Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Photocatalysts
2.2. Analysis of Optical and Photoelectrochemical Performances
2.3. Photocatalytic Performance Analysis
3. Experimental
3.1. Chemicals
3.2. Preparation of β-Bi2O3/TiO2 Photocatalysts
3.3. Characterization
3.4. Photocatalytic Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Luo, J.; Dai, Z.; Feng, M.; Gu, M.; Xie, Y. Graphitic carbon nitride/ferroferric oxide/reduced graphen oxide nanocomposite as highly active visible light photocatalyst. Nano Res. 2022, 16, 371–376. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Y.; Mei, J.; Li, Z.; Xu, S.; Yao, C. Synthesis of CdS/BiOBr nanosheets composites with efficient visible-light photocatalytic activity. J. Phys. Chem. Solids 2018, 112, 80–87. [Google Scholar] [CrossRef]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Verhaeghe, D.; Weng, B.; Ghosh, B.; Zhang, H.; Hofkens, J.; Steele, J.A.; Roeffaers, M.B.J. Metal Halide Perovskite-Based Heterojunction Photocatalysts. Angew. Chem. Int. Ed. 2022, 134, e202203261. [Google Scholar]
- Liao, H.; Liu, C.; Zhong, J.; Li, J. Fabrication of BiOCl with adjustable oxygen vacancies and greatly elevated photocatalytic activity by using bamboo fiber surface embellishment. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127892. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, Y.; Pan, J.; Zhao, J.; Ling, Y.; Xie, Y.; Zhou, Y.; Zhao, J. N, P, O co-doped carbon filling into carbon nitride microtubes to promote photocatalytic hydrogen production. Sci. Total Environ. 2022, 809, 151114. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Weng, B.; Lai, F.; Zhang, M.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A.; Long, J. Site-Sensitive Selective CO2 Photoreduction to CO over Gold Nanoparticles. Angew. Chem. Int. Ed. 2022, 134, e202204563. [Google Scholar]
- Wu, W.; Sun, Y.; Zhou, H. In-situ construction of β-Bi2O3/Ag2O photocatalyst from deactivated AgBiO3 for tetracycline degradation under visible light. Chem. Eng. J. 2022, 432, 134316. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, Y.; Guo, Y.; Zhao, J.; Song, J.; Xie, Y.; Ling, Y.; Zhang, W. Tuning the concentration of surface/bulk oxygen vacancies in CeO2 nanorods to promote highly efficient photodegradation of organic dyes. Chin. Chem. Lett. 2021, 32, 2524–2528. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Mahmood, K.; Batool, S.; Hasan, M.; ur Rehman, K.; Iqbal, F. Enhancement in carrier separation of ZnO-Ho2O3-Sm2O3 hetrostucturednanocomposite with rGO and PANI supported direct dual Z-scheme for antimicrobial inactivation and sunlight driven photocatalysis. Adv. Powder Technol. 2021, 32, 3770–3787. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Batool, S.; Hasan, M.; Riaz, M.; ur Rehman, K.; Iqbal, F. Highly efficient tri-phase TiO2–Y2O3–V2O5 nanocomposite: Structural, optical, photocatalyst, and antibacterial studies. J. Nanostruct. Chem. 2022, 12, 547–564. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, X.-L.; Ren, J.-T.; Yuan, Z.-Y. Precisely modifying Co2P/black TiO2 S-scheme heterojunction by in situ formed P and C dopants for enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2022, 315, 121546. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Zhu, G.; Feng, S.; Chao, J.; Zheng, W.; Shao, C. One-pot synthesis of C-dots modified TiO2 nanosheets/UiO-66-NH2 with improved photocatalytic activity under visible light. Ceram. Int. 2020, 46, 2530–2537. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, W.; Wang, J.; Ren, F.; Chen, Z.; Sun, Z.; Ren, P.-G. Construction of biomass derived carbon quantum dots modified TiO2 photocatalysts with superior photocatalytic activity for methylene blue degradation. J. Alloys Compd. 2023, 932, 167627. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Albukhari, S.M.; Ismail, A.A. Visible-light driven of heterostructured LaFeO3/TiO2 photocatalysts for degradation of antibiotics: Ciprofloxacin as case study. J. Photochem. Photobiol. A Chem. 2022, 432, 114078. [Google Scholar] [CrossRef]
- Wang, H.; Song, L.; Yu, L.; Xia, X.; Bao, Y.; Lourenco, M.; Homewood, K.; Gao, Y. Charge transfer between Ti4+, Sn4+ and Pt in the tin doped TiO2 photocatalyst for elevating the hydrogen production efficiency. Appl. Surf. Sci. 2022, 581, 152202. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Niu, M.; Cao, D.; Sui, K.; Liu, C. InP/TiO2 heterojunction for photoelectrochemical water splitting under visible-light. Int. J. Hydrogen Energy 2020, 45, 11615–11624. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, J.; Xie, Y.; Ling, Y.; Zhao, J.; Ye, H.; Chen, T. TiO2@ZnFe2O4 heterojunctions for effecicent photocatalytic degradation of persistent pollutants and hydrogen evolution. Mater. Chem. Phys. 2022, 277, 125462. [Google Scholar] [CrossRef]
- Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-Scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst. ChemCatChem 2019, 11, 6301–6309. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Liu, X.; Li, J.; Xu, H.; Ding, H.; Li, G. Facile Assembly of InVO4/TiO2 Heterojunction for Enhanced Photo-Oxidation of Benzyl Alcohol. Nanomaterials 2022, 12, 1544. [Google Scholar] [CrossRef]
- Lu, H.; Hao, Q.; Chen, T.; Zhang, L.; Chen, D.; Ma, C.; Yao, W.; Zhu, Y. A high-performance Bi2O3/Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B Environ. 2018, 237, 59–67. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.-T.; Zhong, Z.; Wang, R.; Hu, X. Acetic acid-assisted fabrication of hierarchical flower-like Bi2O3 for photocatalytic degradation of sulfamethoxazole and rhodamine B under solar irradiation. J. Colloid Interface Sci. 2017, 505, 489–499. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, L.; Han, Y.; Feng, J.; Zhang, J. One-pot hydrothermal synthesis of Bi2O3-WO3 p-n heterojunction film for photoelectrocatalytic degradation of norfloxacin. Sep. Purif. Technol. 2020, 238, 116428. [Google Scholar] [CrossRef]
- Tang, X.; Ma, C.; Liu, N.; Liu, C.; Liu, S. Visible light β-Bi2O3/BiOCl heterojunction photocatalyst with highly T enhanced photocatalytic activity. Chem. Phys. Lett. 2018, 709, 82–87. [Google Scholar] [CrossRef]
- Majumder, S.; Quang, N.D.; Hien, T.T.; Chinh, N.D.; Yang, H.; Kim, C.; Kim, D. Nanostructured β-Bi2O3/PbS heterojunction as np-junction photoanode for enhanced photoelectrochemical performance. J. Alloys Compd. 2021, 870, 159545. [Google Scholar] [CrossRef]
- Liu, X.; Kang, Y.; Wang, Y. Novel high-efficiency visible-light-driven p-n heterojunction beta-Bi2O3/Ag2WO4 photocatalysts. Chem. Phys. Lett. 2022, 790, 139347. [Google Scholar] [CrossRef]
- Lou, B.; Chen, C.; Liu, J.; Zou, S.; Xiao, L.; Fan, J. Selectively depositing Bi2O3 quantum dots on TiO2 nanotubes for efficient visible-light-driven photocatalysis. Mater. Lett. 2021, 288, 129413. [Google Scholar] [CrossRef]
- Reddy, N.L.; Emin, S.; Valant, M.; Shankar, M.V. Nanostructured Bi2O3@TiO2 photocatalyst for enhanced hydrogen production. Int. J. Hydrogen Energy 2017, 42, 6627–6636. [Google Scholar] [CrossRef]
- Wei, K.; Armutlulu, A.; Wang, Y.; Yao, G.; Xie, R.; Lai, B. Visible-light-driven removal of atrazine by durable hollow core-shell TiO2@LaFeO3 heterojunction coupling with peroxymonosulfate via enhanced electron-transfer. Appl. Catal. B Environ. 2022, 303, 120889. [Google Scholar] [CrossRef]
- Shamaila, S.; Sajjad, A.K.L.; Chen, F.; Zhang, J. Study on highly visible light active Bi2O3 loaded ordered mesoporous titania. Appl. Catal. B Environ. 2010, 94, 272–280. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Wang, J.; Zhang, D.; Li, H. Highly active and durable Bi2O3/TiO2 visible photocatalyst in flower-like spheres with surface-enriched Bi2O3 quantum dots. Appl. Catal. B Environ. 2011, 102, 120–125. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, C.M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef]
- Chen, J.; Tang, T.; Feng, W.; Liu, X.; Yin, Z.; Zhang, X.; Chen, J.; Cao, S. Large-Scale synthesis of p−n Heterojunction Bi2O3/TiO2 nanostructures as photocatalysts for removal of antibiotics under visible light. ACS Appl. Nano Mater. 2022, 5, 1296–1307. [Google Scholar] [CrossRef]
- Xu, D.; Hai, Y.; Zhang, X.; Zhang, S.; He, R. Bi2O3 cocatalyst improving photocatalytic hydrogen evolution performance of TiO2. Appl. Surf. Sci. 2017, 400, 530–536. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, R.; Li, H.; Xu, Z.; Dai, H.; Gao, H.; Yu, H.; Wang, Z.; Wang, Y.; Liu, Y.; et al. Boosting visible light photocatalysis in an Au@TiO2 yolk-in-shell nanohybrid. Appl. Catal. B Environ. 2022, 303, 120869. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-Khathami, N.D.; Mohamed, R.M. Co3O4 Nanoparticles Accommodated Mesoporous TiO2 framework as an Excellent Photocatalyst with Enhanced Photocatalytic Properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Huang, Y.; Zhang, J.; Dai, K.; Liang, C.; Dawson, G. Efficient solar-driven CO2 reduction on aminated 2D/2D BiOBr/CdS-diethylenetriamine S-scheme heterojunction. Ceram. Int. 2022, 48, 8423–8432. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, G.; Li, C.; Zhong, S.; Zhang, F. One-step synthesis of a sulfur doped Bi2WO6/Bi2O3 composite with enhanced visible-light photocatalytic activity. Mater. Lett. 2015, 138, 81–84. [Google Scholar] [CrossRef]
- Zhu, P.; Xu, J.; Xie, L.; Duan, M.; Wu, X.; Xiao, X.; Liu, M. Preparation and characterization of highly efficient Z-scheme oxygen vacancy-BiOBr/CoFe2O4 heterojunction photocatalyst driven by visible light for antibiotic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128810. [Google Scholar] [CrossRef]
- Huang, Y.; Weib, Y.; Wang, J.; Luo, D.; Fan, L.; Wu, J. Controllable fabrication of Bi2O3/TiO2 heterojunction with excellent visible-light responsive photocatalytic performance. Appl. Surf. Sci. 2017, 423, 119–130. [Google Scholar] [CrossRef]
- Jing, H.; Gao, Y.; Li, L.; Wang, X.; Pei, W.; Yang, X. Synthesis of a Novel Double Z-Scheme TiO2/Bi2O3-g-C3N4 Photocatalyst with Enhanced Photocatalytic Performance to Rhodamine B Under Sunlight. J. Clust. Sci. 2022, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Yang, Y.; Rodriguez, R.D.; Lipovka, A.; Lu, Y.; Huang, H.; Chen, J. Ag nanoparticle-decorated Bi2O3-TiO2 heterogeneous nanotubular photocatalysts for enhanced degradation of organic contaminants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129233. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.; Koutavarapu, R. Enhanced photocatalytic activity of ZnO–CdS composite nanostructures towards the degradation of Rhodamine B under solar Light. Catalysts 2022, 12, 84. [Google Scholar] [CrossRef]


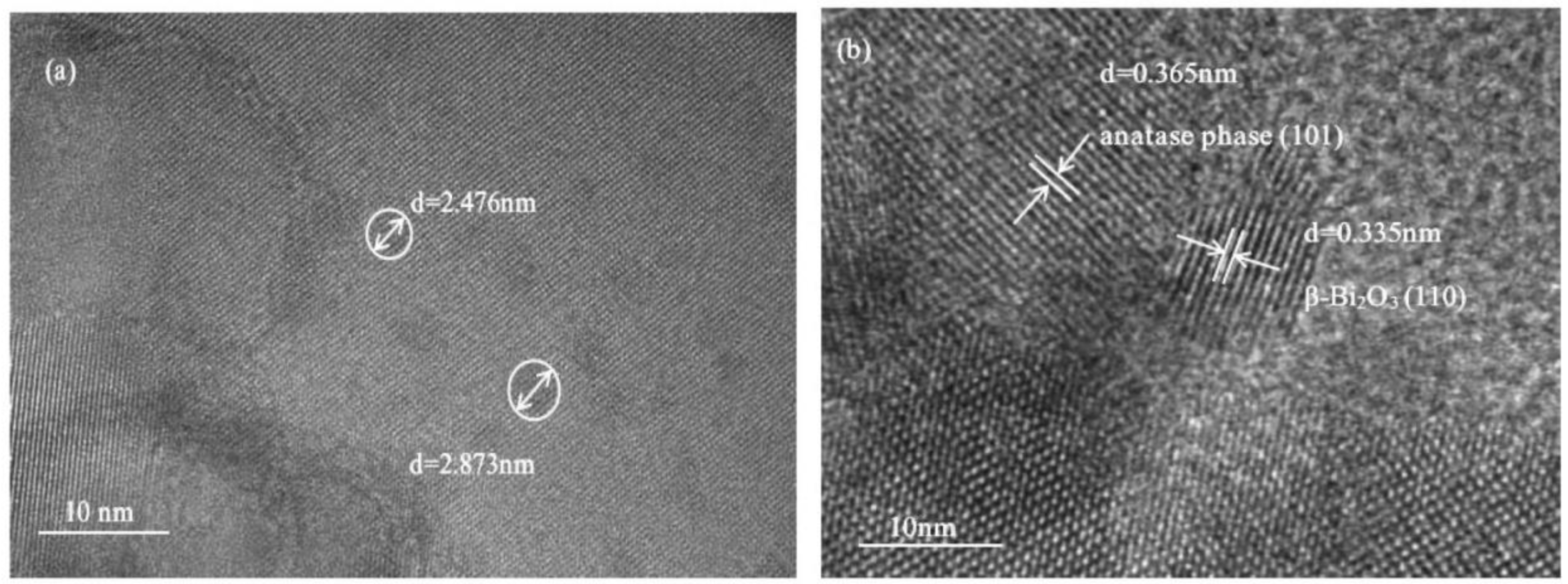

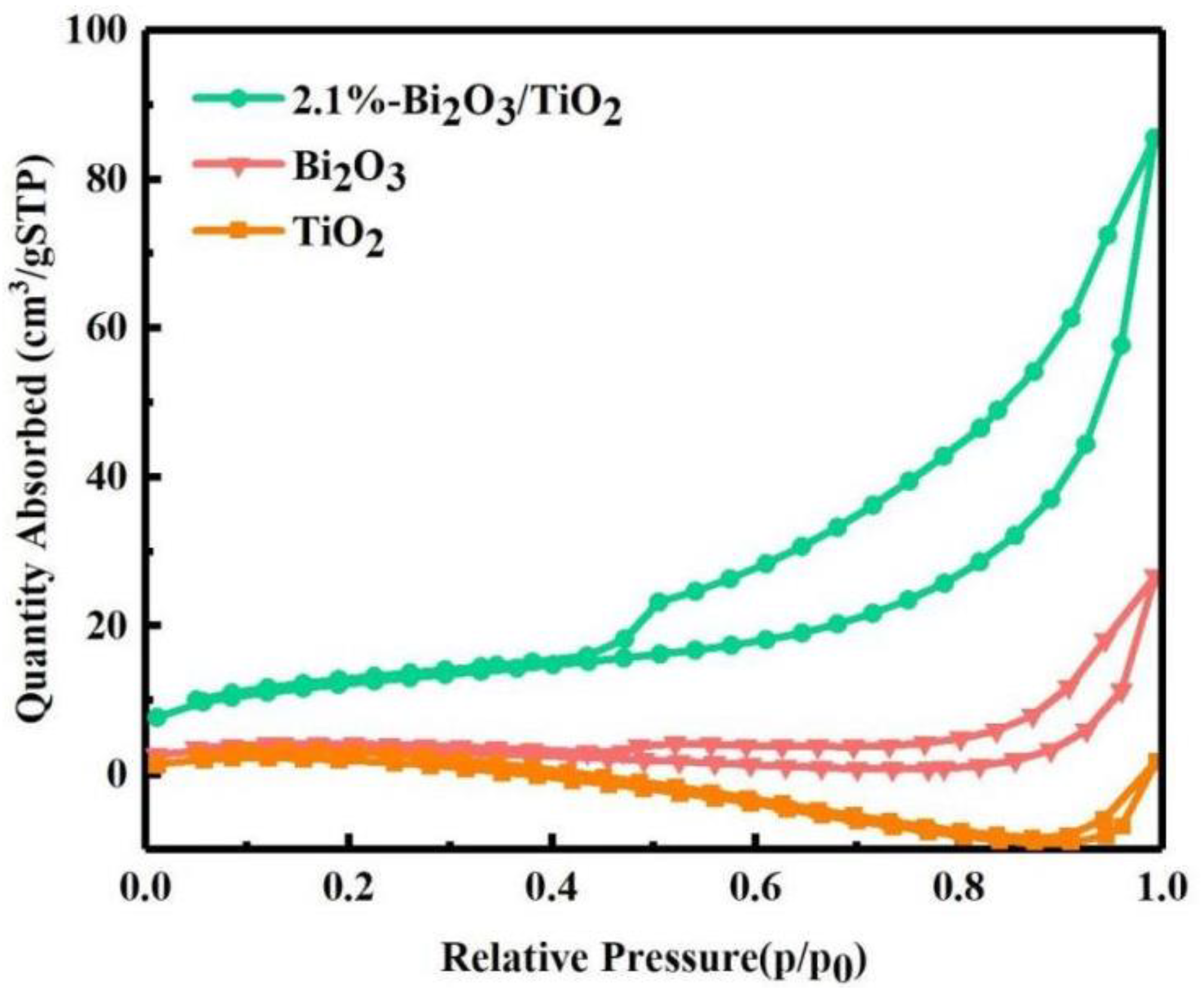
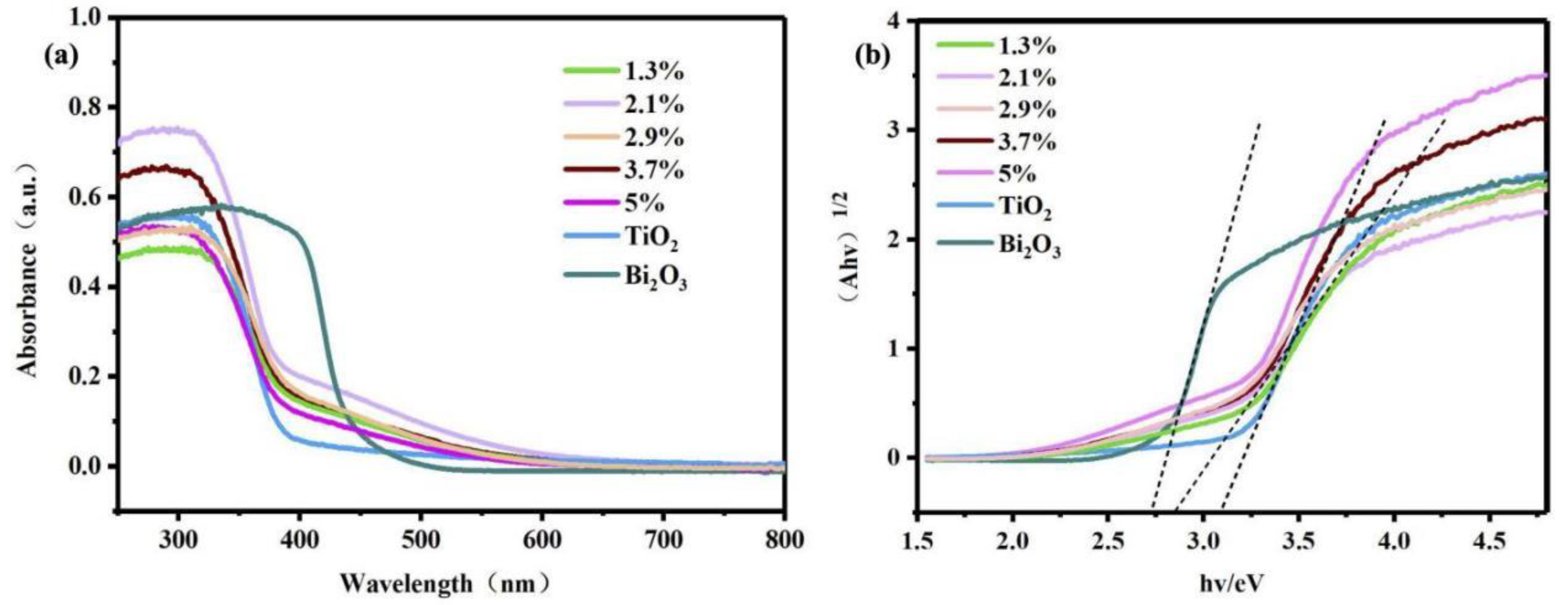



| Element | Atomic Fraction (%) | Atomic Error (%) | Mass Fraction (%) | Mass Error (%) | Fit Error (%) |
|---|---|---|---|---|---|
| O | 61.88 | 1.29 | 38.71 | 0.1 | 0.61 |
| Ti | 37.06 | 0.38 | 52.26 | 0.18 | 2.57 |
| Bi | 1.06 | 9.03 | 8.03 | 9.23 | 0.19 |
| Catalyst | Degradation Time (min) | Performance (Efficiency (%)) | Light Source | Reference |
|---|---|---|---|---|
| β-Bi2O3/TiO2 | 60 min | 100% | Simulated sunlight | this work |
| Bi2O3/TiO2 nanofiber | 120 min | 65% | Simulated sunlight | [45] |
| Bi2O3/TiO2-Ph | 120 min | 87% | Visible light | [35] |
| Bi2O3/TiO2 | 75 min | 99% | Visible light | [49] |
| TiO2/Bi2O3-g-C3N4 | 120 min | 98% | Ultraviolet light/sunlight | [50] |
| Ag-Bi2O3-TiO2 | 90 min | 100% | Full-spectrum light irradiation | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules 2023, 28, 882. https://doi.org/10.3390/molecules28020882
Wang M, Li C, Liu B, Qin W, Xie Y. Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules. 2023; 28(2):882. https://doi.org/10.3390/molecules28020882
Chicago/Turabian StyleWang, Mingjun, Che Li, Bingfang Liu, Wenzhen Qin, and Yu Xie. 2023. "Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB" Molecules 28, no. 2: 882. https://doi.org/10.3390/molecules28020882
APA StyleWang, M., Li, C., Liu, B., Qin, W., & Xie, Y. (2023). Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules, 28(2), 882. https://doi.org/10.3390/molecules28020882







