Using Microstructures and Composition to Decipher the Alterations of Rodent Teeth in Modern Regurgitation Pellets—A Good News-Bad News Story
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
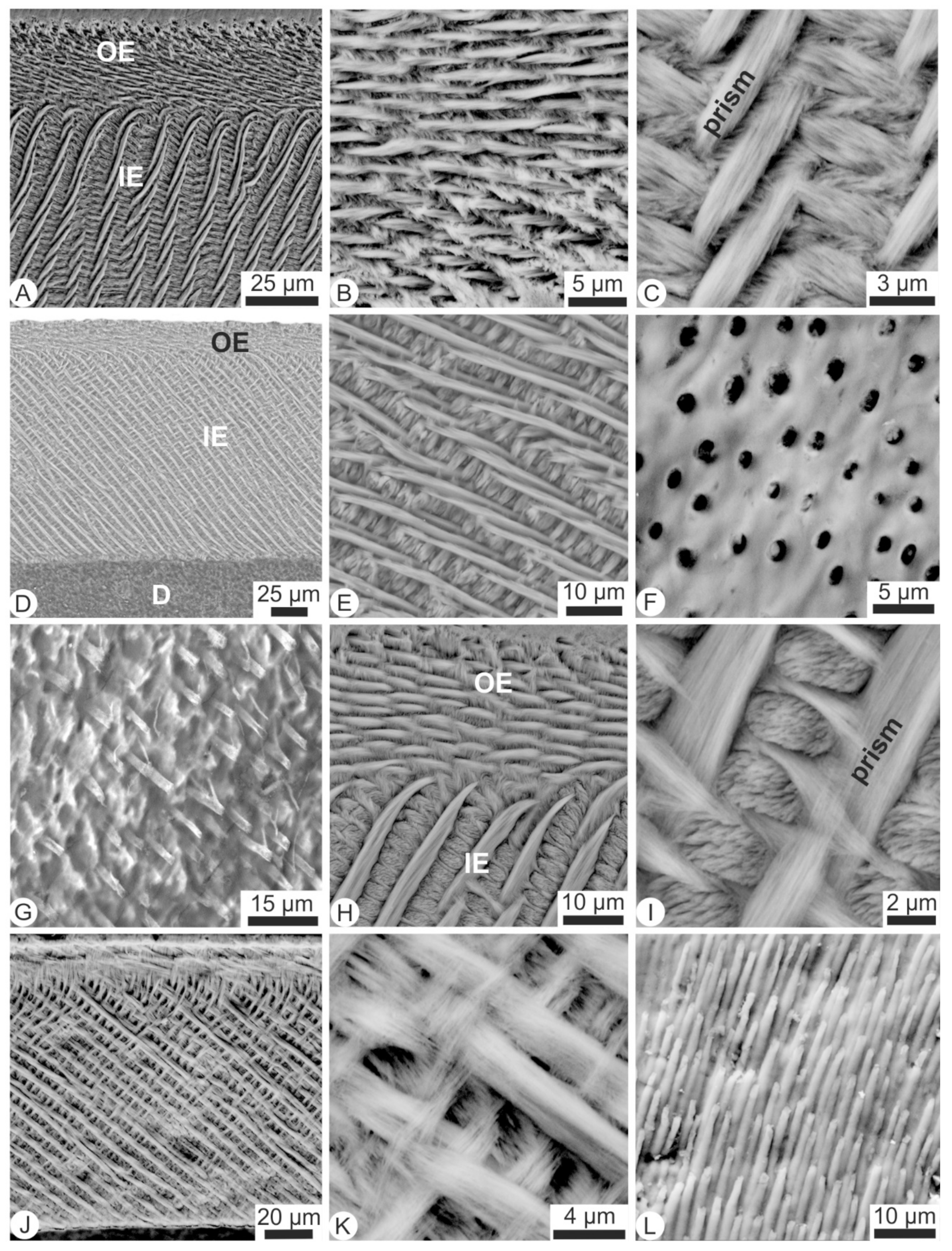
2.2.1. Scanning Electron Microscopy (SEM)
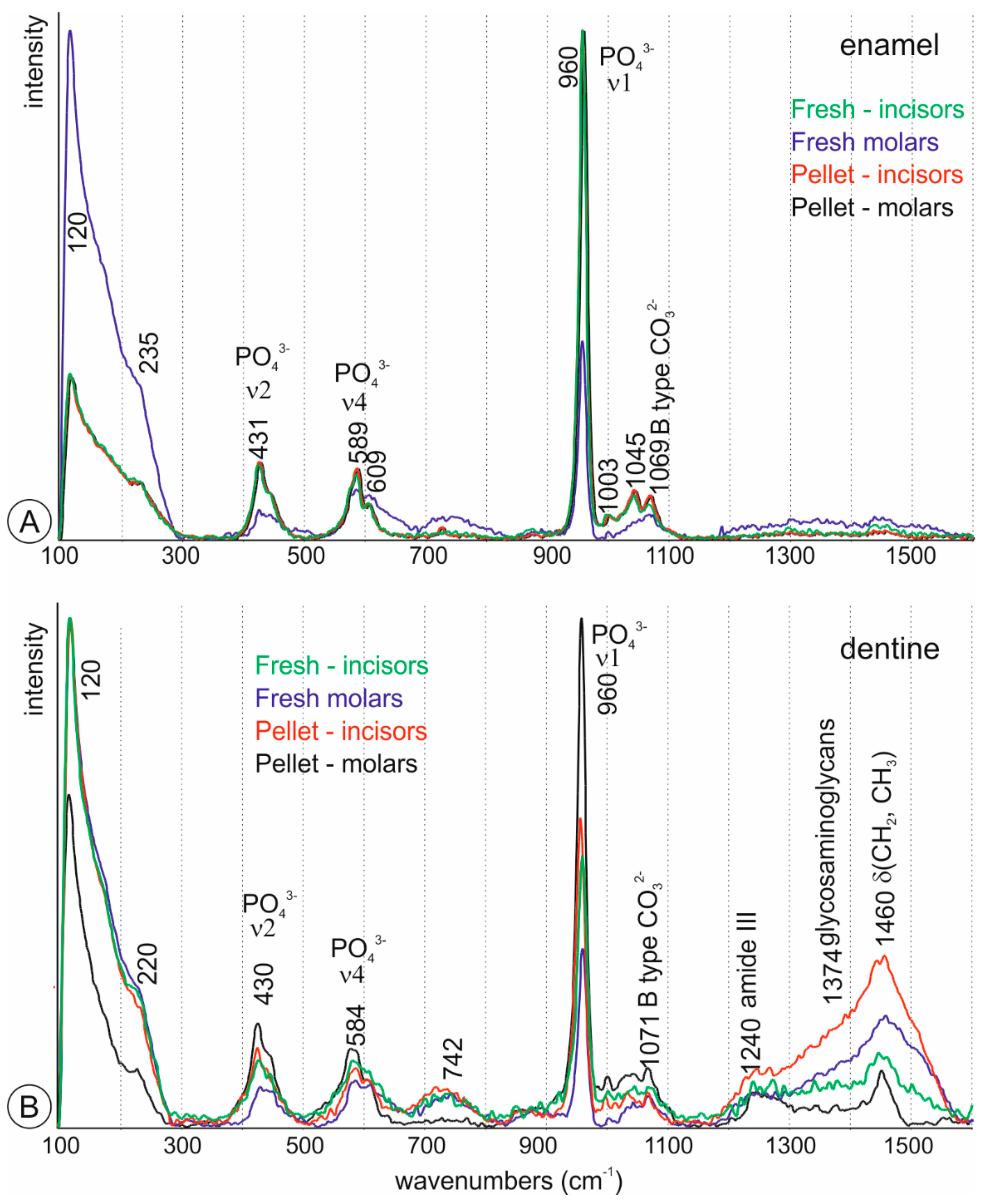
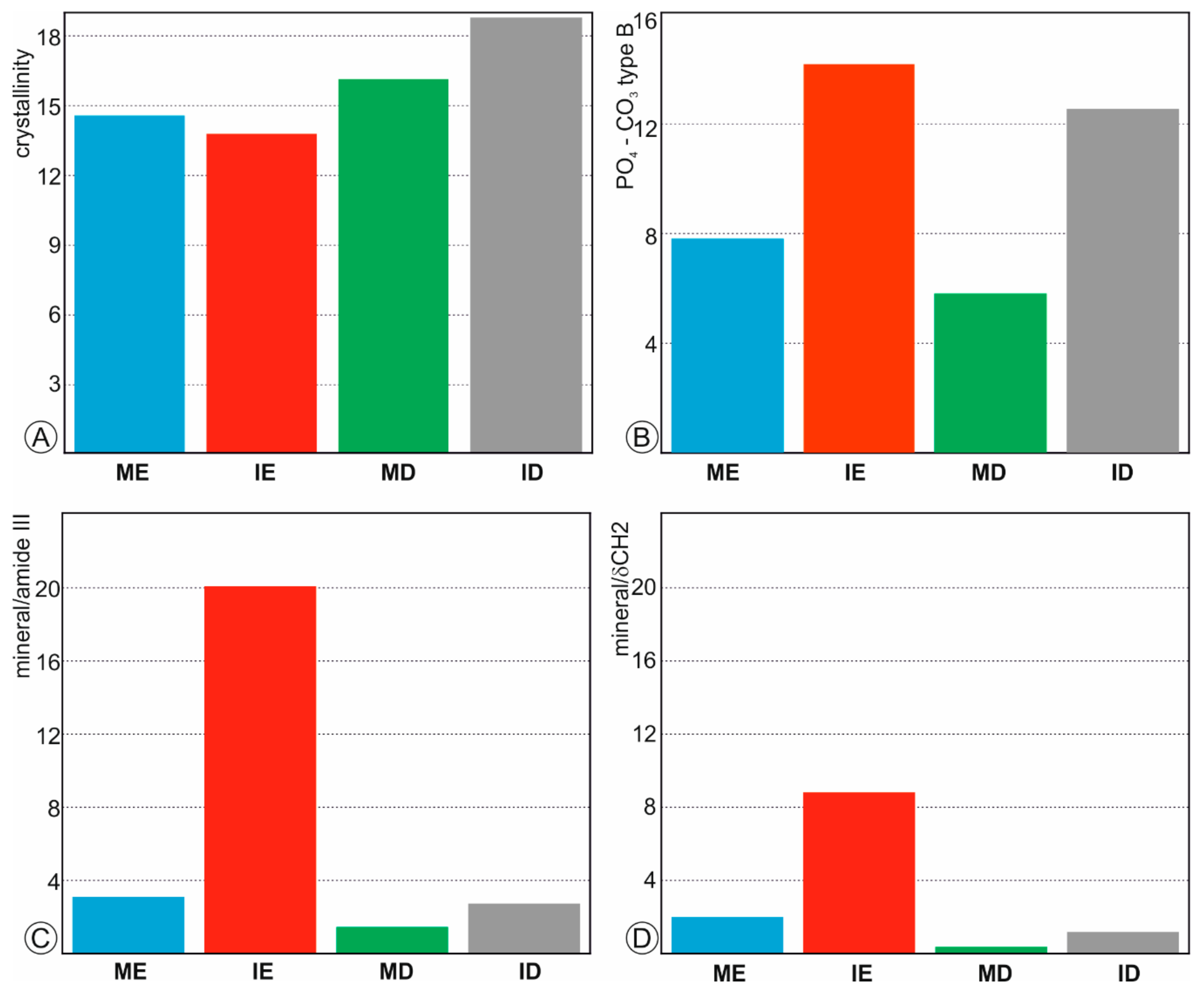
2.2.2. Raman Spectrometry
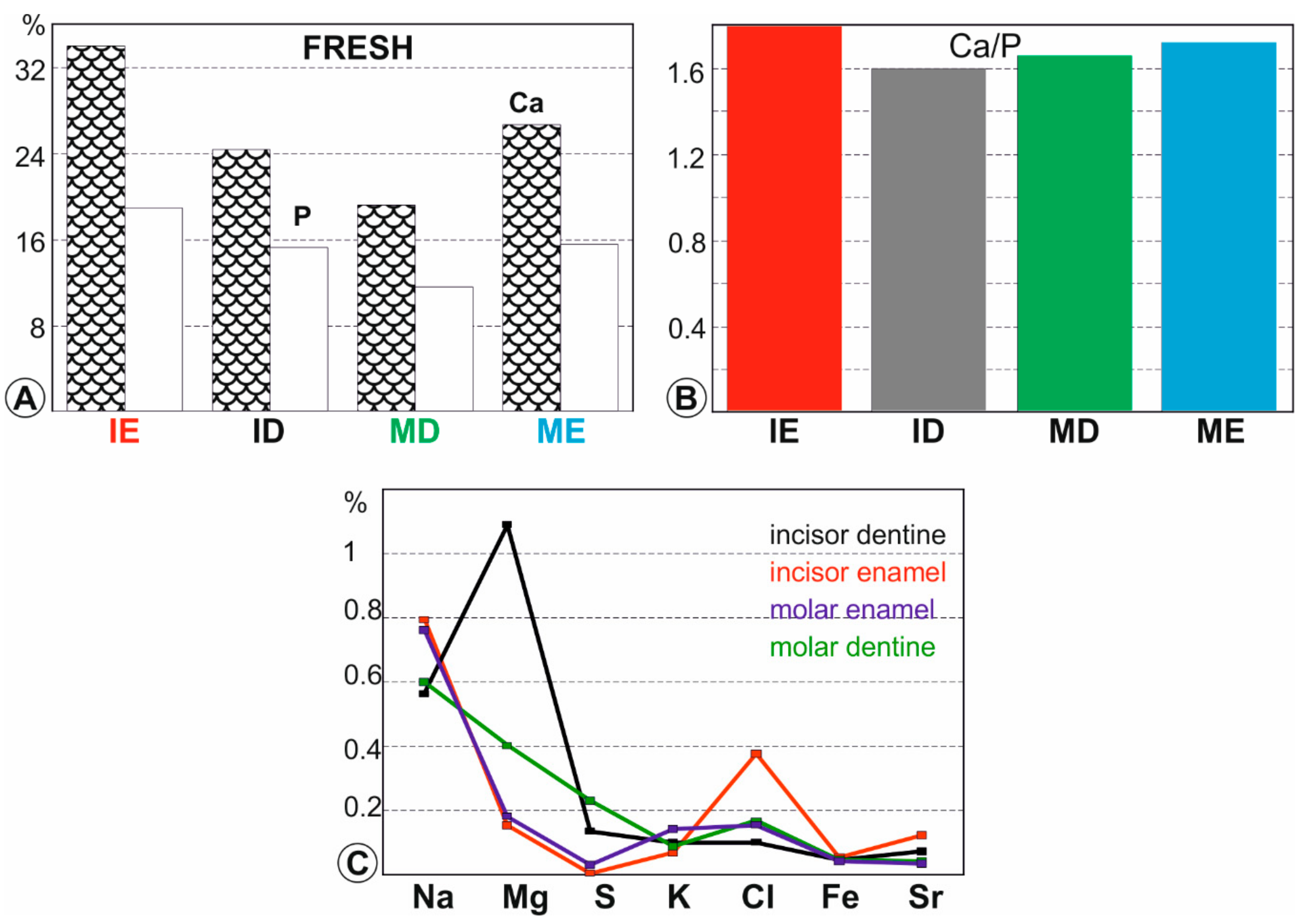
2.2.3. Chemical Analyses
2.2.4. Statistical Analyses
3. Results
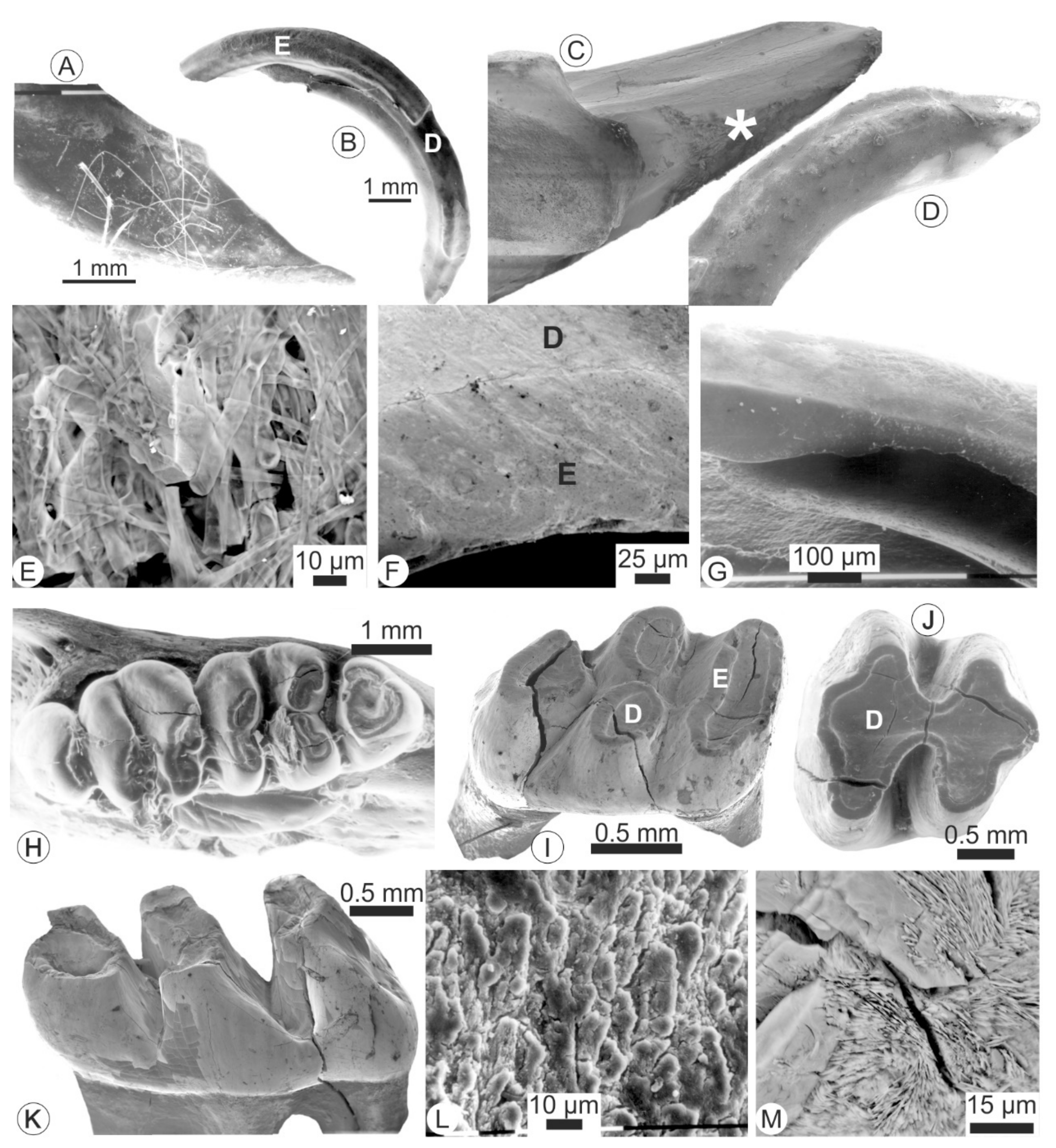
3.1. Microstructures and Composition of Fresh Teeth (i.e., Not Extracted from Pellets)
3.2. Mineralogy and Bulk Composition of Fresh Teeth
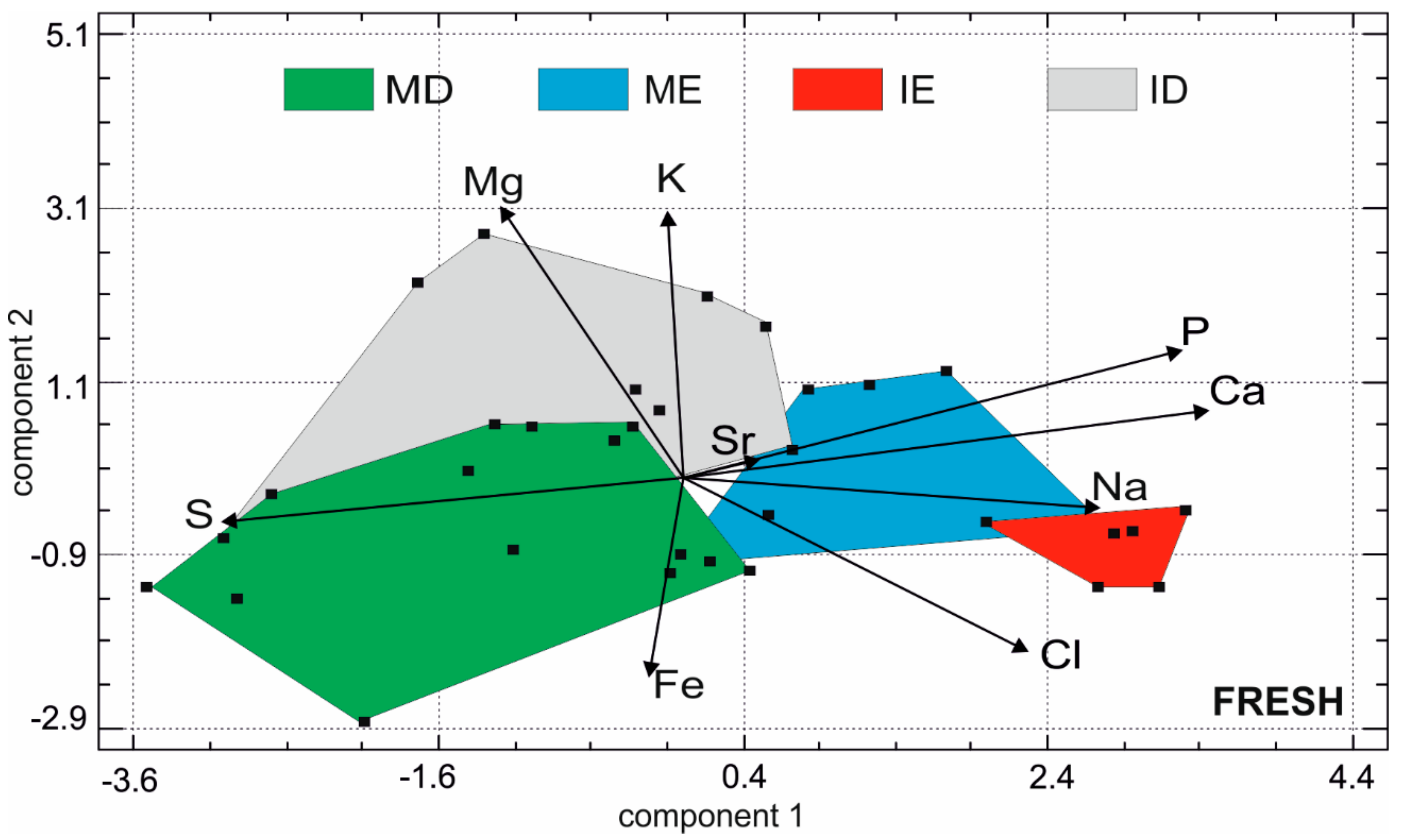
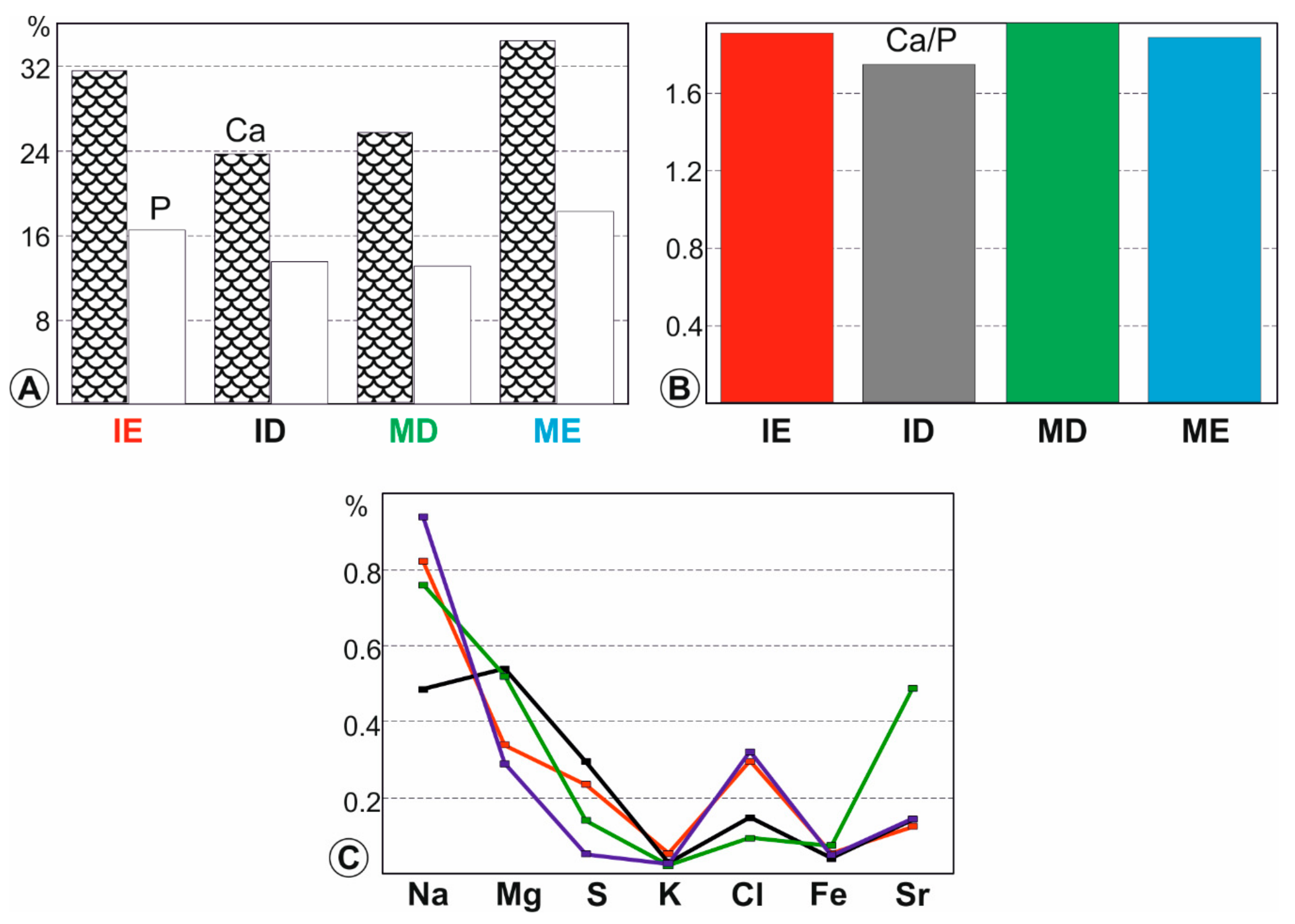
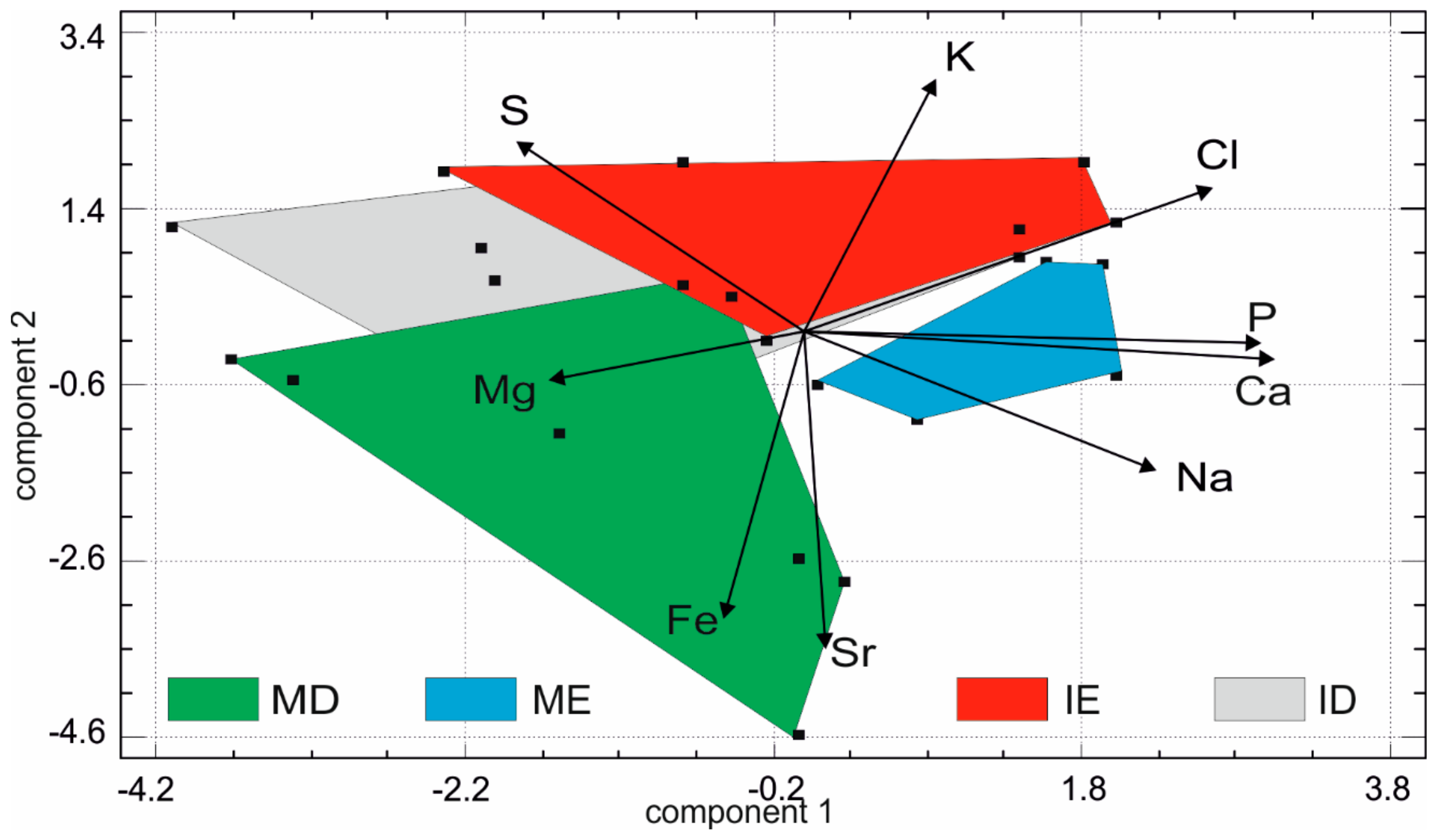
3.3. Elemental Composition of Fresh Teeth
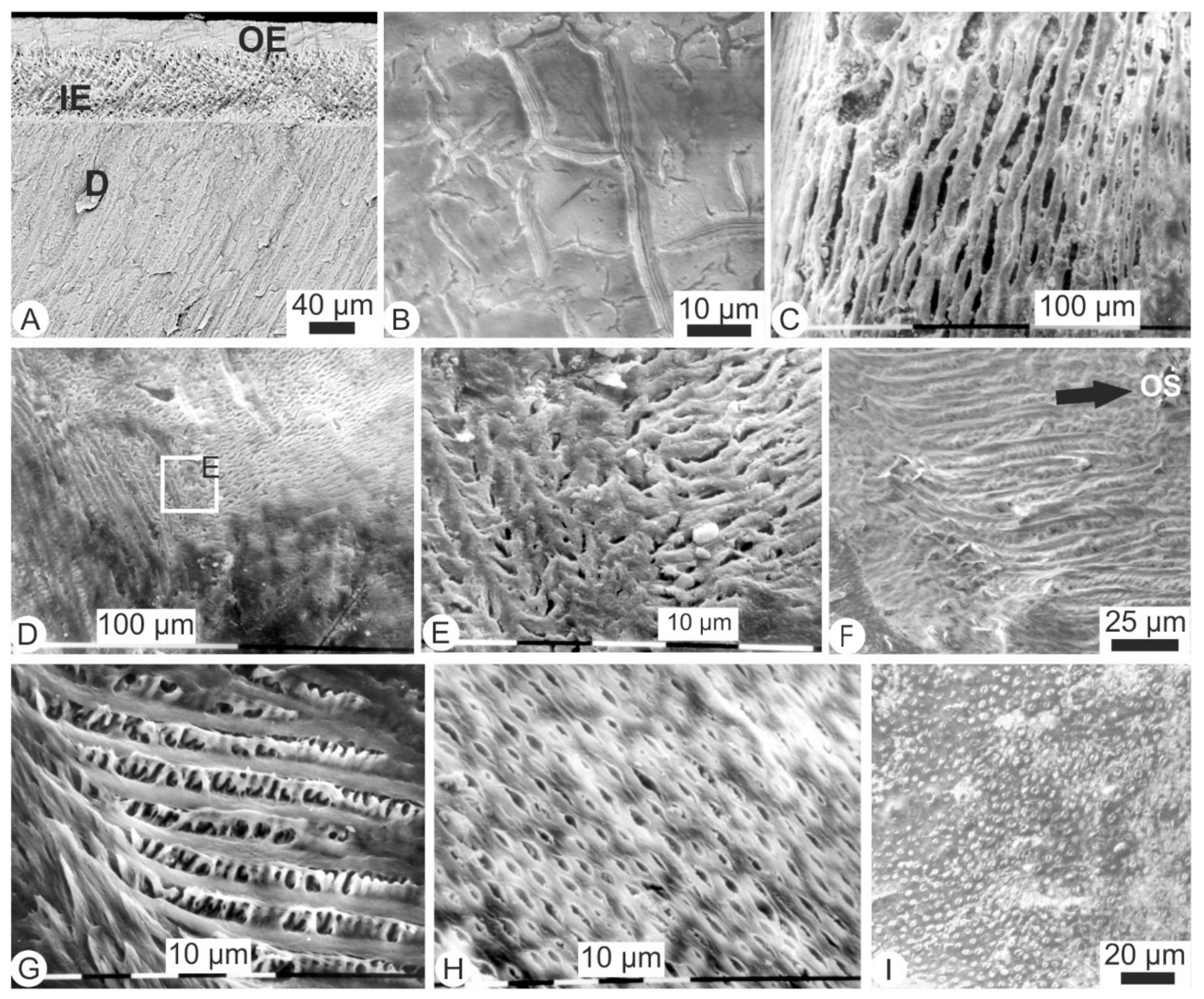
3.4. Surficial, Microstructural Alterations of Digested Teeth
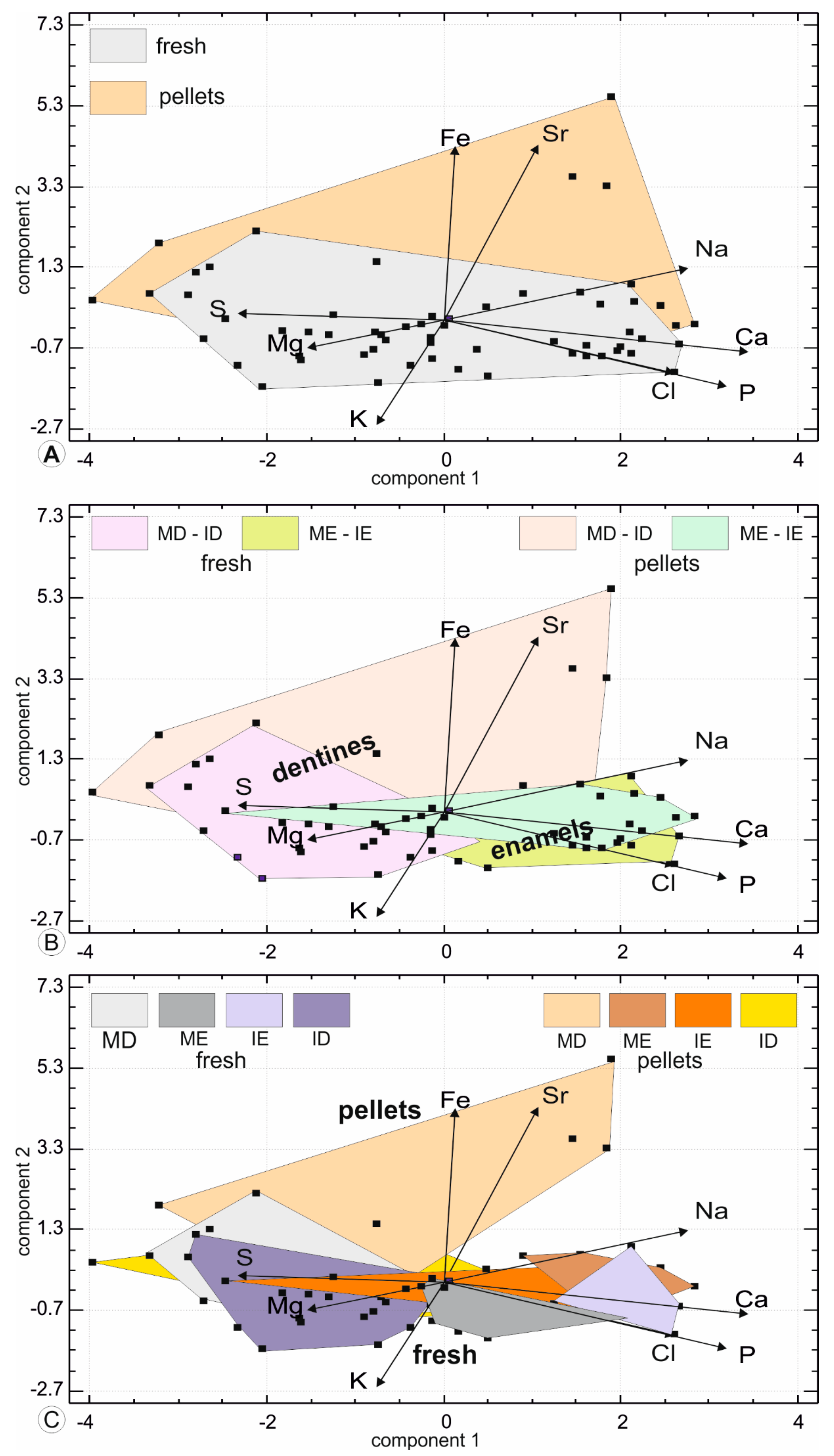
3.5. Compositional Alterations of Digested Teeth
4. Discussion
4.1. The Used Techniques
4.2. Comparisons between Fresh and Pellet Teeth
4.3. Comparisons between Fresh and Digested Bones from the Same Site
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Mayhew, D.F. Avian predators as accumulators of fossil mammal material. Boreas 1977, 6, 25–31. [Google Scholar] [CrossRef]
- Shipman, P. Applications of scanning electron microscopy to taphonomic problems. Ann. N. Y. Acad. Sci. 1981, 376, 357–385. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P. Owls, Caves and Fossils; Natural History Museum Publications: London, UK, 1990. [Google Scholar]
- Fernandez-Jalvo, Y.; Andrews, P. Small mammal taphonomy of Gran Dolina, Atapuerca (Burgos), Spain. J. Archaeol. Sci. 1992, 19, 407–428. [Google Scholar] [CrossRef]
- Matthews, T. Taphonomic characteristics of micromammals predated by small mammalian carnivores in South Africa: Application to fossil accumulations. J. Taphon. 2006, 4, 143–161. [Google Scholar]
- Bochensky, Z.M.; Tomek, T. Preservation of bird bones: Erosion versus digestion by owls. Int. J. Osteoarchaeol. 1997, 7, 372–387. [Google Scholar] [CrossRef]
- Bochensky, Z.M.; Korodin, V.A.; Nekrasov, A.E.; Tomek, T. Fragmentation of bird bones in food remains of imperial eagles (Aquila heliaca). Int. J. Osteoarchaeol. 1997, 7, 165–171. [Google Scholar] [CrossRef]
- Fernandez-Jalvo, Y.; Andrews, P.; Sevilla, P.; Requejo, V. Digestion versus abrasion features in rodent bones. Lethaia 2017, 47, 323–336. [Google Scholar] [CrossRef]
- Fernandez-Jalvo, Y.; Andrews, P. Atlas of Taphonomic Identifications; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2016. [Google Scholar]
- Dauphin, Y.; Denis, A.; Denys, C. Les mécanismes de formation des gisements de microvertébrés: Modifications de la composition chimique des os et dents de rongeurs issus de pelotes de régurgitation de rapaces. C. R. Acad. Sci. Paris 1988, 307, 603–608. [Google Scholar]
- Dauphin, Y.; Denys, C.; Denis, A. Les mécanismes de formation des gisements de microvertébrés. 2. Composition chimique élémentaire des os et dents de rongeurs provenant de pelotes de régurgitation. Bull. Mus. Natl. Hist. Nat. Paris 1989, 11, 253–269. [Google Scholar]
- Dauphin, Y.; Denys, C.; Kowalski, K. Analysis of accumulations of rodent remains: Role of the chemical composition of skeletal elements. N. Jahrb. Geol. Paläontol. Abh. 1997, 203, 295–315. [Google Scholar] [CrossRef]
- Dauphin, Y.; Castillo-Michel, H.; Farre, B.; Mataame, A.; Rbii, K.; Rihane, A.; Stoetzel, E.; Denys, C. Identifying predation on rodent teeth through structure and composition: A case from Morocco. Micron 2015, 75, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Toots, H.; Voorhies, M.R. Strontium in fossil bones and the reconstruction of food chains. Science 1965, 149, 545–855. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, R.W.G.; Doberenz, A.R. The strontium content of fossil teeth and bones. Geochim. Cosmochim. Acta 1968, 32, 109–115. [Google Scholar] [CrossRef]
- Henderson, P.; Marlow, C.A.; Molleson, T.I.; Williams, C.T. Patterns of chemical change during bone fossilization. Nature 1983, 306, 358–360. [Google Scholar] [CrossRef]
- Williams, C.T. Trace elements in fossil bone. Appl. Geochem. 1989, 4, 247–248. [Google Scholar] [CrossRef]
- Williams, C.T.; Marlow, C.A. Uranium and thorium distribution in fossil bones from Olduvai Gorge, Tanzania and Kanam, Kenya. J. Archaeol. Sci. 1987, 14, 297–309. [Google Scholar] [CrossRef]
- Williams, C.T.; Potts, P.J. Element distribution maps in fossil bones. Archaeometry 1988, 30, 237–247. [Google Scholar] [CrossRef]
- Dauphin, Y.; Denys, C. Diagenèse différentielle chez les rongeurs fossiles—Validité des paramètres géochimiques pour les reconstitutions des régimes alimentaires. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 99, 213–223. [Google Scholar] [CrossRef]
- Kendall, C.; Høier Eriksen, A.M.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef]
- Garcia-Alix, A.; Minwer-Barakat, R.; Martin Suarez, E.; Matthijs Freudenthal, M.; Delgado Huertas, A. Cinnabar mineralization in fossil small mammal remains as a consequence of diagenetic processes. Lethaia 2013, 46, 1–6. [Google Scholar] [CrossRef]
- Dauphin, Y.; Massard, P. Diagenèse des os de rongeurs fossiles d’El Harhoura 2 (Maroc): Microstructure versus composition globale. Trav. Inst. Sci. Sér. Gén. 2015, 8, 31–42. [Google Scholar]
- Keenan, S.W.; Engel, A.D. Early diagenesis and recrystallization of bone. Geoch. Cosmoch. Acta 2017, 196, 209–223. [Google Scholar] [CrossRef]
- McMillan, R.; Weis, D.; Amini, M.; Bonjean, D. Identifying the reworking and stratigraphic provenance of bones by exploring multivariate geochemical relationships with the ‘Perio-spot’ technique. J. Archaeol. Sci. 2017, 88, 1–13. [Google Scholar] [CrossRef]
- Dauphin, Y.; Castillo-Michel, H.; Denys, C.; El Hajiraoui, M.A.; Nespoulet, R.; Stoetzel, E. Diagenetic alterations of Meriones incisors (Rodentia) of El Harhoura 2 cave, Morocco (Late Pleistocene-middle Hococene). PalZ 2018, 92, 163–177. [Google Scholar] [CrossRef]
- Thomas, D.B.; Chinsamy, A.; Conard, N.J.; Kandel, A.W. Chemical investigation of mineralisation categories used to assess taphonomy. Palaeogrogr. Palaeoclimatol. Palaeoecol. 2012, 361, 104–110. [Google Scholar] [CrossRef]
- Williams, J.P. Bones of comprehension—The analysis of small mammal predator-prey interactions. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewski, M., Hansen, T.A., Eds.; Kluwer Acad Plenum: New York, NY, USA, 2003. [Google Scholar]
- Jaeger, J.J. (Université de Poitiers, Poitiers, France). Personal communication, 1989.
- Açil, Y.; Mobasseri, A.E.; Warnke, P.H.; Terheyden, H.; Wiltfang, J.; Springer, I. Detection of mature collagen in human dental enamel. Calc. Tissue Intern. 2005, 76, 121–126. [Google Scholar]
- Duverger, O.; Beniash, E.; Morasso, M.I. Keratins as components of the enamel organic matrix. Matrix Biol. 2016, 52–54, 260–265. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Nik Hassan, N.F.; Arya, N. Evaluation of Raman spectroscopy and application of chemometric methods for the differentiation of contemporary ivory specimens I: Elephant and mammalian species. J. Raman Spectr. 2006, 37, 353–360. [Google Scholar] [CrossRef]
- France, C.A.M.; Thomas, D.B.; Doney, C.R.; Madden, O. FT-Raman spectroscopy as a method for screening collagen diagenesis in bone. J. Archaeol. Sci. 2014, 42, 346–355. [Google Scholar] [CrossRef]
- Coello, B.; Lopez-Alvarez, M.; Rodriguez-Dominguez, M.; Serra, J.; Gonzalez, P. Quantitative evaluation of the mineralization level of dental tissues by Raman spectroscopy. Biomed. Phys. Eng. Express 2015, 1, 045204. [Google Scholar] [CrossRef]
- Korvenkontio, V.A. Mikroskopische Untersuchungen an Nagerincisiven, unter Hinweis auf die Schmelzstruktur der Backenzahne. Ann. Zool. Soc. Zool. Bot. Fenn. Vanamo 1934, 2, i-xiv: 1–274. [Google Scholar]
- Wahlert, J.H. Variability of rodent incisor enamel as viewed in thin section, and the microstructure of the enamel in fossil and recent rodent groups. Breviora 1968, 309, 1–18. [Google Scholar]
- Risnes, D. A scanning electron microscope study of aberrations in the prism pattern of rat incisor inner enamel. Am. J. Anat. 1979, 54, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.J.; Wopenka, B.; Silva, M.J.; Pasteris, J.D. Raman spectroscopic detection of changes in bioapatite in mouse femora as a function of age and in vitro fluoride treatment. Calcif. Tissue Int. 2002, 68, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Slimani, A.; Nouioua, F.; Desoutter, A.; Levallois, B.; Cuisinier, F.J.G.; Tassery, H.; Terrer, E.; Salehia, H. Confocal Raman mapping of collagen cross-link and crystallinity of human dentin-enamel junction. J. Biomed. Opt. 2017, 22, 086003. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, E.V.; Timchenko, P.E.; Volova, J.T.; Rosenbaum, A.Y.; Kulabukhova, A.Y. Analysis of tooth tissues using Raman spectroscopy. J. Phys. Conf. Ser. 2016, 769, 012047. [Google Scholar] [CrossRef]
- Pindborg, J.J. The pigmentation of the rat incisor as an index of metabolic disturbances. Oral Surg. Oral Med. Oral Pathol. 1953, 6, 780–789. [Google Scholar] [CrossRef]
- McKee, M.D.; Zerounian, C.; Martineau-Doizé, B.; Warshawsky, H. Specific binding sites for transferrin on ameloblasts of the enamel maturation zone in the rat incisor. Anat. Rec. 1987, 218, 123–127. [Google Scholar] [CrossRef]
- Soulier, M.C.; Costamagno, S. Let the cutmarks speak! Experimental butcheries to reconstruct carcass processing. J. Archaeol. Sci. Rep. 2017, 11, 782–802. [Google Scholar] [CrossRef]
- Weiner, S. Microarchaeology—Beyond the Visible Archaeological Record; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Dauphin, Y.; Nespoulet, R.; Stoetzel, E.; el Haijraoui, M.A.; Denys, C. Can colour be used as a proxy for paleoenvironmental reconstuctions based on archaeological bones? El Harhoura 2 (Morocco) case study. J. Taphon. 2012, 10, 69–84. [Google Scholar]
- Epstein, A.G.; Epstein, J.B.; Harris, L.D. Conodont color alteration: An index to organic metamorphism. Geol. Survey Profes. Paper 1977, 995, 1–27. [Google Scholar]
- Conard, N.J.; Walker, S.J.; Kandel, A.W. How heating and cooling and wetting and drying can destroy dense faunal elements and lead to differential preservation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 236–245. [Google Scholar] [CrossRef]
- Termine, J.D.; Posner, A.S. Infra-red determination of the percentage of crystallinity in apatitic calcium phosphates. Nature 1966, 211, 268–270. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.; Trautz, O.R.; Klein, E.; LeGeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Sillen, A. Biogenic and diagenetic Sr/Ca in Plio-Pleistocene fossils of the Omo Shungura formation. Paleobiology 1986, 12, 311–323. [Google Scholar] [CrossRef]
- Shemesh, A. Crystallinity and diagenesis of sedimentary apatites. Geochim. Cosmochim. Acta 1990, 545, 2433–2438. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- El Feki, H.; Rey, C.; Vignoles, M. Carbonate ions in apatites: Infrared investigations in the 4 CO3 domain. Calcif. Tissue Int. 1991, 49, 269–274. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishnan, V.; Collins, B.; Glimcher, M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif. Tissue Int. 1991, 46, 251–258. [Google Scholar] [CrossRef]
- Botha, J.; Lee-Thorp, J.; Sponheimer, M. An examination of Triassic cynodont tooth enamel chemistry using Fourier Transform infrared spectroscopy. Calcif. Tissue Int. 2004, 74, 163–169. [Google Scholar] [CrossRef]
- Farre, B.; Massard, P.; Nouet, J.; Dauphin, Y. Preservation of rodent bones from El Harhoura 2 cave (Morocco, Neolithic-Middle Palaeolithic): Microstructure, mineralogy, crystallinity and composition. J. Afr. Earth Sci. 2014, 92, 1–13. [Google Scholar] [CrossRef]
- Wright, L.E.; Schwarcz, H.P. Infrared and isotopic evidence for diagenesis of bone apatite at Dos Pilas, Guatemala: Palaeodietary implications. J. Archaeol. Sc. 1996, 23, 933–944. [Google Scholar] [CrossRef]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; van der Houwen, J.A.M.; Silva, M.J. Lack of OH in nanocrystalline apatite as a function of degree of atomic order: Implications for bone and biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Young, H.A. Implications of atomic substitutions and other structural details in apatite. J. Dent. Res. 1974, 53, 193–203. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Dauphin, Y.; Andrews, P.; Denys, C.; Fernandez-Jalvo, Y.; Williams, C.T. Structural and chemical bone modifications in a modern owl pellet assemblage from Olduvai Gorge (Tanzania). J. Taphon. 2003, 1, 209–231. [Google Scholar]
- Denys, C.; Williams, C.T.; Dauphin, Y.; Andrews, P.; Fernandez-Jalvo, Y. Diagenetical changes in Pleistocene small mammal bones from Olduvai Bed, I. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 126, 121–134. [Google Scholar] [CrossRef]
- Dauphin, Y.; Williams, C.T.; Andrews, P.; Denys, C.; Fernandez-Jalvo, Y. Diagenetic alterations of micromammal fossil bones from Olduvai Bed I of the Lower Pleistocene sequence at Olduvai Gorge, Tanzania. J. Sedim. Res. 1999, 69, 612–621. [Google Scholar] [CrossRef]
- Rink, W.J.; Schwarcz, H.P.; Weiner, S.; Goldberg, P.; Meignen, L.; Bar-Yosef, O. Age of the Mousterian industry at Hayonim Cave, Northern Israel, using electron spin resonance and 230Th/234U methods. J. Archaeol. Sci. 2004, 31, 953–964. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauphin, Y. Using Microstructures and Composition to Decipher the Alterations of Rodent Teeth in Modern Regurgitation Pellets—A Good News-Bad News Story. Minerals 2020, 10, 63. https://doi.org/10.3390/min10010063
Dauphin Y. Using Microstructures and Composition to Decipher the Alterations of Rodent Teeth in Modern Regurgitation Pellets—A Good News-Bad News Story. Minerals. 2020; 10(1):63. https://doi.org/10.3390/min10010063
Chicago/Turabian StyleDauphin, Yannicke. 2020. "Using Microstructures and Composition to Decipher the Alterations of Rodent Teeth in Modern Regurgitation Pellets—A Good News-Bad News Story" Minerals 10, no. 1: 63. https://doi.org/10.3390/min10010063
APA StyleDauphin, Y. (2020). Using Microstructures and Composition to Decipher the Alterations of Rodent Teeth in Modern Regurgitation Pellets—A Good News-Bad News Story. Minerals, 10(1), 63. https://doi.org/10.3390/min10010063



