Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications
Abstract
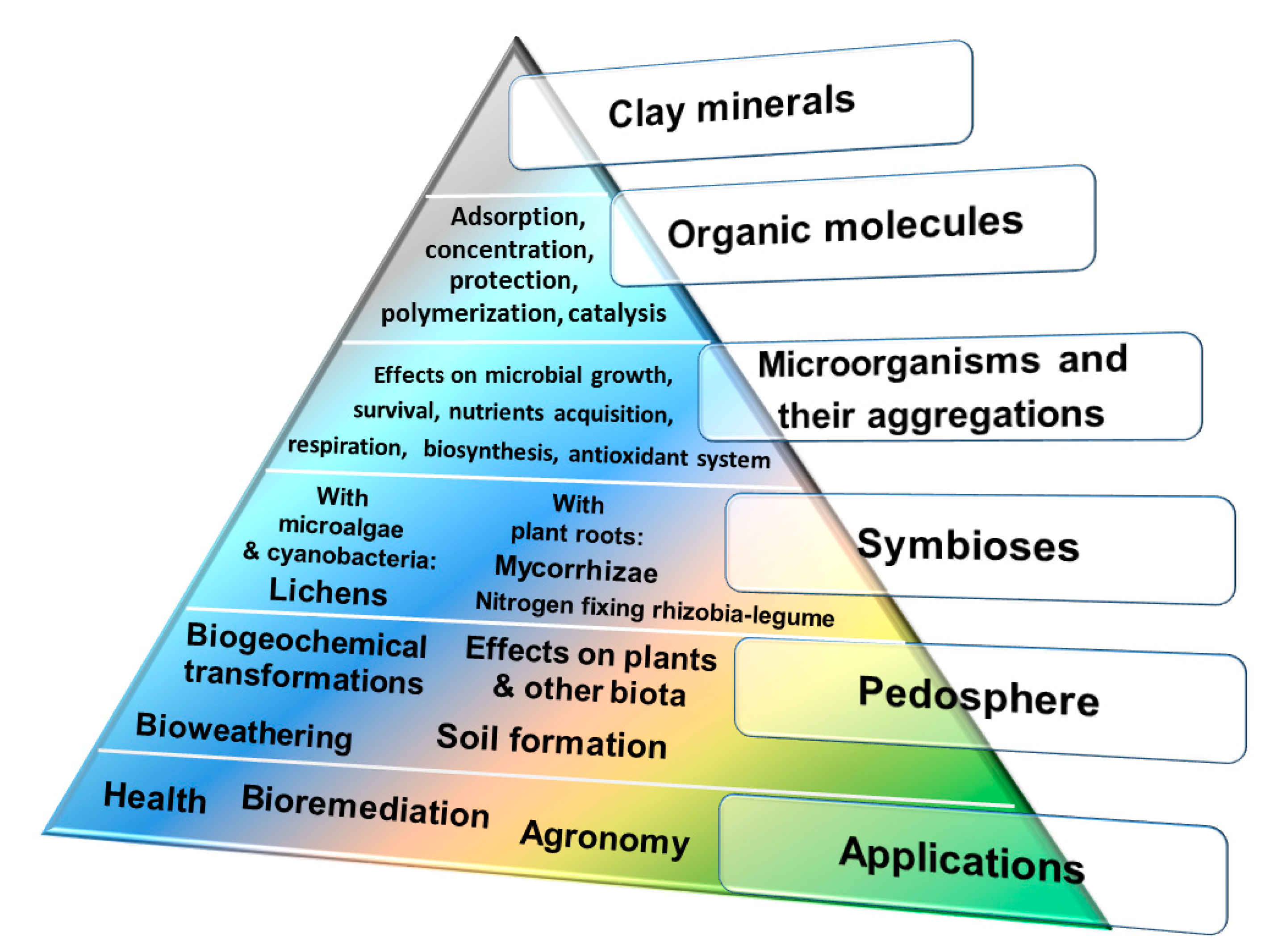
1. Introduction
2. Co-Occurrence of Clays and Microorganisms in Nature
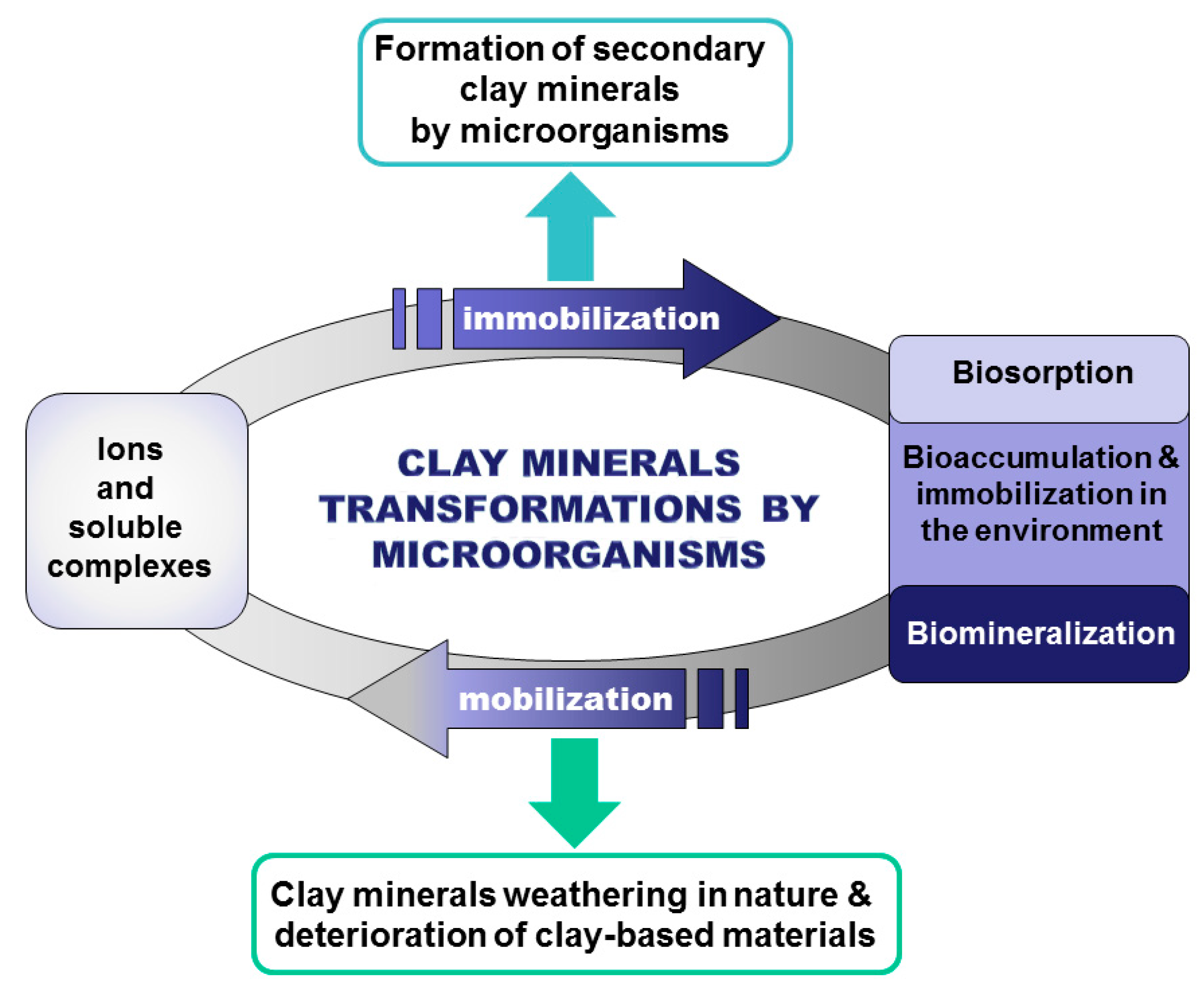
3. Biogeochemical Transformations of Clay Minerals by Microbes
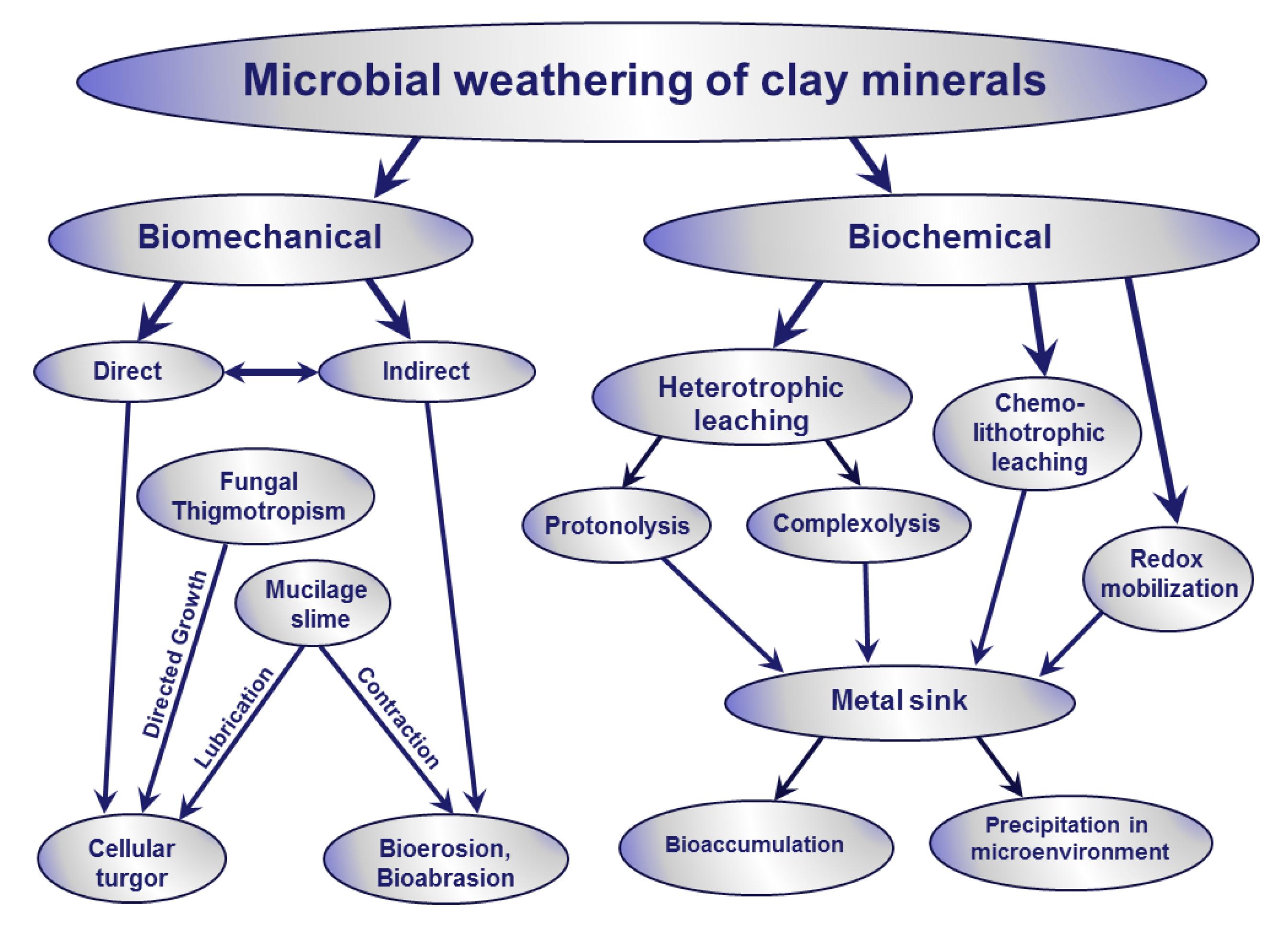
3.1. Microbial Weathering in Clay Minerals and Silicate Transformations
3.2. The Processes of Immobilization and Secondary Clay Minerals’ Formation by Microbes
4. Biological Effects of Clay Minerals on Microbial Growth and Biosynthetic Activity
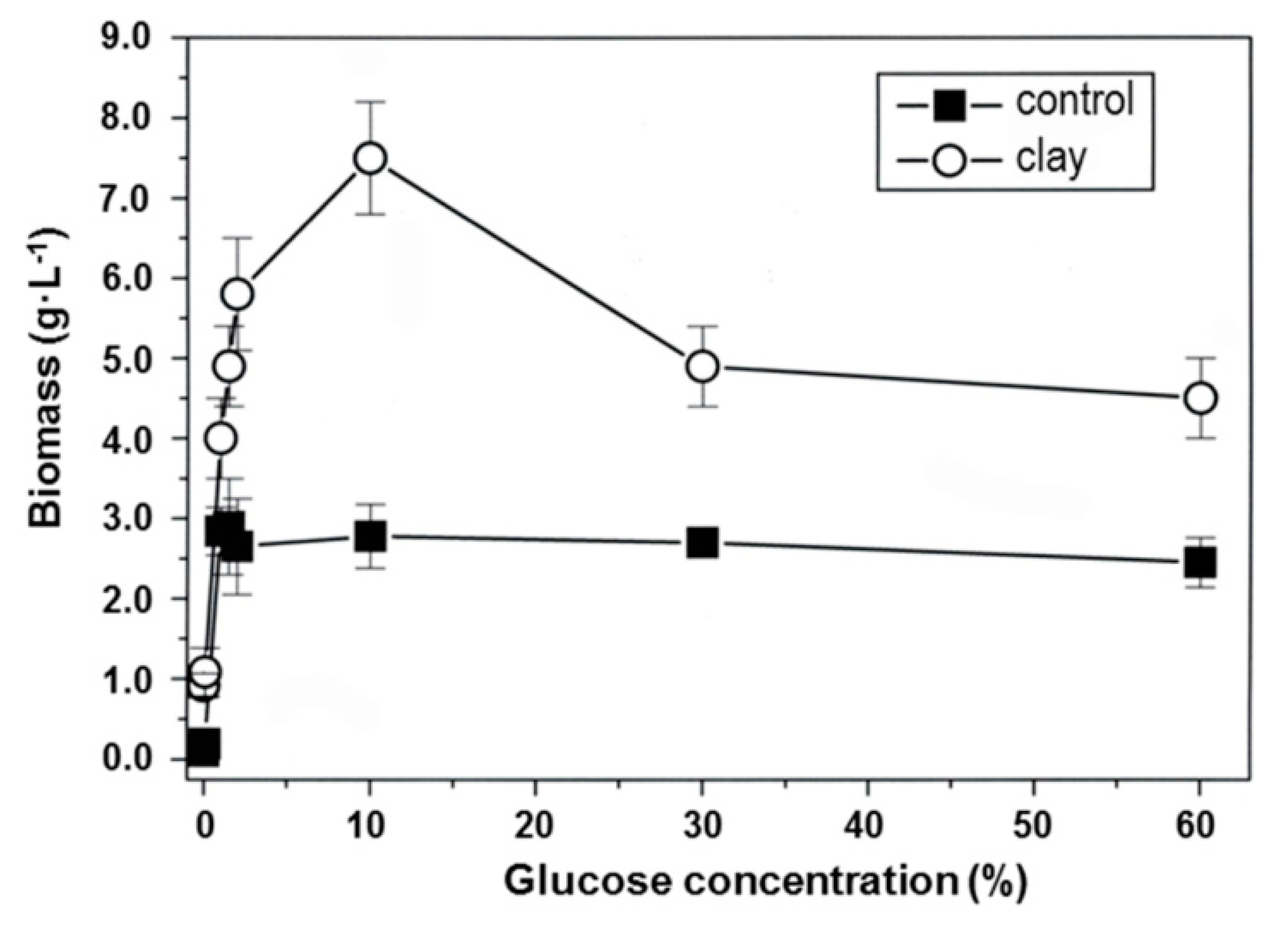
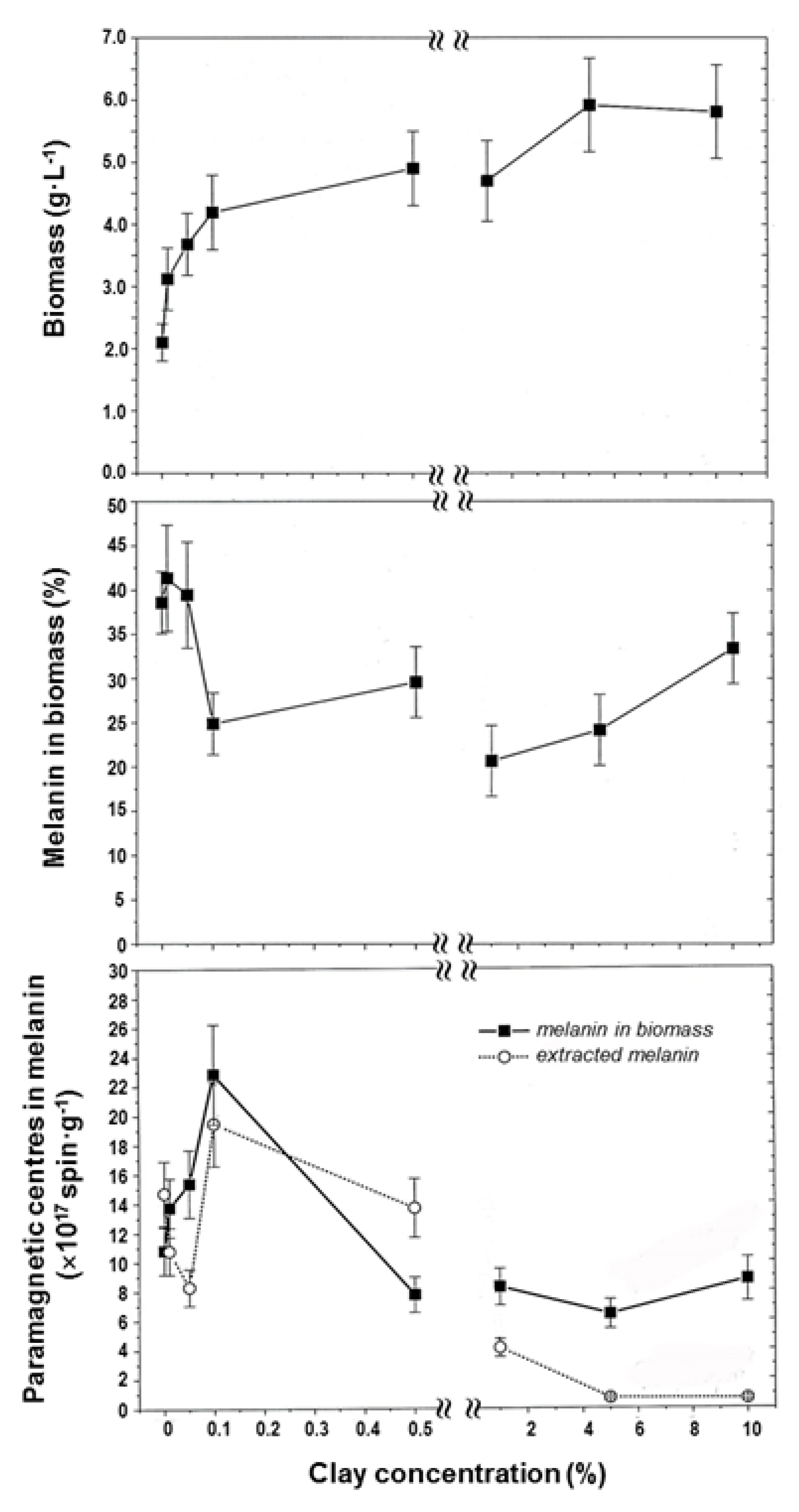
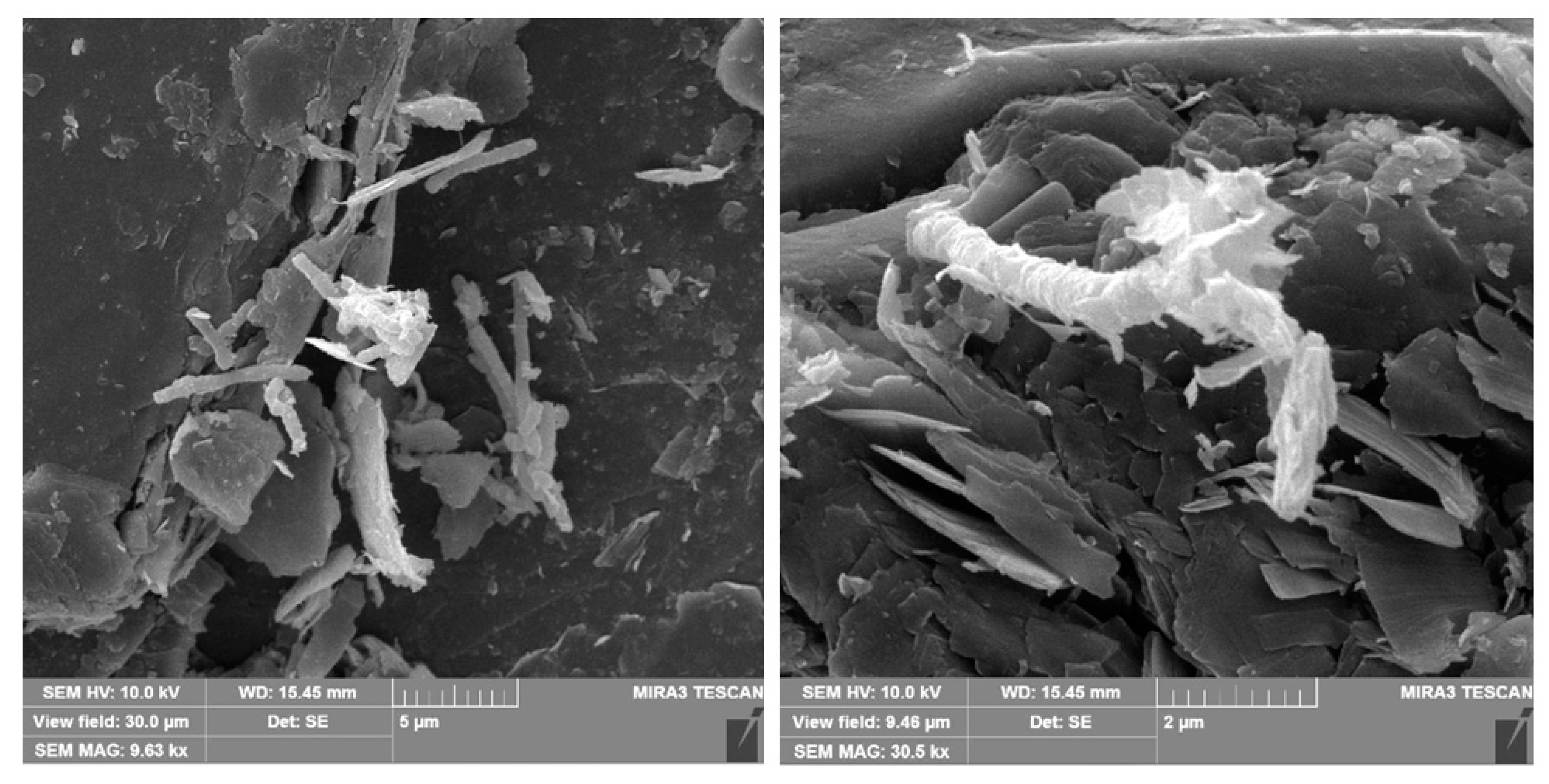
4.1. Clay Mineral Effects on Melanin-Producing Fungi
4.2. Influence of Clay Minerals on Plant Growth-Promoting Rhizobacteria (PGPR)
4.3. Interactions between Clay Minerals, Viruses and Microorganisms
5. Applied and Biotechnological Aspects of Microbial Interactions with Clay Minerals
5.1. Clay–Microbial Interactions in Human and Animal Health
5.2. Applications of Clay Minerals in Microbial Bioremediation and Biodegradation
5.3. Microbial–Clay Interactions in Agronomic Biotechnology
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA and CMS nomenclature committees. Clay Miner. 1995, 30, 257–259. [Google Scholar] [CrossRef]
- Bibi, I.; Ikenhower, J.; Niazi, N.K.; Naz, T.; Shahid, M.; Bashir, S. Clay minerals: Structure, chemistry, and significance in contaminated environments and geological CO2 sequestration. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; Chapter 21; pp. 543–567. [Google Scholar] [CrossRef]
- Johnston, C.T. Probing the nanoscale architecture of clay minerals. Clay Miner. 2010, 45, 245–279. [Google Scholar] [CrossRef]
- Schulze, D. An Introduction to Soil Mineralogy. In Minerals in Soil Environments, 2nd ed.; Dixon, J., Weed, S., Eds.; Soil Science Society of America: Madison, WI, USA, 1989. [Google Scholar]
- Amonette, J.; Zelazny, L. Handbook of Soil Science; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Murray, H. Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays. In Applied Clay Mineralogy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2. [Google Scholar]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Al-Ani, T.; Sarapaa, O. Clay and clay mineralogy. Physical-chemical properties and industrial uses. Geolog. Surv. Finland. 2008, 1, 3–4. [Google Scholar]
- Drits, V.A.; Sokolova, G.V. Structure of palygorskite. Sov. Phys. Crystallogr. 1971, 16, 183–185. [Google Scholar]
- Guggenheim, S.; Krekeler, M. The structures and microtextures of the palygorskite-sepiolite group minerals. In Developments in Palygorskite-Sepiolite Research, 1st ed.; Singer, A., Galan, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 3–32. [Google Scholar]
- Yuan, P.; Thill, A.; Bergaya, F. Halloysite and Imogolite. In Nanosized Tubular Clay Mineral, 1st ed.; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, p. 778. [Google Scholar]
- Wang, W.; Wang, A. Nanoscale Clay Minerals for Functional Ecomaterials: Fabrication, Applications, and Future Trends. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2019; pp. 2409–2490. [Google Scholar] [CrossRef]
- Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 2017, 52, 235–261. [Google Scholar] [CrossRef]
- Bernal, J. The Physical Basis of Life; Routledge and Kegan Paul: London, UK, 1951. [Google Scholar]
- McSween, H.; Richardson, S.; Uhle, M. Geochemistry: Pathways and Processes, 2nd ed.; Columbia University Press: New York, NY, USA, 2003. [Google Scholar]
- Balland, C.; Poszwa, A.; Leyval, C.; Mustin, C. Dissolution rates of phyllosilicates as a function of bacterial metabolic diversity. Geochim. Cosmochim. Acta 2010, 74, 5478–5493. [Google Scholar] [CrossRef]
- Hazen, R.; Downs, R.; Jones, A.; Kah, L.; Oganov, A. The mineralogy of carbon. In Carbon in Earth; Hazen, Baross, J., Hemley, R., Jones, A., Eds.; Mineralogical Society of America: Chantilly, Virginia, 2013. [Google Scholar]
- Golden, J.; McMillan, M.; Downs, R.T.; Hystad, G.; Goldstein, I.; Stein, H.J.; Zimmerman, A.; Sverjensky, D.A.; Armstrong, J.; Hazen, R.M. Rhenium variations in molybdenite (MoS2): Evidence for progressive subsurface oxidation. Earth Planet. Sci. Lett. 2013, 366, 1–5. [Google Scholar] [CrossRef]
- Elmore, S.C. Clay Mineral Evolution. Master’s Thesis, George Mason University, Fairfax, Virginia, 2009. [Google Scholar]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Hazen, R.M.; Ewing, R.C.; Sverjensky, D.A. Evolution of uranium and thorium minerals. Am. Mineral. 2009, 94, 1293–1311. [Google Scholar] [CrossRef]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Droser, M.; Mayer, L.M.; Pevear, D.; Mrofka, D. Late Precambrian oxygenation: Inception of the clay mineral factory. Science 2006, 311, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Burford, E.P.; Fomina, M.; Gadd, G.M. Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineral. Mag. 2003, 67, 1127–1155. [Google Scholar] [CrossRef]
- Tazaki, K. Clays, Microorganisms, and Biomineralization In Handbook of Clay Science, Developments in Clay Science, 1st ed.; Bergaya, F., Theng, B., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 477–497. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of Mineral Surfaces in Prebiotic Chemical Evolution. In Silico Quantum Mechanical Studies. Life 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Negron-Mendoza, A.; Ramos-Bernal, S. The role of clays in the origin of life. In Origins. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer (Kluwer Academic Publishers): Dordrecht, The Netherlands, 2004; Volume 3, pp. 181–194. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. The Origin of life and the nature of the primitive gene. J. Theor. Biol. 1966, 10, 53–88. [Google Scholar] [CrossRef]
- Swartzen-Allen, S.L.; Matijevic, E. Surface and colloid chemistry. Chem. Rev. 1974, 74, 385–400. [Google Scholar] [CrossRef]
- Weiss, A. Organic derivatives of clay minerals, zeolites and related minerals. In Organic Geochemistry; Eglinton, G., Murphy, M., Eds.; Springer: New York, NY, USA, 1969; pp. 737–781. [Google Scholar]
- Theng, B. The Chemistry of Clay-Organic Reactions; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Mortland, M.M. Clay-organic complexes and interactions. Adv. Agron. 1970, 22, 75–117. [Google Scholar] [CrossRef]
- Hsu, S.-C. The Adsorption of Peptides on Montmorillonite. Ph.D. Thesis, Polytechnic Institute of New York, Brooklyn, NY, USA, 1977. [Google Scholar]
- Guzmán, A.; Ramos-Bernal, S.; Negrón-Mendoza, A. Irradiation of adenine adsorbed in Na-montmorillonite. Implication to chemical evolution studies. In Astrobiology: Origins from the Big Bang to Civilization; Chela-Flores, J., Lemarchand, G.J., Oró, J., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2000; pp. 271–273. [Google Scholar]
- Gilbert, W. Origin of life: The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Miyakawa, S.; Ferris, J.P. Sequence-and Regioselectivity in the Montmorillonite-Catalyzed Synthesis of RNA. J. Am. Chem. Soc. 2003, 125, 8202–8208. [Google Scholar] [CrossRef]
- Miyakawa, S.; Joshi, P.C.; Gaffey, M.J.; Gonzalez-Toril, E.; Hyland, C.; Ross, T.; Rybij, K.; Ferris, J.P. Studies in the Mineral and Salt-Catalyzed Formation of RNA Oligomers. Orig. Life Evol. Biosph. 2006, 36, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-F. Origins of life: From the mineral to the biochemical world. BIO Web Conf. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H. Role of Clay Minerals in Chemical Evolution and the Origins of Life. In Clay Minerals in Nature–Their Characterization, Modification and Application; Valaskova, M., Martynkova, G., Eds.; Intech Open Limited: London, UK, 2012; Chapter 10; pp. 191–208. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interaction between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Miner. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Schaetzl, R.; Thompson, M. Soils: Genesis and Geomorphology, 2nd ed.; Cambridge University Press: New York, NY, USA, 2015. [Google Scholar]
- Belnap, J.; Büdel, B.; Lange, O. Biological soil crusts: characteristics and distribution. In Biological Soil Crusts: Structure, Function, and Management; Belnap, J., Lange, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 150, pp. 3–30. [Google Scholar] [CrossRef]
- Belnap, J. The world at your feet: Desert biological soil crusts. Front. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Dröge, M.; Pühler, A.W.; Selbitschka, W. Horizontal gene transfer among bacteria in terrestrial and aquatic habitats as assessed by microcosm and field studies. Biol. Fertil. Soils 1999, 29, 221–245. [Google Scholar]
- Kelleher, B.P.; Simpson, A.J. Humic Substances in Soils: Are They Really Chemically Distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar]
- Jackson, T. Effects of clay minerals, oxyhydroxides and humic matter on the microbial communities of soil, sedimets and water. In Environmental Impacts of Soil Component Interactions: Metals, Other Inorganics, and Microbial Activities; Huang, P., Berthelin, J., Bollag, J.-M., McGill, W., Page, A., Huang, P., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 165–200. [Google Scholar]
- Lorenz, M.; Wackernagel, W. DNA Binding to Various Clay Minerals and Retarded Enzymatic Degradation of DNA in a Sand/Clay Microcosm. In Gene Transfers and Environment; Gauthier, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 103–113. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Q.Y.; Zhang, X.W. Interactions of DNA with Clay Minerals and Soil Colloidal Particles and Protection against Degradation by DNase. Environ. Sci. Technol. 2006, 40, 2971–2976. [Google Scholar] [CrossRef]
- Heberling, C.; Lowell, R.P.; Liu, L.; Fisk, M.R. Extent of the microbial biosphere in the oceanic crust. Geochem. Geophys. Geosyst. 2010, 11, 1–15. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Mancinelli, R. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Fomina, M.; Podgorsky, V.S.; Olishevska, S.V.; Kadoshnikov, V.M.; Pisanska, I.R.; Hiller, S.; Gadd, G.M. Fungal deterioration of barrier concrete used in nuclear waste disposal. Geomicrobiol. J. 2007, 24, 643–653. [Google Scholar] [CrossRef]
- Coutinho, M.L.; Miller, A.Z.; Macedo, M.F. Biological Colonization and Biodeterioration of Architectural Ceramic Materials: An Overview. J. Cult. Herit. 2015, 16, 759–777. [Google Scholar] [CrossRef]
- Drake, H.; Varsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K. Fungi as geologic agents. Geomicrobiol. J. 2000, 17, 97–124. [Google Scholar] [CrossRef]
- Gadd, G.; Burford, E.; Fomina, M.; Melville, K. Mineral transformations and biogeochemical cycles: A geomycological perspective. In Fungi in the Environment; Gadd, G., Dyer, P., Watkinson, S., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 78–111. [Google Scholar]
- Fomina, M.; Burford, E.; Gadd, G.M. Toxic metals and fungal communities. In the Fungal Community. Its Organization and Role in the Ecosystem; Dighton, J., White, J., Oudemans, P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 733–758. [Google Scholar]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.H.; Jellison, J. Calcium translocation, calcium oxalate accumulation, and hyphal sheath morphology in the white rot fungus Resinicium bicolor. Can. J. Bot. 1995, 73, 927–936. [Google Scholar] [CrossRef]
- Gutierrez, A.; Martinez, M.J.; Almendros, G.; Gonzalez-Vila, F.J.; Martinez, A.T. Hyphal-sheath polysaccharides in fungal deterioration. Sci. Total Environ. 1995, 167, 315–328. [Google Scholar] [CrossRef][Green Version]
- Welch, S.A.; Barker, W.W.; Banfield, J.F. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 1999, 63, 1405–1419. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Smith, S.E.; Rengasamy, P. Aggregation of soil by fungal hyphae. Aust. J. Soil. Res. 1997, 35, 55–60. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Prevost, D.; Vezina, L.P.; Chalifour, F.P. Soil aggregation and fungal and bacterial biomass under annual and perennial cropping systems. Soil Sci. Soc. Am. J. 1997, 61, 262–267. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Whitehead, K.; Dornieden, T.; Niesse, A.; Schulte, A.; Hedges, J.I. Black fungal colonies as units of survival: Hyphal mycosporines synthesized by rock-dwelling microcolonial fungi. Can. J. Bot. 2003, 81, 131–138. [Google Scholar] [CrossRef]
- Knabe, N.; Gorbushina, A.A. Territories of Rock-Inhabiting Fungi: Survival on and Alteration of Solid Air-Exposed Surfaces. Methods Microbiol. 2018, 45, 145–169. [Google Scholar] [CrossRef]
- Moshynets, O.; Spiers, A. Viewing biofilms within the larger context of bacterial aggregations. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; IntechOpen: London, UK, 2016; Chapter 1; pp. 3–22. [Google Scholar] [CrossRef][Green Version]
- De los Ríos, A.; Wierzchos, J.; Sancho, L.G.; Ascaso, C. Acid microenvironments in microbial biofilms of antarctic endolithic microecosystems. Environ. Microbiol. 2003, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, J.; Afsin, B.; Jadubansa, P.; Ardakani, M.; Ascaso, C.; Wierzchos, J. Microbial and inorganic control on the composition of clay from volcanic glass alteration experiments. Am. Mineral. 2013, 98, 319–334. [Google Scholar] [CrossRef]
- Cuadros, J.; Afsin, B.; Jadubansa, P.; Ardakani, M.; Ascaso, C.; Wierzchos, J. Pathways of volcanic glass alteration in laboratory experiments through inorganic and microbially-mediated processes. Clay Miner. 2013, 48, 423–445. [Google Scholar] [CrossRef]
- Ransom, B.; Bennett, R.H.; Baerwald, R.; Hulbert, M.H.; Burkett, P.J. In situ conditions and interactions between microbes and minerals in fine-grained marine sediments: A TEM microfabric perspective. Am. Mineral. 1999, 84, 183–192. [Google Scholar] [CrossRef]
- Lünsdorf, H.; Erb, R.W.; Abraham, W.R.; Timmins, K.N. “Clay Hutches”: A novel interaction between bacteria and clay minerals. Environ. Microbiol. 2000, 2, 161–168. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Warr, L.N.; Perdrial, J.N.; Lett, M.-C.; Heinrich-Salmeron, A.; Khodja, M. Clay mineral-enhanced bioremediation of marine oil pollution. Appl. Clay Sci. 2009, 46, 337–345. [Google Scholar] [CrossRef]
- Banfield, J.P.; Barker, W.W.; Welch, S.A.; Taunton, A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: London, UK, 1997. [Google Scholar]
- Chen, J.; Blume, H.-P.; Beyer, L. Weathering of rocks induced by lichen colonization–a review. CATENA 2000, 39, 121–146. [Google Scholar] [CrossRef]
- Meharg, A.A.; Cairney, J.W.G. Co-evolution of mycorrhizal symbionts and their hosts to metal-contaminated environments. Adv. Ecol. Res. 2000, 30, 69–112. [Google Scholar] [CrossRef]
- Lundstrom, U.S.; Van Breemen, N.; Bain, D. The podzolization process. A review. Geoderma 2000, 94, 91–107. [Google Scholar] [CrossRef]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agronom. 2000, 69, 99–151. [Google Scholar]
- Barker, W.; Welch, S.; Banfield, J. Biogeochemical weathering of silicate minerals. In Geomicrobiology: Interactions between Microbes and Minerals, Reviews in Mineralogy; Benfield, J.K., Nealson, H., Eds.; Mineralogical Society of America: Chelsea, MI, USA, 1997; Volume 35, pp. 391–428. [Google Scholar]
- Haas, J.; Purvis, O. Lichen biogeochemistry. In Fungi in Biogeochemical Cycles; Gadd, G., Ed.; Cambridge University Press: Cambridge, UK, 2006; Chapter 15; pp. 344–376. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Viles, H.A.; Gorbushina, A.A. Soiling and microbial colonization on urban roadside limestone: A three year study in Oxford, England. Build. Environ. 2003, 38, 1217–1224. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- May, E. Microbes on building stone for good or bad? Culture 2003, 24, 4–8. [Google Scholar]
- Ehrlich, H. Geomicrobiology, 4th ed.; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Gorbushina, A.A.; Krumbein, W.E.; Hamann, R.; Panina, L.; Soucharjevsky, S.; Wollenzien, U. On the role of black fungi in colour change and biodeterioration of antique marbles. Geomicrobiol. J. 1993, 11, 205–221. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A. Biodeterioration of Stone in Tropical Environments: An Overview; The J. Paul Getty Trust: Madison, WI, USA, 1999. [Google Scholar]
- Verrecchia, E.P. Fungi and Sediments. In Microbial Sediments; Riding, R., Awramik, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 69–75. [Google Scholar]
- Ivarsson, M.; Schnürer, A.; Bengtson, S.; Neubeck, A. Anaerobic fungi: A potential source of biological H2 in the oceanic crust. Front. Microbiol. 2016, 7, 674. [Google Scholar] [CrossRef]
- Scappini, F.; Casadei, F.; Zamboni, R.; Franchi, M.; Gallori, E.; Monti, S. Protective effect of clay minerals on adsorbed nucleic acid against UV radiation: Possible role in the origin of life. Int. J. Astrobiol. 2004, 3, 17–19. [Google Scholar] [CrossRef]
- Alimova, A.; Katz, A.; Steiner, N.; Rudolph, E.; Wei, H.; Steiner, J.C.; Gottlieb, P. Bacteria-clay interaction: Structural changes in smectite induced during biofilm formation. Clays Clay Miner. 2009, 57, 205–212. [Google Scholar] [CrossRef]
- Cuadros, J.; Diaz-Hernandez, J.L.; Sanchez-Navas, A.; Garcia-Casco, A. Role of clay minerals in the formation of atmospheric aggregates of Saharan dust. Atmos. Environ. 2015, 120, 160–172. [Google Scholar] [CrossRef]
- Cuadros, J.; Andrade, G.; Ferreira, T.O.; Partiti, C.S.M.; Cohen, R.; Vidal-Torrado, P. The mangrove reactor: Fast clay transformation and potassium sink. Appl. Clay Sci. 2017, 140, 50–58. [Google Scholar] [CrossRef]
- Ehrlich, H.L. How microbes influence mineral growth and dissolution. Chem. Geol. 1996, 132, 5–9. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Arocena, J.M.; Glowa, K.R.; Massicotte, H.B.; Lavkulich, L. Chemical and mineral composition of ectomycorrhizosphere soils of subalpine fir (Abies lasiocarpa (Hook.) Nutt.) in the AE horizon of a Luvisol. Can. J. Soil. Sci. 1999, 79, 25–35. [Google Scholar] [CrossRef]
- Arocena, J.M.; Zhu, L.P.; Hall, K. Mineral accumulations induced by biological activity on granitic rocks in Qinghai Plateau, China. Earth Surf. Process. Landf. 2003, 28, 1429–1437. [Google Scholar] [CrossRef]
- Arocena, J.M.; Siddique, T.; Thring, R.W.; Kapur, S. Investigation of lichens using molecular techniques and associated mineral accumulations on a basaltic flow in a Mediterranean environment. CATENA 2007, 70, 356–365. [Google Scholar] [CrossRef]
- Pinzari, F.; Cuadros, J.; Napoli, R.; Canfora, L.; Baussà Bardají, D. Routes of phlogopite weathering by three fungal strains. Fungal Biol. 2016, 120, 1582–1599. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G. Metal and mineral transformations: A mycoremediation perspective. In Exploitation of Fungi; Robson, G., Van West, P., Gadd, G., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 236–254. [Google Scholar] [CrossRef]
- Fomina, M.; Bowen, A.D.; Charnock, J.M.; Podgorsky, V.S.; Gadd, G.M. Biogeochemical spatio-temporal transformation of copper in Aspergillus niger colonies grown on malachite with different inorganic nitrogen sources. Environ. Microbiol. 2017, 19, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Hong, J.W.; Gadd, G.M. Effect of depleted uranium on a soil microcosm fungal community and influence of a plant-ectomycorrhizal association. Fungal Biol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomicrobiology of the built environment. Nat. Microbiol. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bergna, H.E. Colloid chemistry of silica–an overview. Adv. Chem. 1994, 234, 1–47. [Google Scholar] [CrossRef]
- Amores, D.R.; Warren, L.A. Identifying when microbe’s biosilicify: The interconnected requirements of acidic pH, colloidal SiO2 and exposed microbial surface. Chem. Geol. 2007, 240, 298–312. [Google Scholar] [CrossRef]
- Burgstaller, W.; Schinner, F. Leaching of metals with fungi. J. Biotechnol. 1993, 27, 91–116. [Google Scholar] [CrossRef]
- Money, N.P. The fungal dining habit–a biomechanical perspective. Mycologist 2004, 18, 71–76. [Google Scholar] [CrossRef]
- Fomina, M.; Burford, E.P.; Hillier, S.; Kierans, M.; Gadd, G.M. Rock-building fungi. Geomicrobiol. J. 2010, 27, 624–629. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Boneville, S.; Morgan, D.J.; Schmalenberger, A.; Bray, A.; Brown, A.; Banwart, S.A.; Benning, L.G. Tree-mycorrhiza symbiosis accelerate mineral weathering: Evidence from nanometer-scale elemental fluxes at the hypha-mineral interface. Geochim. Cosmochim. Acta 2011, 75, 6988–7005. [Google Scholar] [CrossRef]
- Lian, B.; Wang, B.; Pan, M.; Liu, C.; Teng, H.H. Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim. Cosmochim. Acta 2008, 72, 87–98. [Google Scholar] [CrossRef]
- Bowen, A.D.; Davidson, F.A.; Keatch, R.; Gadd, G.M. Induction of contour sensing in Aspergillus niger by stress and its relevance to fungal growth mechanics and hyphal tip structure. Fung. Genet. Biol. 2007, 44, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Watts, H.J.; Very, A.A.; Perera, T.H.S.; Davies, J.M.; Gow, N.A.R. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology 1998, 144, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Krumbein, W.E. Biodeterioration processes on inorganic materials and means of counter measures. Mater. Corros. 1994, 45, 105–113. [Google Scholar]
- Gaylarde, P.; Gaylarde, C. Deterioration of siliceous stone monuments in Latin America: Microorganisms and mechanisms. Corros. Rev. 2004, 22, 395–415. [Google Scholar] [CrossRef]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Sand, W.; Bock, E. Biodeterioration of mineral materials by microorganisms: Biogenic sulphuric and nitric-acid corrosion of concrete and natural stone. Geomicrobiol. J. 1991, 9, 129–138. [Google Scholar] [CrossRef]
- Sand, W.; Bock, E. Biodeterioration of ceramic materials by biogenic acids. Inter. Biodeterior. 1991, 27, 175–183. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar] [CrossRef]
- Devevre, O.; Garbaye, J.; Botton, B. Release of complexing organic acids by rhizosphere fungi as a factor in Norway Spruce yellowing in acidic soils. Mycol. Res. 1996, 100, 1367–1374. [Google Scholar] [CrossRef]
- Dong, H. Clay-microbe interactions and implications for environmental mitigation. Elements 2012, 8, 113–118. [Google Scholar] [CrossRef]
- Strasser, H.; Burgstaller, W.; Schinner, F. High yield production of oxalic acid for metal leaching purposes by Aspergillus niger. FEMS Microbiol. Lett. 1994, 119, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Drever, J.I.; Stillings, L.L. The role of organic acids in mineral weathering. Coll. Surf. 1997, 120, 167–181. [Google Scholar] [CrossRef]
- Ehrlich, H. Geomicrobiology, 3rd ed.; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Lloyd, J.R.; Lovley, D.R. Microbial detoxification of metals and radionuclides. Curr. Opin. Biotechnol. 2001, 12, 248–253. [Google Scholar] [CrossRef]
- Lloyd, J.R. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Tazaki, K. Clays, Microorganisms and Biomineralization. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 613–653. [Google Scholar] [CrossRef]
- Bennett, P.C.; Hiebert, F.K.; Choi, W.J. Microbial colonization and weathering of silicates in petroleum-contaminated groundwater. Chem. Geol. 1996, 132, 45–53. [Google Scholar] [CrossRef]
- Bennett, P.C.; Rogers, J.A.; Hiebert, F.K.; Choi, W.J. Silicates, silicate weathering, and microbial ecology. Geomicrobiol. J. 2001, 18, 3–19. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Jongmans, A.G.; Van Breemen, N.; Lungstrom, U.; Van Hees, P.A.W.; Finlay, R.D.; Srinivasan, M.; Unestam, T.; Giesler, R.; Melkerud, P.A.; Olsson, M. Rock-eating fungi. Nature 1997, 389, 682–683. [Google Scholar] [CrossRef]
- Van Breemen, N.; Lundstrom, U.S.; Jongmans, A.G. Do plants drive podzolization via rock-eating mycorrhizal fungi? Geoderma 2000, 94, 163–171. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; Van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Cockell, C.S.; Herrera, A. Why are some microorganisms boring? Trends Microbiol. 2008, 16, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Brehm, U.; Gorbushina, A.; Mottershead, D. The role of microorganisms and biofilms in the breakdown and dissolution of quartz and glass. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 117–129. [Google Scholar] [CrossRef]
- Aristovskaya, T. Microbiology of the Processes of Soil Formation; Nauka: Leningrad, Russia, 1980. (In Russian) [Google Scholar]
- Song, W.; Ogawa, N.; Oguchi, C.T.; Hatta, T.; Matsukura, Y. Effect of Bacillus subtilis on granite weathering: A laboratory experiment. CATENA 2007, 70, 275–281. [Google Scholar] [CrossRef]
- Barker, W.W.; Banfield, J.F. Biologically versus inorganically mediated weathering reactions: Relationships between minerals and extracellular microbial polymers in lithobiotic communities. Chem. Geol. 1996, 132, 55–69. [Google Scholar] [CrossRef]
- Barker, W.W.; Welch, S.A.; Chu, S.; Banfield, J.F. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Mineral. 1998, 83, 1551–1563. [Google Scholar] [CrossRef]
- Barker, W.W.; Banfield, J.F. Zones of chemical and physical interaction at interfaces between microbial communities and minerals: a model. Geomicrobiol. J. 1998, 15, 223–244. [Google Scholar] [CrossRef]
- Mehta, A.P.; Torma, A.E.; Murr, L.E. Effect of environmental parameters on the efficiency of biodegradation of basalt rock by fungi. Biotechnol. Bioeng. 1979, 21, 875–885. [Google Scholar] [CrossRef]
- Rossi, G. Potassium recovery through leucite bioleaching: Possibilities and limitations. In Metallurgical Applications of Bacterial Leaching and Related Phenomena; Murr, L., Torma, A., Brierley, J., Eds.; Academic Press: New York, NY, USA, 1979; pp. 279–319. [Google Scholar]
- Callot, G.; Maurette, M.; Pottier, L.; Dubois, A. Biogenic etching of microfractures in amorphous and crystalline silicates. Nature 1987, 328, 147–149. [Google Scholar] [CrossRef]
- Kutuzova, R.S. The release of silica from minerals as a result of microbial activity. Microbiologiya 1969, 38, 714–721. (In Russian) [Google Scholar]
- Webley, D.M.; Henderson, M.E.F.; Taylor, I.F. The microbiology of rocks and weathered stones. J. Soil Sci. 1963, 14, 102–112. [Google Scholar] [CrossRef]
- Henderson, M.E.K.; Duff, R.B. The release of metallic and silicate ions from minerals, rocks and soils by fungal activity. J. Soil Sci. 1963, 14, 236–246. [Google Scholar] [CrossRef]
- Cromack, K., Jr.; Solkins, P.; Grausten, W.C.; Speidel, K.; Todd, A.W.; Spycher, G.; Li, C.Y.; Todd, R.L. Calcium oxalate accumulation and soil weathering in mats of the hypogeous fungus Hysterangium crassum. Soil Biol. Biochem. 1979, 11, 463–468. [Google Scholar] [CrossRef]
- Mandal, S.K.; Roy, A.; Banerjee, P.C. Iron leaching from China clay by fungal strains. Trans. Indian Inst. Met. 2002, 55, 1–7. [Google Scholar]
- De la Torre, M.A.; Gomez-Alarcon, G.; Vizcaino, C.; Garcia, M.T. Biochemical mechanisms of stone alteration carried out by filamentous fungi living on monuments. Biogeochemistry 1993, 19, 129–147. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Chen, J.; Teng, H.H. Cellular dissolution at hypha- and spore-mineral interfaces revealing unrecognized mechanisms and scales of fungal weatering. Geology 2016, 44, 319–322. [Google Scholar] [CrossRef]
- Kuhn, K.M.; DuBois, J.L.; Maurice, P.A. Strategies of aerobic microbial Fe acquisition from Fe-bearing montmorillonite clay. Geochim. Cosmochim. Acta 2013, 117, 191–202. [Google Scholar] [CrossRef]
- Balogh-Brunstad, Z.; Keller, C.K.; Dickinson, J.T.; Stevens, F.; Li, C.Y.; Bormann, B.T. Biotite weathering and nutrient uptake by ectomycorrhizal fungus, Suillus tomentosus, in liquid-culture experiments. Geochim. Cosmochim. Acta 2008, 72, 2601–2618. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Metal sorption by biomass of melanin-producing fungi grown in clay-containing medium. J. Chem.Technol. Biotechnol. 2002, 78, 23–34. [Google Scholar] [CrossRef]
- Sarret, G.; Manceau, A.; Spadini, L.; Roux, J.R.; Hazemann, J.L.; Soldo, Y.; Eybert-BÉrard, L.; Menthonnex, J.-J. Structural determination of Zn and Pb binding sites in Penicillium chrysogenum cell walls by EXAFS spectroscopy. Environ. Sci. Technol. 1998, 32, 1648–1655. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Pearce, C.I.; Coker, V.S.; Pattrick, R.A.D.P.; van der Laan, G.; Cutting, R.; Vaughan, D.V.; Paterson-Beedle, M.; Mikheenko, I.P.; Yong, P.; et al. Biomineralization: Linking the fossil record to the production of high value functional materials. Geobiology 2008, 6, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J.; Meloche, J.D.; Fyfe, W.S.; Murray, R.G.E. Diagenesis of metals chemically complexed to bacteria: Laboratory formation of metal phosphates, sulfides and organic condensates in artificial sediments. Appl. Environ. Microbiol. 1983, 45, 1094–1108. [Google Scholar] [PubMed]
- Styriakova, I.; Styriak, I. Iron removal from kaolins by bacterial leaching. Ceram. Silik. 2000, 44, 135–141. [Google Scholar]
- Ritz, K.; Young, I.M. Interaction between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Tazaki, K. Microbial formation of a halloysite-like mineral. Clays Clay Miner. 2005, 53, 224–233. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Fyfe, W.S.; Ferris, F.G.; Beveridge, T.J. Metal sorption and mineral precipitation by bacteria in two Amazonian river systems: Rio Solimoes and Rio Negro, Brazil. Geology 1993, 21, 1103–1106. [Google Scholar] [CrossRef]
- Konhauser, K.; Urrutia, M. Bacterial clay authigenesis: A common biogeochemical process. Chem. Geol. 1999, 161, 399–413. [Google Scholar]
- Kawano, M.; Tomita, K. Microbial biomineralization in weathered volcanic ash deposit and formation of biogenic minerals by experimental incubation. Am. Mineral. 2001, 86, 400–410. [Google Scholar] [CrossRef]
- Ueshima, M.; Tazaki, K. Possible role of microbial polysaccharides in nontronite formation. Clays Clay Miner. 2001, 49, 292–299. [Google Scholar] [CrossRef]
- Konhauser, K.; Schiffman, P.; Fisher, Q. Microbial mediation of authigenic clays during hydrothermal alteration of basaltic tephra, Kilauea Volcano. Geochem. Geophys. Geosyst. 2002, 3, 1075. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C. Morphological and chemical features of bioweathered granitic biotite induced by lichen activity. Clays Clay Miner. 1996, 44, 653–657. [Google Scholar] [CrossRef]
- Arocena, J.M.; Velde, B.; Robertson, S.J. Weathering of biotite in the presence of arbuscular mycorrhizae in selected agricultural crops. Appl. Clay Sci. 2012, 64, 12–17. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, H.; Kim, J.; Eberl, D. Microbial reduction of structural Fe3+ in nontronite by a thermophilic bacterium and its role in promoting the smectite to illite reaction. Am. Mineral. 2007, 92, 1411–1419. [Google Scholar] [CrossRef]
- Ascaso, C.; Galvan, J. Studies on the pedogenetic action of lichen acids. Pedobiologia 1976, 16, 321–331. [Google Scholar]
- Prieto Lamas, B.; Rivas Brae, M.T.; Silva Hermo, B.M. Colonization by lichens of granite churches in Galicia northwest Spain. Sci. Total Environ. 1995, 167, 343–351. [Google Scholar] [CrossRef]
- Naimark, E.B.; Erouchev-Snack, V.A.; Chizhikova, N.P.; Kompantseva, E.I. Interaction of clay minerals with microorganisms: A review of experimental data. J. Gen. Biol. 2009, 70, 155–167. [Google Scholar]
- Fomina, M.; Gadd, G.M. Influence of clay minerals on the morphology of fungal pellets. Mycol. Res. 2002, 106, 107–117. [Google Scholar] [CrossRef]
- ZoBell, C.E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943, 46, 39–58. [Google Scholar]
- Stotzky, G. Influence of clay minerals on microorganisms—II. Effect of various clay species, homoionic clays, and other particles on bacteria. Can.J. Microbiol. 1966, 12, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Stotzky, G. Persistence and biological activity in soil of insecticidal proteins from Bacillus thuringiensis and of bacterial DNA bound on clays and humic acids. J. Environ. Qual. 2000, 29, 691–705. [Google Scholar] [CrossRef]
- Haider, K.; Filip, Z.; Martin, J.P. Einfluß von montmorillonit auf die bildung von biomasse und stoffwechselproducten durch einige mikroorganismen. Arch. Microbiol. 1970, 73, 201–215. [Google Scholar]
- Martin, J.P.; Filip, Z.; Haider, K. Effect of montmorillonite and humate on growth and metabolic activity of some actinomyces. Soil Biol. Biochem. 1976, 8, 409–413. [Google Scholar] [CrossRef]
- Fletcher, M. How do bacteria attach to solid surfaces? Microbiol. Sci. 1987, 4, 133–136. [Google Scholar]
- Zvyagintsev, D. Soil and Microorganisms; Moscow University: Moscow, Russia, 1987. [Google Scholar]
- Marshall, K.C. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can. J. Microbiol. 1988, 34, 593–606. [Google Scholar] [CrossRef]
- Claus, H.; Filip, Z. Effects of clay and other solids on the activity of phenoloxidases produced by some fungi and actinomycetes. Soil Biol. Biochem. 1990, 22, 483–488. [Google Scholar] [CrossRef]
- Filip, Z.; Haider, K.; Martin, J.P. Influence of clay minerals on growth and metabolic activity of Epicoccum nigrum and Stachybotrys chartarum. Soil Biol. Biochem. 1972, 4, 135–145. [Google Scholar] [CrossRef]
- Filip, Z.; Haider, K.; Martin, J.P. Influence of clay minerals on the formation of humic substances by Epicoccum nigrum and Stachybotrys chartarum. Soil Biol. Biochem. 1972, 4, 147–154. [Google Scholar] [CrossRef]
- Bondietti, E.; Martin, J.P.; Haider, K. Influence of nitrogen source and clay on growth and phenolic polymer production by Stachybotrys species, Hendersonula toruloidea, and Aspergillus sydowii. Soil Sci. Soc. Am. Proc. 1971, 35, 917–922. [Google Scholar]
- Kurdish, I.K.; Kigel, N.F. Effect of palygorskite, clay mineral, on physiological activity and adhesion of methanotrophic bacteria. Microbiol. J. 1992, 54, 73–78. [Google Scholar]
- Lee, G.H.; Stotzky, G. Transformation and survival of donor, recepient, and transformants of Bacillus subtilis in vitro and insoil. Soil Biol. Biochem. 1999, 31, 1499–1508. [Google Scholar] [CrossRef]
- Lotareva, O.V.; Prozorov, A.A. Effect of the clay minerals montmorillonite and kaolinite on the generic transformation of competent Bacillus subtilis cells. Microbiology 2000, 69, 571–574. [Google Scholar] [CrossRef]
- Demaneche, S.; Jocteur-Monrozier, L.; Quiquampoix, H.; Simonet, P. Evaluation of biological and physical protection against nuclease degradation of clay-bo und plasmid DNA. Appl. Environ. Microbiol. 2001, 67, 293–299. [Google Scholar] [CrossRef]
- Courvoisier, E.; Dukan, S. Improvement of Escherichia coli growth by kaolinite. Appl. Clay Sci. 2009, 44, 67–70. [Google Scholar] [CrossRef]
- Chobotarov, A.Y.; Gordienko, A.S.; Samchuk, A.I.; Kurdish, I.K. Influence of silicon dioxide and saponite on growth of Bacillus subtilis ІМV В-7023. Microbiol. J. 2010, 72, 33–39. [Google Scholar]
- Xiao, B.; Lian, B.; Sun, L.; Shao, W. Gene transcription response to weathering of K-bearing minerals by Aspergillus fumigatus. Chem. Geol. 2012, 306–307, 1–9. [Google Scholar] [CrossRef]
- Kang, C.; Wu, P.; Li, Y.; Ruan, B.; Li, L.; Tran, L.; Zhu, N.; Dang, Z. Understanding the role of clay minerals in the chromium(VI) bioremoval by Pseudomonas aeruginosa CCTCC AB93066 under growth condition: Microscopic, spectroscopic and kinetic analysis. World J. Microbiol. Biotechnol. 2015, 31, 1765–1779. [Google Scholar] [CrossRef]
- Fomina, M.A.; Gromosova, E.N.; Podgorsky, V.S. Light influence on melaninogenesis of Cladosporium cladosporioides (Fresen) deVries. Biopolym. Cell. 1996, 12, 58–63. [Google Scholar] [CrossRef][Green Version]
- Zhdanova, N.N.; Zakharchenko, V.A.; Vember, V.V.; Nakonechnaya, L.T. Fungi from Chernobyl: Mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol. Res. 2000, 104, 1421–1426. [Google Scholar] [CrossRef]
- Zhdanova, N.; Fomina, M.; Redchitz, T.; Olsson, S. Chernobyl effects: Growth characteristics of soil fungi Cladosprorium cladosporioides (Fresen) de Vries with and without positive radiotropism. Polish J. Ecol. 2001, 49, 309–318. [Google Scholar]
- Blois, M.S.; Zahlan, A.B.; Maling, J.E. Electron spin resonance studies on melanin. Biophys. J. 1964, 4, 471–490. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Rittenour, W.R.; Ciaccio, C.E.; Barnes, C.S. Internal transcribed spacer rRNA gene sequencing analysis of fungal diversity in Kansas City indoor environments. Environ. Sci. Process. Impacts 2014, 16, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Unković, N.; Ljaljević Grbić, M.; Subakov-Simić, G.; Stupar, M.; Vukojević, J.; Jelikić, A.; Stanojević, D. Biodeteriogenic and toxigenic agents on 17th century mural painting sand façade of the old church of the Holy Ascension (Veliki Krcimir, Serbia). Indoor Built Environ. 2015, 25, 826–837. [Google Scholar] [CrossRef]
- Zhdanova, N.N.; Melezhik, A.V.; Shkolny, A.T. Production and characterization of melanin of fungus Cladosporium cladosporioides (Fresen) de Vries. Mikrobiol. J. 1993, 55, 79–85. [Google Scholar]
- Zhdanova, N.; Izzheurova, V.V.; Artyshkova, L.V.; Vasilevska, A.I. A Method for Producing Melanin-Containing Biomass Using Micromycetes Strain. Ukrainian Patent UA 57317, 16 June 2003. (In Ukrainian). [Google Scholar]
- Fomina, M.A.; Kadoshnikov, V.M.; Zlobenko, B.P. Fungal Biomass Grown on Media Containing Clay as a Sorbent of Radionuclides.Biohydrometallurgy and the Environment toward the Mining of the 21st Century’–Proceedings of the International Biohydrometallurgy Symposium (IBS’99), Madrid, Spain, 20–23 June 1999; Amils, R., Ballester, A., Eds.; San Lorenzo de El Escorial: Madrid, Spain, 1999. [Google Scholar] [CrossRef]
- Fomina, M. Clay effect on growth and melaninogenesis of Cladosporium cladosporioides (Fresen) de Vries. Microbiol. J. Under review.
- Pirt, S. Principles of Microbe and Cell Cultivation; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Kovarova-Kovar, K.; Egli, T. Growth Kinetics of Suspended Microbial Cells: From Single Substrate-Controlled Growth to Mixed-Substrate Kinetics. Microbiol. Mol. Biol. Rev. 1998, 62, 646–666. [Google Scholar] [CrossRef]
- Liu, S. How Cells Grow. In Bioprocess Engineering, Kinetics, Sustainability, and Reactor Design, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands; Boston/Heidelberg, Germany, 2017; pp. 629–697. [Google Scholar]
- Delgado-Jarana, J.; Moreno-Mateos, M.A.; Benítez, T. Glucose Uptake in Trichoderma harzianum: Role of gtt1. Eukaryot. Cell 2003, 2, 708–717. [Google Scholar] [CrossRef]
- Gadd, G.M.; Fomina, M.; Burford, E. Fungal roles and functions in rock, mineral and soil transformations. In Microorganisms and Earth Systems—Advances in Geomicrobiology; Gadd, G., Semple, K., Lappin-Scott, H., Eds.; UNESCO/Cambridge University Press: Cambridge, UK, 2005; Volume 65, pp. 201–232. [Google Scholar] [CrossRef]
- Skujins, J. Enzymes in soil. In Soil Biochemistry; McLaren, A., Peterson, G., Eds.; Marcel Dekker: New York, NY, USA, 1967; pp. 371–414. [Google Scholar]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Baham, J. Adsorption of Cd(II) and Cu(II) by Na-montmorillonite at low surface coverage. Soil Sci. Soc. Am. J. 1983, 47, 660–665. [Google Scholar] [CrossRef]
- Farrah, H.; Pickering, W.F. The sorption of copper species by clays. 2. Illite and montmorillonite. Austral. J. Chem. 1976, 29, 1177–1184. [Google Scholar] [CrossRef]
- Farrah, H.; Pickering, W.F. The sorption of zinc species by clay minerals. Aust. J. Chem. 1976, 29, 1649–1656. [Google Scholar] [CrossRef]
- Fujiyoshi, R.; Eugene, A.S.; Katayama, M. Behavior of radionuclides in the environment. 1. Sorption of Zn(II) on clay minerals. Int. J. Rad. Appl. Instrum. A 1992, 43, 1223–1226. [Google Scholar] [CrossRef]
- Van Bladel, R.; Halen, H.; Cloose, P. Calcium-zinc and calcium-cadmium exchange in suspensions of various types of clays. Clay Min. 1993, 28, 33–38. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, R.S. The role of clay minerals and the effect of H+ ions on removal of heavy metal (Pb2+) from contaminated soils. Can. Geotech. J. 2000, 37, 296–307. [Google Scholar] [CrossRef]
- Dinelli, E.; Tateo, F. Sheet silicates as effective carriers of heavy metals in the ophiolitic mine area of Vigonzano (Northern Italy). Mineral. Mag. 2001, 65, 121–132. [Google Scholar] [CrossRef]
- Dong, W.M.; Wang, X.K.; Bian, X.Y.; Wang, A.X.; Du, J.Z.; Tao, Z.Y. Comparative study on the sorption/desorption of radioeuropium on alumina, bentonite and red earth: Effect of pH, ionic strength, fulvic acid, and iron oxide in red earth. Appl. Radiat. Isot. 2001, 54, 603–610. [Google Scholar] [CrossRef]
- Garnham, G.W.; Codd, G.A.; Gadd, G.M. Uptake of cobalt and cesium by microalgal- and cyanobacterial–clay mixtures. Microb. Ecol. 1991, 25, 71–82. [Google Scholar] [CrossRef]
- Kadoshnikov, V.; Golovko, N.; Fomina, M.; Zlobenko, B.; Pisanskya, J. On the melanin and humic acids interaction with clay minerals. Process Metall. 1995, 9, 289–297. [Google Scholar] [CrossRef]
- Morley, G.F.; Gadd, G.M. Sorption of toxic metals by fungi and clay minerals. Mycol. Res. 1995, 99, 1429–1438. [Google Scholar] [CrossRef]
- Dumat, C.; Quiquampoix, H.; Staunton, S. Adsorption of cesium by synthetic clay-organic matter complexes: Effect of the nature of organic polymers. Environ. Sci. Technol. 2000, 34, 2985–2989. [Google Scholar] [CrossRef]
- Leboda, R.; Chodorowsky, S.; Skubiszewska-Zieba, J.; Tarasevich, Y.I. Effect of the carbonaceous matter deposition on the textural and surface properties of complex carbon-mineral adsorbents prepared on the basis of palygorskite. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 113–128. [Google Scholar] [CrossRef]
- Neubauer, U.; Nowack, B.; Furrer, G.; Schulin, R. Heavy metal sorption on clay minerals affect ed by siderophore desferrioxamine B. Environ. Sci. Technol. 2000, 34, 2749–2755. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient Soil Microorganisms: A New Dimension for Sustainable Agriculture and Environmental Development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Müller, B. Experimental Interactions between Clay Minerals and Bacteria: A Review. Pedosphere 2015, 25, 799–810. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud. Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Kurdish, I.K.; Тitova, L.V. Use of high-dispersed materials for culturing and obtaining granular Agrobacterium radiobacter preparation. Appl. Biochem. Microbiol. 2001, 37, 318–321. [Google Scholar] [CrossRef]
- Guida, L.; Saidi, Z.; Hughes, M.N.; Poole, R.K. Aluminum toxicity and binding to Escherichia coli. Arch. Microbiol. 1991, 56, 507–512. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Influence of nutrient solution on tolerance of Pseudomonas sp. FEMS Microbiol. Let. 1999, 170, 187–190. [Google Scholar] [CrossRef][Green Version]
- Wong, D.; Sujlita, J.M.; McKinley, J.P.; Krumholz, L.R. Impact of Clay Minerals on Sulfate-Reducing Activity in Aquifers. Microb. Ecol. 2004, 47, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kurdish, I.K.; Melnykova, N.M. Influence of clay minerals on growth and nodulation activity of Bradyrhizobium japonicum. Microbiol. J. 2011, 73, 527–531. [Google Scholar]
- Kurdish, I.K.; Antonyuk, T.S. Effect of clay minerals on viability of some bacteria a high temperatures. Microbiol. J. 1999, 61, 3–8. [Google Scholar]
- Kurdish, I.K.; Roy, A.O. Strain of Bacteria Bacillus subtilis for Bacterial Fertilizer Obtaining for Plant-Growing. Ukrainian Patent No. 54923А, 17 March 2003. (In Ukrainian). [Google Scholar]
- Kurdish, І.К.; Bega, Z.Т. Strain of bacteria Azotobacter vinelandii for Obtaining of Bacterial Fertilizer for Plant-Growing. Ukrainian Patent No. 72856, 15 August 2006. (In Ukrainian). [Google Scholar]
- Kurdish, I. Introduction of Microorganisms in Agroecosystems; Naukova Dumka: Kyiv, Ukraine, 2010. [Google Scholar]
- Filip, Z. Clay minerals as a factor influencing the biochemical activity of soil microorganisms. Folia Microbiol. 1973, 18, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. Effect of solid surfaces on activity of attached bacteria. In Bacterial Adhesion; Plenum Press: New York, NY, USA; London, UK, 1985; pp. 339–362. [Google Scholar]
- Chobotarov, A.Y.; Gordienko, A.S.; Kurdish, I.K. Influence of natural minerals on growth of Azotobacter vinelandii IMV B-7076. Microbiol. J. 2010, 72, 27–31. [Google Scholar]
- Globa, L.I.; Gordienko, A.S.; Garbara, S.V.; Rotmistrov, M.N. Bacterial interaction with natural Cherkassy palygorskite at different pH values of the medium. Microbiol. J. 1983, 45, 22–26. [Google Scholar]
- Kurdish, I.K.; Bega, Z.T. Effect of argillaceous minerals on growth of phosphate-mobilizing bacteria Bacillus subtilis. Appl. Biochem. Microbiol. 2006, 42, 388–391. [Google Scholar] [CrossRef]
- Kameneva, I.A. Vermiculite influence on growth and storage of Mesorhizobium ciceri H-12 in heterophase preparation. Agric. Microbiol. Interag. Them. Res. Collect. 2009, 10, 91–96. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2003, 3, 3–8. [Google Scholar]
- Kibanova, D.; Nieto-Camacho, A.; Cervini-Silva, J. Lipid peroxidation induced by expandable clay minerals. Environ. Sci. Technol. 2009, 43, 7550–7555. [Google Scholar] [CrossRef] [PubMed]
- Cervini-Silva, J.; Nieto-Camacho, A.; Gómez-Vidales, V. Oxidative stress and inhibition and antioxidant activity by fibrous clays. Colloids Surf. B Biointerfaces 2015, 133, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Skorochod, I.O.; Kurdish, I.K. Influence of nanoparticles of silica and vermiculite on activity of enzymes of antioxidant defense. Міcrobiol. Biotechnol. 2013, 1, 59–67. [Google Scholar]
- Gerashchenko, I.I. Membranotropic properties of nano-sized silica. Surface 2009, 1, 288–306. [Google Scholar]
- Gmoshinski, I.V.; Smirnova, V.V.; Hotimchenko, S.A. The current state of the problem of safety assessment of nanomaterials. Nanotechnol. Russ. 2010, 5, 6–10. [Google Scholar]
- Naclerio, G.; Baccigalupi, L.; Caruso, C.; De Felice, M.; Ricca, E. Bacillus subtilis vegetative catalase is an extracellular enzyme. Appl. Environ. Microbiol. 1995, 61, 4471–4473. [Google Scholar] [CrossRef]
- Kenya, M.V.; Lukash, A.I.; Gusykov, E.P. The role of low molecular weight antioxidants in oxidative stress. Usp. Sovrem. Biol. 1993, 113, 456–470. [Google Scholar]
- Chobotarov, A.Y. Physiological activity of Azotobacter vinelandii IMV B-7076 and Bacillus subtilis IMV B-7023 under Condition of Dispersed Materials Action. Ph.D. Thesis, Zabolotny Institute of Microbiology and Virology, National Academy of Sciences of Ukraine, Kyiv, Ukraine, 2015. [Google Scholar]
- Davies, P. Plant Hormones—Biosynthesis, Signal Transduction, Action; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Leyser, O.; Day, S. Mechanisms in Plant Development; Wiley-Blackwell: Padstow, UK, 2003. [Google Scholar]
- Chobotarov, A.; Volkogon, M.; Voytenko, L.; Kurdish, I. Accumulation of phytohormones by soil bacteria Azotobacter vinelandii and Bacillus subtilis under the influence of nanomaterials. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 271–274. [Google Scholar] [CrossRef]
- Kurdish, I.; Roy, A.; Skorochod, I.; Chobotarov, A.; Herasimenko, I. Elaboration of a free-flowing complex bacterial preparation for cereal crops. In Nanosize System and Nanomaterials: Studies in Ukraine; Akademperiodika: Kiev, Ukraine, 2014; pp. 610–614. [Google Scholar]
- Titova, L.V.; Antipchuk, A.F.; Kurdish, I.K.; Skocinskaya, N.N.; Tantsyurenko, E.V. The effect of highly dispersed materials on the physiological activity of bacteria of the genus Azotobacter. Microbiol. J. 1994, 56, 60–65. [Google Scholar]
- Skorochod, I.O.; Kurdish, I.K. Change of Qualitative Composition of the Phenolic Compounds at Cultural Medium of the Azotobacter Vinelandii IMV B-7076 and Their Cultivation with Palygorskite Nanoparticles, Proceedings of Ukrainian Conference with International Participation «Chemistry, Physics and Technology of Surface» and Workshop «Metal-Based Biocompatible Nanoparticles: Synthesis and Applications», Kyiv, Ukraine, 15–17 May 2019; Chuiko Institute of Surface Chemistry of National Academy of Sciences of Ukraine: Kyiv, Ukraine, 2019. [Google Scholar]
- Al-Futaisi, A.; Jamrah, A.; Al-Rawas, A.; Al-Hanai, S. Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ. Geol. 2007, 51, 1317–1327. [Google Scholar] [CrossRef]
- Evanko, C.R.; Dzombak, D.A. Influence of structural features on sorption of NOM-analogue organic acids to goethite. Environ. Sci. Technol. 1998, 32, 2846–2855. [Google Scholar] [CrossRef]
- Tarasevich, Y. Adsorption and Its Application in Industry and Environmental Protection. In Application in Environmental Protection; Dabrowski, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 2, p. 659. [Google Scholar]
- Skorochod, I.O.; Kurdish, I.K. Changes in the quantitative composition of phenol carboxylic acids in the cultural medium of Bacillus subtilis IMV B-7023 during its cultivation with bentonite nanoparticles. In Abstract Book of the 7th International Conference, Proceedings of the Nanotechnologies and Nanomaterials, NANO-2019, Lviv, Ukraine, 27–30 August 2019; Lviv University: Lviv, Ukraine, 2019. [Google Scholar]
- Skorochod, I.O.; Kurdish, I.K. Influence of vermiculite particles on antioxidant properties of cultural medium of Bacillus subtilis IMV B-7023. Ukr. Biochem. J. 2014, 86, 61–68. [Google Scholar] [CrossRef]
- Okamura, Y. Competitive adsorption of phenolic acids on allophanic, halloysitic, and illitic clays. Clay Sci. 1990, 8, 37–44. [Google Scholar]
- Hyman, P.; Abedon, S.T. Viruses of microorganisms: What are They and Why Care. In Viruses of Microorganisms; Hyman, P., Abedon, S.T., Eds.; Caister Academic Press: Norfolk, UK, 2018; Part 1; pp. 1–14. [Google Scholar] [CrossRef][Green Version]
- Ripp, S.; Miller, R.V. Effects of suspended particulates on the frequency of transduction among Pseudomonas aeruginosa in a freshwater environment. Appl. Environ. Microbiol. 1995, 61, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Starr, E.P.; Nuccio, E.E.; Patt-Ridge, J.; Banfield, J.F.; Firestone, M.K. Metatranscriptomic reconstruction reveals RNA viruses with the potential to shape carbon cycling in soil. Proc. Natl. Acad. Sci. USA 2019, 116, 25900–25908. [Google Scholar] [CrossRef]
- Kimura, M.; Jia, Z.-J.; Nakayama, N.; Asakawa, S. Ecology of viruses in soils: Past, present and future perspectives. Soil Sci. Plant Nutr. 2008, 54, 1–32. [Google Scholar] [CrossRef]
- Michen, B.; Graule, T. Isoelectric points of viruses. J. Appl. Microbiol. 2010, 109, 388–397. [Google Scholar] [CrossRef]
- Park, J.-A.; Kang, J.-K.; Kim, J.-H.; Kim, S.-B.; Yu, S.; Kim, T.-H. Bacteriophage removal in various clay minerals and clay-amended soils. Environ. Eng. Res. 2015, 20, 133–140. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chattopadhyay, S.; Lyon, W.G.; Wilson, J.T. Effect of surfactants on the survival and sorption of viruses. Environ. Sci. Technol. 2002, 36, 4017–4024. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Interaction between virusesand clays in static and dynamic batch systems. Environ. Sci.Technol. 2010, 44, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Schiffenbauer, M.; Stotzky, G. Adsorption of coliphages T1 andT7 to clay minerals. Appl. Environ. Microbiol. 1982, 43, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bystricky, V.; Stotzky, G.; Schiffenbauer, M. Electron microscopy of T1-bacteriophage adsorbed to clay minerals: Application of the critical point drying method. Can. J. Microbiol. 1975, 21, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Puls, R.W. Adsorption of bacteriophages on clay minerals. Environ. Sci. Technol. 1999, 33, 3609–3614. [Google Scholar] [CrossRef]
- Carlson, G.F., Jr.; Woodard, F.E.; Wentworth, D.F.; Sproul, O.J. Virus inactivation on clay particles in natural waters. J. Water Poll. Control Fed. 1968, 40, 98–106. [Google Scholar]
- Bales, R.C.; Hinkle, S.R.; Kroeger, T.W.; Stocking, K.; Gerba, C.P. Bacteriophage adsorption during transport through porous media: Chemical perturbations and reversibility. Environ. Sci. Technol. 1991, 25, 2088–2095. [Google Scholar] [CrossRef]
- Sobeck, D.C.; Higgins, M.J. Examination of three theories for mechanisms of cation induced bioflocculation. Water Res. 2002, 36, 527–538. [Google Scholar] [CrossRef]
- Stotzky, G.; Schiffenbauer, M.; Lipson, S.M.; Yu, B.H. Surface Interactions between Viruses and Clay Minerals and Microbes: Mechanisms and Implications; Viruses and Wastewater Treatment. In Proceedings of the International Symposium on Viruses and Wastewater Treatment, Guildford, UK, 15–17 September 1991. [Google Scholar]
- Vettori, C.; Gallori, E.; Stotzky, G. Clay minerals protect bacteriophage PBS1 of Bacillus subtilis against inactivation and loss of transducing ability by UV radiation. Can. J. Microbiol. 2000, 46, 770–773. [Google Scholar] [CrossRef]
- Roper, M.M.; Marshall, K.C.J. Effect of clay particle size on clay–Escherichia coli–bacteriophage interactions. J. Gen. Microbiol. 1978, 106, 187–189. [Google Scholar] [CrossRef]
- Peterson, C.T.; Sharma, V.; Elmen, L.; Peterson, S.N. Immunehomeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grewal, S.; Vakhlu, J. Phylogenetic diversity and metabolic potential of microbiome of natural healing clay from Chamliyal (J&K). Arch. Microbiol. 2018, 200, 1333–1343. [Google Scholar] [CrossRef]
- Falkinham, J.O.; Wall, T.E.; Tanner, J.R.; Tawaha, K.; AlaliF, Q.; Li, C.; Oberlies, N.H. Proliferation of antibiotic-producing bacteria and concomitant antibiotic production as the basis for the antibiotic activity of Jordan’s red clays. Appl. Environ. Microbiol. 2009, 75, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, K.R.; Anderson, N.H.; McCaigue, M.D.; Erwin, P.J.; Halliday, M.I.; Rowlands, B.J. Adsorbents as antiendotoxin agents in experimental colitis. Gut 1993, 34, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Bisceglia, M.; Castellucci, G. Smectite in the treatment of acute diarrhea: A nationwide randomized controlled study of the Italian Society of Pediatric Gastroenterology and Hepatology (SIGEP) in collaboration with primary care pediatricians. SIGEP Study Group for Smectite in Acute Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 71–75. [Google Scholar] [CrossRef]
- Du, K.; Wang, C.; Liu, P.; Li, Y.; Ma, X. Effects of dietary mycotoxins on gut microbiome. Protein Peptide Lett. 2017, 24, 397–405. [Google Scholar] [CrossRef]
- Wang, M.; Maki, C.R.; Deng, Y.; Tian, Y.; Phillips, T.D. Development of high capacity enterosorbents for aflatoxin B1 and other hazardous chemicals. Chem. Res. Toxicol. 2017, 30, 1694–1701. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Xue, K.S.; Lin, S.; Marroquin-Cardona, A.; Brown, K.A.; Elmore, S.E.; Tang, L.; Romoser, A.; Gelderblom, W.C.; Wang, J.S.; et al. Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. J. Appl. Toxicol. 2014, 34, 795–804. [Google Scholar] [CrossRef]
- Phillips, T.D. Dietary clay in the chemoprevention of aflatoxine-induced disease. Toxicol. Sci. 1999, 52, 118–126. [Google Scholar] [CrossRef]
- Wang, J.P.; Chi, F.; Kim, I.H. Effects of montmorillonite clay on growth performance, nutrient digestibility, vulva size, faecal microflora, and oxidative stress in weaning gilts challenged with zearalenone. Anim. Feed Sci. Technol. 2012, 178, 158–166. [Google Scholar] [CrossRef]
- Xia, M.S.; Hu, C.H.; Xu, Z.R.; Ye, Y.; Zhou, Y.H.; Xiong, L. Effects of copper–bearing montmorillonite (Cu-MMT) on Escherichia coli and diarrhea on weanling pigs. Asian Australas. J. Anim. Sci. 2004, 17, 1712–1716. [Google Scholar] [CrossRef]
- Xia, M.S.; Hu, C.H.; Xu, Z.R. Effects of copper bearing montmorillonite on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2005, 118, 307–317. [Google Scholar] [CrossRef]
- Lee, E.S.; Song, E.J.; Lee, S.Y.; Park, S.L.; Kim, D.; Kim, D.; Kim, J.H.; Lim, S.I.; Nam, Y.D. Effects of bentonite Bgp35b-p on the gut microbiota of mice fed a high-fat diet. J. Sci. Food Agric. 2018, 98, 4369–4373. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hong, F.; Wang, J.; Cong, Y.; Dai, S.; Wang, S.; Wang, J.; Jin, X.; Wang, F.; Liu, J.; et al. Microbiome remodelling via the montmorillonite adsorption-excretion axis prevents obesity-related metabolic disorders. EBioMedicine 2017, 16, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chalvatzi, S.; Kalamaki, M.S.; Arsenos, G.; Fortomaris, P. Dietary supplementation with the clay mineral palygorskite affects performance and beneficially modulates caecal microbiota in laying pullets. J. Appl. Microbiol. 2016, 120, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Long, L.; Liu, T.; Cao, Y. Montmorillonite adsorbs creatinine and accelerates creatinine excretion from the intestine. J. Pharm. Pharmacol. 2009, 61, 459–464. [Google Scholar] [CrossRef]
- Li, J.; Kim, I.H. Effects of dietary supplementation of sericite on growth performance, nutrient digestibility, blood profiles and fecal microflora shedding in growing pigs. Anim. Feed Sci. Technol. 2013, 184, 100–104. [Google Scholar] [CrossRef]
- Thacker, P.A. Performance of growing-finishing pigs fed diets containing graded levels of biotite, aluminosilicate clay. Asian Australas. J. Anim. Sci. 2003, 16, 1666–1672. [Google Scholar] [CrossRef]
- Garmasheva, I.L.; Kovalenko, N.K.; Pidgorskyi, V.S.; Livins’ka, O.P.; Voychuk, S.I.; Oleschenko, L.T.; Tomila, T.V.; Lobunets, Т.F. Interaction of Lactobacillus plantarum 337D UKMB-2627 strain cells with clay minerals in vitro. Microb. J. 2016, 78, 11–24. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nogina, T.; Fomina, M.; Dumanskaya, T.; Zelena, L.; Khomenko, L.; Mikhalovsky, S.; Podgorskyi, V.; Gadd, G.M. A new Rhodococcus aetherivorans strain isolated from lubricant-contaminated soil as a prospective phenol-biodegrading agent. Appl. Microbiol. Biotechnol. 2020, 104, 3689–3690. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Bioremediation of PAHs and VOCs: Advances in clay mineral-microbial interaction. Environ. Int. 2015, 85, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Willaert, R. Immobilization and Its Applications in Biotechnology: Current Trends and Future Prospects. In Fermentation Microbiology and Biotechnology, 3rd ed.; El-Mansi, E.M.T., Bruce, C.F.A., Dahhou, B., Sanchez, S., Demain, A.L., Allman, A.R., Eds.; CRC Press/Taylor & Francis Group: Philadelphia, PA, USA, 2011; Chapter 12; pp. 313–367. [Google Scholar]
- Omar, S.H.; Rehm, H.J. Degradation of n-alkanes by Candida parapsilosis and Penicillium frequentans immobilized on granular clay and aquifer sand. Appl. Microbiol. Biotechnol. 1988, 28, 103–108. [Google Scholar] [CrossRef]
- Taoufik, J.; Zeroual, Y.; Moutaouakkil, A.; Moussaid, S.; Dzairi, F.Z.; Talbi, M.; Hammoumi, A.; Belghmi, K.; Lee, K.; Loutfi, M.; et al. Aromatic hydrocarbons removal by immobilized bacteria (Pseudomonas sp., Staphylococcus sp.) influidized bed bioreactor. Ann. Microbiol. 2004, 54, 189–200. [Google Scholar]
- Su, D.; Li, P.J.; Frank, S.; Xiong, X.Z. Biodegradation of benzo[a]pyrene in soil by Mucor sp. SF06 and Bacillus sp. SB02 co-immobilized on vermiculite. J. Environ. Sci. 2006, 18, 1204–1209. [Google Scholar] [CrossRef]
- Shumkova, E.S.; Solyanikova, I.P.; Plotnikova, E.G.; Golovleva, L.A. Phenol degradation by Rhodococcus opacusstrain 1G. Appl. Biochem. Microbiol. 2009, 45, 43–49. [Google Scholar] [CrossRef]
- Gong, B.; Wu, P.; Huang, Z.; Li, Y.; Dang, Z.; Ruan, B.; Kang, C.; Zhu, N. Enhanced degradation of phenol by Sphingomonas sp. GY2B with resistance towards suboptimal environment through adsorption on kaolinite. Chemosphere 2016, 148, 388–394. [Google Scholar] [CrossRef]
- Froehner, S.; Martins, R.F.; Furukawa, W.; Errera, M.R. Water remediation by adsorption of phenol onto hydrophobic modified clay. Water Air Soil Pollut. 2009, 199, 107–113. [Google Scholar] [CrossRef]
- Ruan, B.; Wu, P.; Chen, M.; Lai, X.; Chen, L.; Yu, L.; Gong, B.; Kang, C.; Dang, Z.; Shi, Z.; et al. Immobilization of Sphingomonas sp. GY2B in polyvinyl alcohol-alginate-kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotoxicol. Environ. Saf. 2018, 162, 103–111. [Google Scholar] [CrossRef]
- Atlas, R.M. Microbial hydrocarbon degradation—bioremediation of oil spills. Chem. Technol. Biotechnol. 1991, 52, 149–156. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K.; Asada, R.; Kogure, K. Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: Isolation and characterization of hydrocarbon-degrading bacteria. Environ. Int. 2004, 30, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Chaerun, S.K.; Tazaki, K.; Asada, R.; Kogure, K. Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: Implications for bioremediation. Clay Miner. 2005, 40, 105–114. [Google Scholar] [CrossRef]
- Warr, L.N.; Friese, A.; Schwarz, F.; Schaurer, F.; Portier, R.J.; Bacirico, L.M.; Olson, G.M. Experimental study of clay-hydrocarbon interactions relevant to the biodegradation of the Deepwater Horizon oil from the Gulf of Mexico. Chemosphere 2016, 162, 208–221. [Google Scholar] [CrossRef]
- Tazaki, K.; Chaerun, S.K. Life in oil: Hydrocarbon-degrading bacterial mineralization in oil spill-polluted marine environment. Front. Mater. Sci. China 2008, 2, 120–133. [Google Scholar] [CrossRef]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Material, 1st ed.; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 113–127. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Naidu, R. Bacterial mineralization of phenanthrene on thermally activated palygorskite: A 14C radiotracer study. Sci. Total Environ. 2016, 579, 709–717. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation and adsorption of crude oil hydrocarbons supported on “homoionic” montmorillonite clay minerals. Appl. Clay Sci. 2014, 87, 81–86. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Manning, D.A.C.; Fialips, C.I. Microbial degradation of crude oil hydrocarbons on organoclay minerals. J. Environ. Manag. 2014, 144, 197–202. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef]
- Bishop, M.E.; Dong, H.; Kukkadapu, R.K.; Lin, C.; Edelmann, R.E. Bioreduction of Fe-bearing clay minerals and their reactivity toward pertechnetate (Tc-99). Geochim. Cosmochim. Acta 2011, 75, 5229–5246. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Dong, H.; Plymale, A.E.; Fredrickson, J.K.; Zachara, J.M.; Heald, S.; Liu, C.X. Reduction and long-term immobilization of technetium by Fe(II) associated with clay mineral nontronite. Chem. Geol. 2009, 264, 127–138. [Google Scholar] [CrossRef]
- Zhang, G.; Senko, J.M.; Kelly, S.D.; Tan, H.; Kemner, K.M.; Burgos, W.D. Microbial reduction of iron(III)-rich nontronite and uranium(VI). Geochim. Cosmochim. Acta 2009, 73, 3523–3538. [Google Scholar] [CrossRef]
- Taylor, R.W.; Shen, S.; Bleam, W.F.; Tu, S.-I. Chromate removal by dithionitereduced clays: Evidence from direct X-ray adsorption near edge spectroscopy (XANES) of chromate reduction at clay surfaces. Clay Miner. 2000, 48, 648–654. [Google Scholar] [CrossRef]
- Stucki, J.W.; Kostka, J.E. Microbial reduction of iron in smectite. Comptes Rendus Geosci. 2006, 338, 468–475. [Google Scholar] [CrossRef]
- Fialips, C.I.; Cooper, N.G.A.; Jones, D.M.; White, M.L.; Gray, N.D. Reductive degradation of p,p’-DDT by Fe(II) in nontronite NAu-2. Clay Miner. 2010, 58, 821–836. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Heijnen, C.E.; Hok-A-Hin, C.H.; Van Veen, J.A. Improvements to the use of bentonite clay as a protective agent increasing survival levels of bacteria introduced into soil. Soil Biol. Biochem. 1992, 24, 533–538. [Google Scholar] [CrossRef]
- Skorokhod, I.O.; Tserkovniak, L.S.; Kurdish, I.K.; Plotnikov, V.V.; Gylchuk, V.G.; Korniychuk, O.V. Influence of granulated bacterial preparation complex action on the growth and yield of barley. Microbiol. J. 2012, 74, 23–28. [Google Scholar]
- Kurdish, I.; Roy, A.; Skorochod, I.; Chobotarov, A.; Herasimenko, I.; Plotnikov, V.; Gylchuk, V.; Korniychuk, A. Free-flowing complex bacterial preparation for crop and efficiency of its use in agroecosystems. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 527–531. [Google Scholar] [CrossRef]
- Kurdish, I.K.; Drobitko, A.V.; Shevchenko, T.V.; Maryushkin, V.F. The interaction of Bradyrhizobium japonicum with clay minerals. Microbiol. J. 2000, 62, 45–50. [Google Scholar]
- Kurdish, I.K.; Titova, L.V. Granular preparations of Azotobacter based in a clay mineral-base. Appl. Biochem. Microbiol. 2000, 36, 484–487. [Google Scholar] [CrossRef]
- Kurdish, І.К.; Roy, A.O. The Method of the Reception of Free-Flowing Complex Bacterial Preparation for Crop Production. Ukrainian Patent No. 106135C2, 25 July 2014. (In Ukrainian). [Google Scholar]
- Kurdish, І.K.; Tserkovniak, L.S.; Tsvey, Y.P.; Chernata, D.М. Perspectives and introduction problems of microbial preparation in agrocenosis. Sci. Bull. Chernivtsy Univ. 2005, 252, 126–131. [Google Scholar]
- Roy, А.A.; Pasichnyk, L.А.; Tserkovniak, L.S.; Chodos, S.F.; Kurdish, I.K. Influence of bacteria of Bacillus subtilis on the agent of bacterial cancer of tomatoes. Microbiol. J. 2012, 74, 74–80. [Google Scholar]
- Roy, A.A.; Matselyukh, O.V.; Zubko, P.D.; Varbanets, L.D.; Kurdish, I.K. Proteolytic activity of phosphorous mobilizing bacteria of Bacillus genus and their influence on some phytophages. Agric. Microbiol. 2014, 20, 66–73. [Google Scholar]
- Skorochod, I.O.; Roy, A.A.; Melentiev, A.I.; Kurdish, I.K. Influence of bioactive substances of phosphate-mineralizing strains of genus Bacillus on plant seeds affected by oxidative stress. Microbiol. Biotechnol. 2013, 2, 41–51. [Google Scholar]
- Tserkovniak, L.S.; Bega, Z.T.; Ostapchuk, A.N.; Kuz’min, V.E.; Kurdish, I.K. Production of biologically active substances of indol nature by bacteria of Azotobacter genus. Ukr. Biochem. J. 2009, 81, 122–128. [Google Scholar]
- Tserkovniak, L.S.; Kurdish, I.K. Phosphate-mobilizing bacteria Bacillus subtilis as phenolic producers. Appl. Biochem. Microbiol. 2009, 45, 311–317. [Google Scholar] [CrossRef]
- Kurdish, I.K.; Bеgа, Z.Т.; Gordienko, А.S.; Dyrenko, D.I. The effect of Azotobacter vinelandii on plant seed germination and adhesion of these bacteria to cucumber roots. Appl. Biochem. Microbiol. 2008, 45, 400–404. [Google Scholar] [CrossRef]
- Kurdish, I.K.; Chuiko, N.V.; Bulavenko, L.V.; Dyrenko, D.I. Efficiency of the introduction of granular bacterial preparations into the agroecosystem of flowering plants. In Proceedings of the Uman National University of Horticulture: The Basis for the Formation of Productivity of Agricultural Crop with Intensive Cultivation Technologies; Uman National University of Horticulture: Uman, Ukraine, 2008; pp. 186–192. [Google Scholar]
- Kurdish, I.; Roy, A.; Chuiko, N.; Belogubova, O.; Bulavenko, L.; Bega, Z.; Dyrenko, D.; Chobotarjov, A. The application of granulated bacterial preparation of complex action in crop production. Agroecol. J. 2008, 2, 141–142. [Google Scholar]
- Graham-Weiss, L.; Bennett, M.L.; Paau, A.S. Production of bacterial inoculants by direct fermentation on nutrient-supplemented vermiculite. Appl. Environ. Microbiol. 1987, 53, 2138–2141. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomina, M.; Skorochod, I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals 2020, 10, 861. https://doi.org/10.3390/min10100861
Fomina M, Skorochod I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals. 2020; 10(10):861. https://doi.org/10.3390/min10100861
Chicago/Turabian StyleFomina, Marina, and Iryna Skorochod. 2020. "Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications" Minerals 10, no. 10: 861. https://doi.org/10.3390/min10100861
APA StyleFomina, M., & Skorochod, I. (2020). Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals, 10(10), 861. https://doi.org/10.3390/min10100861




