.0 | |||
| Parameter | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 33.75 | 17.46 | 25.96 | 10.00 | 3.98 | 2.84 | 4.25 | 1.00 | 0.70 | 0.0573 |
| Sample | Cr | Cu | Li | Mn | Ni | Pb | Zn | Cd | Sn | Sb | Ag |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 138 | 51 | 31 | 600 | 110 | 480 | 36 | 2.2 | <5 | 160 | 5.4 |
| Limit value * | 70 | 40 | 50 | 5.0 |
| Released Element | Concentration | Allowed Values * |
|---|---|---|
| Cr | 75 | 4000–12,000 |
| Cu | 81 | 6000–12,000 |
| Mn | 114 | - |
| Ni | 154 | 3000–7500 |
| Pb | 563 | 5000–15,000 |
| Zn | 668 | 6000–20,000 |
| Cd | 29 | 50–200 |
| Ag | 21 | - |
| Weight Loss, % | |||||
|---|---|---|---|---|---|
| Temperature | 25–150 °C | 200–650 °C | 650–800 °C | 800–1000 °C | 25–1000 °C |
| Slag | 4.00 | 32.78 | 0.61 | 0.001 | 38.31 |
| Temperature | 25–400 °C | 400–1000 °C | 25–1000 °C | ||
| CEM I 42.5. | 0.65 | 6.87 | 7.52 | ||
| Sample | SBET, m2/g | Vmeso, cm3/g | Vmic, cm3/g |
|---|---|---|---|
| Slag | 25.0 | 0.020 | 0.010 |
| Spectrum Number | Element Concentration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | Si | Fe | Ti | Ca | Mg | K | Na | S | |
| 1 | 38.57 | 33.15 | 6.1 | 11.34 | 5.03 | 0.31 | 3.43 | 0.70 | 0.70 | - | 0.67 |
| 2 | 27.96 | 42.24 | 7.83 | 14.86 | 2.98 | 0.32 | 1.85 | 0.69 | 0.64 | 0.13 | 0.52 |
| 3 | 41.92 | 35.25 | 5.43 | 8.1 | 3.08 | 0.25 | 3.44 | 0.9 | 0.5 | - | 1.14 |
| 4 | 34.13 | 36.25 | 7.12 | 13.02 | 4,00 | 1.75 | 2.07 | 0.65 | 0.68 | - | 0.33 |
| 5 | 12.62 | 35.52 | 13.22 | 24.99 | 6.4 | 0.32 | 3.38 | 1.16 | 1.59 | - | 0.8 |
| Properties | 100% CEM | 75% CEM + 25% Slag | Requirements EN 450-1 |
|---|---|---|---|
| Activity index, % | - | 28 days–76.9 90 days–76.4 | 28 days–75 * 90 days–85 * |
| Water Requirement, % | - | 113 | <95% of CEM * |
| Setting time, min | initial 115 final 165 | initial 170 final 215 | <2 × 100% CEM ** - |
| Soundness, mm | 1.0 | 1.5 | <10.0 ** |
| Parameter | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Silica fume | 93.60 | 0.21 | 0.27 | 0.05 | 0.05 | 0.80 | 0.23 | 0.50 | - | 2.40 |
| Mark | E | P10-3 | P15-3 | P20-3 | P25-3 |
|---|---|---|---|---|---|
| Description | Etalon (without CEM replacement) | CEM replacement with 8.9% of slag and 1.1% silica fume. Total replacement of CEM = 10% | CEM replacement with 13.3% of slag and 1.7% silica fume. Total replacement of CEM = 15% | CEM replacement with 17.8% of slag and 2.2% silica fume. Total replacement of CEM = 20% | CEM replacement with 22.2% of slag and 2.8% silica fume. Total replacement of CEM = 25% |
| Mix Code | Activity Index/% | Requirements EN 450-1/% |
|---|---|---|
| P10 | 28 days–95.1 90 days–93.2 | 28 days–75 90 days–85 |
| P15 | 28 days–90.8 90 days–89.1 | |
| P20 | 28 days–86.9 90 days–85.2 | |
| P25 | 28 days–82.3 90 days–81.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeljković, A.; Stojmenović, M.; Gulicovski, J.; Ristić, N.; Milićević, S.; Krstić, J.; Kragović, M. Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals 2020, 10, 992. https://doi.org/10.3390/min10110992
Nedeljković A, Stojmenović M, Gulicovski J, Ristić N, Milićević S, Krstić J, Kragović M. Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals. 2020; 10(11):992. https://doi.org/10.3390/min10110992
Chicago/Turabian StyleNedeljković, Andrijana, Marija Stojmenović, Jelena Gulicovski, Nenad Ristić, Sonja Milićević, Jugoslav Krstić, and Milan Kragović. 2020. "Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag" Minerals 10, no. 11: 992. https://doi.org/10.3390/min10110992
APA StyleNedeljković, A., Stojmenović, M., Gulicovski, J., Ristić, N., Milićević, S., Krstić, J., & Kragović, M. (2020). Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals, 10(11), 992. https://doi.org/10.3390/min10110992






