Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization
Abstract
1. Introduction
2. Stable Isotopes in ARD-Related Processes
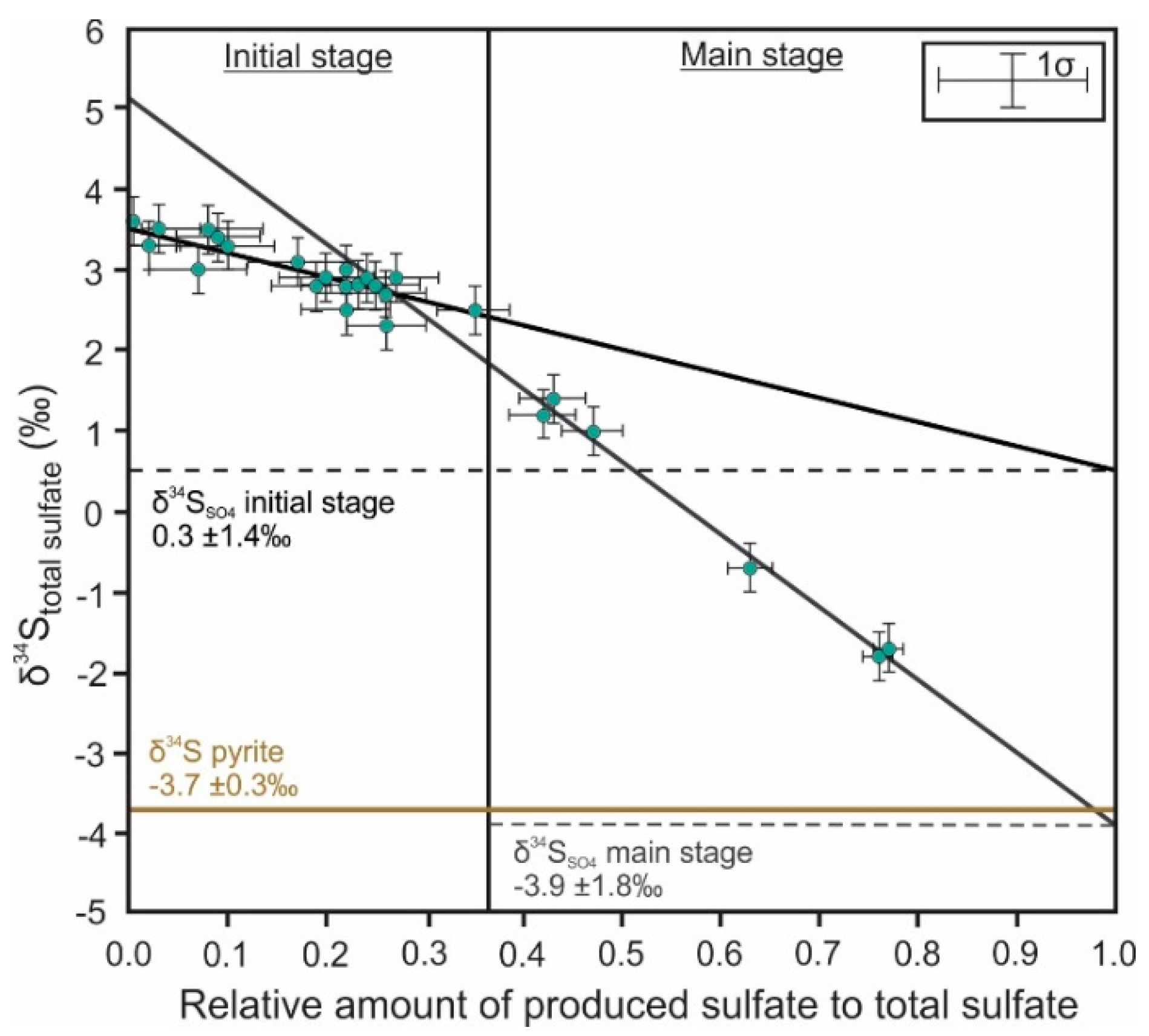
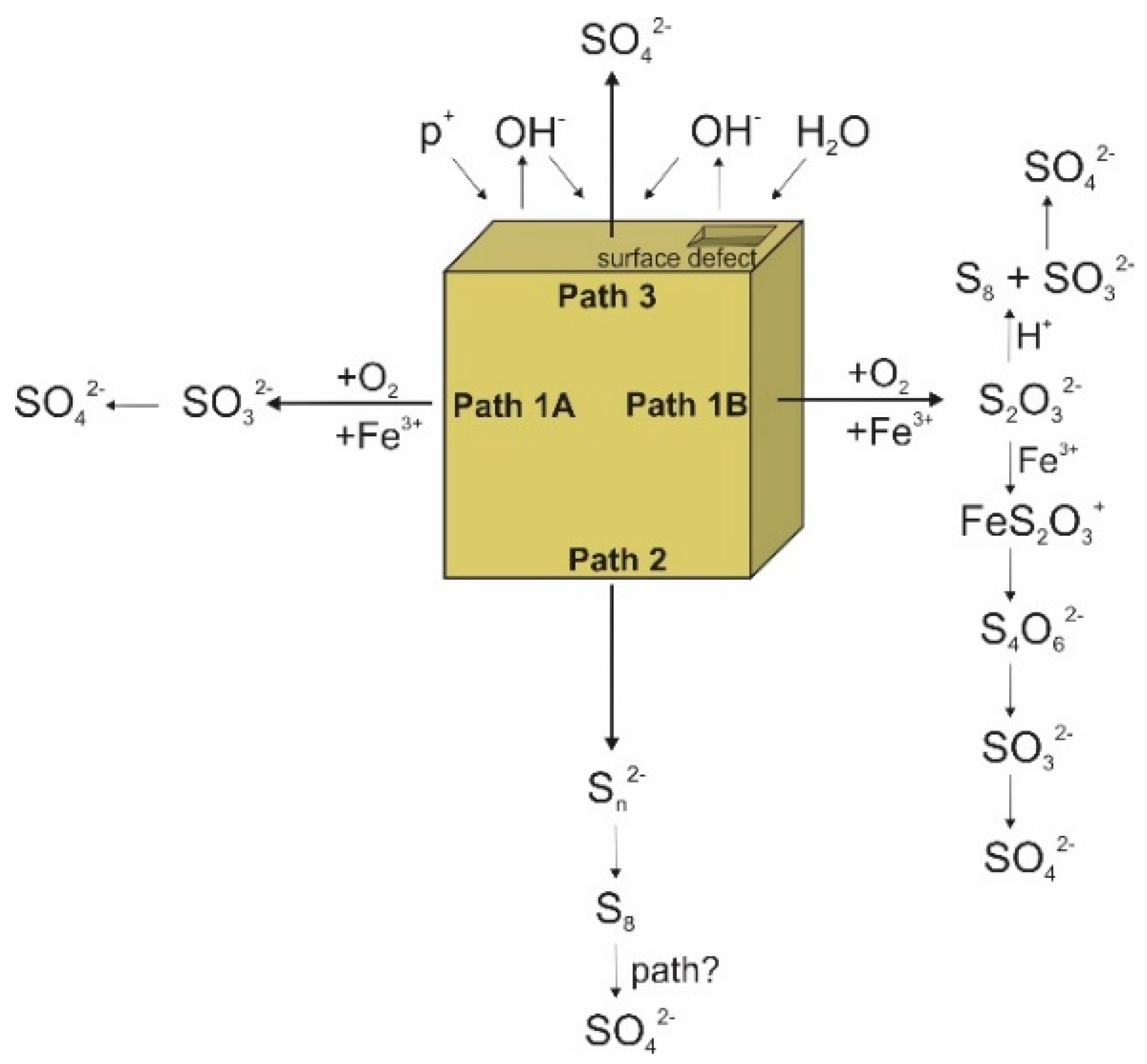
3. Sulfur Isotope Signatures of Pyrite Leaching
4. Oxygen Isotope Signatures of Pyrite Leaching
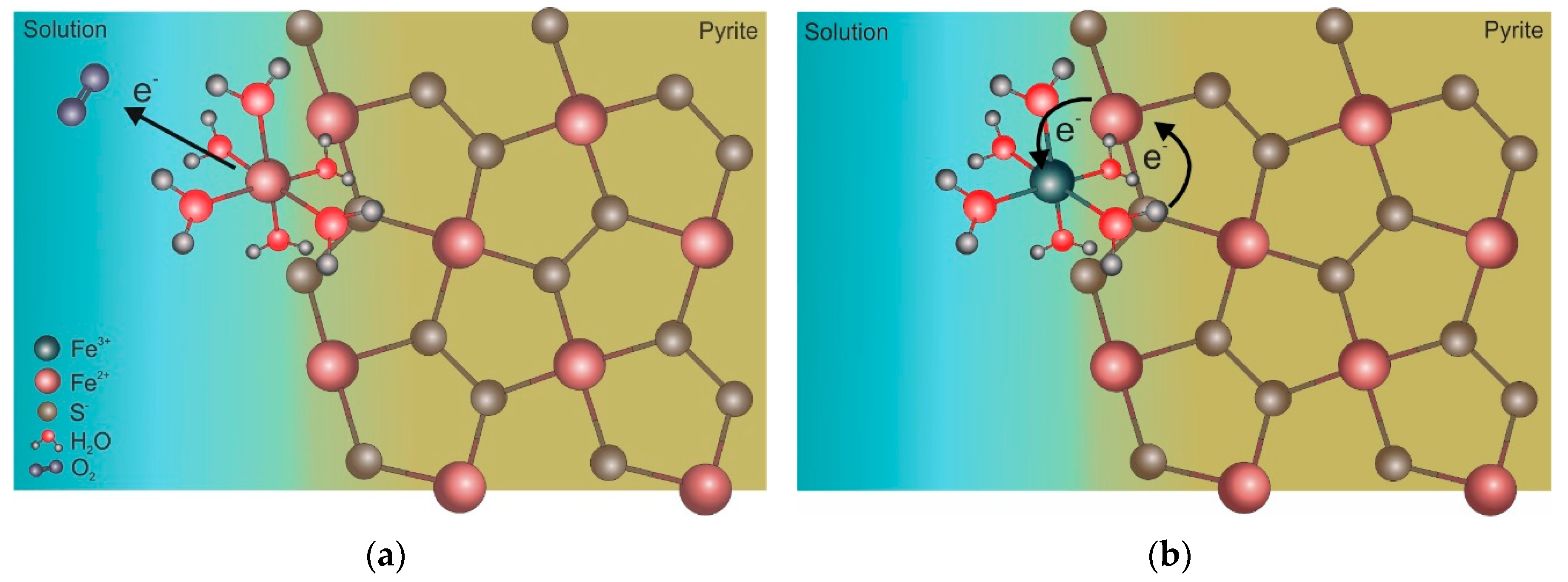
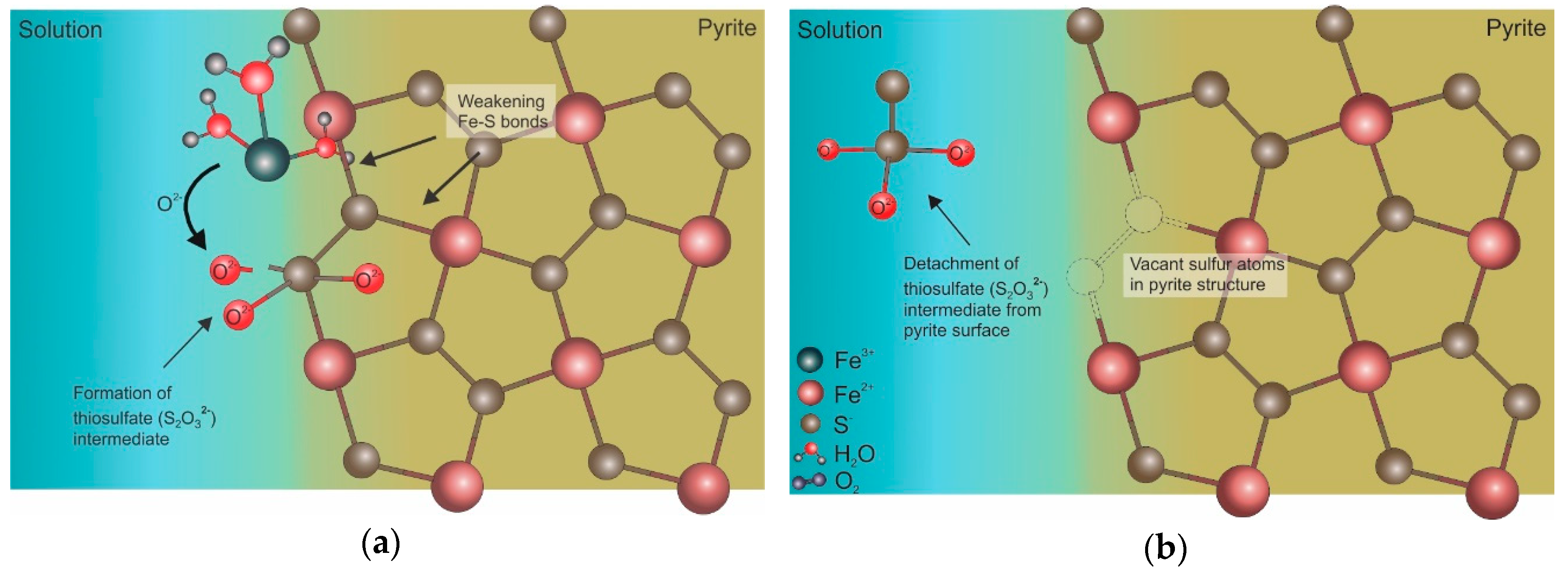
5. Iron Isotope Signatures of Pyrite Leaching
Influence of Microorganisms on Iron Isotope Fractionation
6. Summary and Future Perspective
- Understanding the role of pyrite type: Most laboratory experiments have used hydrothermal pyrite, even though it is well established that pyrites from different geological environments, morphologies, or electrochemical properties [37] show distinctly different oxidation rates and dissolution signatures [147,148]. Consequently, there is a need to extend the database of isotope fractionation factors to include all forms and physical parameters of pyrites in order to apply the most appropriate fractionation factors in field studies.
- Understanding acid neutralization processes: An understudied but promising area in the application of isotope geochemistry involves the isotope signatures of neutralization processes. ARD-related carbon isotope fractionation would not only provide information on the mechanisms of neutralization reactions, but also has the potential to determine the relative proportion of the main neutralizing carbonate phases using mixing calculations. This may provide more accurate Acid Neutralizing Capacity (ANC) calculations, as currently calcite is considered as the most dominant neutralizing mineral [3] without its quantified relative contribution to neutralization.
- Understanding the role of different mineral processing and metallurgical activities on isotope processes: Although several isotope investigations have been undertaken in mining areas to track ARD-related processes in underground and surface water bodies, the method still has not become common practice. In addition, most of these field studies are focused on underground mine workings, pit lakes, as well as waste and water storage facilities that are collectively considered as the major environments of ARD generation. To our knowledge, no studies have examined the role of ore beneficiation processes in ARD formation using the isotope techniques in detail. The water consumption of different processing steps such as milling, dense media separation, or flotation may also provide the required conditions of sulfide oxidation, allowing the initial steps of ARD generation to take place. Tracking the sulfide oxidation pathways and sulfur transformations during various beneficiations steps would help to assess the relative contribution of mineral processing to ARD generation. Eliminating the ARD potential before waste disposal would have a significant beneficial impact on mine water quality. In addition, the quantification of mineral oxidation rates of certain processing steps via isotopes could add to process optimization by the evaluation of more accurate residence times.
- ARD prevention, mitigation, and management: In order to minimize the potential impacts of ARD on natural water systems, it is necessary to understand (i) the mechanisms taking place during ARD formation and (ii) the mechanisms that control the mobility of contaminants. Although isotopes are successfully used in pollution source-tracking and transportation modelling, the applications of isotopes in ARD generation processes are not yet fully explored. To create more accurate prediction models and thus be able to select the most optimal pollution emission control strategies, more accurate isotope fractionation factors and the identification of causative mechanisms are required. Accurate measurements of fractionation would allow the estimation of various reaction rates with greater certainty. This would help to improve our ability to evaluate the chemical evolution of both mine waste material and the impacted water systems, potentially informing ARD control measures in a timely manner. In addition to tracking ARD generation and transportation, isotopes also can be used in evaluating the effectiveness of different remediation methods, for example, passive treatment systems [75,149].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egiebor, N.O.; Oni, B. Acid Rock Drainage Formation and Treatment: A Review. Asia Pac. J. Chem. Eng. 2007, 2, 47–62. [Google Scholar] [CrossRef]
- Moncur, M.C.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Mine Drainage from the Weathering of Sulfide Minerals and Magnetite. Appl. Geochem. 2009, 24, 2362–2373. [Google Scholar] [CrossRef]
- Dold, B. Basic Concepts in Environmental Geochemistry of Sulfidic Mine-Waste Management. In Waste Management; Kumar, E.S., Ed.; InTech: Rijeka, Croatia, 2010; Available online: http://www.intechopen.com/books/waste-management/basic-concepts-in-environmental-geochemistry-ofsulfidic-mine-waste-management (accessed on 22 May 2020).
- Chopard, A.; Benzaazoua, M.; Bouzahzah, H.; Plante, B.; Marion, P. A Contribution to Improve the Calculation of the Acid Generating Potential of Mining Wastes. Chemosphere 2017, 175, 97–107. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Larsen, D.; Mann, R. Origin of High Manganese Concentrations in Coal Mine Drainage, Eastern Tennessee. J. Geochem. Explor. 2005, 86, 143–163. [Google Scholar] [CrossRef]
- Cravotta, C.A. Dissolved Metals and Associated Constituents in Abandoned Coal-Mine Discharges, Pennsylvania, USA. Part 1: Constituent Quantities and Correlations. Appl. Geochem. 2008, 23, 166–202. [Google Scholar] [CrossRef]
- Vyawahre, A.; Rai, S. Acid Mine Drainage: A Case Study of An Indian Coal Mine. Int. J. Sci. Res. Sci. Eng. Technol. 2016, 2, 1297–1301. [Google Scholar]
- Bell, F.G.; Bullock, S.E.T.; Hälbich, T.F.; Lindsay, P. Environmental Impacts Associated with an Abandoned Mine in The Witbank Coalfield, Sout Africa. Int. J. Coal Geol. 2001, 45, 195–216. [Google Scholar] [CrossRef]
- Casiot, C.; Egal, M.; Elbaz-Poulichet, F.; Bruneel, O.; Bancon-Montigny, C.; Cordier, M.-A.; Gomez, E.; Aliaume, C. Hydrological and Geochemical Control of Metals and Arsenic in a Mediterranean River Contaminated by Acid Mine Drainage (the Amous River, France); Preliminary Assessment of Impacts on Fish (Leuciscus Cephalus). Appl. Geochem. 2009, 24, 787–799. [Google Scholar] [CrossRef]
- Olenici, A.; Blanco, S.; Borrego-Ramos, M.; Momeu, L.; Baciu, C. Exploring the Effects of Acid Mine Drainage on Diatom Teratology Using Geometric Morphometry. Ecotoxicology 2017, 26, 1018–1030. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L. Competitive Sorption and Desorption of Heavy Metals in Mine Soils: Influence of Mine Soil Characteristics. J. Colloid Interface Sci. 2006, 298, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Anderson, T.-H. Comparison of Soil Fungal/Bacterial Ratios in a PH Gradient Using Physiological and PLFA-Based Techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy Metal Distribution and Chemical Speciation in Tailings and Soils around a Pb–Zn Mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Clarke, L.B. Coal Mining and Water Quality; Technical Report; IEA Coal Research: London, UK, 1995. [Google Scholar]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-Sulfate Salts from Sulfide Mineral Oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Nicholson, R.Y. Iron-Sulfide Oxidation Mechanisms; Laboratory Studies. In The Environmental Geochemistry of Sulfide, Mine-Wastes; Short Course Handbook; Blowes, D.W., Jambor, J.L., Eds.; Mineralogical Association of Canada: Nepean, ON, Canada, 1994; Volume 22, pp. 163–183. [Google Scholar]
- Chandra, A.P.; Gerson, A.R. The Mechanisms of Pyrite Oxidation and Leaching: A Fundamental Perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid Mine Drainage Formation and Arsenic Mobility under Strongly Acidic Conditions: Importance of Soluble Phases, Iron Oxyhydroxides/Oxides and Nature of Oxidation Layer on Pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.G. A Critical Review of Acid Rock Drainage Prediction Methods and Practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Dold, B. Acid Rock Drainage Prediction: A Critical Review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Brady, K.B.C.; Perry, E.F.; Beam, R.L.; Bisko, D.C.; Gardner, M.D.; Tarantino, J.M. Evaluation of Acid-Base Accounting to Predict the Quality of Drainage at Surface Coal Mines in Pennsylvania, U.S.A. J. Am. Soc. Min. Reclam. 1994, 1, 138–147. [Google Scholar] [CrossRef]
- Paktunc, A.D. Mineralogical Constraints on the Determination of Neutralization Potential and Prediction of Acid Mine Drainage. Environ. Geol. 1999, 39, 103–112. [Google Scholar] [CrossRef]
- Verburg, R.; Bezuidenhout, N.; Chatwin, T.; Ferguson, K. The Global Acid Rock Drainage Guide (GARD Guide). Mine Water Environ. 2009, 28, 305–310. [Google Scholar] [CrossRef]
- Morin, K.A.; Hutt, N.M.; Ferguson, K.D. Measured Rates of Sulfide Oxidation and Neutralization in Kinetic Tests: Statistical Lessons from the Database. In Proceedings of the Sudbury ’95: Mining and the Environment, Sudbury, ON, Canada, 23 May–1 June 1995; pp. 525–536. [Google Scholar]
- Banerjee, D. Acid Drainage Potential from Coal Mine Wastes: Environmental Assessment through Static and Kinetic Tests. Int. J. Environ. Sci. Technol. 2014, 11, 1365–1378. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, Selenium, Boron, Lead, Cadmium, Copper, and Zinc in Naturally Contaminated Rocks: A Review of Their Sources, Modes of Enrichment, Mechanisms of Release, and Mitigation Strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Becker, M.; Dyantyi, N.; Broadhurst, J.L.; Harrison, S.T.L.; Franzidis, J.P. A Mineralogical Approach to Evaluating Laboratory Scale Acid Rock Drainage Characterisation Tests. Miner. Eng. 2015, 80, 33–36. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical Characterization of Mine Waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Guseva, O.; Opitz, A.; Broadhurst, J.; Harrison, S.; Becker, M. Characterisation and prediction of acid rock drainage in waste rock: Value of integrating quantitative mineralogical and textural measurements. Miner. Eng. Under review.
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally Sustainable Acid Mine Drainage Remediation: Research Developments with a Focus on Waste/by-Products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the Compositional, Textural and Electrical Properties of Natural Pyrite: A Review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Savage, K.S.; Stefan, D.; Lehner, S.W. Impurities and Heterogeneity in Pyrite: Influences on Electrical Properties and Oxidation Products. Appl. Geochem. 2008, 23, 103–120. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Tamoto, S.; Takahashi, R. The Roles of Pyrite and Calcite in the Mobilization of Arsenic and Lead from Hydrothermally Altered Rocks Excavated in Hokkaido, Japan. J. Geochem. Explor. 2012, 119–120, 17–31. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J.; Barnes, A.R. Classification of the Major Copper Sulphides into Semiconductor Types, and Associated Flotation Characteristics. Miner. Eng. 2016, 96–97, 177–184. [Google Scholar] [CrossRef]
- Miljević, N.; Golobočanin, D. Potential Use of Environmental Isotopes in Pollutant Migration Studies. Arh. Hig. Rada Toksikol. 2007, 58, 251–262. [Google Scholar] [CrossRef]
- Wiederhold, J.G. Metal Stable Isotope Signatures as Tracers in Environmental Geochemistry. Environ. Sci. Technol. 2015, 49, 2606–2624. [Google Scholar] [CrossRef]
- Seal, R.R.; Alpers, C.N.; Rye, R.O. Stable Isotope Systematics of Sulfate Minerals. Rev. Mineral. Geochem. 2000, 40, 541–602. [Google Scholar] [CrossRef]
- Skierszkan, E.; Mayer, U.; Beckie, R.; Weis, D. Tracking the Fate of Metals in Mining Waste Rock Using Metal Stable Isotopes. In Proceedings of the Agreeing on Solutions for more Sustainable Mine Water Management, 10th International Conference on Acid Rock Drainage and IMWA Annual Conference, Santiago, Chile, 21–24 April 2015; Brown, A., Bucknam, C., Burgess, J., Carballo, M., Castendyk, D., Figueroa, L., Kirk, L., McLemore, V., McPhee, J., O’Kane, M., et al., Eds.; p. 236. [Google Scholar]
- Michener, R.H.; Lajtha, K. Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 6–11. [Google Scholar]
- Weiss, D.J.; Rehkämper, M.; Schoenberg, R.; McLaughlin, M.; Kirby, J.; Campbell, P.G.C.; Arnold, T.; Chapman, J.; Peel, K.; Gioia, S. Application of Non-traditional Stable-isotope Systems to the Study of Sources and Fate of Metals in the Environment. Environ. Sci. Technol. 2008, 42, 655–664. [Google Scholar] [CrossRef]
- Young, E.D.; Galy, A.; Nagahara, H. Kinetic and Equilibrium Mass-dependent Isotope Fractionation Laws in Nature and Their Geochemical and Cosmochemical Significance. Geochim. Cosmochim. Acta 2002, 66, 1095–1104. [Google Scholar] [CrossRef]
- Sharp, Z. Principles of Stable Isotope Geochemistry, 2nd ed.; Pearson Education: Upper Saddle River, NJ, USA, 2017. [Google Scholar]
- Garrels, R.; Thompson, M. Oxidation of Pyrite by Iron Sulfate Solutions. Am. J. Sci. 1960, 258, 57–67. [Google Scholar]
- Nordstrom, K.D.; Alpers, C.N. Geochemistry of Acid Mine Waters. In The Environmental Geochemistry of Mineral Deposits. Part A: Processes, Techniques, and Health Issues; Plumlee, G.D., Logsdon, M.J., Filipek, L.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6, pp. 133–160. [Google Scholar]
- Smart, M.; Huddy, R.J.; Edward, C.J.; Fourie, C.; Shumba, T.; Iron, J.; Harrison, S.T.L. Linking Microbial Community Dynamics in BIOX® Leaching Tanks to Process Conditions: Integrating Lab and Commercial Experience. Solid State Phenom. 2017, 262, 38–42. [Google Scholar] [CrossRef]
- Tupikina, O.V.; Minnaar, S.H.; van Hille, R.P.; van Wyk, N.; Rautenbach, G.F.; Dew, D.; Harrison, S.T.L. Determining the Effect of Acid Stress on the Persistence and Growth of Thermophilic Microbial Species after Mesophilic Colonisation of Low Grade Ore in a Heap Leach Environment. Miner. Eng. 2013, 53, 152–159. [Google Scholar] [CrossRef]
- Nordstrom, K.D.; Southam, G. Geomicrobiology of Sulfide Mineral Oxidation. In Geomicrobiology: Interactions between Microbes and Minerals: Reviews in Mineralogy; Banfield, J.F., Nealson, K.H., Eds.; Mineralogical Society of America: Washington, DC, USA, 1997; Volume 35, pp. 361–390. [Google Scholar]
- Mustin, C.; Berthelin, J.; Marion, P.; de Donato, P. Corrosion and Electrochemical Oxidation of a Pyrite by Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1992, 58, 1175–1182. [Google Scholar] [CrossRef]
- Rimstidt, D.D.; Vaughan, D.J. Pyrite Oxidation: A State-of-the-Art Assessment of the Reaction Mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Moses, C.O.; Herman, J.S. Pyrite Oxidation at Circumneutral pH. Geochim. Cosmochim. Acta 1991, 55, 471–482. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Yin, Q.; Vaughan, D.J.; England, K.E.R.; Brandon, N.P. Electrochemical Oxidation of Pyrite (FeS2) in Aqueous Electrolytes. J. Electroanal. Chem. 1999, 471, 116–125. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite Oxidation in the Presence of Hematite and Alumina: II. Effects on the Cathodic and Anodic Half-Cell Reactions. Sci. Total Environ. 2017, 581–582, 126–135. [Google Scholar] [CrossRef]
- Tu, Z.; Wan, J.; Guo, C.; Fan, C.; Zhang, T.; Lu, G.; Reinfelder, J.R.; Dang, Z. Electrochemical Oxidation of Pyrite in PH 2 Electrolyte. Electrochim. Acta 2017, 239, 25–35. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Hiroyoshi, N.; Tabelin, C.B.; Taketsugu, T.; Ito, M. Suppression of Pyrite Oxidation by Ferric-Catecholate Complexes: An Electrochemical Study. Miner. Eng. 2019, 138, 226–237. [Google Scholar] [CrossRef]
- Nourmohamadi, H.; Aghazadeh, V.; Esrafili, M.D. A Comparative DFT Study of Fe3+ and Fe2+ Ions Adsorption on (100) and (110) Surfaces of Pyrite: An Electrochemical Point of View. Surf. Interface Anal. 2020, 52, 110–118. [Google Scholar] [CrossRef]
- Jerz, J.K.; Rimstidt, J.D. Pyrite Oxidation in Moist Air. Geochim. Cosmochim. Acta 2004, 68, 701–714. [Google Scholar] [CrossRef]
- Borda, M.J.; Strongin, D.R.; Schoonen, M.A.A. Vibrational Spectroscopic Study of Pyrite Oxidation. Am. Mineral. 2003, 88, 1318–1323. [Google Scholar] [CrossRef]
- Nordstrom, K.D.; Wright, W.G.; Mast, A.M.; Bove, D.J.; Rye, R.O. Aqueous-Sulfate Stable Isotopes-A Study of Mining-Affected and Undisturbed Acidic Drainage. In Integrated Investigations of Environmental Effects of Historical Mining in the Animas River Watershed, San Juan County, Colorado; Church, S.E., von Guerard, P., Finger, S.E., Eds.; U.S. Geological Survey Professional Paper 1651; USGS: Lawrence, KS, USA, 2007; Volume 1, pp. 387–416. [Google Scholar]
- Field, C.W. Sulfur Isotopic Method for Discriminating between Sulfates of Hypogene and Supergene Origin. Econ. Geol. 1966, 61, 1428–1435. [Google Scholar] [CrossRef]
- Taylor, B.E.; Wheeler, M.C.; Nordstrom, D.K. Stable Isotope Geochemistry of Acid Mine Drainage: Experimental Oxidation of Pyrite. Geochim. Cosmochim. Acta 1984, 48, 2669–2678. [Google Scholar] [CrossRef]
- Taylor, B.E.; Wheeler, M.C. Sulfur- and Oxygen-isotope Geochemistry of Acid Mine Drainage in the Western United States. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society Symposium Series: Washington, DC, USA, 1993; Volume 550, pp. 481–514. [Google Scholar]
- Van Stempvoort, D.R.; Krouse, H.R. Controls of δ18O in Sulfate: Review of Experimental Data and Application to Specific Environments. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society Symposium Series: Washington, DC, USA, 1993; Volume 550, pp. 446–480. [Google Scholar]
- Krouse, H.R.; Mayer, B. Sulphur and Oxygen Isotopes in Sulphate. In Environmental Tracers in Subsurface Hydrology; Cook, P., Herczeg, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 195–231. [Google Scholar]
- Otero, N.; Soler, A.; Canals, À. Controls of δ34S and δ18O in Dissolved Sulphate: Learning from a Detailed Survey in the Llobregat River (Spain). Appl. Geochem. 2008, 23, 1166–1185. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Hałas, S.; Dołegowska, S.; Dabek, J.; Starnawska, E. Geochemistry and Stable Sulfur and Oxygen Isotope Ratios of the Podwiśniówka Pit Pond Water Generated by Acid Mine Drainage (Holy Cross Mountains, South-Central Poland). Appl. Geochem. 2008, 23, 3620–3634. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Michalik, A.; Dołegowska, S.; Migaszewski, A.; Hałas, S.; Trembaczowski, A. The Use of Stable Sulfur, Oxygen and Hydrogen Isotope Ratios as Geochemical Tracers of Sulfates in the Podwiśniówka Acid Drainage Area (South-Central Poland). Aquat. Geochem. 2013, 19, 261–280. [Google Scholar] [CrossRef]
- Nakai, N.; Jensen, M.L. The Kinetic Isotope Effect in the Bacterial Reduction and Oxidation of Sulfur. Geochim. Cosmochim. Acta 1964, 28, 1893–1912. [Google Scholar] [CrossRef]
- McCready, R.G.L.; Krouse, H.R. Sulfur Isotope Fractionation during the Oxidation of Elemental Sulfur by Thiobacilli in a Solonetzic Soil. Can. J. Soil Sci. 1982, 62, 105–110. [Google Scholar] [CrossRef]
- Balci, N.; Shanks, W.C.; Mayer, B.; Mandernack, K.W. Oxygen and Sulfur Isotope Systematics of Sulfate Produced by Bacterial and Abiotic Oxidation of Pyrite. Geochim. Cosmochim. Acta 2007, 71, 3796–3811. [Google Scholar] [CrossRef]
- Pisapia, C.; Chaussidon, M.; Mustin, C.; Humbert, B. O and S Isotopic Composition of Dissolved and Attached Oxidation Products of Pyrite by Acidithiobacillus ferrooxidans: Comparison with Abiotic Oxidations. Geochim. Cosmochim. Acta 2007, 71, 2474–2490. [Google Scholar] [CrossRef]
- Heidel, C.; Tichomirowa, M. The Isotopic Composition of Sulfate from Anaerobic and Low Oxygen Pyrite Oxidation Experiments with Ferric Iron—New Insights into Oxidation Mechanisms. Chem. Geol. 2011, 281, 305–316. [Google Scholar] [CrossRef]
- Lefticariu, L.; Behum, P.; Bender, K.; Lefticariu, M. Sulfur Isotope Fractionation as an Indicator of Biogeochemical Processes in an AMD Passive Bioremediation System. Minerals 2017, 7, 41. [Google Scholar] [CrossRef]
- Alvaro, A.; Roldán, F.V. Stable Isotope Composition of Acid Mine Drainage Minerals from San Miguel Massive Sulphide Mine Waste. In Proceedings of the Securing the Future: Mining, Metals and the Environment in a Sustainable Society, 8th International Conference on Acid Rock Drainage, Skellefteå, Sweden, 22–26 June 2009; pp. 166–176. [Google Scholar]
- Gallo, A.A.; Roldán, F.V. Stable Isotope Study in Iron-Rich Sulphates from San Miguel Mine Wastes (Iberian Pyrite Belt, Spain). Rev. Soc. Española Mineral. 2009, 11, 27–28. [Google Scholar]
- Brunner, B.; Yu, J.Y.; Mielke, R.E.; MacAskill, J.A.; Madzunkov, S.; McGenity, T.J.; Coleman, M. Different Isotope and Chemical Patterns of Pyrite Oxidation Related to Lag and Exponential Growth Phases of Acidithiobacillus ferrooxidans Reveal a Microbial Growth Strategy. Earth Planet. Sci. Lett. 2008, 270, 63–72. [Google Scholar] [CrossRef]
- Yu, J.Y.; McGenity, T.J.; Coleman, M.L. Solution Chemistry during the Lag Phase and Exponential Phase of Pyrite Oxidation by Thiobacillus ferrooxidans. Chem. Geol. 2001, 175, 307–317. [Google Scholar] [CrossRef]
- Descostes, M.; Vitorge, P.; Beaucaire, C. Pyrite Dissolution in Acidic Media. Geochim. Cosmochim. Acta 2004, 68, 4559–4569. [Google Scholar] [CrossRef]
- Granger, H.C.; Warren, C.G. Unstable Sulfur Compounds and the Origin of Roll-Type Uranium Deposits. Econ. Geol. 1969, 64, 160–171. [Google Scholar] [CrossRef]
- Basolo, F.; Pearson, R.G. Mechanisms of Inorganic Reactions—A Study of Metal Complexes in Solution, 2nd ed.; John Wiley and Sons Ltd.: New York, NY, USA, 1967. [Google Scholar]
- Goldhaber, M.B. Experimental Study of Metastable Sulfur Oxyanion Formation during Pyrite Oxidation at pH 6–9 and 30 °C. Am. J. Sci. 1983, 283, 193–217. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.G.; Sand, W. Sulfur Chemistry in the Leaching of Pyrite. Appl. Environm. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [CrossRef]
- Toran, L.; Harris, R.F. Interpretation of Sulfur and Oxygen Isotopes in Biological and Abiological Sulfide Oxidation. Geochim. Cosmochim. Acta 1989, 53, 2341–2348. [Google Scholar] [CrossRef]
- McKibben, M.A.; Barnes, H.L. Oxidation of Pyrite in Low Temperature Acidic Solutions: Rate Laws and Surface Textures. Geochim. Cosmochim. Acta 1986, 50, 1509–1520. [Google Scholar] [CrossRef]
- Luther, G.W. Pyrite Oxidation and Reduction: Molecular Orbital Theory Considerations. Geochim. Cosmochim. Acta 1987, 51, 3193–3199. [Google Scholar] [CrossRef]
- Moses, C.O.; Nordstrom, K.D.; Herman, J.S.; Mills, A.L. Aqueous Pyrite Oxidation by Dissolved Oxygen and by Ferric Iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Demoisson, F.; Mullet, M.; Humbert, B. Pyrite Oxidation in Acidic Medium: Overall Reaction Pathway. Surf. Interface Anal. 2008, 40, 343–348. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial Leaching of Metal Sulfides Proceeds by Two Indirect Mechanisms via Thiosulfate or via Polysulfides and Sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.G.; Schippers, A. (Bio)Chemistry of Bacterial Leaching—Direct vs. Indirect Bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching Review Part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef]
- Borda, M.J.; Elsetinow, A.R.; Strongin, D.R.; Schoonen, M.A.A. Mechanism for the Production of Hydroxyl Radical at Surface Defect Sites on Pyrite. Geochim. Cosmochim. Acta 2003, 67, 935–939. [Google Scholar] [CrossRef]
- Druschel, G.; Borda, M. Comment on “Pyrite Dissolution in Acidic Media” by M. Descostes, P. Vitorge, and C. Beaucaire. Geochim. Cosmochim. Acta 2006, 70, 5246–5250. [Google Scholar] [CrossRef]
- Krouse, H.R.; McCready, R.G.L. Reductive Reactions in the Sulfur Cycle. In Biochemical Cycling of Mineral-Forming Elements, 1st ed.; Trudinger, P., Swaine, D., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1979; Volume 3, pp. 315–368. [Google Scholar]
- Edraki, M.; Golding, S.D.; Baublys, K.A.; Lawrence, M.G. Hydrochemistry, Mineralogy and Sulfur Isotope Geochemistry of Acid Mine Drainage at the Mt. Morgan Mine Environment, Queensland, Australia. Appl. Geochem. 2005, 20, 789–805. [Google Scholar] [CrossRef]
- Canfield, D.; Thamdrup, B. The Production of 34S-Depleted Sulfide during Bacterial Disproportionation of Elemental Sulfur. Science 1994, 266, 1973–1975. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Thamdrup, B.; Vennemann, T.W. Oxygen and Sulfur Isotope Fractionation during Anaerobic Bacterial Disproportionation of Elemental Sulfur. Geochim. Cosmochim. Acta 2001, 65, 1601–1609. [Google Scholar] [CrossRef]
- Balci, N.; Brunner, B.; Turchyn, A.V. Tetrathionate and Elemental Sulfur Shape the Isotope Composition of Sulfate in Acid Mine Drainage. Front. Microbiol. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Müller, I.A.; Brunner, B.; Coleman, M. Isotopic Evidence of the Pivotal Role of Sulfite Oxidation in Shaping the Oxygen Isotope Signature of Sulfate. Chem. Geol. 2013, 354, 186–202. [Google Scholar] [CrossRef]
- Kokh, M.A.; Assayag, N.; Mounic, S.; Cartigny, P.; Gurenko, A.; Pokrovski, G.S. Multiple Sulfur Isotope Fractionation in Hydrothermal Systems in the Presence of Radical Ions and Molecular Sulfur. Geochim. Cosmochim. Acta 2020, 285, 100–128. [Google Scholar] [CrossRef]
- Lloyd, R.M. Oxygen Isotope Behavior in the Sulfate-Water System. J. Geophys. Res. 1968, 73, 6099–6110. [Google Scholar] [CrossRef]
- Chiba, H.; Sakai, H. Oxygen Isotope Exchange Rate between Dissolved Sulfate and Water at Hydrothermal Temperatures. Geochim. Cosmochim. Acta 1985, 49, 993–1000. [Google Scholar] [CrossRef]
- Seal, R.R.; Wandless, G.A. Sulfur Isotope Evidence for Sea-floor Mineralizing Processes at the Bald Mountain and Mount Chase Massive Sulfide Deposits, Northern Maine. In Massive Sulfide Deposits of the Bathurst Mining Camp, New Brunswick, and Northern Maine; Goodfellow, W.D., McCutcheon, S.R., Peter, J.M., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2003; Volume 11, pp. 567–587. [Google Scholar]
- Dole, M.; Lane, G.A.; Rudd, D.P.; Zaukelies, D.A. Isotopic Composition of Atmospheric Oxygen and Nitrogen. Geochim. Cosmochim. Acta 1954, 6, 65–78. [Google Scholar] [CrossRef]
- Kroopnick, P.; Craig, H. Atmospheric Oxygen: Isotopic Composition and Solubility Fractionation. Science 1972, 175, 54–55. [Google Scholar] [CrossRef]
- Van Everdingen, R.O.; Krouse, H.R. Isotope Composition of Sulphates Generated by Bacterial and Abiological Oxidation. Nature 1985, 315, 395–396. [Google Scholar] [CrossRef]
- Epstein, S.; Mayeda, T. Variations in the 18O Content of Waters from Natural Sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- Rose, A.W.; Cravotta, C.A. Geochemistry of Coal Mine Drainage. In Coal Mine Drainage Prediction and Pollution Prevention in Pennsylvania; Brady, K.B.C., Smith, M.W., Schueck, J., Eds.; Pennsylvania Department of Environmental Protection: Harrisburg, PA, USA, 1998; pp. 1.1–1.22. [Google Scholar]
- Gammons, C.H.; Duaime, T.E.; Parker, S.R.; Poulson, S.R.; Kennelly, P. Geochemistry and Stable Isotope Investigation of Acid Mine Drainage Associated with Abandoned Coal Mines in Central Montana, USA. Chem. Geol. 2010, 269, 100–112. [Google Scholar] [CrossRef]
- Salifu, M.; Aiglsperger, T.; Mörth, C.-M.; Alakangas, L. Stable Sulphur and Oxygen Isotopes as Indicators of Sulphide Oxidation Reaction Pathways and Historical Environmental Conditions in a Cu–W–F Skarn Tailings Piles, South-Central Sweden. Appl. Geochem. 2019, 110, 104426. [Google Scholar] [CrossRef]
- Butler, T.W. Isotope Geochemistry of Drainage from an Acid Mine Impaired Watershed, Oakland, California. Appl. Geochem. 2007, 22, 1416–1426. [Google Scholar] [CrossRef]
- Qureshi, R.M. The Isotopic Composition of Aqueous Sulfate (A Laboratory Investigation). Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 1986. [Google Scholar]
- Sasaki, K.; Tsunekawa, M.; Ohtsuka, T.; Konno, H. Confirmation of a Sulfur-rich Layer on Pyrite after Oxidative Dissolution by Fe(III) Ions around pH2. Geochim. Cosmochim. Acta 1995, 59, 3155–3158. [Google Scholar] [CrossRef]
- Krouse, H.R.; Gould, W.D.; McCready, R.G.L.; Rajan, S. 18O Incorporation into Sulphate during the Bacterial Oxidation of Sulphide Minerals and the Potential for Oxygen Isotope Exchange between O2, H2O and Oxidized Sulphur Intermediates. Earth Planet. Sci. Lett. 1991, 107, 90–94. [Google Scholar] [CrossRef]
- Holt, B.D.; Kumar, R.; Cunningham, P.T. Oxygen-18 Study of the Aqueous-Phase of Sulfur Dioxide. Atmos. Environ. 1981, 15, 557–566. [Google Scholar] [CrossRef]
- Viers, J.; Grande Gil, J.A.; Zouiten, C.; Freydier, R.; Masbou, J.; Valente, T.; de la Torre, M.L.; Destrigneville, C.; Pokrovsky, O.S. Are Cu Isotopes a Useful Tool to Trace Metal Sources and Processes in Acid Mine Drainage (AMD) Context? Chemosphere 2018, 193, 1071–1079. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guo, Y.; Williams, R.J.P.; O’Nions, R.K.; Matthews, A.; Belshaw, N.S.; Salvato, B. Mass Fractionation Processes of Transition Metal Isotopes. Earth Planet. Sci. Lett. 2002, 200, 47–62. [Google Scholar] [CrossRef]
- Ehrlich, S.; Butler, I.; Halicz, L.; Rickard, D.; Oldroyd, A.; Matthews, A. Experimental Study of the Copper Isotope Fractionation between Aqueous Cu(II) and Covellite, CuS. Chem. Geol. 2004, 209, 259–269. [Google Scholar] [CrossRef]
- Fantle, M.S.; De Paolo, D.J. Iron Isotopic Fractionation during Continental Weathering. Earth Planet. Sci. Lett. 2004, 228, 547–562. [Google Scholar] [CrossRef]
- Borrok, D.M.; Nimick, D.A.; Wanty, R.B.; Ridley, W.I. Isotopic Variations of Dissolved Copper and Zinc in Stream Waters Affected by Historical Mining. Geochim. Cosmochim. Acta 2008, 72, 329–344. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Forstner, U. Binding and Mobilization of Heavy Metals in Contaminated Sediments Affected by PH and Redox Potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar] [CrossRef]
- Bourg, A.C.M.; Loch, J.P.G. Mobilization of Heavy Metals as Affected by pH and Redox Conditions. In Biogeodynamics of Pollutants in Soils and Sediments: Risk Assessment of Delayed and Non-Linear Responses; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 87–102. [Google Scholar]
- Schott, J.; Pokrovsky, O.S.; Oelkers, E.H. The Link between Mineral Dissolution/Precipitation Kinetics and Solution Chemistry. Rev. Mineral. Geochem. 2009, 70, 207–258. [Google Scholar] [CrossRef]
- Wiederhold, J. Iron Isotope Fractionation in Soils from Phenomena to Process Identification. Ph.D. Thesis, Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland, 2006. [Google Scholar]
- Matthews, A.; Zhu, X.K.; O’Nions, K. Kinetic iron stable isotope fractionation between iron (-II) and (-III) complexes in solution. Earth Planet. Sci. Lett. 2001, 192, 81–92. [Google Scholar] [CrossRef]
- Welch, S.A.; Beard, B.L.; Johnson, C.M.; Braterman, P.S. Kinetic and equilibrium Fe isotope fractionation between aqueous Fe(II) and Fe(III). Geochim. Cosmochim. Acta. 2003, 67, 4231–4250. [Google Scholar] [CrossRef]
- Johnson, C.M.; Skulan, J.L.; Beard, B.L.; Sun, H.; Nealson, K.H.; Braterman, P.S. Isotopic fractionation between Fe(III) and Fe(II) in aqueous solutions. Earth Planet. Sci. Lett. 2002, 195, 141–153. [Google Scholar] [CrossRef]
- Anbar, A.D.; Jarzecki, A.A.; Spiro, T.G. Theoretical investigation of iron isotope fractionation between Fe(H2O)63+ and Fe(H2O)62+: Implications for iron stable isotope geochemistry. Geochim. Cosmochim. Acta 2005, 69, 825–837. [Google Scholar] [CrossRef]
- Skulan, J.L.; Beard, B.L.; Johnson, C.M. Kinetic and equilibrium Fe isotope fractionation between aqueous Fe(III) and hematite. Geochim. Cosmochim. Acta 2002, 66, 2995–3015. [Google Scholar] [CrossRef]
- Fernandez, A.; Borrok, D.M. Fractionation of Cu, Fe, and Zn Isotopes during the Oxidative Weathering of Sulfide-Rich Rocks. Chem. Geol. 2009, 264, 1–12. [Google Scholar] [CrossRef]
- Rodríguez, N.P.; Engström, E.; Rodushkin, I.; Nason, P.; Alakangas, L.; Öhlander, B. Copper and Iron Isotope Fractionation in Mine Tailings at the Laver and Kristineberg Mines, Northern Sweden. Appl. Geochem. 2013, 32, 204–215. [Google Scholar] [CrossRef]
- Balci, N.; Bullen, T.D.; Witte-Lien, K.; Shanks, W.C.; Motelica, M.; Mandernack, K.W. Iron Isotope Fractionation during Microbially Stimulated Fe(II) Oxidation and Fe(III) Precipitation. Geochim. Cosmochim. Acta 2006, 70, 622–639. [Google Scholar] [CrossRef]
- Brantley, S.L.; Liermann, L.; Bullen, T. Fractionation of Fe Isotopes by Soil Microbes and Organic Acids. Geology 2001, 29, 535–538. [Google Scholar] [CrossRef]
- Beard, B.L.; Johnson, C.M.; Skulan, J.L.; Nealson, K.H.; Cox, L.; Sun, H. Application of Fe Isotopes to Tracing the Geochemical and Biological Cycling of Fe. Chem. Geol. 2003, 195, 87–117. [Google Scholar] [CrossRef]
- Mathur, R.; Ruiz, J.; Titley, S.; Liermann, L.; Buss, H.; Brantley, S. Cu Isotopic Fractionation in the Supergene Environment with and without Bacteria. Geochim. Cosmochim. Acta 2005, 69, 5233–5246. [Google Scholar] [CrossRef]
- Johnson, C.M.; Beard, B.L.; Roden, E.E. The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annu. Rev. Earth Planet. Sci. 2008, 36, 457–493. [Google Scholar] [CrossRef]
- Rodríguez, N.P.; Khoshkhoo, M.; Sandström, Å.; Rodushkin, I.; Alakangas, L.; Öhlander, B. Isotopic Signature of Cu and Fe during Bioleaching and Electrochemical Leaching of a Chalcopyrite Concentrate. Int. J. Miner. Process. 2015, 134, 58–65. [Google Scholar] [CrossRef]
- Falco, L.; Pogliani, C.; Curutchet, G.; Donati, E. A Comparison of Bioleaching of Covellite Using Pure Cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a Mixed Culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2003, 71, 31–36. [Google Scholar] [CrossRef]
- Garcia, O.; Bigham, J.M.; Tuovinen, O.H. Oxidation of Isochemical FeS2 (Marcasite-Pyrite) by Acidithiobacillus Thiooxidans and Acidithiobacillus ferrooxidans. Miner. Eng. 2007, 20, 98–101. [Google Scholar] [CrossRef]
- Tributsch, H. Direct versus indirect bioleaching. Hydrometallurgy 2001, 59, 177–185. [Google Scholar] [CrossRef]
- Natarajan, K.A. Microbial Aspects of Acid Mine Drainage and Its Bioremediation. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2008, 18, 1352–1360. [Google Scholar] [CrossRef]
- Brantley, S.L.; Liermann, L.J.; Guynn, R.L.; Anbar, A.; Icopini, G.A.; Barling, J. Fe Isotopic Fractionation during Mineral Dissolution with and without Bacteria. Geochim. Cosmochim. Acta 2004, 68, 3189–3204. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Anbar, A.D.; Liermann, L.J.; Mathur, R.; Gordon, G.W.; Brantley, S.L. Isotope Fractionation during Microbial Metal Uptake Measured by MC-ICP-MS. J. Anal. At. Spectrom. 2007, 22, 905. [Google Scholar] [CrossRef]
- Croal, L.R.; Johnson, C.M.; Beard, B.L.; Newman, D.K. Iron isotope fractionation by Fe(II)-oxidizing photoautotrophic bacteria 1. Geochim. Cosmochim. Acta 2004, 68, 1227–1242. [Google Scholar] [CrossRef]
- Hubbard, C.G.; Black, S.; Coleman, M.L. Aqueous Geochemistry and Oxygen Isotope Compositions of Acid Mine Drainage from the Río Tinto, SW Spain, Highlight Inconsistencies in Current Models. Chem. Geol. 2009, 265, 321–334. [Google Scholar] [CrossRef]
- Malmström, M.E.; Destouni, G.; Banwart, S.A.; Strömberg, B.H.E. Resolving the Scale-Dependence of Mineral Weathering Rates. Environ. Sci. Technol. 2000, 34, 1375–1378. [Google Scholar] [CrossRef]
- Liu, R.; Wolfe, A.L.; Dzombak, D.A.; Stewart, B.W.; Capo, R.C. Comparison of Dissolution under Oxic Acid Drainage Conditions for Eight Sedimentary and Hydrothermal Pyrite Samples. Environ. Geol. 2008, 56, 171–182. [Google Scholar] [CrossRef]
- Matthies, R.; Aplin, A.C.; Boyce, A.J.; Jarvis, A.P. Geochemical and Stable Isotopic Constraints on the Generation and Passive Treatment of Acidic, Fe–SO4 Rich Waters. Sci. Total Environ. 2012, 420, 238–249. [Google Scholar] [CrossRef]
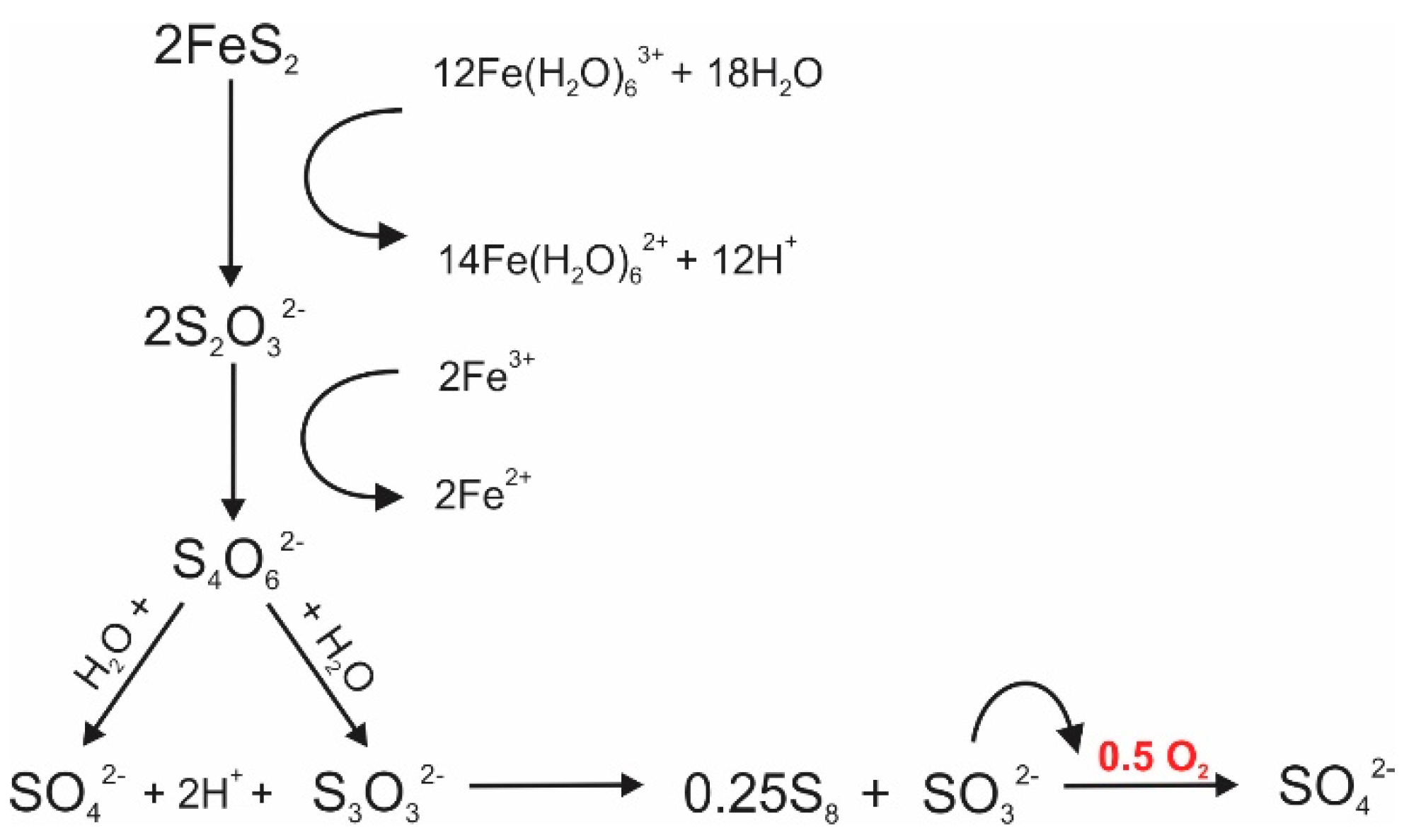
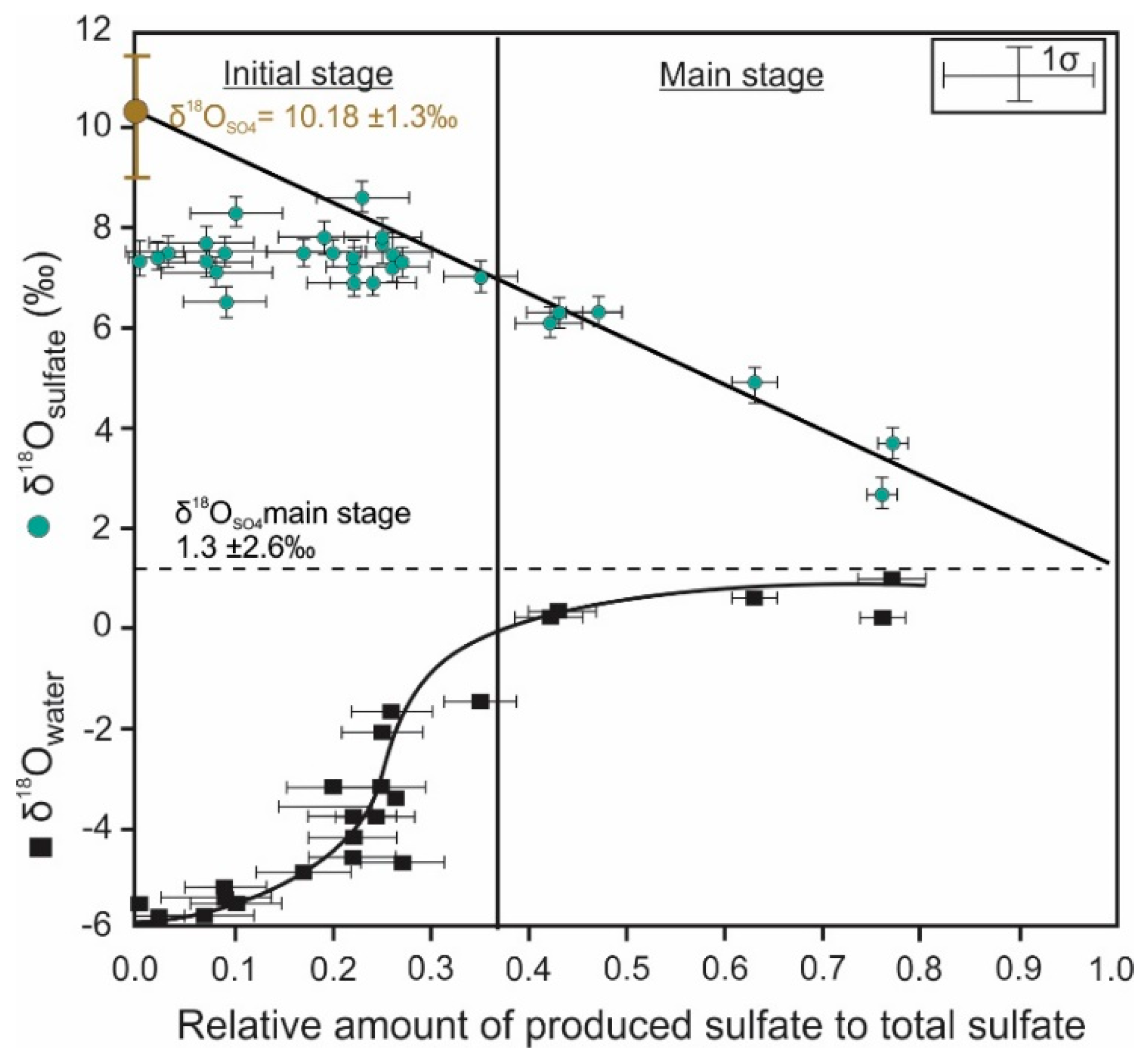
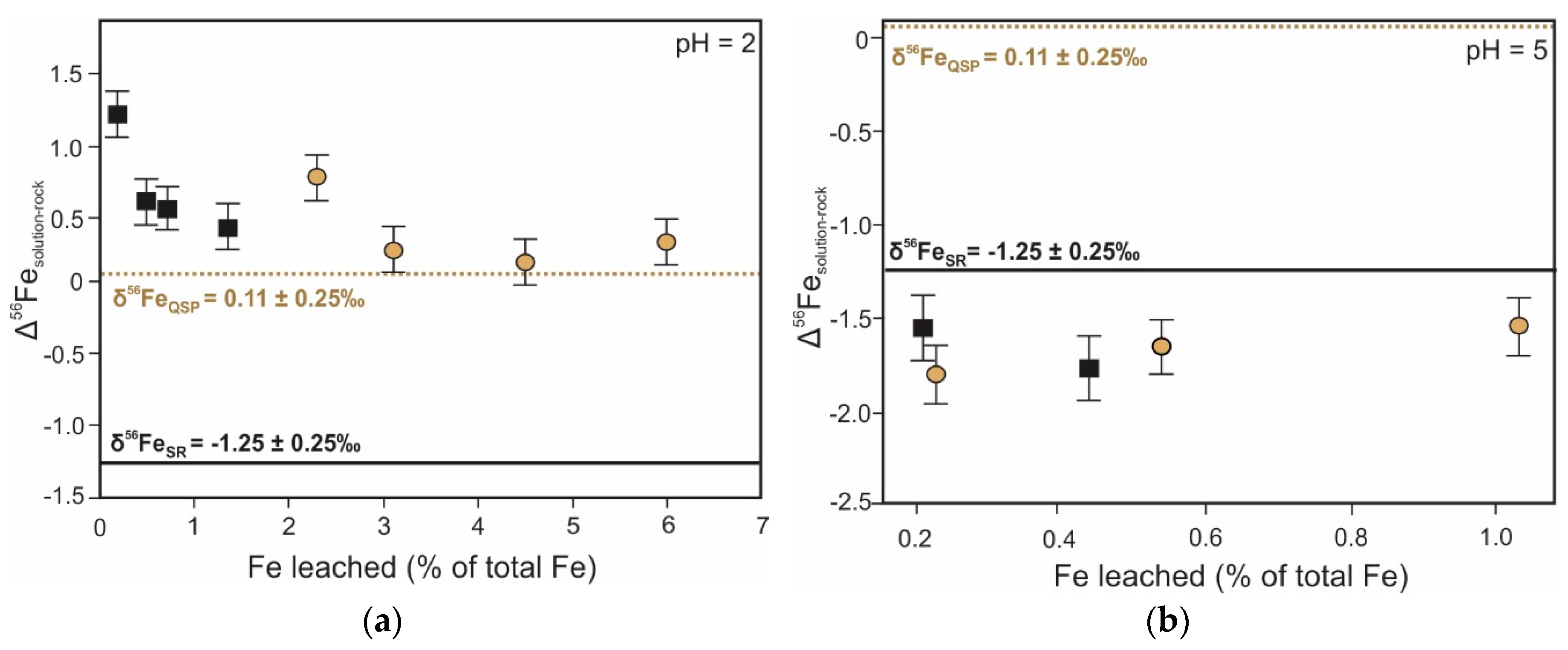
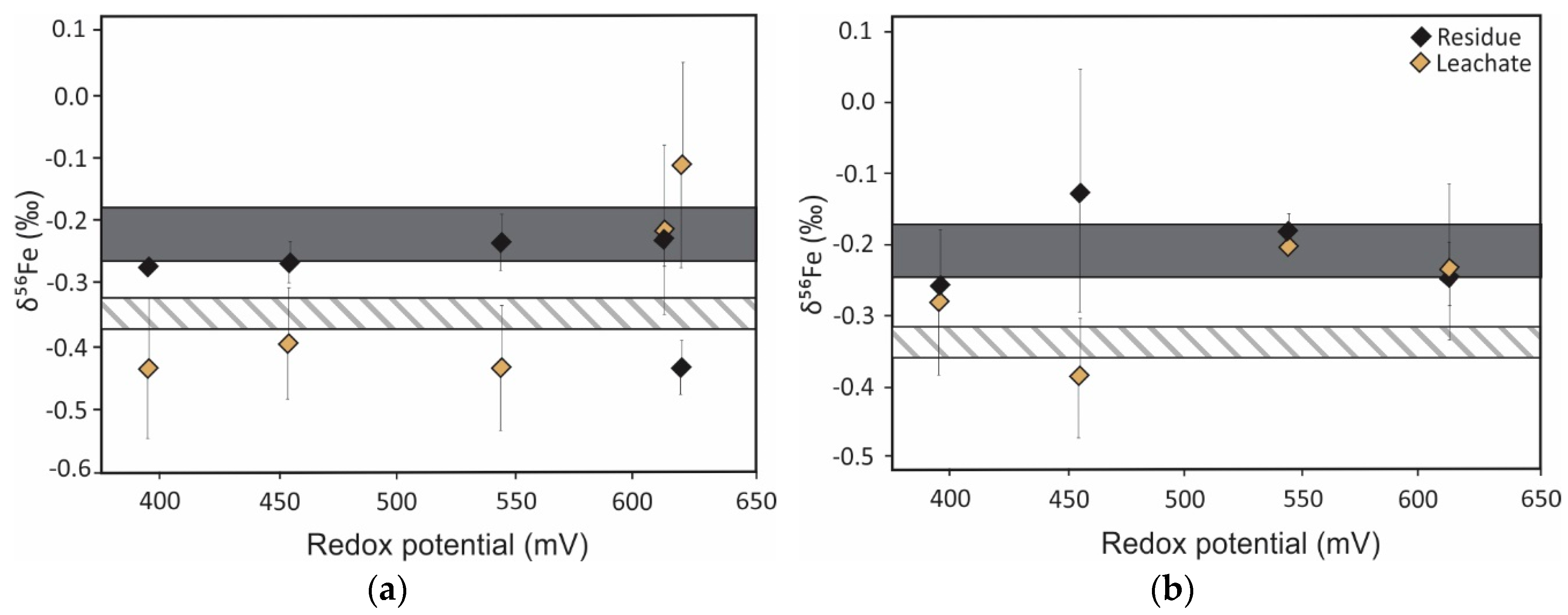
| Isotope | Process | Isotopic Signature of the Process | Role in ARD Characterization | Notes |
|---|---|---|---|---|
| Sulfur | Quantitative conversion of pyrite to sulfate | Fractionation ranges between −1.3 ‰ and +0.4 ‰ [63,70,71,72,73,74] | Allows the identification of pyrite as the source of sulfur | Inconsistent sulfate δ34S values during pyrite dissolution due to SO2 degassing [78] |
| Stepwise or incomplete conversion of pyrite to sulfate | Degree of fractionation varies with the oxidation state of sulfur [72,74,97] | Provide information about the pathways of sulfur oxidation or reduction | 34S enrichment follows the general trend: SO42− > SO32− > S2O32− > S0 > S2− [96] | |
| Oxygen | Quantitative conversion of pyrite to sulfate | Sulfate δ18O mainly depends on the relative contribution of oxygen sources [68,109,110,111,112] | Indicative of the dominant reaction mechanism that is responsible for pyrite oxidation | Inconsistent sulfate δ18O values during pyrite dissolution due to prolonged oxygen isotope equilibration [78] |
| Stepwise or incomplete conversion of pyrite to sulfate | Sulfate δ18O is influenced by the presence of sulfur intermediates [61] | Provide insights into intermediate mechanisms that control pyrite oxidation | Oxygen exchange kinetics between sulfur oxyanions and water varies with the oxidation state of sulfur [146] | |
| Iron | Oxidative dissolution of pyrite | Fractionation between Fe2+FeS2 and Fe3+precipitate ranges from −1.7 ‰ to 3.0 ‰ [128,129,131] 1 | Provide insights into source and (bio)geochemical cycling of iron | Inconsistent solution δ56Fe values at pH 2 due to dissolution of air oxidized layer [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ódri, Á.; Becker, M.; Broadhurst, J.; Harrison, S.T.L.; Edraki, M. Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization. Minerals 2020, 10, 982. https://doi.org/10.3390/min10110982
Ódri Á, Becker M, Broadhurst J, Harrison STL, Edraki M. Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization. Minerals. 2020; 10(11):982. https://doi.org/10.3390/min10110982
Chicago/Turabian StyleÓdri, Ágnes, Megan Becker, Jennifer Broadhurst, Susan T. L. Harrison, and Mansour Edraki. 2020. "Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization" Minerals 10, no. 11: 982. https://doi.org/10.3390/min10110982
APA StyleÓdri, Á., Becker, M., Broadhurst, J., Harrison, S. T. L., & Edraki, M. (2020). Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization. Minerals, 10(11), 982. https://doi.org/10.3390/min10110982








