Abstract
Hematite is a potential mineral for reconstructing the oxygen isotope composition and paleotemperature of paleowater. A highly accurate analysis of oxygen isotopes is essential. However, relative to other oxygenated minerals, we lack hematite reference materials that allow for internationally comparable analyses between different laboratories. To address this issue, we attempted to perform bulk rock oxygen isotope analysis on five hematite reference materials (GBW07223a, GBW07825, YSBC28740-95, YSBC28756-2008, Harvard 92649). Meanwhile, the oxygen isotope ratios of iron oxides (GBW07223a, GBW07825, YSBC28740-95, YSBC28756-2008) were obtained by mass balance involving other oxygen-bearing minerals such as quartz and silicates. In addition, the oxygen isotope ratios of iron oxides in an oolitic hematite (ca. 1.65 billion years ago) are consistent with the results of previous analyses of this class of minerals.
1. Introduction
Hematite, siderite, goethite and magnetite are iron-bearing minerals that are relatively widely distributed in nature. The oxygen isotope of iron oxides is an important tool for reconstructing the paleo-temperature, oxygen isotopes of fluids, etc. during mineral formation or climate on land [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Hematite is a relatively common iron-bearing mineral, especially from the Precambrian period, and is the most important constituent mineral of Banded Iron Formation (BIF) [16,17,18,19,20,21,22]. Among these iron-bearing minerals, an oxygen isotope of hematite has been used to reconstruct paleotemperature variations during the Paleocene–Eocene Thermal Maximum (PETM) [1]. More recently, a systematic analysis of iron oxides of oxygen isotopes in hematite over 2 billion years was performed [7].
To date, the oxygen isotope of a large number of hematite samples has been analyzed by the laser fluorination method [23], with a few laboratories testing for hematite oxygen isotopes using secondary-ion mass spectrometry (SIMS) [24,25]. However, many laboratories lack an intercomparable reference material (RM) for hematite oxygen isotopes. To address the lack of stable isotope analysis RMs for hematite, we attempted to standardize the oxygen isotope values of a number of hematite RMs procured for principal analysis to assess whether they could be used as potential reference material for comparison between laboratories.
2. Experimental Section
2.1. Samples
In this study, we selected five samples of hematite. Two (GBW07223a, GBW07825) were purchased from the China National Standards, and two (YSBC28740-95, YSBC28756-2008) were purchased from NCS Testing Technology Co. They were previously used as reference materials for primary or trace element analysis (see https://www.ncrm.org.cn/Web/Home/Index; http://icloud.ncscrm.com/catalog.aspx). One is a granular reference material of hematite (Harvard 92649), which has previously been used as an RM for electron probe analysis [21]. We also collected a piece of oolitic hematite (Chuan), as shown in Figure 1, from the Chuanlinggou Formation (ca. 1.65 Ga.) in North China, which was crushed into < 63 µm powder. Oxygen isotope composition of hematite has been analyzed on similar such samples by previous researchers [7].
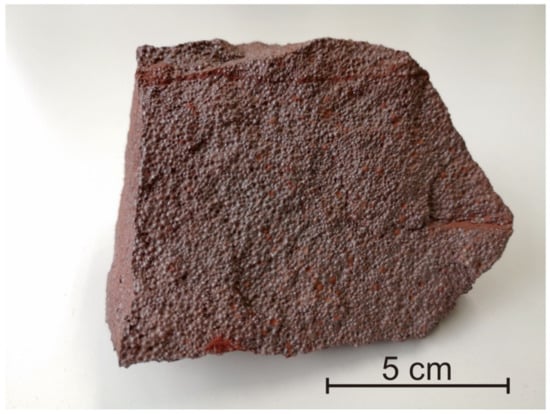
Figure 1.
Oolitic hematite from the Chuanlinggou formation (ca. 1.65 Ga), North China.
2.2. Laser Fluorination Extraction System and Oxygen Isotope Analysis
We designed and built a laser-based fluorination oxygen extraction line (Figure 2) at the Institute of Geology and Geophysics, Chinese Academy of Sciences. This line was made with a stainless steel tube. BrF5 was purchased from Tianjin Special Gas Company and placed in Kel-F Teflon distillation traps (Figure 2). The laser fluorination system utilizes a New Wave Research MIR10-30 laser coupled to a vacuum extraction system. The sample chamber in the laser fluorination system is made of nickel metal and has 36 circular cavities with diameters of 3 mm. The whole system consists of 2 cold fingers, 2 cold traps and one hot trap. Both the cold fingers and the cold traps were immersed in liquid nitrogen during the reaction. KBr was added to the hot trap and heated to 100 °C to react with the F2 possibly present in BrF5 and to collect the Br2 in the later cold trap.
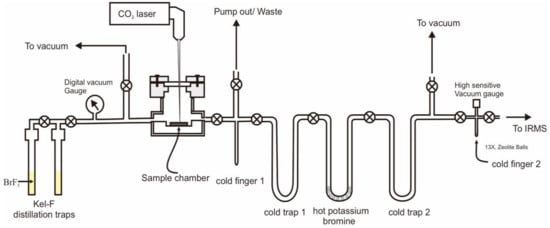
Figure 2.
BrF5 laser oxygen isotope fluoridation preparation system.
One zircon, Penglai (PL) (IGGCAS; δ18O = +5.17‰) [26] and one garnet, 04BXL07 garnet (University of Science and Technology of China; δ18O = +3.70‰) [27] were used in the standard set and were ± 0.08‰ and ± 0.05‰, respectively. The measured 18O/16O ratios were normalised using the value for Standard Mean Ocean Water (SMOW). A ca. 2 mg sample was weighed and placed into the sample chamber. The sample chamber is designed to hold up to 36 samples at a time, with a batch of 10 RMs, including garnet (04BXL07) and zircon (PL) for the analytical test procedure. An excess of BrF5 was added, and the reaction took approximately 10 minutes to remove adsorbed moisture from the air. The reaction gas was purified and withdrawn from the sample. Usually, two PL zircon samples are analyzed consecutively until the specific value of the PL sample reaches its given value (+5.17‰) before the other samples are analyzed.
The fluorination of minerals is usually performed by adjusting the diameter of the CO2 laser beam to 3 mm and the power of the reaction to between 10 and 20 W. In the presence of BrF5, the reaction usually emits a bright white light, and the reaction time is usually 5 minutes, until no white light is emitted and the oxygen of the sample is completely released. Furthermore, the amount of oxygen obtained from the reaction is used to calculate the yield, which is usually > 99%, indicating a complete reaction. The remaining BrF5 from the reaction is first collected in cold finger 1 and cold trap 1, and trace F2 gas passes through a hot well containing KBr at 100 °C to produce Br2, which is collected in cold trap 2 (Figure 2). The reference O2 gas used was purchased high-purity oxygen (99.999%). The oxygen isotope values (δ18O) were −9.91‰, which ere obtained by calibrating to the international RM quartz (NBS-28). The O2 gas was analyzed on a MAT 252 mass spectrometer. The mass spectrometer is equipped with three Faraday cups for simultaneous measurement of 32, 33 and 34.
2.3. Chemical Treatment
The fluorination of natural ironstones releases oxygen not only from iron-bearing minerals like hematite but also from other oxygen-bearing minerals like quartz and silicate minerals. Therefore, in order to analyze oxygen isotopes of iron oxides in natural ironstones, it is necessary to analyze the oxygen content and isotope of quartz and silicate minerals present in natural ironstones.
Sedimentary hematite usually contains significant amounts of silicates, including quartz, and different oxygen-bearing silicate minerals have a significant effect on the determination of oxygen isotopes of iron oxides. Methods for the assessment of oxygen isotopes of iron oxides for hematite are numerous [1,7,11]. In this study, we used the 12N HCl dissolution treatment method. About 1.5 g of the powder sample was weighed and placed in a 15 mL centrifuge tube, and 10 mL HCl was added. To avoid the possible exchange of oxygen from the reagent with the silicate as much as possible, the reaction condition was room temperature at 25 °C for 24 hours. However, we found that at room temperature, even for pure ferric oxide (metal basis), complete dissolution was still not achieved (only 83.4% dissolution), whereas at 90 °C, complete dissolution can be achieved. Therefore, the reaction temperature is very critical for the dissolution of hematite. Previous studies have also shown that a temperature range of 60–80 °C ensures the complete dissolution of hematite in the presence of sufficient HCl [28]. After the 24-hour reaction is completed, the tube was centrifuged for 10 minutes at 4000 rpm. After pouring off the upper clear liquid, secondary ionized water was added for repeated washing and centrifugation more than four times. The residue was then dried in a freeze dryer or oven (60 °C). The weight of the residue as a percentage of the whole rock sample was calculated (Table 1).

Table 1.
Oxygen isotope (δ18OResidue) and oxygen content of residue after HCl dissolution treatment and oxygen isotope values of iron oxides (δ18OOxides).
2.4. Measument of CO32−
In natural hematite samples, CO32−-bearing minerals, such as siderite, calcite or dolomite are common. Therefore, it is necessary to measure the oxygen content of these kinds of minerals. In this study, we used GasBench II system (ThermoFisher company) which is commercial equipment to measure carbon and oxygen isotope of carbonate.
In order to measure the total inorganic carbon, approximately 300 µg of pure carbonate RM and 4 mg of hematite samples were reacted with 100% phosphoric acid at 70 ℃ within a 12 mL vial tube filled with He, and generated CO2 gas was introduced into an isotope ratio mass spectrometer.
3. Results and Discussions
3.1. Oxygen Isotope of Bulk Rocks (δ18OBulk)
The oxygen isotopes of bulk rocks tested in this study were mainly in the range of −1.12 to 2.25‰ (Table 2). The two RM of GBW07223a and GBW07825 had consistent bulk rock δ18O values within the error ranges of 2.20 ± 0.02‰ and 2.25 ± 0.08‰, respectively. Whereas YSBC28740-95 has a slightly lower value of 0.76 ± 0.26‰, it is less reproducible relative to the oxygen isotope values of the other samples. This indicates its possible inhomogeneity. There are two extremes of δ18O values: one sample (YSBC28756-2008) has a high δ18O value (9.51 ± 0.03‰), and the hematite RM (Harvard 92649) has the lowest δ18O value (−1.12 ± 0.07‰). With the exception of RM YSBC28740-95, the analytical repeatability of the oxygen isotopes of the other whole rock samples analyzed in this analysis is high, which also indicates that the samples have good homogeneity, at least within the 2 mg sample size range.

Table 2.
Oxygen isotopes and oxygen content data in bulk rock samples.
The bulk rock δ18O values for the newly collected samples (Chuan) are −0.57 ± 0.05‰. In addition, it is very noteworthy that this sample (Chuan) has the highest oxygen content (34.09 ± 0.76%) compared to the other standard samples, which indicates that the sample contains more silicates or quartz.
We should note that one of the selected samples (GBW07825) contained high CO32− content, with 3.9% (GBW07825) of its oxygen from CO32−. Since this sample contains low Ca and Mg, the mineral could not have been calcite or dolomite, but rather siderite for GBW07825, given that these samples were rich in iron. The oxygen content from CO32− in the other four samples (YSBC28756-2008, GBW07223a, YSBC28740-95, Chuan) was less than 0.45%, and the oxygen content from CO32− in YSBC28740-95 was not detectable at all. Because the oxygen content of the CO32−-bearing minerals could not be detected through faraday cup (32, 33, 34) of MS, the presence of CO32−-bearing minerals also does not affect the bulk oxygen isotope of the sample to be tested [30,31,32]. The oxygen content of iron-bearing mineral obtained by the fluoridation method will underestimate the total oxygen content of the sample. The sum of the oxygen content obtained by fluoridation and the oxygen content of the carbonate minerals is representative of the total oxygen content of the sample.
3.2. Oxygen Isotopes of Residues After HCl Dissolution
Dissolution of hematite at room temperature was used to avoid possible oxygen isotope exchange as much as possible. However, our HCl dissolution experiments for pure hematite (metal basis) showed that pure hematite could not be completely dissolved at 25 °C, and about 16.6% could not be dissolved. For samples (GBW07223a, YSBC28740-95, Chuan), the red mineral (hematite) was clearly present in the residue, which indicates that room temperature is not sufficient to completely dissolve hematite. In contrast, the red hematite residue was clearly not present in the residue, and the pure hematite was completely dissolved at 90 °C, indicating that the temperature of the reaction directly determined the degree of dissolution of hematite. Oxygen isotope analysis of the residues at both temperatures also showsed that 90 °C is the more valid temperature for the complete dissolution of iron oxides.
Except for hematite RM (Harvard 92649), we performed HCl dissolution treatment on all other samples (GBW07223a, GBW07825, YSBC28740-95, YSBC28756-2008, Chuan). The four samples (GBW07223a, GBW07825, YSBC28740-95, YSBC28756-2008, Chuan) were treated with HCl under 25 °C and 90 °C conditions. The percentage content of the residue of hematite at 25 °C was significantly higher than that at 90 °C. The oxygen content of the residue nferred from its molar amount of oxygen (Table 1) is significantly higher at 90 °C than that at 25 °C due to the high oxygen content of silicate minerals compared to Fe2O3 (30.06%).
It is also important to note that because we used two temperature conditions (25 °C and 90 °C) for hematite dissolution, δ18O values for both residues were obtained for each sample (Table 1, Figure 3). It is noteworthy that the content of residue at 90 °C is lower than that at 25 °C (Table 1), and the content of residue in these two samples (GBW07223a, YSBC28740-95) is significantly lower than that in the other two samples (GBW07825, YSBC28756-2008). Correspondingly, the sample with the largest change in residue amount (GBW07223a, YSBC28740-95) had the largest change in oxygen isotope value (Figure 3, Table 1). The residue δ18O values at 25 °C are much closer to those of the bulk rock samples (Figure 3, Table 1), which also indicates that hematite is not completely dissolved at 25 °C. The oxygen isotope ratios of the residues of the two samples (GBW07825, YSBC28756-2008) with small residue variation are very similar, which indicates that the dissolution of Fe2O3 was nearly achieved in these two samples at 25 °C. However, we can also see that the δ18O values of the residues at 25 °C are obviously lower, which also reflects that a small portion of the Fe2O3 exists in the residue. In addition, we noted that the δ18O values of the residues of three (GBW07223a, GBW07825, YSBC28756-2008) of the four RMs and one other collected sample (Chuan) were all in the positive range of 10–13‰ at 90 °C (Table 1). The δ18O values of the residues of one RM (YSBC28740-95) were relatively low (5.59‰) (Table 1).
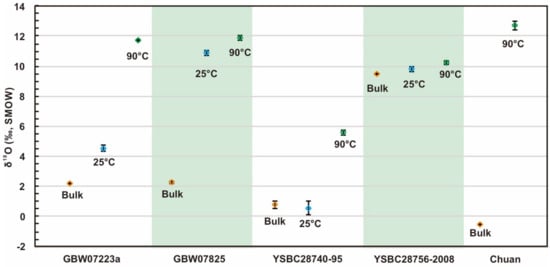
Figure 3.
Oxygen isotope ratios of iron oxide reference materials (RMs) and residues after the HCl dissolution treatment at room temperature (25 °C) and 90 °C. The orange rhombi represent the bulk rock δ18O. The blue rhombi represent the reaction at room temperature. The green rhombi represent the 90 °C condition. The error bars represent 1σ.
The Hematite RM (Harvard 92649) is known from previous testing that this RM is a relatively pure hematite and that total iron and oxygen are essentially the main elemental composition of this sample, so the low and negligible amount of residue can be expected.
3.3. Oxygen Isotope of Iron Oxides (δ18OOxides)
By measuring the moles of O2 gas released from oxides during fluorination, we can calculate the oxygen content of the bulk rock from the oxygen production. In addition, the oxygen content of the residue from the oxygen yield was obtained. In this way, without distinguishing the specific mineral composition of the residue, mass balance calculation was expressed as
where NBulk refers to the moles of oxygen in the bulk rock, NResidue refers to the moles of oxygen in the residue and NOxides refers to the moles of oxygen in iron oxides, which can be calculated from Equation (2). δ18OResidue refers to the oxygen isotope of the residue remaining after HCl dissolution of hematite.
With Equation (2) we can obtain the oxygen isotope values for iron oxides by following equation
In addition, we also tried to perform a calculation of δ18OOxides by Equation (3) after HCl dissolution treatment at 25 °C (Table 1), although the residue under 25 °C includes part of the iron oxides.
Ironstones in nature are not composed of iron-containing minerals such as hematite and magnetite, but also contain other oxygen-containing minerals such as quartz, calcite and other minerals. Thus, it is impossible to directly get the oxygen isotope values of iron oxides. In contrast, a pure hematite RM (Harvard 92649) is composed mainly of Fe and O. The oxygen isotope analysis of iron oxides shows a good homogeneity (1σ = 0.07‰). It is an ideal RM for inter-laboratory comparisons.
The other samples also had more than 16% of the residue undissolved in HCl, so the oxygen isotopes of the residue and the percentage content of the residue need to be evaluated for calculating the oxygen isotope of iron oxides. In sum, the δ18OOxides values of hematite obtained at 25 °C were all higher than those at 90 °C (Table 1, Figure 4). The difference between the δ18OOxides value (−0.89 ± 0.11‰) and the bulk rock δ18O is the largest in GBW07825, an RM, relative to the small deviation between δ18OBulk and δ18OOxides of the other samples.
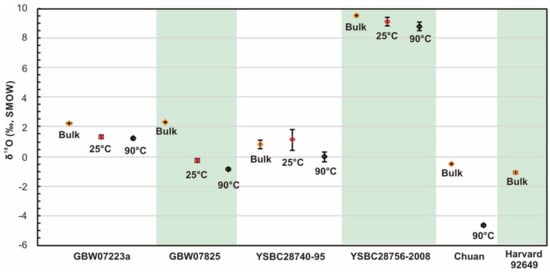
Figure 4.
Ratio of bulk rock δ18O and corrected δ18OOxides for hematite RMs. The orange rhombi represent the bulk rock δ18O. The red rhombi represent the corrected δ18OOxides after HCl dissolution treatment at room temperature (25 °C). The black rhombi represent the δ18OOxides after HCl dissolution treatment at 90 °C. The error bars represent 1σ.
In this study, the δ18OOxides value of hematite sample (Chuan) is −4.71 ‰ (Table 1). It is worth noting that the δ18OOxides values of some samples collected from the same location range from −3.41‰ to −5.67‰, which is close to those of the present study [7].
4. Conclusions
The hematite sample (Harvard 92649) is an ideal hematite RM for inter-laboratory comparisons, and our study shows that it not only has good primary element homogeneity but also has good oxygen isotope homogeneity.
In addition, we analyzed other samples from China (GBW07223a, GBW07825, YSBC28740-95, YSBC28756-2008, Chuan), three of which (GBW07223a, GBW07825, YSBC28756-2008, Chuan) had better homogeneity, while YSBC28740-95 had poorer homogeneity.
In the present study, ironstones were subjected to HCl dissolution chemistry. By further analysis of the oxygen content and oxygen isotopes of the insoluble residue, the oxygen isotope ratios of iron oxides can be obtained by mass balance calculations.
Author Contributions
Conceptualization, L.F.; Funding acquisition, L.F.; Methodology, L.F., H.L. and T.L.; Writing—original draft, L.F., H.L. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (No.2018YFF0215400), Instrument Function Developing Project of the Chinese Academy of Sciences (No.IGG201603), CAS Key Technology Talent Program and the Key Research Program of the Institute of Geology & Geophysics, CAS, Grant No. IGGCAS-201905.
Acknowledgments
Thanks to Chaofeng Li for providing the sample GBW07825 and to Lanzhen Guo for his help in the sample analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bao, H.; Koch, P.L.; Rumble, D. Paleocene–Eocene climatic variation in western North America: Evidence from the δ18O of pedogenic hematite. GSA Bull. 1999, 111, 1405–1415. [Google Scholar] [CrossRef]
- Yapp, C.J. Oxygen and hydrogen isotope variations among goethites (α-FeOOH) and the determination of paleotemperatures. Geochim. Cosmochim. Acta 1987, 51, 355–364. [Google Scholar] [CrossRef]
- Yapp, C.J. The stable isotope geochemistry of low temperature Fe(III) and Al “oxides” with implications for continental paleoclimates. In Climate Change in Continental Isotopic Records; American Geophysical Union (AGU): Washington, DC, USA, 1993; pp. 285–294. ISBN 978-1-118-66402-5. [Google Scholar]
- Yapp, C.J. Oxygen isotopes in synthetic goethite and a model for the apparent pH dependence of goethite–water 18O/16O fractionation. Geochim. Cosmochim. Acta 2007, 71, 1115–1129. [Google Scholar] [CrossRef]
- Yapp, C.J. Oxygen isotopes in iron (III) oxides: 2. Possible constraints on the depositional environment of a Precambrian quartz-hematite banded iron formation. Chem. Geol. 1990, 85, 337–344. [Google Scholar] [CrossRef]
- Gutzmer, J.; Mukhopadhyay, J.; Beukes, N.J.; Pack, A.; Hayashi, K.; Sharp, Z.D. Oxygen isotope composition of hematite and genesis of high-grade BIF-hosted iron ores. In Evolution of Early Earth’s Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits; Geological Society of America: Washington, DC, USA, 2006; ISBN 978-0-8137-1198-0. [Google Scholar]
- Galili, N.; Shemesh, A.; Yam, R.; Brailovsky, I.; Sela-Adler, M.; Schuster, E.M.; Collom, C.; Bekker, A.; Planavsky, N.; Macdonald, F.A.; et al. The geologic history of seawater oxygen isotopes from marine iron oxides. Science 2019, 365, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Yapp, C.J. Recovery and interpretation of the 18O/16O of Miocene oolitic goethites in multi-generational mixtures of Fe (III) oxides from a channel iron deposit of Western Australia. Geochim. Cosmochim. Acta 2020, 279, 143–164. [Google Scholar] [CrossRef]
- Miller, H.B.D.; Farley, K.A.; Vasconcelos, P.M.; Mostert, A.; Eiler, J.M. Intracrystalline site preference of oxygen isotopes in goethite: A single-mineral paleothermometer. Earth Planet. Sci. Lett. 2020, 539, 116237. [Google Scholar] [CrossRef]
- Sutherland, K.M.; Wostbrock, J.A.G.; Hansel, C.M.; Sharp, Z.D.; Hein, J.R.; Wankel, S.D. Ferromanganese crusts as recorders of marine dissolved oxygen. Earth Planet. Sci. Lett. 2020, 533, 116057. [Google Scholar] [CrossRef]
- Gao, B.F.; Wu, C.Z.; Yang, T.; Santosh, M.; Dong, L.H.; Zhao, T.Y.; Ye, H.; Lei, R.X.; Li, W. The neoproterozoic “blood falls” in Tarim Craton and their possible connection with snowball earth. J. Geophys. Res. Earth Surf. 2019, 124, 229–244. [Google Scholar] [CrossRef]
- Mandernack, K.W.; Bazylinski, D.A.; Shanks, W.C.; Bullen, T.D. Oxygen and iron isotope studies of magnetite produced by magnetotactic bacteria. Science 1999, 285, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Nyström, J.O.; Billström, K.; Henríquez, F.; Fallick, A.E.; Naslund, H.R. Oxygen isotope composition of magnetite in iron ores of the Kiruna type in Chile and Sweden. GFF 2010. [Google Scholar] [CrossRef]
- Sjostrom, D.J.; Hren, M.T.; Chamberlain, C.P. Oxygen isotope records of goethite from ferricrete deposits indicate regionally varying holocene climate change in the Rocky Mountain region, U.S.A. Quat. Res. 2004, 61, 64–71. [Google Scholar] [CrossRef]
- Troll, V.R.; Weis, F.A.; Jonsson, E.; Andersson, U.B.; Majidi, S.A.; Högdahl, K.; Harris, C.; Millet, M.-A.; Chinnasamy, S.S.; Kooijman, E.; et al. Global Fe–O isotope correlation reveals magmatic origin of Kiruna-type apatite-iron-oxide ores. Nat. Commun. 2019, 10, 1712. [Google Scholar] [CrossRef]
- Feng, L.; Huang, J.; Lu, D.; Zhang, Q. Major and trace element geochemistry of the Neoproterozoic syn-glacial Fulu iron formation, South China. Geol. Mag. 2017, 154, 1371–1380. [Google Scholar] [CrossRef]
- Dymek, R.F.; Klein, C. Chemistry, petrology and origin of banded iron-formation lithologies from the 3800 MA isua supracrustal belt, West Greenland. Precambrian Res. 1988, 39, 247–302. [Google Scholar] [CrossRef]
- Cloud, P. Paleoecological significance of the banded iron-formation. Econ. Geol. 1973, 68, 1135–1143. [Google Scholar] [CrossRef]
- Gole, M.J.; Klein, C. Banded iron-formations through much of Precambrian time. J. Geol. 1981, 89, 169–183. [Google Scholar] [CrossRef]
- Becker, R.H.; Clayton, R.N. Oxygen isotope study of a Precambrian banded iron-formation, Hamersley Range, Western Australia. Geochim. Cosmochim. Acta 1976, 40, 1153–1165. [Google Scholar] [CrossRef]
- Hagemann, S.G.; Angerer, T.; Duuring, P.; Rosière, C.A.; e Silva, R.F.; Lobato, L.; Hensler, A.S.; Walde, D.H.G. BIF-hosted iron mineral system: A review. Ore Geol. Rev. 2016, 76, 317–359. [Google Scholar] [CrossRef]
- Pecoits, E.; Gingras, M.K.; Barley, M.E.; Kappler, A.; Posth, N.R.; Konhauser, K.O. Petrography and geochemistry of the Dales Gorge banded iron formation: Paragenetic sequence, source and implications for palaeo-ocean chemistry. Precambrian Res. 2009, 172, 163–187. [Google Scholar] [CrossRef]
- Sharp, Z.D. A laser-based microanalytical method for the in situ determination of oxygen isotope ratios of silicates and oxides. Geochim. Cosmochim. Acta 1990, 54, 1353–1357. [Google Scholar] [CrossRef]
- Huberty, J.M.; Kita, N.T.; Kozdon, R.; Heck, P.R.; Fournelle, J.H.; Spicuzza, M.J.; Xu, H.; Valley, J.W. Crystal orientation effects in δ18O for magnetite and hematite by SIMS. Chem. Geol. 2010, 276, 269–283. [Google Scholar] [CrossRef]
- Li, W.; Huberty, J.M.; Beard, B.L.; Kita, N.T.; Valley, J.W.; Johnson, C.M. Contrasting behavior of oxygen and iron isotopes in banded iron formations revealed by in situ isotopic analysis. Earth Planet. Sci. Lett. 2013, 384, 132–143. [Google Scholar] [CrossRef]
- Li, X.-H.; Long, W.-G.; Li, Q.-L.; Liu, Y.; Zheng, Y.-F.; Yang, Y.-H.; Chamberlain, K.R.; Wan, D.-F.; Guo, C.-H.; Wang, X.-C.; et al. Penglai zircon megacrysts: A potential new working reference material for microbeam determination of Hf-O isotopes and U-Pb age. Geostand. Geoanal. Res. 2010, 34, 117–134. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, Y.-F.; Chen, R.-X. TC/EA-MS online determination of hydrogen isotope composition and water concentration in eclogitic garnet. Phys. Chem. Miner. 2007, 34, 687–698. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. General preparative techniques. In Iron Oxides in the Laboratory; John Wiley & Sons, Ltd.: London, UK, 2007; pp. 19–25. ISBN 978-3-527-61322-9. [Google Scholar]
- McGuire, A.V.; Francis, C.A.; Dyar, M.D. Mineral standards for electron microprobe analysis of oxygen. Am. Mineral. 1992, 77, 1087–1091. [Google Scholar]
- Smalley, P.C.; Stijfhoorn, D.E.; Råheim, A.; Johansen, H.; Dickson, J.A.D. The laser microprobe and its application to the study of C and O isotopes in calcite and aragonite. Sediment. Geol. 1989, 65, 211–221. [Google Scholar] [CrossRef]
- Dickson, J.A.D.; Smalley, P.C.; Råheim, A.; Stijfhoorn, D.E. Intracrystalline carbon and oxygen isotope variations in calcite revealed by laser microsampling. Geology 1990, 18, 809–811. [Google Scholar] [CrossRef]
- Powell, M.D.; Kyser, T.K. Analysis of δ13C and δ18O in calcite, dolomite, rhodochrosite and siderite using a laser extraction system. Chem. Geol. 1991, 94, 55–66. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).