Strength and Volume Change Characteristics of Clayey Soils: Performance Evaluation of Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Enzymes
2.1.2. Reference Clays
2.1.3. UKM Soil Mixed with 10% Bentonite (S1)
2.2. Samples Preparation and Testing
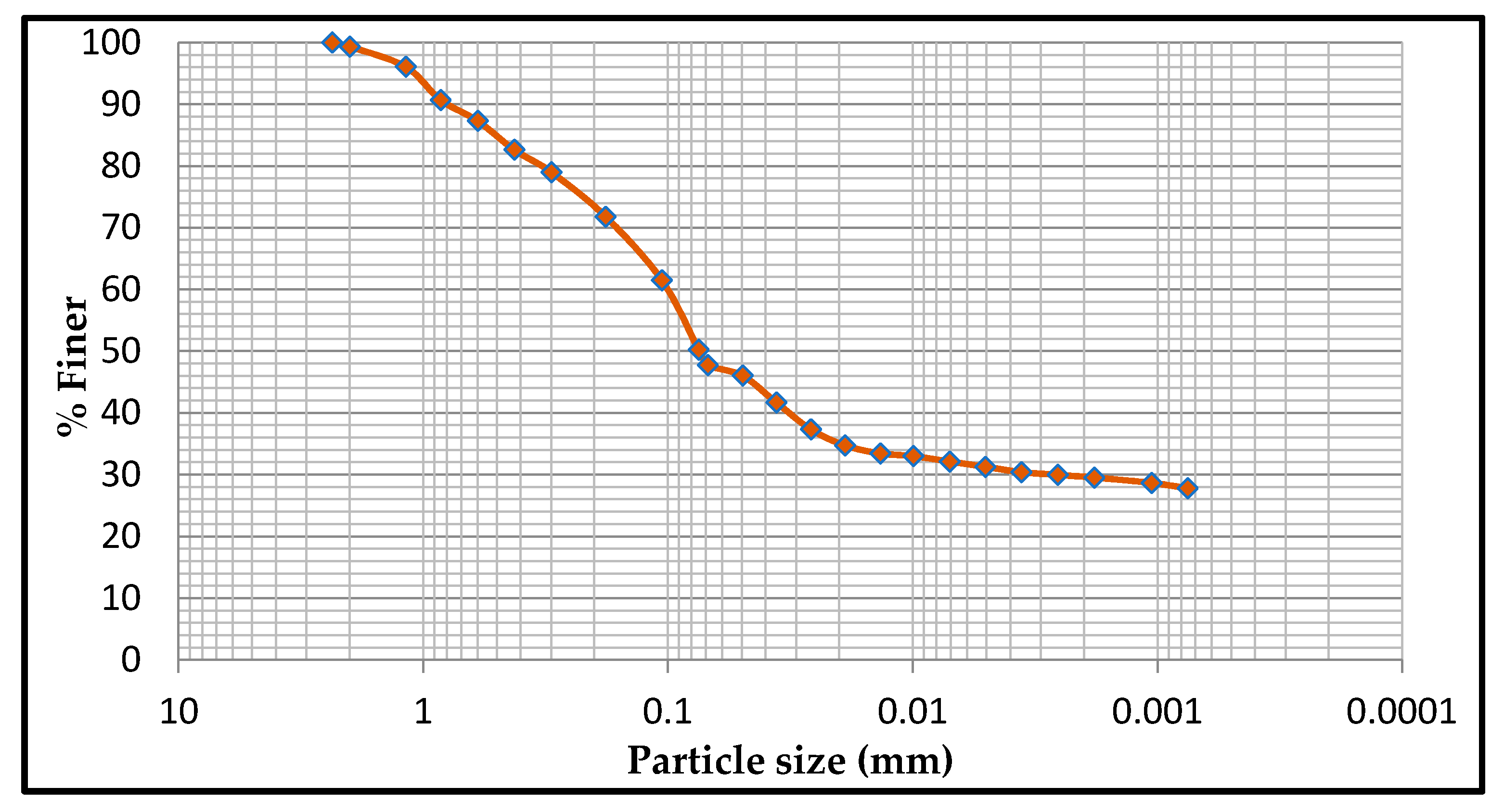
3. Results and Discussion
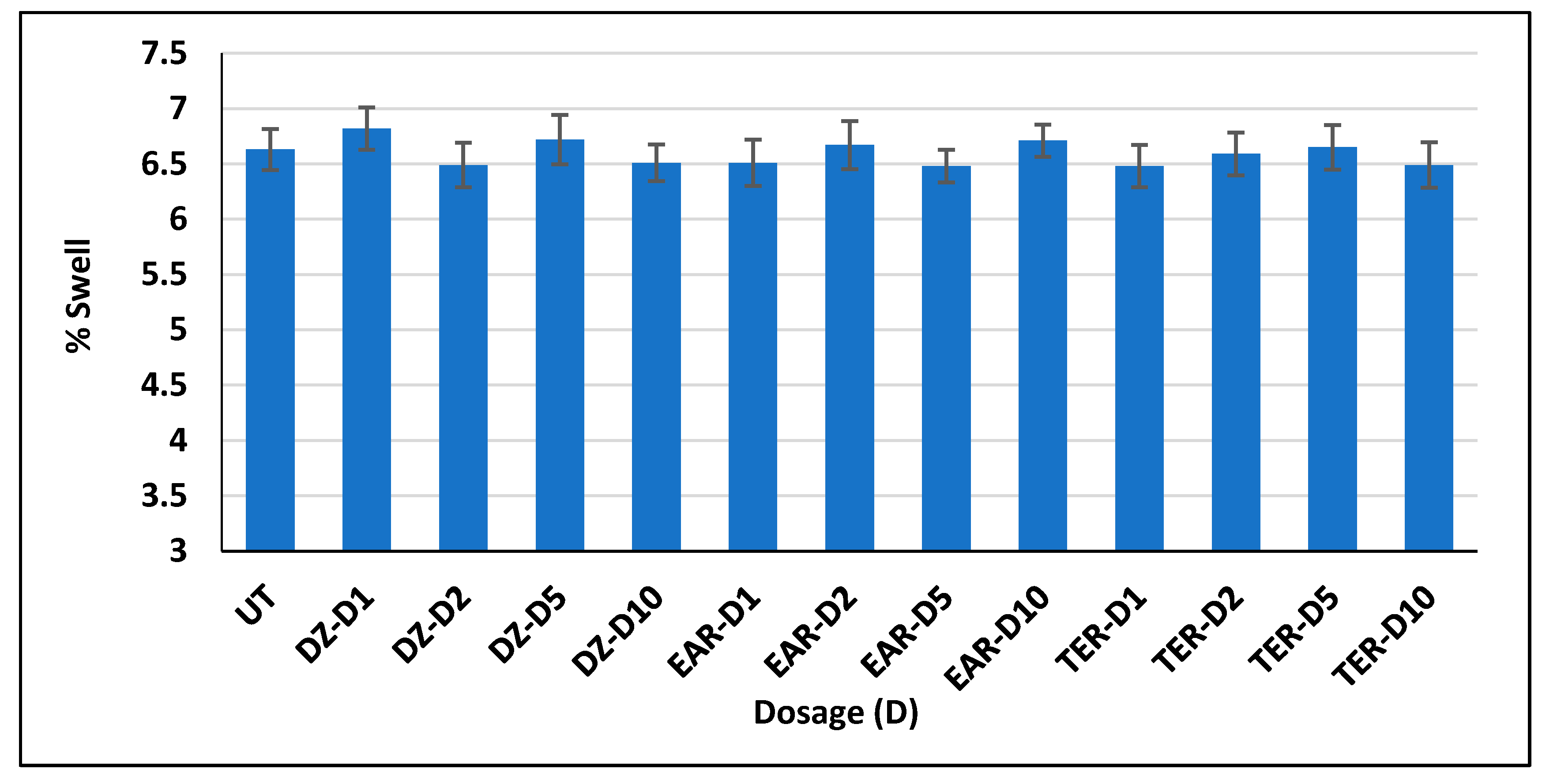
3.1. Three-Dimensional Swell Test
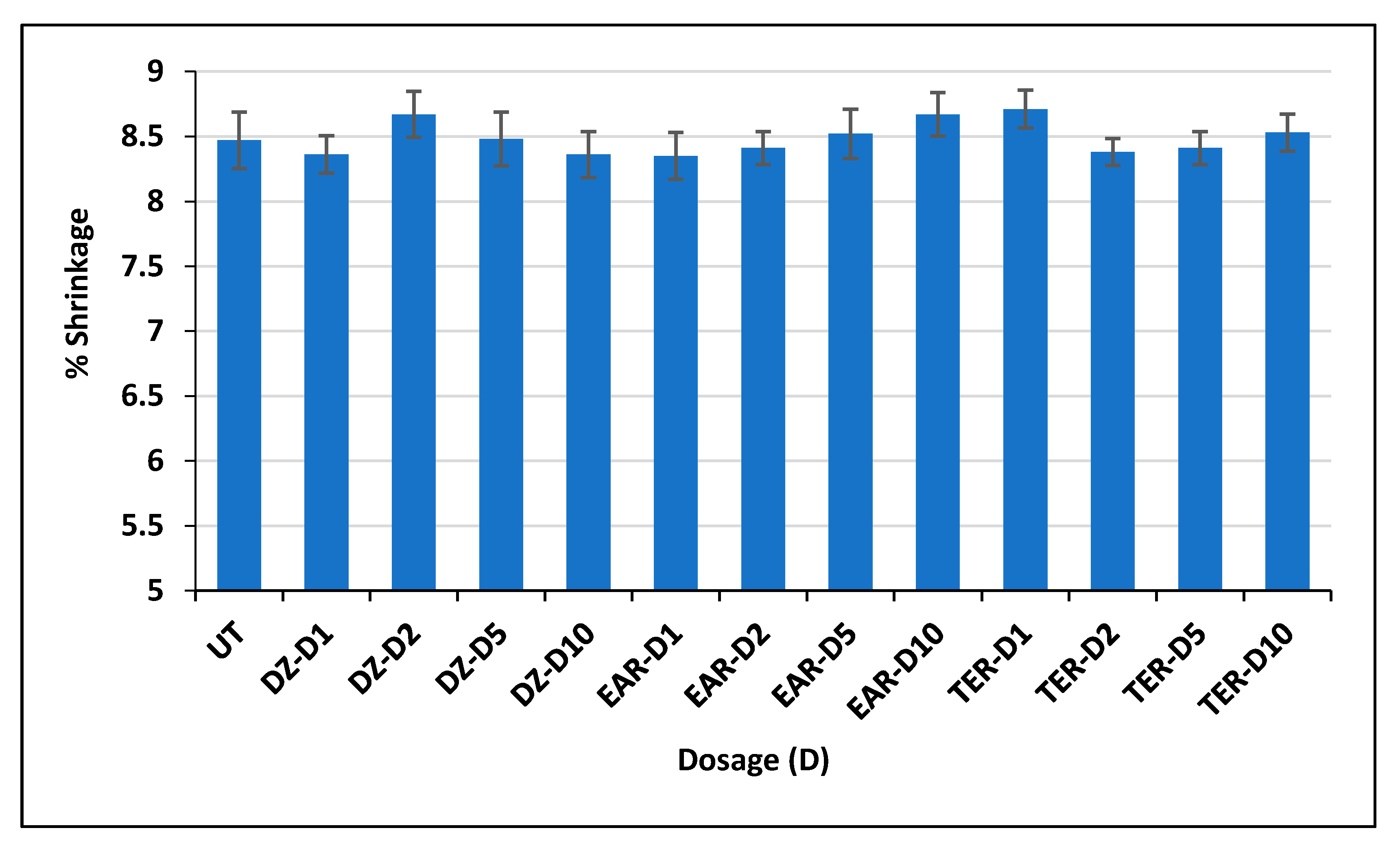
3.2. Shrinkage Test
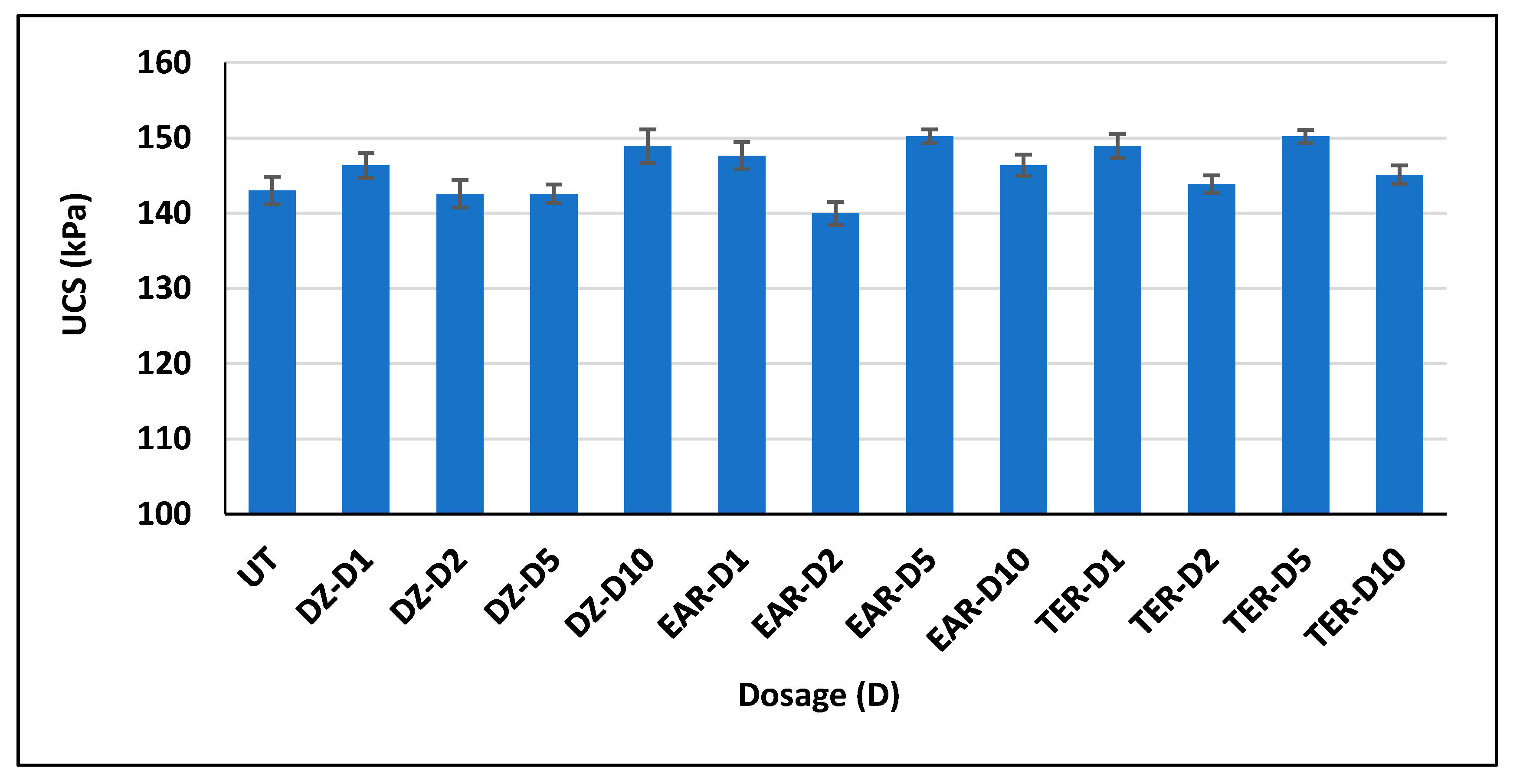
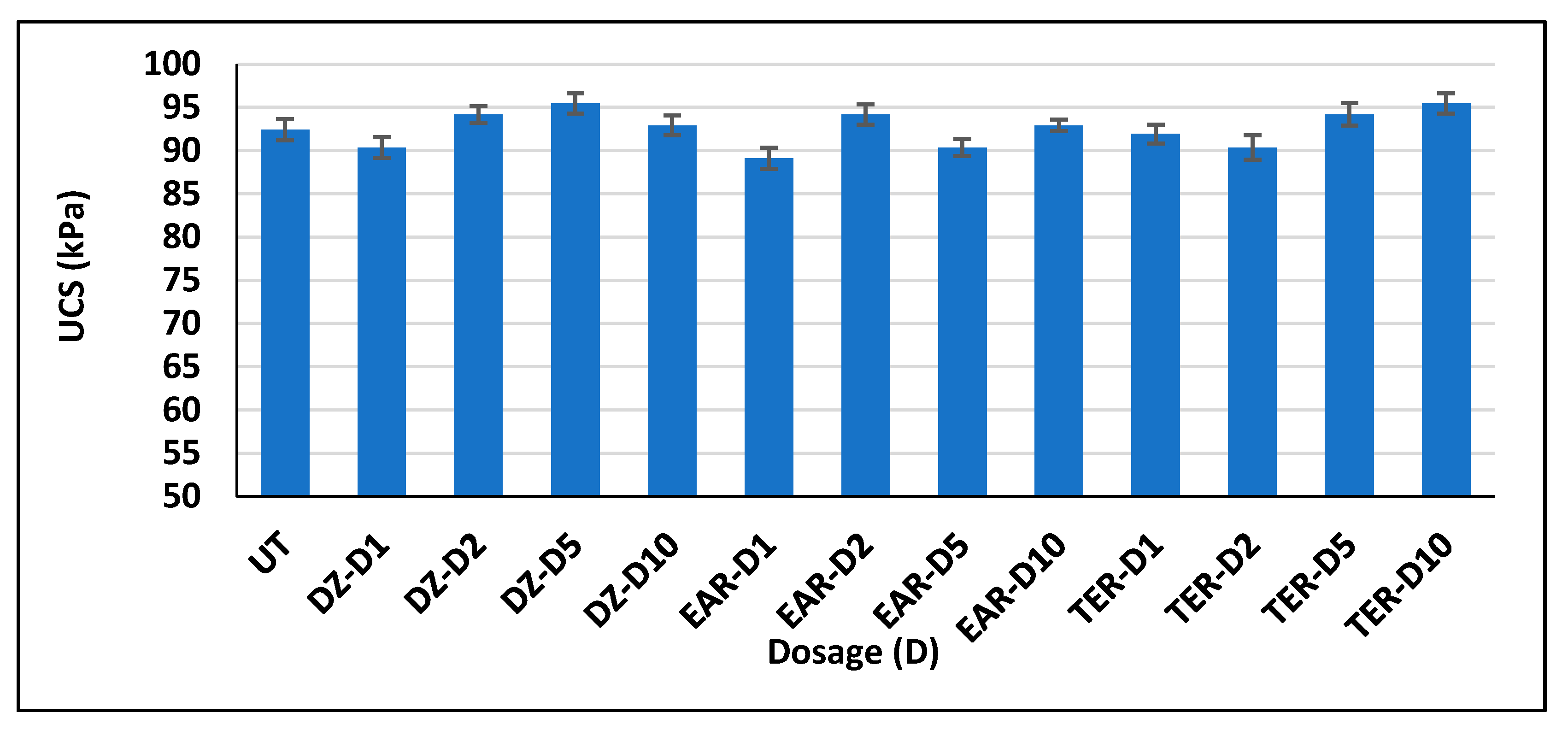
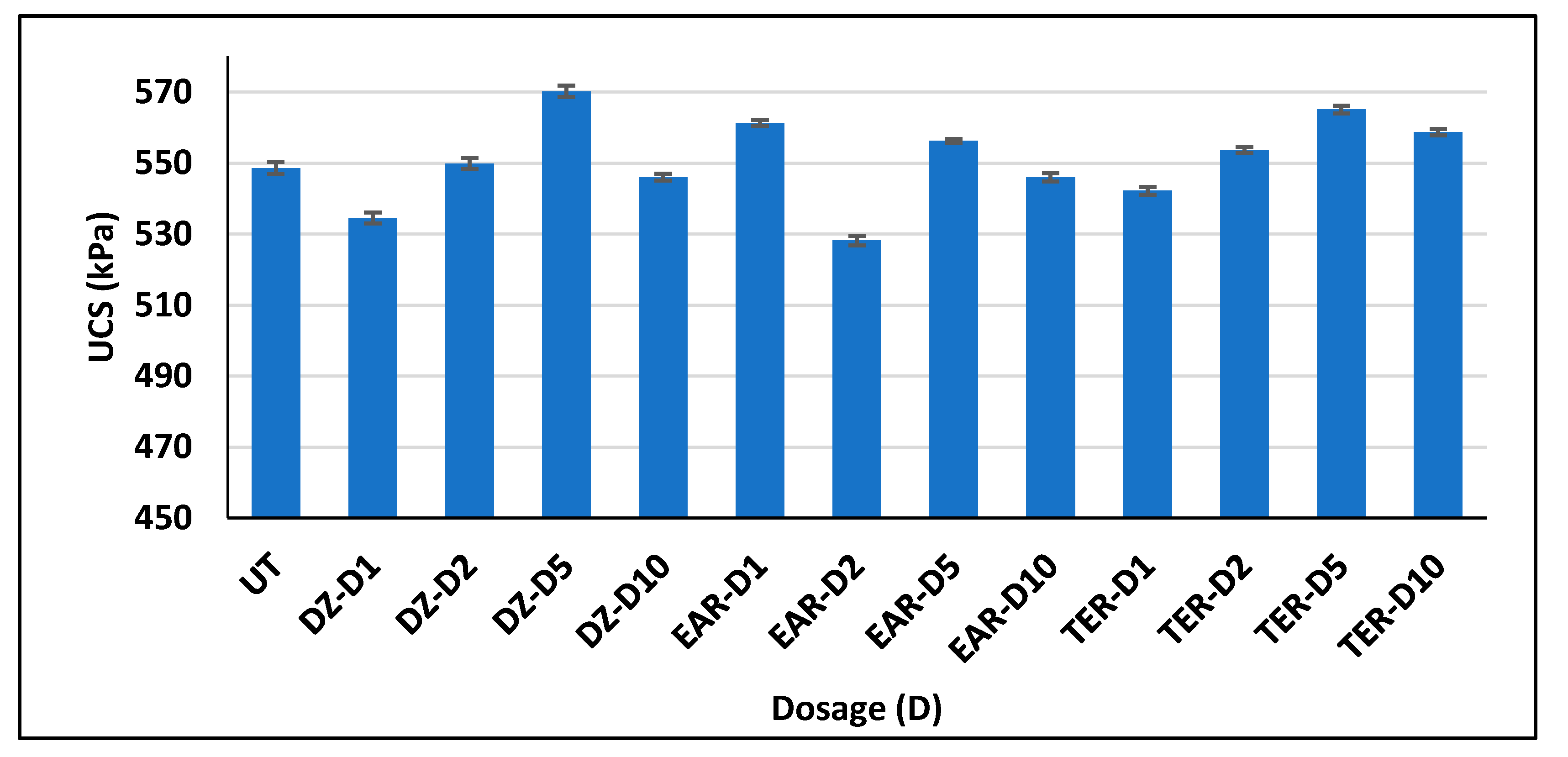
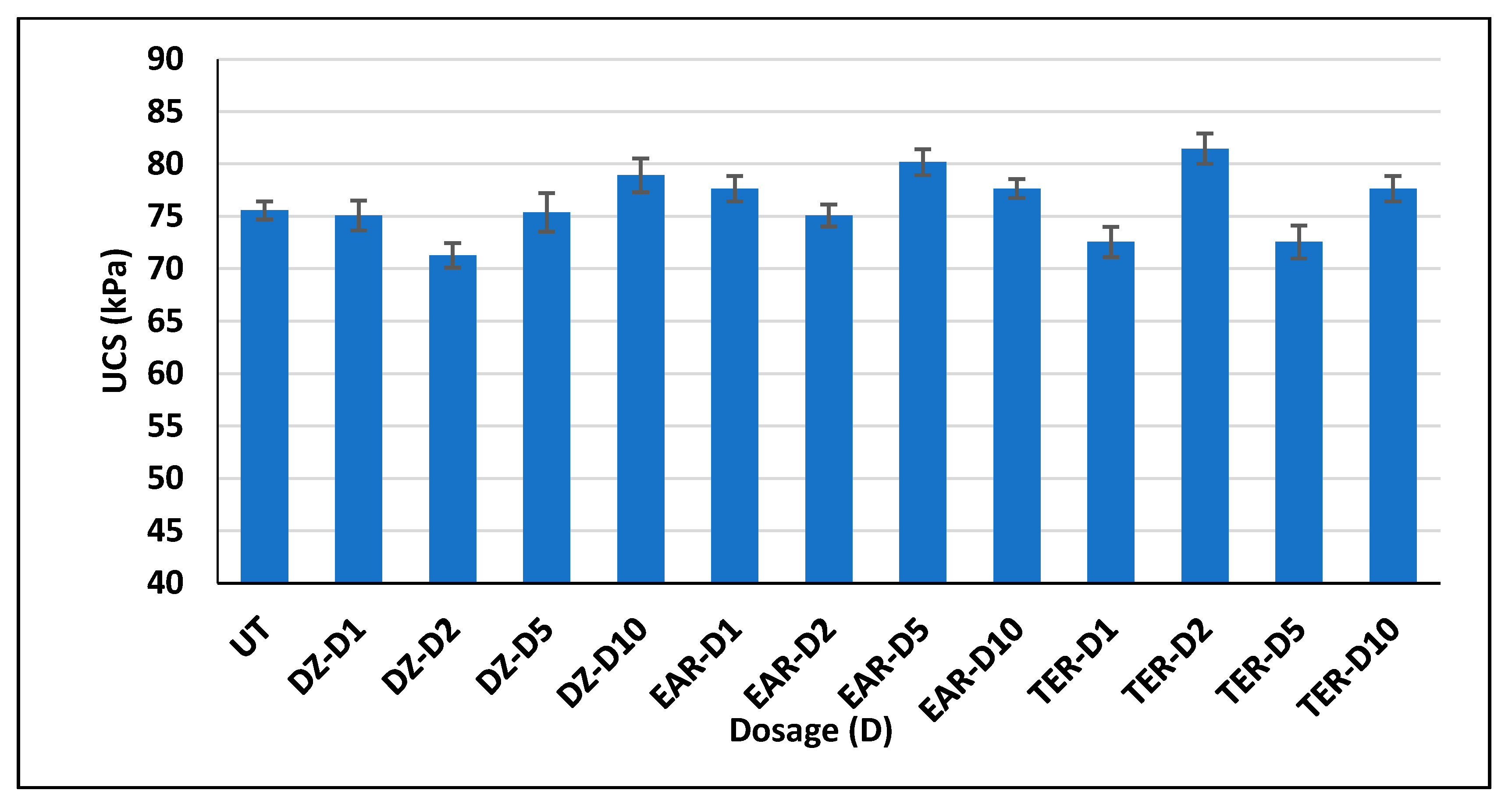
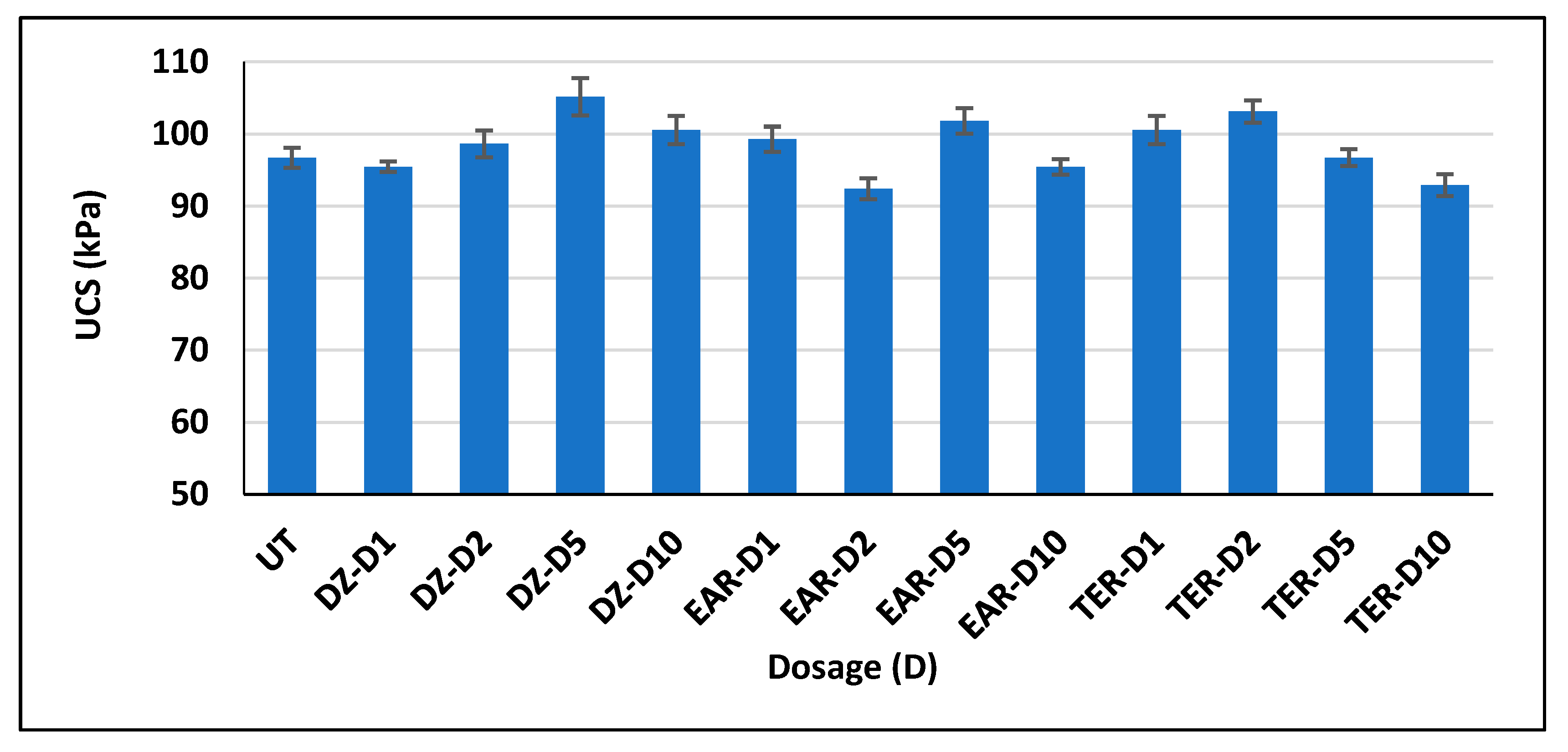
3.3. Unconfined Compressive Strength Test
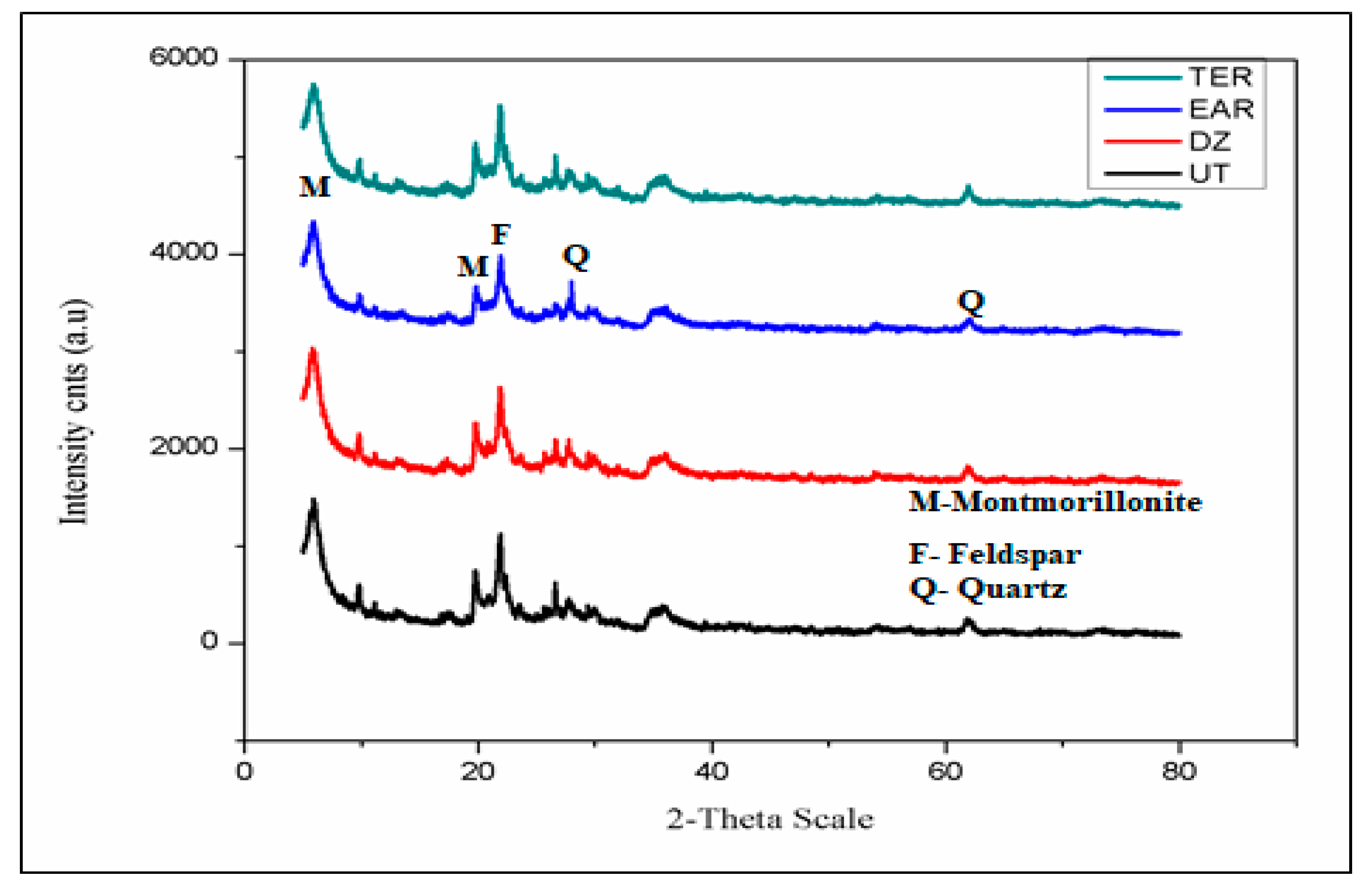
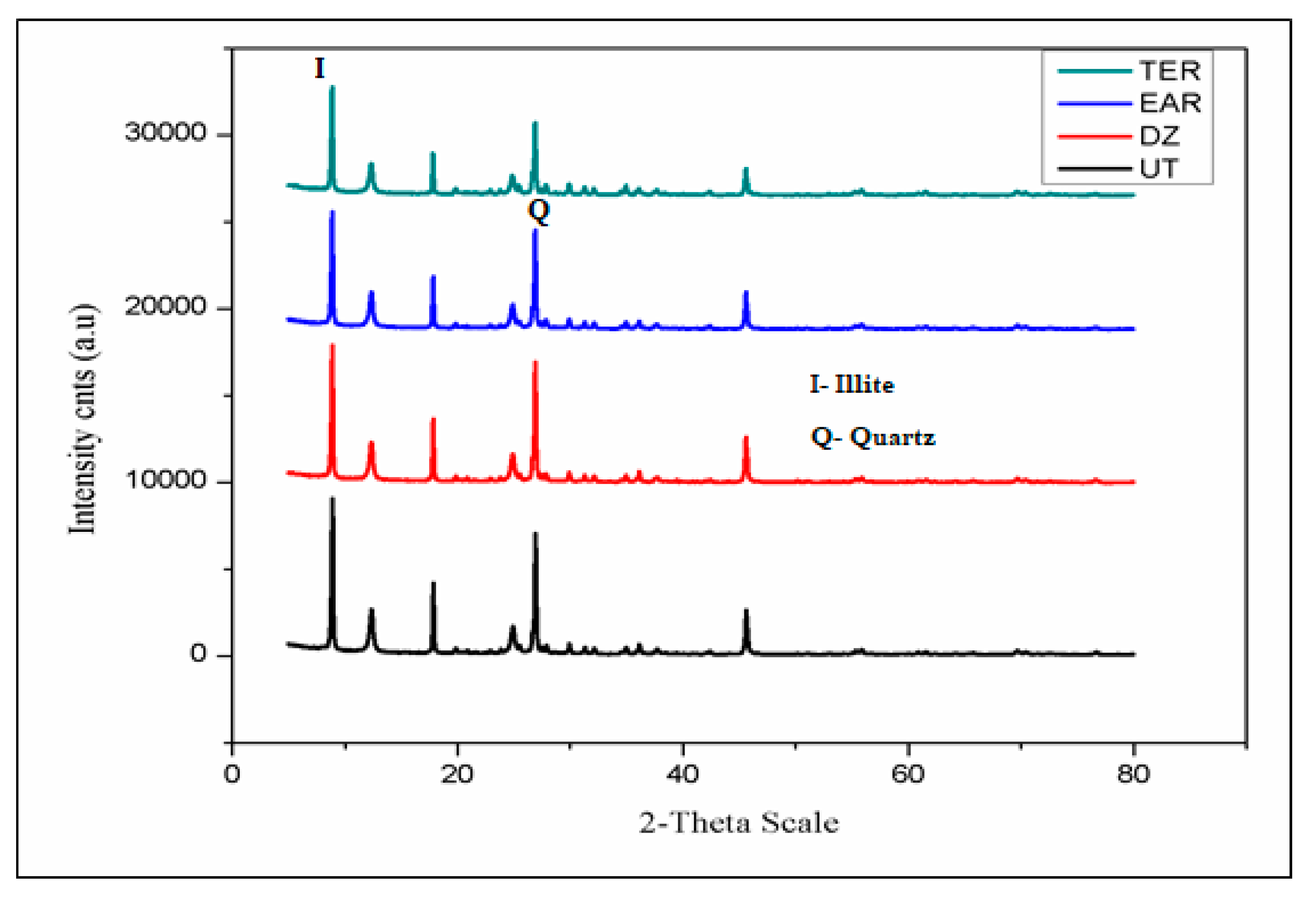
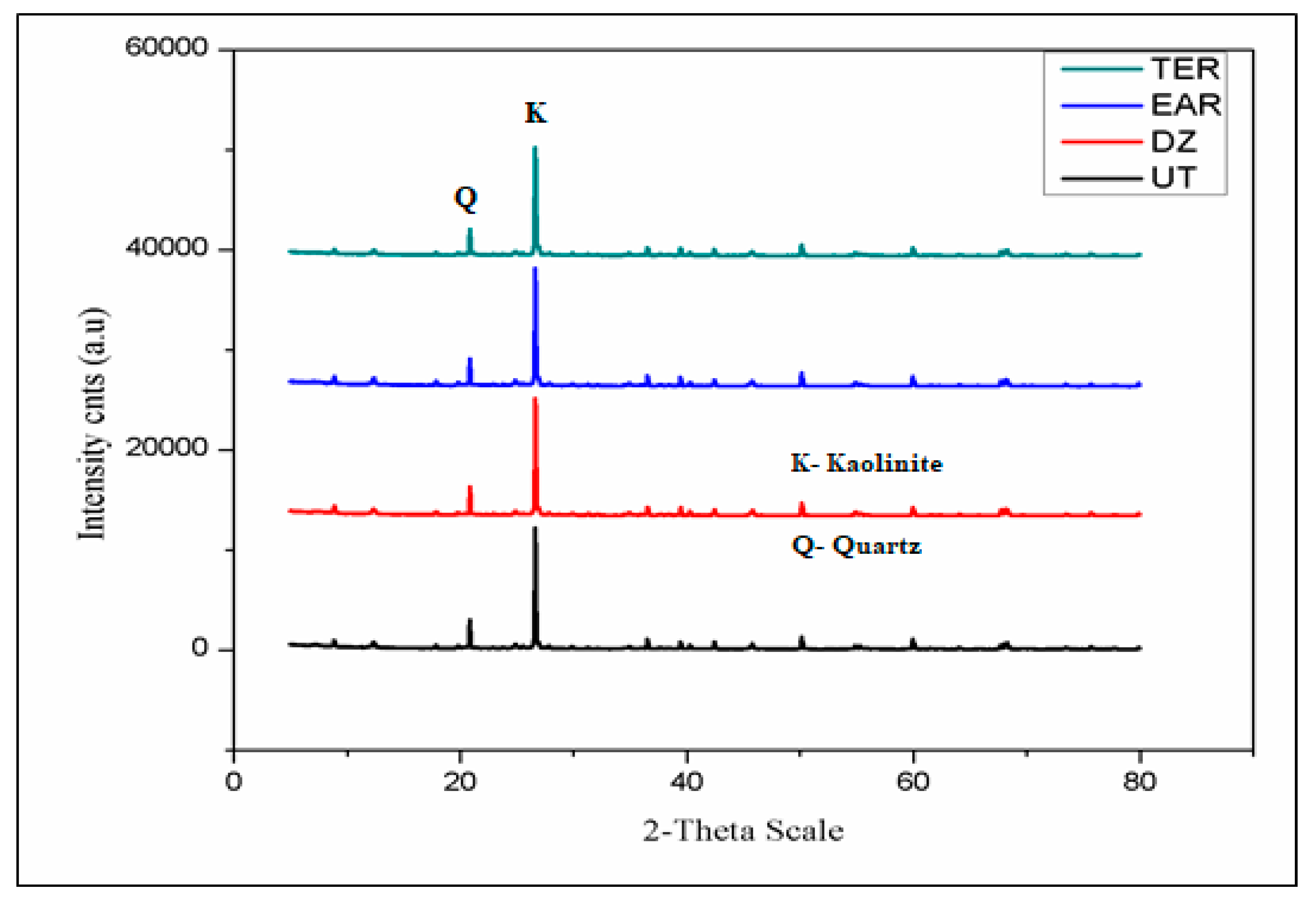
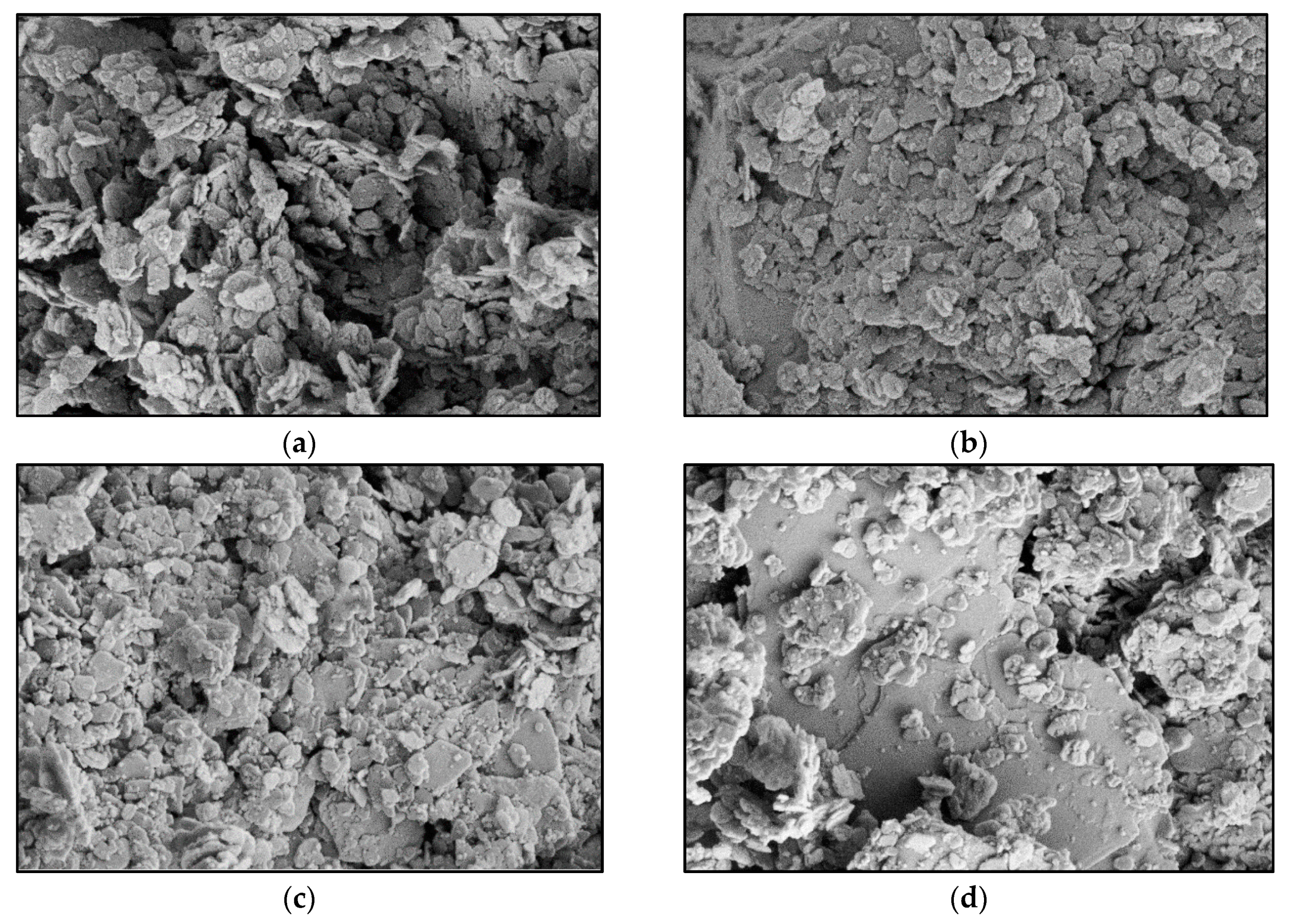
3.4. X-Ray Diffraction (XRD) and Field Emission Scanning Electron Microscope (FESEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muguda, S.; Nagaraj, H. Effect of enzymes on plasticity and strength characteristics of an earthen construction material. Int. J. Geo Eng. 2019, 10, 2. [Google Scholar] [CrossRef]
- Latifi, N.; Vahedifard, F.; Ghazanfari, E.; Horpibulsuk, S.; Marto, A.; Williams, J. Sustainable improvement of clays using low-carbon nontraditional additive. Int. J. Geomech. 2017, 18, 04017162. [Google Scholar] [CrossRef]
- Hoy, M.; Rachan, R.; Horpibulsuk, S.; Arulrajah, A.; Mirzababaei, M. Effect of wetting–drying cycles on compressive strength and microstructure of recycled asphalt pavement–Fly ash geopolymer. Constr. Build. Mater. 2017, 144, 624–634. [Google Scholar] [CrossRef]
- Alazigha, D.P.; Indraratna, B.; Vinod, J.S.; Ezeajugh, L.E. The swelling behaviour of lignosulfonate-treated expansive soil. Mater. Sci. 2016. [Google Scholar] [CrossRef]
- Zhao, Y.; Soltani, A.; Taheri, A.; Karakus, M.; Deng, A. Application of slag–cement and fly ash for strength development in cemented paste backfills. Minerals 2019, 9, 22. [Google Scholar] [CrossRef]
- Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; Soltani, A.; Khayat, N. Stabilization of soft clay using short fibers and poly vinyl alcohol. Geotext. Geomembr. 2018, 46, 646–655. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; Mirzababaei, M. A sulphonated oil for stabilisation of expansive soils. Int. J. Pavement Eng. 2019, 20, 1285–1298. [Google Scholar] [CrossRef]
- Yazdandoust, F.; Yasrobi, S.S. Effect of cyclic wetting and drying on swelling behavior of polymer-stabilized expansive clays. Appl. Clay Sci. 2010, 50, 461–468. [Google Scholar] [CrossRef]
- Mousavi, F.; Abdi, E.; Rahimi, H. Effect of polymer stabilizer on swelling potential and CBR of forest road material. KSCE J. Civ. Eng. 2014, 18, 2064–2071. [Google Scholar] [CrossRef]
- Mirzababaei, M.; Yasrobi, S.; Al-Rawas, A. Effect of polymers on swelling potential of expansive soils. Proc. Inst. Civ. Eng. Ground Improv. 2009, 162, 111–119. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; Mirzababaei, M.; Vanapalli, S.K. Swell-Shrink Behavior of Rubberized Expansive Clays During Alternate Wetting and Drying. Minerals 2019, 9, 224. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; Mirzababaei, M. Rubber powder–polymer combined stabilization of South Australian expansive soils. Geosynth. Int. 2018, 25, 304–321. [Google Scholar] [CrossRef]
- Kestler, M.A. Stabilization Selection Guide for Aggregate-and Native-Surfaced Low-Volume Roads; US Department of Agriculture, Forest Service, National Technology & Development Program: Washington, DC, USA, 2009.
- Khan, T.A.; Taha, M.R. Effect of Three Bioenzymes on Compaction, Consistency Limits, and Strength Characteristics of a Sedimentary Residual Soil. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Sarkar, J.M.; Leonowicz, A.; Bollag, J.-M. Immobilization of enzymes on clays and soils. Soil Biol. Biochem. 1989, 21, 223–230. [Google Scholar] [CrossRef]
- Sravan, M.V.; Nagaraj, H.B. Potential use of enzymes in the preparation of compressed stabilized earth blocks. J. Mater. Civ. Eng. 2017, 29, 04017103. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 25–42. [Google Scholar]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Lacuoture, A.; Gonzalez, H. Usage of Organic Enzymes for the Stabilization of Natural Base Soils and Sub-Bases in Bagota; Faculty of Engineering, Pontificia Universidad Jevariana: Bogota, Colombia, 1995. [Google Scholar]
- Thomas, A.; Tripathi, R.; Yadu, L. A laboratory investigation of soil stabilization using enzyme and alkali-activated ground granulated blast-furnace slag. Arab. J. Sci. Eng. 2018, 43, 5193–5202. [Google Scholar] [CrossRef]
- Shukla, M.; Bose, S.; Sikdar, P. Bio-enzyme for stabilization of soil in road construction a cost effective approach. In Proceedings of the IRC Seminar Integrated Development of Rural and Arterial Road Networks for Socio-Economic Development, New Delhi, India, 5–6 December 2003. [Google Scholar]
- Venkatasubramanian, C.; Dhinakaran, G. Effect of Bio-Enzymatic Soil Stabilisation on Uneonfined Compressive Strength and California Bearing Ratio. J. Eng. Appl. Sci. 2011, 6, 295–298. [Google Scholar]
- Shankar, A.; Rai, H.K.; Mithanthaya, R. Bio-enzyme stabilized lateritic soil as a highway material. Indian Roads Congr. J. 2009, 70, 143–151. [Google Scholar]
- Hitam, A.; Yusof, A.Z.; Samad, O. Soil stabilizer for plantation road. In Proceedings of the National Seminar on Mechanization in Oil Palm Plantation, Palm Oil Research Institute of Malaysia (PORIM), Bangi, Malaysia, 30 June–1 July 1998. [Google Scholar]
- Milburn, J.P.; Parsons, R. Performance of Soil Stabilization Agents; Kansas Department of Transportation: Topeka, KS, USA, 2004.
- Brandon, F.; Ding, C.; Gary, H.; Charles, R. Permazyme Testing Volume I: Final Testing Summary Report; California Pavement Preservation Center: Chico, CA, USA, 2010. [Google Scholar]
- Mgangira, M. Evaluation of the Effects of Enzyme-Based Liquid Chemical Stabilizers on Subgrade Soils. In Proceedings of the 28th Southern African Transport Conference, Pretoria, South Africa, 6–9 July 2009. [Google Scholar]
- Rauch, A.F.; Katz, L.E.; Liljestrand, H.M. An Analysis of the Mechanisms and Efficacy of Three Liquid Chemical Soil Stabilizers; Research report 1993-1; Center for Transportation Research, The University of Texas at Austin: Austin, TX, USA, 1993; Volume 1. [Google Scholar]
- Tingle, J.S.; Santoni, R.L. Stabilization of clay soils with nontraditional additives. Transp. Res. Rec. 2003, 1819, 72–84. [Google Scholar] [CrossRef]
- Velasquez, R.; Marasteanu, M.O.; Hozalski, R. Investigation of the effectiveness and mechanisms of enzyme products for subgrade stabilization. Int. J. Pavement Eng. 2006, 7, 213–220. [Google Scholar] [CrossRef]
- Scholen, D. Non-Standard Stabilizers; Report FHWA-FLP-92-011; FHWA, U.S. Department of Transportation: Washington, DC, USA, 1992.
- Rauch, A. An Analysis of the Mechanisms and Efficacy of Three Liquid Chemical Soil Stabilizers; National Technical Information Service: Alexandria, VA, USA, 2003.
- Parsons, R.; Milburn, J. Engineering behavior of stabilized soils. Transp. Res. Rec. J. Transp. Res. Board 2003, 1837, 20–29. [Google Scholar] [CrossRef]
- Tingle, J.S.; Newman, J.K.; Larson, S.L.; Weiss, C.A.; Rushing, J.F. Stabilization mechanisms of nontraditional additives. Transp. Res. Rec. J. Transp. Res. Board 2007, 1989, 59–67. [Google Scholar] [CrossRef]
- Agarwal, P.; Kaur, S. Effect of bio-enzyme stabilization on unconfined compressive strength of expansive soil. Int. J. Res. Eng. Technol. 2014, 3, 30–33. [Google Scholar]
- Rauch, A.F.; Harmon, J.S.; Katz, L.E.; Liljestrand, H.M. Measured effects of liquid soil stabilizers on engineering properties of clay. Transp. Res. Rec. J. Transp. Res. Board 2002, 1787, 33–41. [Google Scholar] [CrossRef]
- Kabir, H.; Taha, M.R. Sedimentary residual soil as a waste containment barrier material. Soil Sediment Contam. 2004, 13, 407–420. [Google Scholar] [CrossRef]
- Taha, M.R.; Taha, O.M.E. Influence of nano-material on the expansive and shrinkage soil behavior. J. Nanoparticle Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Santoni, R.L.; Tingle, J.S.; Webster, S.L. Stabilization of silty sand with nontraditional additives. Transp. Res. Rec. 2002, 1787, 61–70. [Google Scholar] [CrossRef]
- NLA. Lime-Treated Soil Construction Manual Lime Stabilization & Lime Modification: Construction Manusal; Bulletin 326; National Lime Association: Arlington, VA, USA, 2004. [Google Scholar]



| Item | DZ-1X | EarthZyme | TerraZyme |
|---|---|---|---|
| Water | --- | 21.06% | >50% |
| Alcohols, C12-C16, ethoxylated | --- | --- | <30% |
| Fermented vegetable extract | --- | --- | <20% |
| Non-ionic surfactants | --- | 55% | --- |
| Polysaccharides | --- | 2% | --- |
| Oligosaccharides | --- | 3% | --- |
| Disaccharides | --- | 5% | --- |
| Monosaccharide | --- | 8% | --- |
| Lactic acid | --- | 3.5% | --- |
| Potassium as the chloride | --- | 1.2% | --- |
| Aluminum as the sulfate | --- | 0.04% | --- |
| Magnesium as the sulfate | --- | 1.2% | --- |
| Total | --- | 100% | --- |
| Specific gravity | 1.0 | 1.0 to 1.1 | 1.0 to 1.1 |
| pH (neat) | 4.5 | 3 to 6 | 2.8 to 3.5 |
| Boiling point | >100 °C | >100 °C | >100 °C |
| Ultimate biodegradability | --- | Dissolved organic content reduction >90% after 28 days | --- |
| Composition | --- | A blend of fermented carbohydrates, inorganic salts, and surfactants | --- |
| Stabilizer | DZ | EAR | TER |
|---|---|---|---|
| Suppliers recommended dosage | 1 L per 4.2 m3 | 1 L per 33 m3 | 1 L per 25 m3 |
| Equivalent Dilution Mass Ratio (DMR) | |||
| Equivalent Application Mass Ratio (AMR) * | |||
| Diluted application ratios (DAR) * | 28.5 mL per kg of soil | 18 mL per kg of soil | 24 mL per kg of soil |
| Characteristics | Bentonite | Illite | Kaolinite |
|---|---|---|---|
| SiO2 | 60.85 | 29.43 | 85.76 |
| Al2O3 | 14.82 | 52.37 | 9.11 |
| Fe2O3 | 4.38 | 1.85 | 0.38 |
| K2O | --- | 8.21 | 1.34 |
| CaO | 3.67 | --- | --- |
| MgO | --- | 1.76 | --- |
| Na2O | 3.13 | --- | --- |
| TiO2 | --- | 1.36 | --- |
| MgO | 3.09 | --- | --- |
| Heat loss | 8.22 | 5.02 | 3.41 |
| Specific gravity | 2.66 | 2.46 | 2.54 |
| Liquid Limit (LL) | 466 | 64.8 | 42.8 |
| Plastic limit (PL) | 69.5 | 22.7 | 17.1 |
| Plasticity Index (PI) | 396.5 | 42.1 | 25.7 |
| Optimum moisture content (OMC) | 30.9% | 23.6% | 17.1% |
| Maximum dry density (MDD) | 1.16 g/cm3 | 1.19 g/cm3 | 1.55 g/cm3 |
| Soil classification * | CH | CH | CL |
| Characteristics | Kabir and Taha [38] | Taha and Taha [39] | UKM Soil | S1 |
|---|---|---|---|---|
| Plasticity Index (PI) | 18.8% | 16.96% | 19.5% | 38.3% |
| Liquid limit (LL) | 36% | --- | 38.4% | 70.3% |
| Silt fraction | 18% | 29.16% | 20.64% | 18.77% |
| Clay fraction | 23% | 18% | 29.6% | 36% |
| Optimum moisture content | 15.8% | 14.29% | 16% | 19.4% |
| Maximum dry density | 1.78 g/cm3 | 1.84 g/cm3 | 1.79 g/cm3 | 1.68 g/cm3 |
| Soil classification * | SC | SC | CL | CH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.A.; Taha, M.R.; Khan, M.M.; Shah, S.A.R.; Aslam, M.A.; Waqar, A.; Khan, A.R.; Waseem, M. Strength and Volume Change Characteristics of Clayey Soils: Performance Evaluation of Enzymes. Minerals 2020, 10, 52. https://doi.org/10.3390/min10010052
Khan TA, Taha MR, Khan MM, Shah SAR, Aslam MA, Waqar A, Khan AR, Waseem M. Strength and Volume Change Characteristics of Clayey Soils: Performance Evaluation of Enzymes. Minerals. 2020; 10(1):52. https://doi.org/10.3390/min10010052
Chicago/Turabian StyleKhan, Tanveer Ahmed, Mohd Raihan Taha, Mudasser Muneer Khan, Syyed Adnan Raheel Shah, Muhammad Asif Aslam, Ahsan Waqar, Ahsan Rehman Khan, and Muhammad Waseem. 2020. "Strength and Volume Change Characteristics of Clayey Soils: Performance Evaluation of Enzymes" Minerals 10, no. 1: 52. https://doi.org/10.3390/min10010052
APA StyleKhan, T. A., Taha, M. R., Khan, M. M., Shah, S. A. R., Aslam, M. A., Waqar, A., Khan, A. R., & Waseem, M. (2020). Strength and Volume Change Characteristics of Clayey Soils: Performance Evaluation of Enzymes. Minerals, 10(1), 52. https://doi.org/10.3390/min10010052





