Variability of Carbonate Isotope Signatures in a Hydrothermally Influenced System: Insights from the Pastos Grandes Caldera (Bolivia)
Abstract
1. Introduction
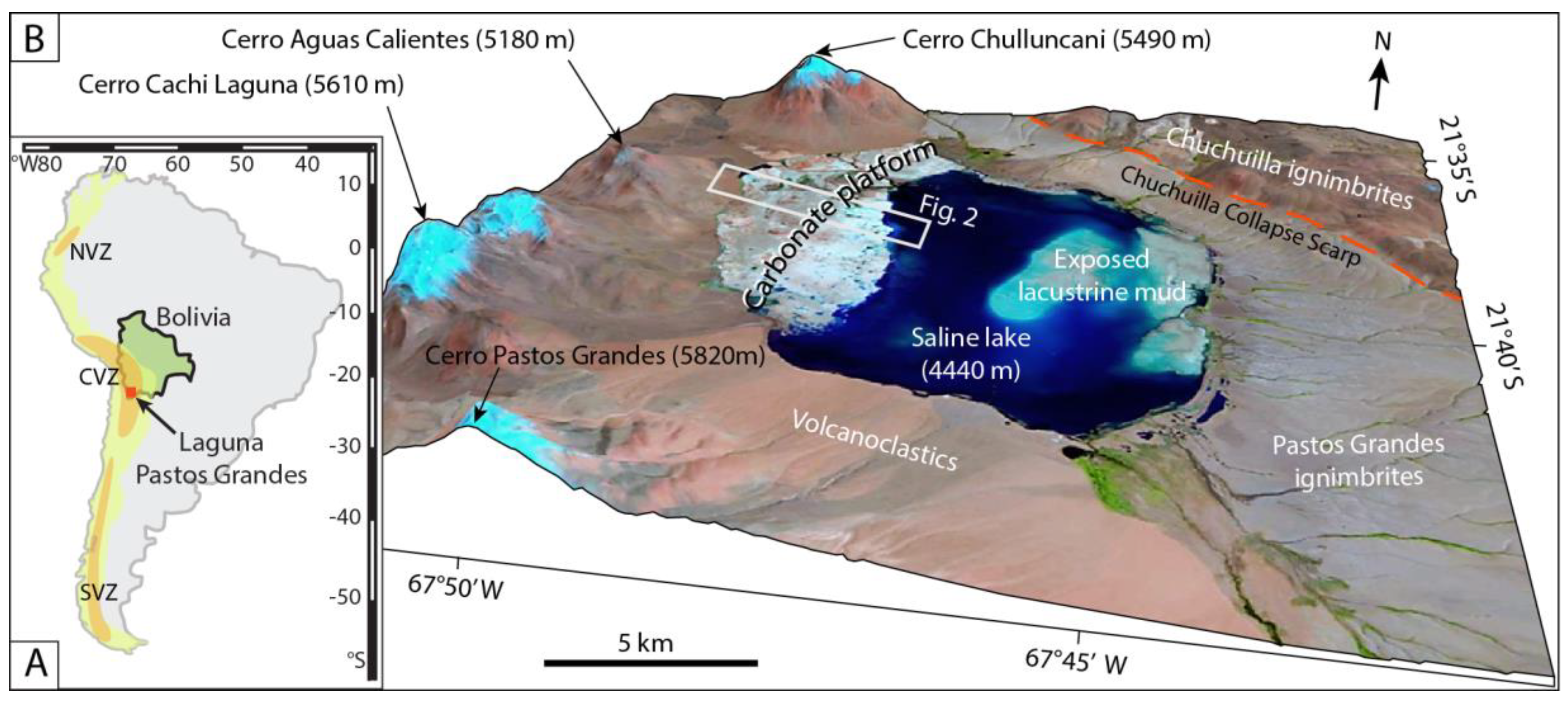
2. Regional Background, Volcanic Setting, and Carbonate Facies of the Pastos Grandes Area
3. Materials and Methods
4. Results
4.1. Carbonates of Laguna Pastos Grandes and Dating
4.2. Modern Calcite Facies and Parent Waters
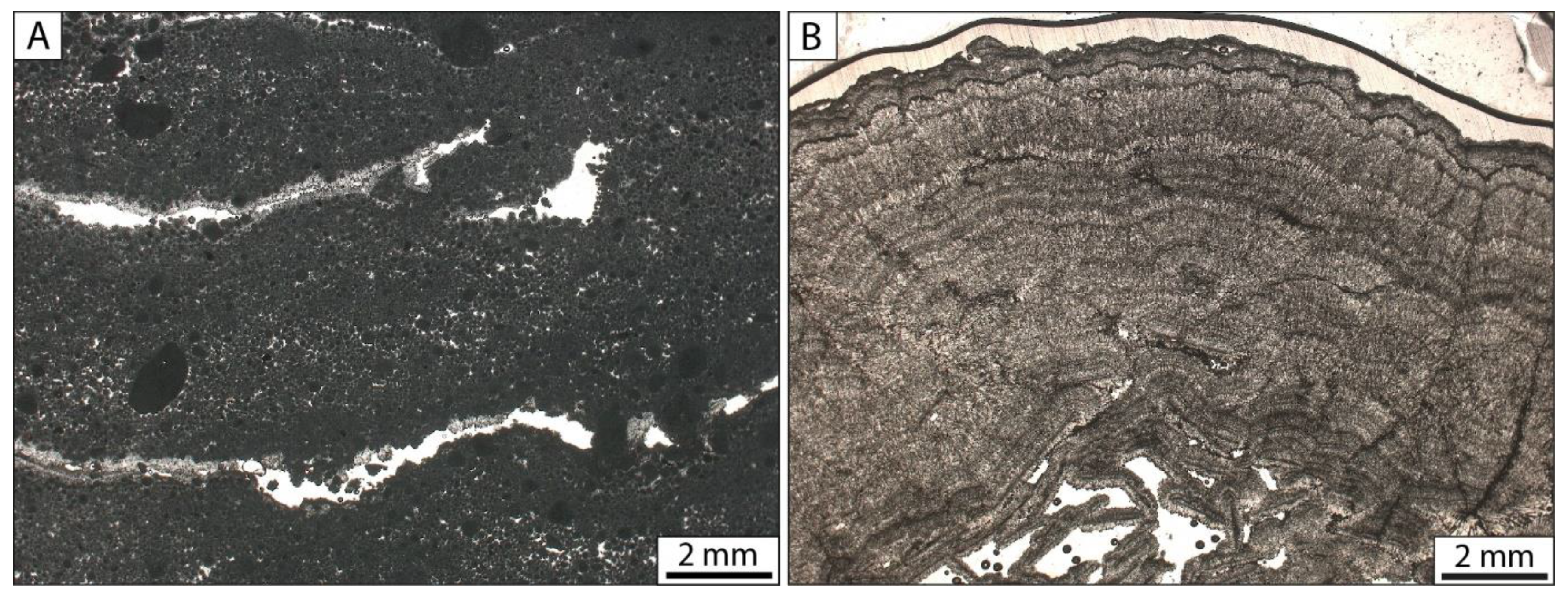
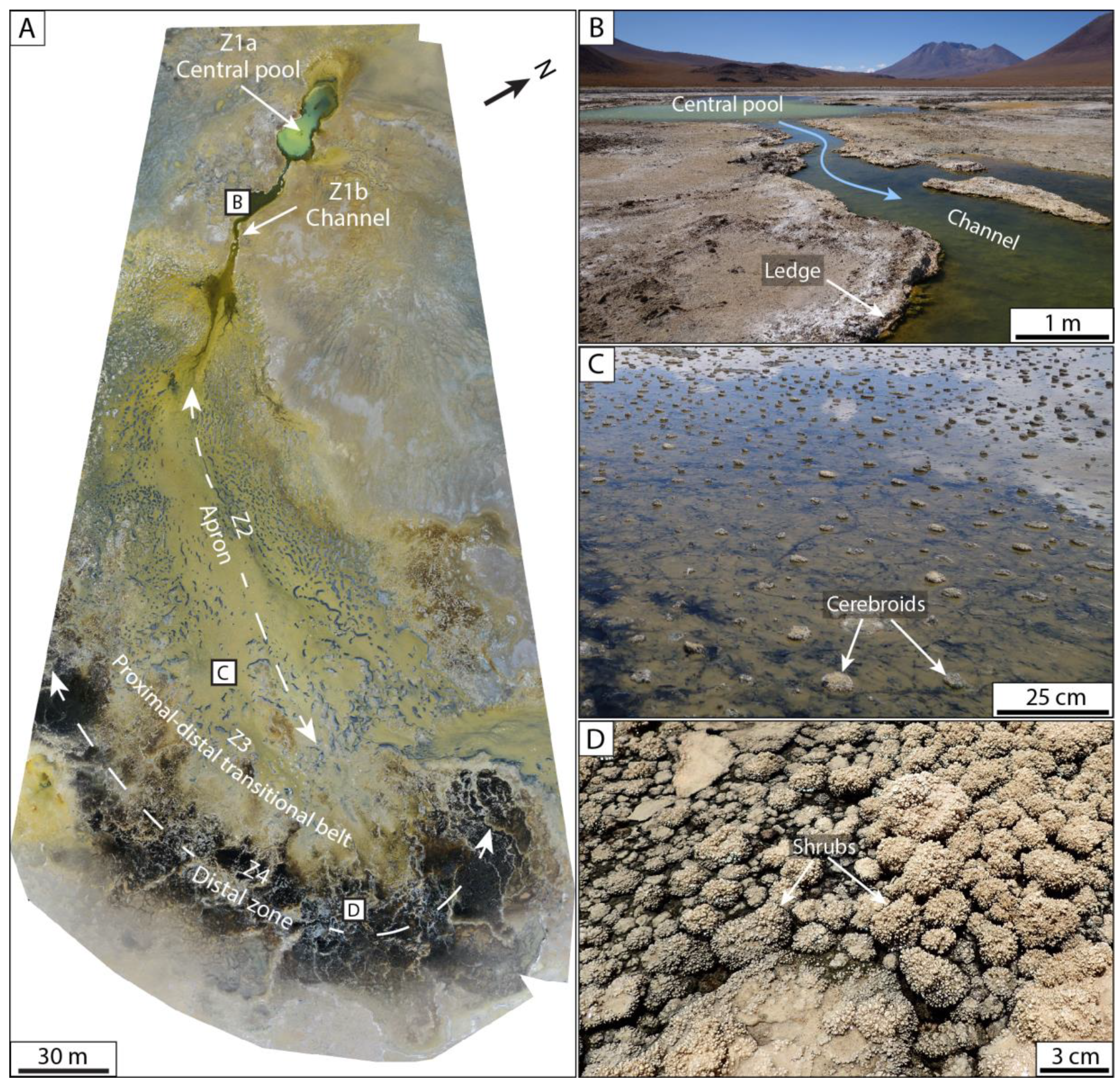
4.2.1. Microbialites
4.2.2. Pisolites
4.2.3. Palmatoid Concretions
4.2.4. Platystromatolites
4.2.5. Calcite Mud
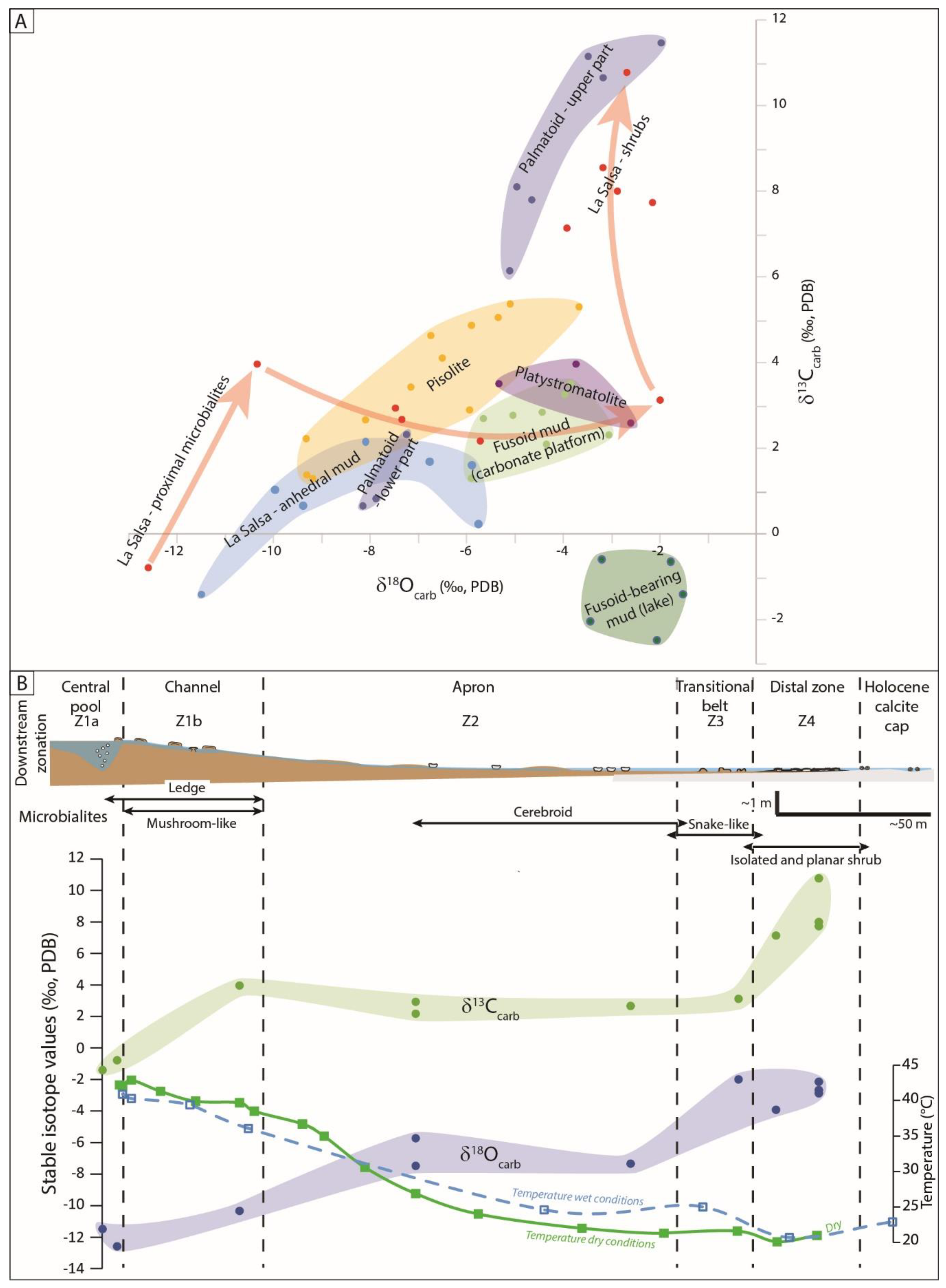
4.3. Isotope Geochemistry of Modern Carbonates
4.3.1. Carbon and Oxygen Stable Isotopes
4.3.2. Fraction Modern Carbon (F14C) from Modern Carbonates
5. Discussion
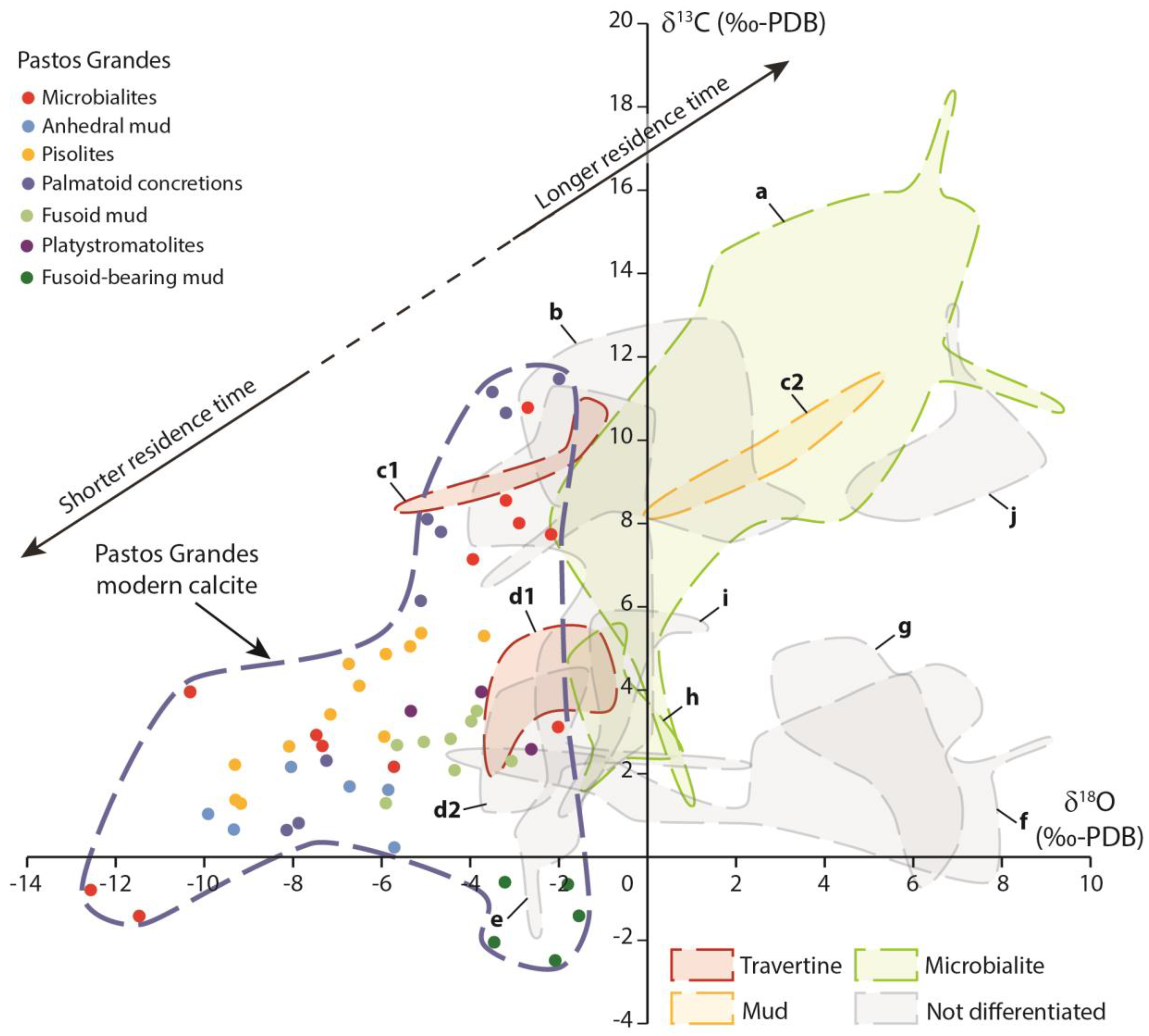
5.1. Using Isotopes for Carbonate Dating in Volcanic Settings
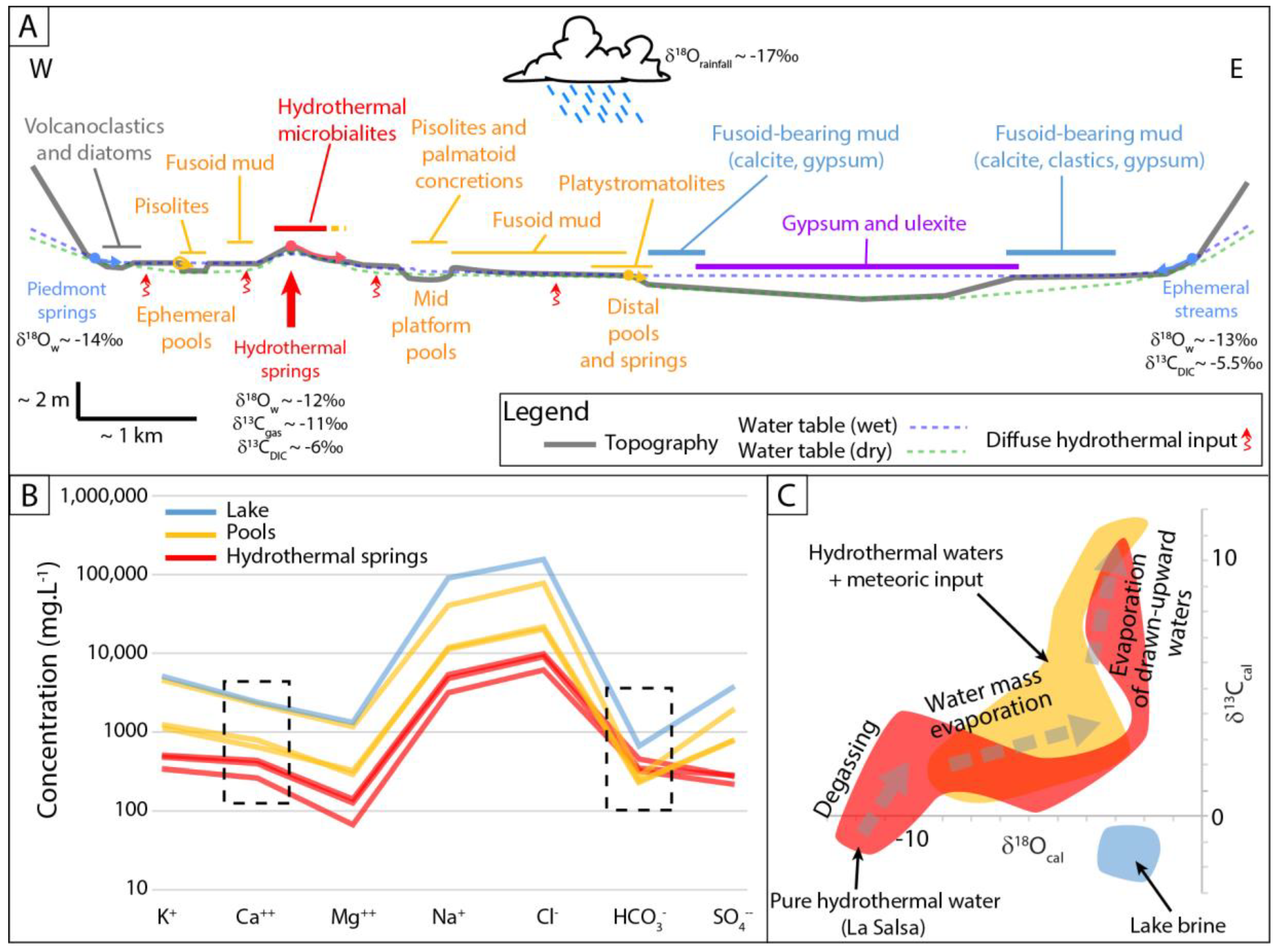
5.2. Factors Controlling the Isotope Record in Each Hydrological Setting
5.2.1. Pure Hydrothermal Systems
5.2.2. Combined Hydrothermal and Meteoric Inputs
5.2.3. The Saline Lake, Mainly Fed by Meteoric Waters
5.3. Laguna Pastos Grandes in Comparison with Other Andean Contexts
6. Conclusions
- The initial isotope composition of parent waters (purely hydrothermal, mixed hydrothermal–meteoric, or meteoric-dominated) is the main extrinsic factor triggering differences in carbon and oxygen isotope values recorded in carbonates from different depositional environments;
- By moving away from hydrothermal inputs, isotopes are increasingly influenced by aridity, causing evaporation, enhanced by wind action, and by capillary rise through the microbial mat, thus triggering major carbon and oxygen isotope enrichments;
- The short water residence time explains the main difference between Laguna Pastos Grandes (with a slightly negative isotope signature of carbonates) and the other Andean lakes (with positive isotope signatures of carbonates), which are all under the influence of an arid climate. The isotope convergence toward high δ13Ccarb from Pastos Grandes and several other Andean lakes is triggered by volcanic CO2 input, and so carbon isotopes can be particularly useful to identify ancient carbonate systems affected by volcanic activity. However, controlling factors triggering 13C enrichment in carbonates corresponded to large-scale processes for Andean lakes (long residence time), and facies-scale processes for Pastos Grandes (evaporation of drawn-upward waters);
- The fraction modern F14C was modified in volcanic settings by the injection of “dead carbon” into the system. This makes 14C dating of carbonates particularly unreliable in Andean lakes enriched in volcanic CO2.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gasse, F.; Fontes, J.-C. Palaeoenvironments and palaeohydrology of a tropical closed lake (Lake Asal, Djibouti) since 10,000 yr B.P. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 69, 67–102. [Google Scholar] [CrossRef]
- Benson, L.; White, L.D.; Rye, R. Carbonate deposition, Pyramid Lake Subbasin, Nevada: 4. Comparison of the stable isotope values of carbonate deposits (tufas) and the Lahontan lake-level record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 122, 45–76. [Google Scholar] [CrossRef]
- Schwalb, A.; Burns, S.J.; Kelts, K. Holocene environments from stable isotope stratigraphy of ostracods and authigenic carbonate in Chilean Altiplano Lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 148, 153–168. [Google Scholar] [CrossRef]
- Valero-Garcés, B.L.; Arenas, C.; Delgado-Huertas, A. Depositional environments of Quaternary lacustrine travertines and stromatolites from high-altitude Andean lakes, northwestern Argentina. Can. J. Earth Sci. 2001, 38, 1263–1283. [Google Scholar] [CrossRef]
- McDermott, F. Palaeo-climate reconstruction from stable isotope variations in speleothems: A review. Quat. Sci. Rev. 2004, 23, 901–918. [Google Scholar] [CrossRef]
- Blard, P.-H.; Sylvestre, F.; Tripati, A.K.; Claude, C.; Causse, C.; Coudrain, A.; Condom, T.; Seidel, J.-L.; Vimeux, F.; Moreau, C.; et al. Lake highstands on the Altiplano (Tropical Andes) contemporaneous with Heinrich 1 and the Younger Dryas: New insights from 14C, U–Th dating and δ18O of carbonates. Quat. Sci. Rev. 2011, 30, 3973–3989. [Google Scholar] [CrossRef]
- Rouchy, J.M.; Servant, M.; Fournier, M.; Causse, C. Extensive carbonate algal bioherms in upper Pleistocene saline lakes of the central Altiplano of Bolivia. Sedimentology 1996, 43, 973–993. [Google Scholar] [CrossRef]
- Sylvestre, F.; Servant, M.; Servant-Vildary, S.; Causse, C.; Fournier, M.; Ybert, J.-P. Lake-Level Chronology on the Southern Bolivian Altiplano (18°–23°) during Late-Glacial Time and the Early Holocene. Quat. Res. 1999, 51, 54–66. [Google Scholar] [CrossRef]
- Buongiorno, J.; Gomez, F.J.; Fike, D.A.; Kah, L.C. Mineralized microbialites as archives of environmental evolution, Laguna Negra, Catamarca Province, Argentina. Geobiology 2019, 17, 199–222. [Google Scholar] [CrossRef]
- Ballivián, O.; Risacher, F. Los Salares del Altiplano Boliviano: Métodos de Estudio y Estimación Económica; ORSTOM: Paris, France, 1981; 246p. [Google Scholar]
- Gomez, F.J.; Kah, L.C.; Bartley, J.K.; Astini, R.A. Microbialites in a high-altitude andean lake: Multiple controls on carbonate precipitation and lamina accretion. Palaios 2014, 29, 233–249. [Google Scholar] [CrossRef]
- Risacher, F.; Eugster, H.P. Holocene pisoliths and encrustations associated with spring-fed surface pools, Pastos Grandes, Bolivia. Sedimentology 1979, 26, 253–270. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Crystal fabrics and microbiota in large pisoliths from Laguna Pastos Grandes, Bolivia. Sedimentology 1994, 41, 1171–1202. [Google Scholar] [CrossRef]
- Bougeault, C.; Vennin, E.; Durlet, C.; Muller, E.; Mercuzot, M.; Chavez, M.; Gérard, E.; Ader, M.; Virgone, A.; Gaucher, E.C. Biotic-abiotic Influences on Modern Ca–Si-Rich Hydrothermal Spring Mounds of the Pastos Grandes Volcanic Caldera (Bolivia). Minerals 2019, 9, 380. [Google Scholar] [CrossRef]
- Soria, M.N.; Lencina, A.I.; Saona, L.A.; Stepanenko, T.M.; Colla, M.F.; Farías, M.E. New Different Modern Microbialites Deposits in Puna of Catamarca, Argentine; M-Fed Congress: Dijon, France, 2019. [Google Scholar]
- Muller, E.; Gaucher, E.C.; Durlet, C.; Moquet, J.; Moreira, M.; Rouchon, V.; Louvat, P.; Bardoux, G.; Noirez, S.; Bougeault, C.; et al. The Origin of Continental Carbonates in Andean Salars: A Multi-Tracer Geochemical Approach in Laguna Pastos Grandes (Bolivia). Geochim. Cosmochim. Acta 2020, 279, 220–237. [Google Scholar] [CrossRef]
- Martinod, J.; Husson, L.; Roperch, P.; Guillaume, B.; Espurt, N. Horizontal subduction zones, convergence velocity and the building of the Andes. Earth Planet. Sci. Lett. 2010, 299, 299–309. [Google Scholar] [CrossRef]
- Thorpe, R.S.; Francis, P.W. Variations in Andean andesite compositions and their petrogenetic significance. Tectonophysics 1979, 57, 53–70. [Google Scholar] [CrossRef]
- Isacks, B.L. Uplift of the Central Andean Plateau and Bending of the Bolivian Orocline. J. Geophys. Res. 1988, 93, 3211–3231. [Google Scholar] [CrossRef]
- De Silva, S.L. Altiplano–Puna volcanic complex of the central Andes. Geology 1989, 17, 1102–1106. [Google Scholar] [CrossRef]
- Salisbury, M.J.; Jicha, B.R.; de Silva, S.L.; Singer, B.S.; Jiménez, N.C.; Ort, M.H. 40Ar/39Ar chronostratigraphy of Altiplano–Puna volcanic complex ignimbrites reveals the development of a major magmatic province. Geol. Soc. Am. Bull. 2011, 123, 821–840. [Google Scholar] [CrossRef]
- De Silva, S.; Zandt, G.; Trumbull, R.; Viramonte, J.G.; Salas, G.; Jiménez, N. Large ignimbrite eruptions and volcano-tectonic depressions in the Central Andes: A thermomechanical perspective. Geol. Soc. Lond. Spec. Publ. 2006, 269, 47–63. [Google Scholar] [CrossRef]
- Kay, S.M.; Coira, B.L. Shallowing and steepening subduction zones, continental lithospheric loss, magmatism, and crustal flow under the central Andean Altiplano-Puna Plateau. In Backbone of the Americas: Shallow Subduction, Plateau Uplift, and Ridge and Terrane Collision; Kay, S.M., Ramos, V.A., Dickinson, W.R., Eds.; The Geological Society of America: Boulder, CO, USA, 2009; Volume 204, pp. 229–260. [Google Scholar]
- De Silva, S.L.; Kay, S.M. Turning up the Heat: High-Flux Magmatism in the Central Andes. Elements 2018, 14, 245–250. [Google Scholar] [CrossRef]
- Chmielowski, J.; Zandt, G.; Haberland, C. The Central Andean Altiplano-Puna Magmatic Body. Geophys. Res. Lett. 1999, 26, 783–786. [Google Scholar] [CrossRef]
- Ward, K.M.; Zandt, G.; Beck, S.L.; Christensen, D.H.; McFarlin, H. Seismic imaging of the magmatic underpinnings beneath the Altiplano-Puna volcanic complex from the joint inversion of the surface wave dispersion and receiver functions. Earth Planet. Sci. Lett. 2014, 404, 43–53. [Google Scholar] [CrossRef]
- Kaiser, J.F.; de Silva, S.; Schmitt, A.K.; Economos, R.; Sunagua, M. Million-year melt-presence in monotonous intermediate magma for a volcanic-plutonic assemblage in the Central Andes: Contrasting histories of crystal-rich and crystal-poor super-sized silicic magmas. Earth Planet. Sci. Lett. 2017, 457, 73–86. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Iltis, A.; Risacher, F.; Servant–Vildary, S. Contribution à l’étude hydrobiologique des lacs salés du sud de l’Altiplano bolivien. Rev. Hydrobiol. Trop. 1984, 17, 259–273. [Google Scholar]
- Servant-Vildary, S.; Roux, M. Multivariate analysis of diatoms and water chemistry in Bolivian saline lakes. Hydrobiologia 1990, 197, 267–290. [Google Scholar] [CrossRef]
- Risacher, F.; Fritz, B. Geochemistry of Bolivian salars, Lipez, southern Altiplano: Origin of solutes and brine evolution. Geochim. Cosmochim. Acta 1991, 55, 687–705. [Google Scholar] [CrossRef]
- Ghaleb, B.; Falguères, C.; Carlut, J.; Pozzi, J.-P.; Mahieux, G.; Boudad, L.; Rousseau, L. Timing of the Brunhes-Matuyama transition constrained by U-series disequilibrium. Sci. Rep. 2019, 9, 6039. [Google Scholar] [CrossRef]
- Edwards, R.L.; Chen, J.H.; Wasserburg, G.J. 238U–234U–230Th–232Th systematics and the precise measurement of time over the past 500,000 years. Earth Planet. Sci. Lett. 1987, 81, 175–192. [Google Scholar] [CrossRef]
- Ludwig, K.R.; Paces, J.P. Uranium-series dating of pedogenic silica and carbonate, Crater Flat, Nevada. Geochim. Cosmochim. Acta 2002, 66, 487–506. [Google Scholar] [CrossRef]
- St-Jean, G.; Kieser, W.E.; Crann, C.A.; Murseli, S. Semi-Automated Equipment for CO2 Purification and Graphitization at the A.E. Lalonde Laboratory (Ottawa, Canada). Radiocarbon 2017, 59, 941–956. [Google Scholar] [CrossRef]
- Placzek, C.; Patchett, P.J.; Quade, J.; Wagner, J.D.M. Strategies for successful U-Th dating of paleolake carbonates: An example from the Bolivian Altiplano. Geochem. Geophys. Geosyst. 2006, 7, Q05024. [Google Scholar] [CrossRef]
- Rucker, J.B.; Carver, R.E. A Survey of the Carbonate Mineralogy of Cheilostome Bryozoa. J. Paleontol. 1969, 43, 791–799. [Google Scholar]
- Geyh, M.A.; Grosjean, M.; Núñez, L.; Schotterer, U. Radiocarbon Reservoir Effect and the Timing of the Late-Glacial/Early Holocene Humid Phase in Atacama Desert (Northern Chile). Quat. Res. 1999, 52, 143–153. [Google Scholar] [CrossRef]
- Oehlerich, M.; Mayr, C.; Griesshaber, E.; Lücke, A.; Oeckler, O.M.; Ohlendorf, C.; Schmahl, W.W.; Zolitschka, B. Ikaite precipitation in a lacustrine environment—Implications for palaeoclimatic studies using carbonates from Laguna Potrok Aike (Patagonia, Argentina). Quat. Sci. Rev. 2013, 71, 46–53. [Google Scholar] [CrossRef]
- Ohlendorf, C.; Fey, M.; Massaferro, J.; Haberzettl, T.; Laprida, C.; Lücke, A.; Maidana, N.; Mayr, C.; Oehlerich, M.; Ramón Mercau, J.; et al. Late Holocene hydrology inferred from lacustrine sediments of Laguna Cháltel (southeastern Argentina). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 411, 229–248. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Discussion Reporting of 14C Data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Mao, X.; Wang, H.; Feng, L. Impact of additional dead carbon on the circulation estimation of thermal springs exposed from deep-seated faults in the Dongguan basin, southern China. J. Volcanol. Geotherm. Res. 2018, 361, 1–11. [Google Scholar] [CrossRef]
- Valero-Garcés, B.L.; Delgado-Huertas, A.; Ratto, N.; Navas, A. Large 13C enrichment in primary carbonates from Andean Altiplano lakes, northwest Argentina. Earth Planet. Sci. Lett. 1999, 171, 253–266. [Google Scholar] [CrossRef]
- Gonfiantini, R.; Panichi, C.; Tongiorgi, E. Isotopic disequilibrium in travertine deposition. Earth Planet. Sci. Lett. 1968, 5, 55–58. [Google Scholar] [CrossRef]
- Pentecost, A. Travertine; Springer: Berlin, Germany, 2005; 445p. [Google Scholar]
- Kele, S.; Özkul, M.; Fórizs, I.; Gökgöz, A.; Baykara, M.O.; Alçiçek, M.C.; Németh, T. Stable isotope geochemical study of Pamukkale travertines: New evidences of low-temperature non-equilibrium calcite-water fractionation. Sediment. Geol. 2011, 238, 191–212. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Compilation of stable isotope fractionation factors of geochemical interest. In Data of Geochemistry, 6th ed.; Geological Survey Professional Paper 440-KK; Fleische, M., Ed.; U.S. Geological Survey: Washington, DC, USA, 1977; pp. KK1–KK12. [Google Scholar]
- Fouke, B.W.; Farmer, J.D.; des Marais, D.J.; Pratt, L.; Sturchio, N.C.; Burns, P.C.; Discipulo, M.K. Depositional facies and aqueous-solid geochemistry of travertine-depositing hot springs (Angel Terrace, Mammoth Hot Springs, Yellowstone National Park, U.S.A.). J. Sed. Res. 2000, 70, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Renaut, R.W.; Jones, B.; Tiercelin, J.-J. Rapid in situ silicification of microbes at Loburu hot springs, Lake Bogoria, Kenya Rift Valley. Sedimentology 1998, 45, 1083–1103. [Google Scholar] [CrossRef]
- Canet, C.; Prol-Ledesma, R.M.; Torres-Alvarado, I.; Gilg, H.A.; Villanueva, R.E.; Lozano-Santa Cruz, R. Silica-carbonate stromatolites related to coastal hydrothermal venting in Bahía Concepción, Baja California Sur, Mexico. Sediment. Geol. 2005, 174, 97–113. [Google Scholar] [CrossRef]
- Hua, Q.; Barbetti, M.; Rakowski, A.Z. Atmospheric radiocarbon for the period 1950-2010. Radiocarbon 2013, 55, 2059–2072. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Göhring, A.; Mauder, M.; Vohberger, M.; Nehlich, O.; von Carnap-Bornheim, C.; Hilberg, V.; Kröger, P.; Grupe, G. Palaeobiodiversity research based on stable isotopes: Correction of the sea spray effect on bone carbonate δ13C and δ18O by Gaussian Mixture Model clustering. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 673–686. [Google Scholar] [CrossRef]
- Deininger, M.; Fohlmeister, J.; Scholz, D.; Mangini, A. Isotope disequilibrium effects: The influence of evaporation and ventilation effects on the carbon and oxygen isotope composition of speleothems—A model approach. Geochim. Cosmochim. Acta 2012, 96, 57–79. [Google Scholar] [CrossRef]
- Yan, H.; Liu, Z.; Sun, H. Large degrees of carbon isotope disequilibrium during precipitation-associated degassing of CO2 in mountain stream. Geochim. Cosmochim. Acta 2020, 273, 244–256. [Google Scholar] [CrossRef]
- Beeler, S.R.; Gomez, F.J.; Bradley, A.S. Controls of extreme isotopic enrichment in modern microbialites and associated abiogenic carbonates. Geochim. Cosmochim. Acta 2020, 269, 136–149. [Google Scholar] [CrossRef]
- Leng, M.J.; Marshall, J.D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quat. Sci. Rev. 2004, 23, 811–831. [Google Scholar] [CrossRef]
- Talbot, M.R. A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. Isot. Geosci. Sect. 1990, 80, 261–279. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Sáez, A.; Giralt, S.; Valero-Garcés, B.L.; Moreno, A.; Bao, R.; Schwalb, A.; Herrera, C.; Klosowska, B.; Taberner, C. Carbonate and organic matter sedimentation and isotopic signatures in Lake Chungará, Chilean Altiplano, during the last 12.3 kyr. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 307, 339–355. [Google Scholar] [CrossRef]



| Sample | Sample type | Position | 238U (ppb) | 232Th (ppb) | 234U/238U | 230Th/234U | 230Th/238U | 232Th/238U | 230Th/232Th | 230Th/U Age ka (uncorr.) | 234U/238Uinit. | 230Th/U Age ka (corr.) | 234U/238Uinit. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PG17-14 | Pisolite core | Upper Platform | 309.488 ± 0.943 | 59.141 ± 0.18 | 1.570 ± 0.006 | 0.043 ± 0.0001 | 0.067 ± 0.0002 | 0.063 ± 0.0003 | 1.068 ± 0.0057 | 4.714 ± 0.024 | 1.578 ± 0.0056 | 1.085 ± 2.009 | 1.603 ± 0.0197 |
| PG17-33 | Pisoidic rudstone | Upper Platform | 690.160 ± 4.21 | 33.395 ± 0.153 | 1.528 ± 0.012 | 0.018 ± 0.0003 | 0.027 ± 0.0004 | 0.016 ± 0.0001 | 1.729 ± 0.0270 | 1.964 ± 0.034 | 1.531 ± 0.0121 | 1.031 ± 0.511 | 1.537 ± 0.0129 |
| 660.145 ± 4.458 | 27.480 ± 0.242 | 1.530 ± 0.013 | 0.016 ± 0.0003 | 0.024 ± 0.0005 | 0.014 ± 0.0002 | 1.775 ± 0.0353 | 1.73 ± 0.036 | 1.533 ± 0.0129 | 0.929 ± 0.439 | 1.538 ± 0.0135 | |||
| PG17-37 | Peloidal grainstone | Upper Platform | 207.194 ± 0.787 | 9.175 ± 0.04 | 1.537 ± 0.011 | 0.026 ± 0.0006 | 0.040 ± 0.0009 | 0.014 ± 0.0001 | 2.726 ± 0.062 | 2.828 ± 0.066 | 1.541 ± 0.0106 | 1.979 ± 0.466 | 1.547 ± 0.0113 |
| 220.421 ± 1.742 | 12.517 ± 0.065 | 1.523 ± 0.014 | 0.027 ± 0.0004 | 0.041 ± 0.0007 | 0.019 ± 0.0002 | 2.211 ± 0.0336 | 2.968 ± 0.055 | 1.528 ± 0.0137 | 1.869 ± 0.601 | 1.534 ± 0.0147 | |||
| PG17-63 | Peloidal grainstone | Lower Platform | 183.093 ± 0.743 | 1.926 ± 0.021 | 1.295 ± 0.007 | 0.276 ± 0.0022 | 0.357 ± 0.0028 | 0.003 ± 0.00004 | 103.752 ± 1.4111 | 34.626 ± 0.379 | 1.328 ± 0.0071 | 34.393 ± 0.396 | 1.326 ± 0.0071 |
| 185.364 ± 0.763 | 1.589 ± 0.01 | 1.286 ± 0.009 | 0.271 ± 0.0023 | 0.348 ± 0.0027 | 0.003 ± 0.00002 | 124.076 ± 1.1664 | 33.899 ± 0.412 | 1.315 ± 0.0093 | 33.707 ± 0.423 | 1.316 ± 0.0093 | |||
| PG17-69 | Botryoidal cement | Lower Platform | 56.647 ± 0.22 | 3.412 ± 0.024 | 1.561 ± 0.011 | 0.042 ± 0.0012 | 0.065 ± 0.0018 | 0.020 ± 0.0002 | 3.323 ± 0.0967 | 4.649 ± 0.138 | 1.568 ± 0.0111 | 3.513 ± 0.629 | 1.576 ± 0.0125 |
| 52.889 ± 0.205 | 1.646 ± 0.007 | 1.547 ± 0.008 | 0.029 ± 0.0005 | 0.045 ± 0.0007 | 0.010 ± 0.0001 | 4.402 ± 0.0736 | 3.191 ± 0.056 | 1.552 ± 0.0081 | 2.600 ± 0.326 | 1.556 ± 0.0086 | |||
| PG17-77 | Fusoid mud | Lower Platform | 175.713 ± 0.909 | 131.034 ± 0.721 | 1.589 ± 0.011 | 0.231 ± 0.0019 | 0.368 ± 0.003 | 0.244 ± 0.0018 | 1.507 ± 0.0137 | 28.148 ± 0.349 | 1.638 ± 0.0119 | 13.737 ± 7.765 | 1.767 ± 0.1078 |
| Facies | Macro to Mesostructures | Microstructures | Depositional Environments | Diurnal Physicochemical Parameters (January 2016 and March 2017) | ||
|---|---|---|---|---|---|---|
| Size | ||||||
| Ledge microbialite | Planar laminated microbialite; lateral development in terraces | 2 to 5 cm thick; 5–20 cm long | Alternating laminae composed of micrite, filament-rich bundles, and diatoms; the upper and lateral parts of the structures (ledge and mushroom-like) show a transition from filament-rich bundles to micrite; sheltered parts are infilled by micrite with diatoms and ostracods; bundles composed of casts and molds of filaments | Main hydrothermal springs located on the UCP (La Salsa spring, see Bougeault et al. [14] | Z1—Central pool and outflow channel | T: 42 to 36 °C pH: 5.8 to 7.2 σ: 25–27 mS·cm−1 |
| Mushroom-like microbialite | Dome-shaped structure developing on intraclasts; planar to laterally curved laminae | 5 to 10 cm in diameter | ||||
| Cerebroid microbialite | Irregular ovoid structure; organized in planar and columnar laminae | 2 to 20 cm in diameter | Z2—Apron | T: 38 to 21 °C pH: 7.1 to 8.2 σ: 26–55 mS·cm−1 | ||
| Snake-like structure | Hemispherical and tortuous structure with white crust on upper part; white crusts locally covered by mm micrite and silica-rich branches; organized in laminae and clots | 10 cm to 1 m long | Alternation of densely and loosely packed peloid-rich laminae with diatoms, top structure showing siliceous cements and gypsum between grains | Z3—Proximal–Distal Transitional belt | T: 25 to 21 °C pH: 8 to 8.8 σ: 32–68 mS·cm−1 | |
| Isolated shrub | Dome-shaped structure covered by millimetric branches; planar to columnar laminae | 2 to 5 cm in diameter | Alternation of micritic, spiritic, and siliceous laminae, coating extraclasts (carbonate pebbles, spherulites, pisolites, detrital grains); millimetric branches (microstromatolites) are composed of micrite and silica | Z4—Distal zone | T: 21 to 20 °C pH: 7.7 to 6.9 σ: 48–225 mS·cm−1 | |
| Planar shrub | Shrub with millimetric branches organized as a ring around a planar central area; planar to columnar laminae | Ranging from 5 to 50 cm | ||||
| Pisolite concretion | Ovoid to spherical concretion; concentric laminae | Few mm to 20 cm in diameter | Alternating micritic, sparitic, and amorphous silica laminae around spherulites, carbonate or detrital grains | Ephemeral to perennial pools on the UCP and the UCP-LCP transition | T: 18.5–21 °C pH: 7.3–7.8 σ: 38.8–73 mS·cm−1 | |
| Palmatoid concretion | Horizontally spreading or columnar structure with branches at the top | 5 to 15 cm long | Micritic, sparitic, and amorphous silica laminae passing into a bud-like shape at the top of the concretion | Perennial pools at the transition between UCP and LCP | T: 18 °C pH: 7.8 σ: 62 mS·cm−1 | |
| Platystromatolite | Plate-shaped structure; wrinkled to columnar laminae | From 5 to 25 cm in diameter | Mainly planar to wavy micritic, with rare sparitic and Fe-oxide layers, growing over and around an extraclast (carbonate pebbles, pisolites) | Distal pools and springs on the LCP and at the transition between the LCP and the ephemeral lake | T: 23°C pH: 7.5 σ: 69–127 mS·cm−1 | |
| Anhedral calcite mud | Mud; structureless, disturbed by bioturbation (flamingos) | - | Anhedral crystals of calcite, associated with diatoms, forming aggregates | Produced in water discharge of hydrothermal springs | T: 42 to 21 °C pH: 5.8 to 8 σ: 25–68 mS·cm−1 | |
| Fusoid mud | Mud; structureless, disrupted by cryoturbation | - | Elongated scalenohedral crystals of calcite (5 to 100 m long, with curved faces and edges resulting in a fusoid shape. Associated with rare diatom frustules and halite crystals | Ephemeral shallow puddles covering the LCP and, to a lesser extent, the UCP | T: 15–24 °C pH: 7.4–8 σ: 48 mS·cm−1 to saturation | |
| Fusoid-bearing mud | Accumulation of beige to brown mud; structureless | - | Mud containing silt- to clay-sized particles with variable proportions of fusoid calcite crystals (5–20%), detrital grains (quartz, feldspar), gypsum, halite, and rare diatoms | Ephemeral central lake; during highstand lake level | T: 15–24 °C pH: 7.4–7.65 σ: 179 mS·cm−1 to saturation | |
| Facies | Sample | Position | Latitude (°N) | Longitude (°E) | δ13C | δ18O | F14C | 14C Age (yr BP) * | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pisolite concretion | PG17-9 | UP | −21.62077 | −67.8499 | 2.66 | −8.09 | 0.0639 | 22,088 (±70) * | ||
| PG17-14 | UP | −21.62077 | −67.8499 | 2.22 | −9.32 | |||||
| PG17-12 | UP | −21.62077 | −67.8499 | 3.43 | −7.15 | |||||
| PG2-7 | UP | −21.61994 | −67.85074 | 2.89 | −5.94 | |||||
| PG2-21 | UP | −21.61961 | −67.85279 | 5.05 | −5.35 | |||||
| PG2-6 | UP | −21.61976 | −67.85204 | 5.37 | −5.10 | |||||
| PG17-54 | UP | −21.59494 | −67.82934 | 1.29 | −9.19 | |||||
| PG17-52 | UP | −21.59301 | −67.8309 | 1.38 | −9.31 | |||||
| PG17-80 | UP | −21.61983 | −67.84538 | 4.63 | −6.74 | |||||
| Fusoid mud | PG17-38 | UP | −21.64951 | −67.83747 | 3.26 | −3.96 | ||||
| PG17-30 | UP | −21.65398 | −67.84463 | 2.31 | −3.05 | |||||
| PG17-34 | UP | −21.65156 | −67.8411 | 2.77 | −5.04 | |||||
| PG17-57 | UP | −21.59722 | −67.82633 | 2.84 | −4.43 | |||||
| PG17-59 | UP | −21.5997 | −67.82579 | 2.69 | −5.65 | |||||
| Microbialites—La Salsa | Z1 | Ledge | PG1−2 | UP | −21.61934 | −67.84836 | −0.79 | −12.59 | ||
| Mushroom | PG1−6 | UP | −21.61951 | −67.84805 | 3.96 | −10.33 | 0.0469 | 24,577 (±94) * | ||
| Z2 | Cerebroid | PG1−11 | UP | −21.61987 | −67.84746 | 2.93 | −7.47 | |||
| PG1−14_1 | UP | −21.61929 | −67.84652 | 2.17 | −5.72 | |||||
| PG1−14_2 | UP | −21.61929 | −67.84652 | 2.67 | −7.34 | |||||
| Z3 | Snake-like | PG2−45 | UP | −21.61955 | −67.84622 | 3.12 | −1.99 | |||
| Z4 | Shrub | PG1−17 | UP | −21.61947 | −67.84598 | 7.14 | −3.92 | |||
| PG1−18_1 | UP | −21.61962 | −67.84782 | 7.74 | −2.15 | |||||
| PG1−18_3 | UP | 8.00 | −2.88 | |||||||
| PG1−18_4 | UP | 10.77 | −2.68 | 0.1068 | 17,965 (±63) * | |||||
| PG17−16 | UP | −21.61957 | −67.8458 | 8.55 | −3.18 | |||||
| Mud—La Salsa | Z1 | Anhedral mud | PG1−S | UP | −21.61932 | −67.84841 | −1.41 | −11.49 | ||
| PG1−5 | UP | −21.61955 | −67.84817 | 0.65 | −9.38 | |||||
| Z2 | Anhedral mud | PG1−8 | UP | −21.61962 | −67.84782 | 0.22 | −5.76 | |||
| PG1−10 | UP | −21.61974 | −67.84759 | 1.61 | −5.89 | |||||
| PG1−11bis | UP | −21.61987 | −67.84746 | 1.03 | −9.96 | |||||
| PG1−12 | UP | −21.61978 | −67.84722 | 1.69 | −6.77 | |||||
| PG1−13 | UP | −21.61955 | −67.84689 | 2.15 | −8.09 | |||||
| Pisolite | PG17-46a | ULT | −21.64688 | −67.83166 | 4.87 | −5.90 | ||||
| PG17-46b | ULT | −21.64688 | −67.83166 | 4.10 | −6.50 | |||||
| PG17-46c | −21.64688 | −67.83166 | 5.30 | −3.67 | ||||||
| Palmatoid concretion | PG17-43a_top | ULT | −21.64674 | −67.83198 | 8.10 | −4.96 | 0.0333 | 27,327 (± 96) * | ||
| PG17-43b_top | ULT | −21.64674 | −67.83198 | 7.80 | −4.65 | |||||
| PG17-43d_top | ULT | −21.64674 | −67.83198 | 6.14 | −5.11 | |||||
| PG17-43d_bot | ULT | −21.64674 | −67.83198 | 0.65 | −8.15 | |||||
| PG17-43c_top | ULT | −21.64674 | −67.83198 | 11.15 | −3.48 | |||||
| PG17-43c_bot | ULT | −21.64674 | −67.83198 | 0.82 | −7.88 | |||||
| PG17-43e_top | ULT | −21.64674 | −67.83198 | 10.65 | −3.17 | |||||
| PG17-43e_bot | ULT | −21.64674 | −67.83198 | 2.32 | −7.24 | |||||
| PG17-48b | ULT | −21.64688 | −67.83166 | 11.46 | −1.97 | |||||
| Fusoid mud | PG17-44 | ULT | −21.64666 | −67.83168 | 3.51 | −3.84 | ||||
| Fusoid mud | PG17-65c | LP | −21.66899 | −67.81787 | 1.30 | −5.90 | ||||
| PG17-76 | LP | −21.65362 | −67.80825 | 2.09 | −4.34 | |||||
| Platystromatolite | PG17-72 | LP | −21.65554 | −67.81396 | 2.59 | −2.60 | 0.1038 | 18,197 (± 52) * | ||
| PG17-75.2 | LP | −21.65362 | −67.80825 | 3.96 | −3.73 | |||||
| PG17-75.1 | LP | −21.65362 | −67.80825 | 3.51 | −5.33 | |||||
| Fusoid-bearing mud | PG2-33 | L | −21.62555 | −67.74513 | −0.65 | −1.78 | ||||
| PG2-2 | L | −21.6957 | −67.80938 | −2.45 | −2.06 | |||||
| PG17-84b | L | −21.69645 | −67.80445 | −2.04 | −3.44 | |||||
| PG17-81 | L | −21.6837 | −67.8222 | −0.60 | −3.20 | |||||
| PG2-16 | L | −21.6975 | −67.7475 | −1.41 | −1.52 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougeault, C.; Durlet, C.; Vennin, E.; Muller, E.; Ader, M.; Ghaleb, B.; Gérard, E.; Virgone, A.; Gaucher, E.C. Variability of Carbonate Isotope Signatures in a Hydrothermally Influenced System: Insights from the Pastos Grandes Caldera (Bolivia). Minerals 2020, 10, 989. https://doi.org/10.3390/min10110989
Bougeault C, Durlet C, Vennin E, Muller E, Ader M, Ghaleb B, Gérard E, Virgone A, Gaucher EC. Variability of Carbonate Isotope Signatures in a Hydrothermally Influenced System: Insights from the Pastos Grandes Caldera (Bolivia). Minerals. 2020; 10(11):989. https://doi.org/10.3390/min10110989
Chicago/Turabian StyleBougeault, Cédric, Christophe Durlet, Emmanuelle Vennin, Elodie Muller, Magali Ader, Bassam Ghaleb, Emmanuelle Gérard, Aurélien Virgone, and Eric C. Gaucher. 2020. "Variability of Carbonate Isotope Signatures in a Hydrothermally Influenced System: Insights from the Pastos Grandes Caldera (Bolivia)" Minerals 10, no. 11: 989. https://doi.org/10.3390/min10110989
APA StyleBougeault, C., Durlet, C., Vennin, E., Muller, E., Ader, M., Ghaleb, B., Gérard, E., Virgone, A., & Gaucher, E. C. (2020). Variability of Carbonate Isotope Signatures in a Hydrothermally Influenced System: Insights from the Pastos Grandes Caldera (Bolivia). Minerals, 10(11), 989. https://doi.org/10.3390/min10110989






