Carbon Nanotube Supported Molybdenum Carbide as Robust Electrocatalyst for Efficient Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.2. Preparation
2.3. Characterization
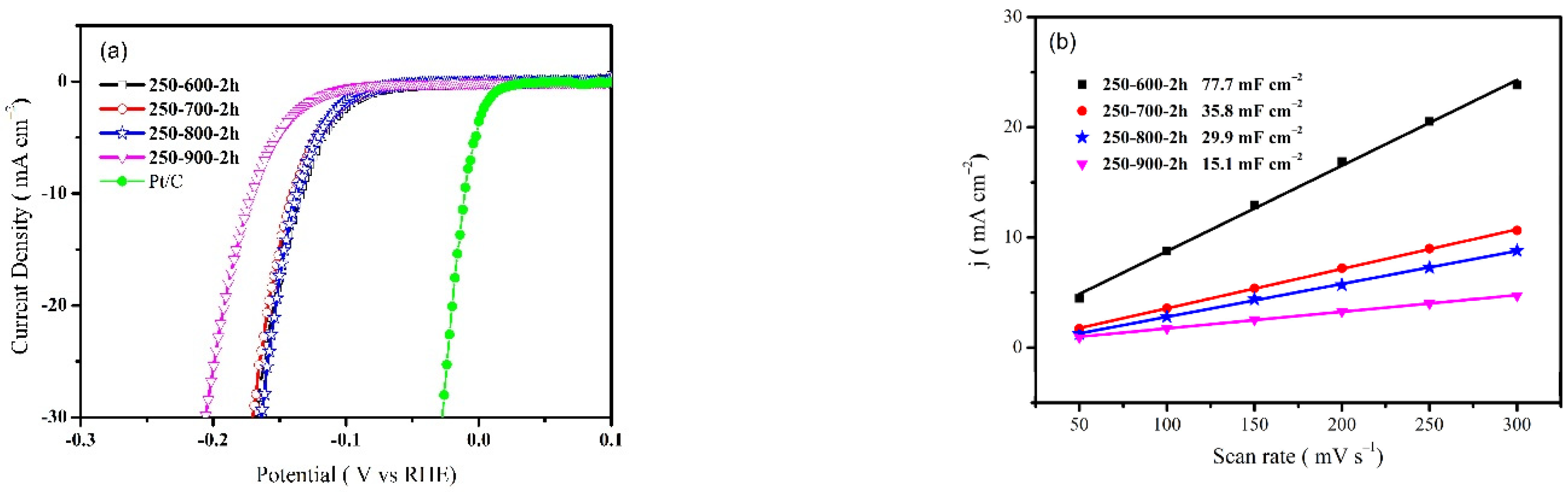
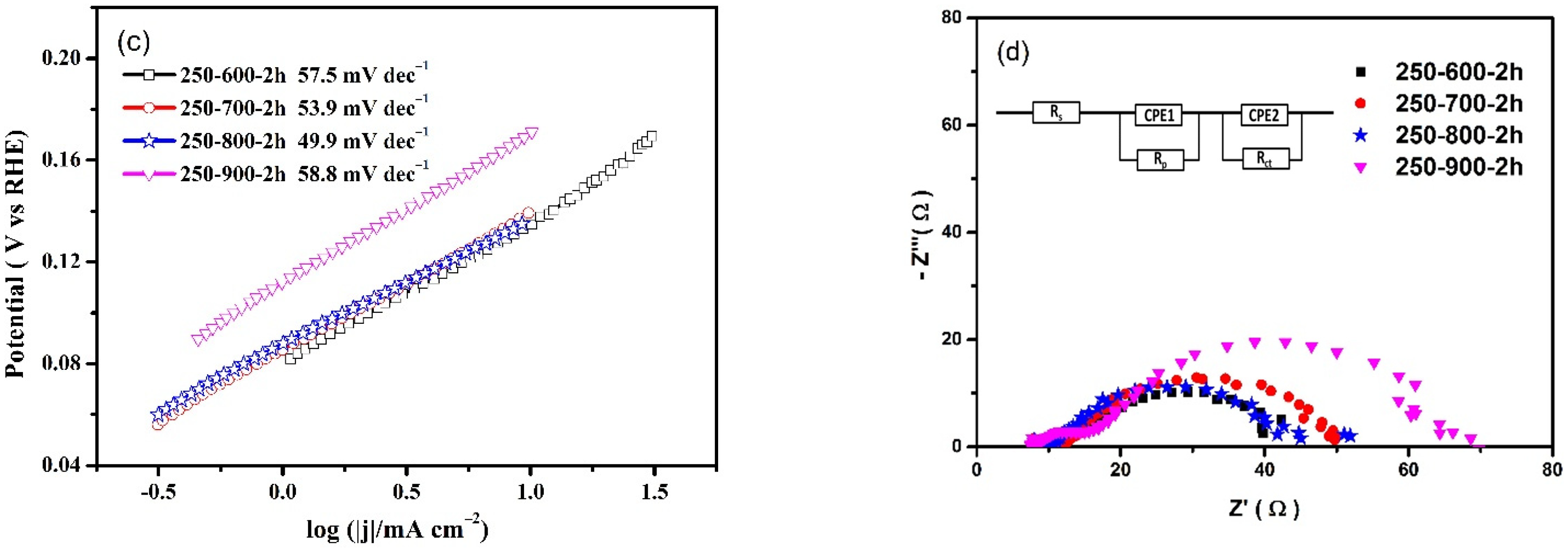
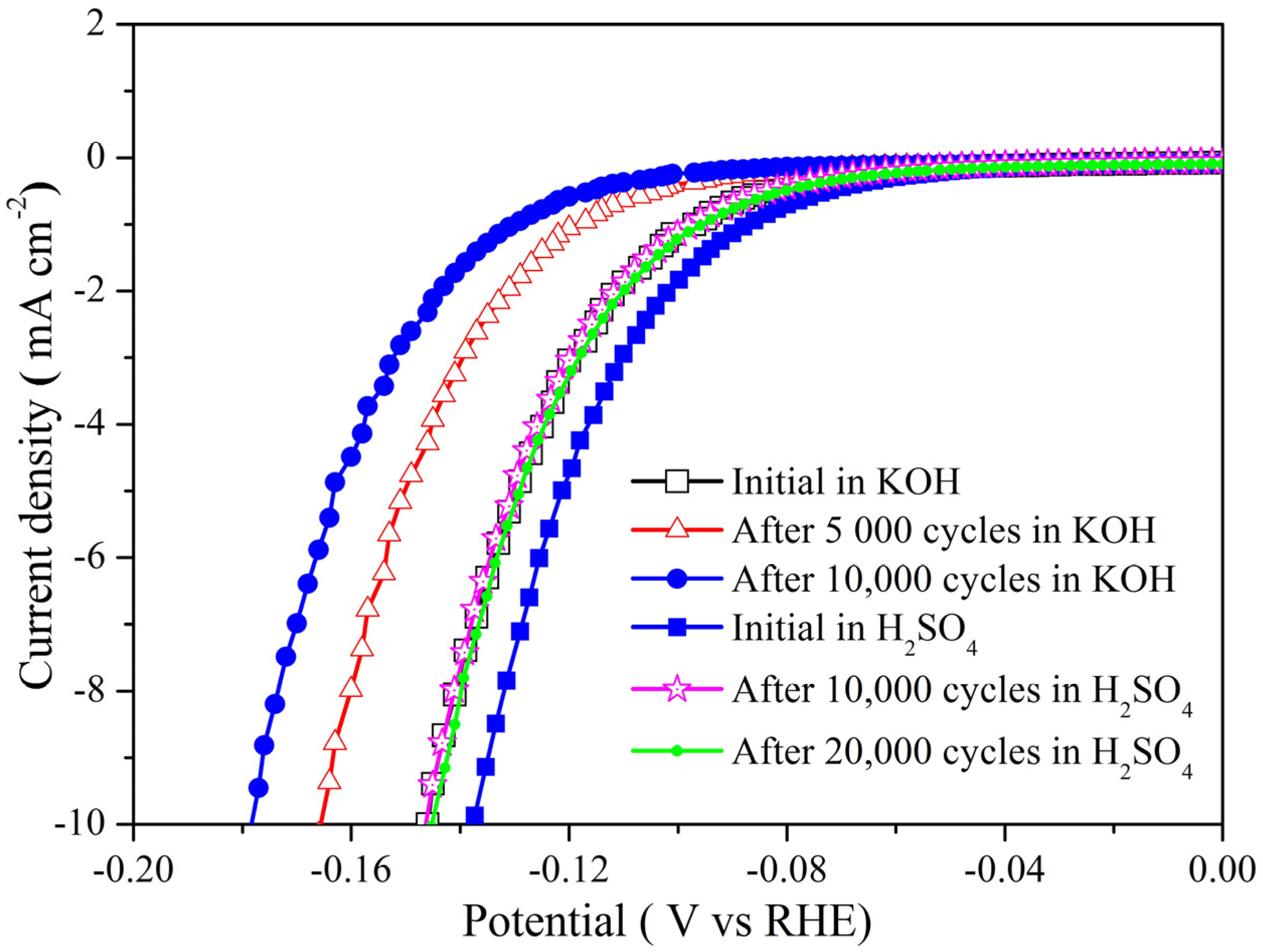
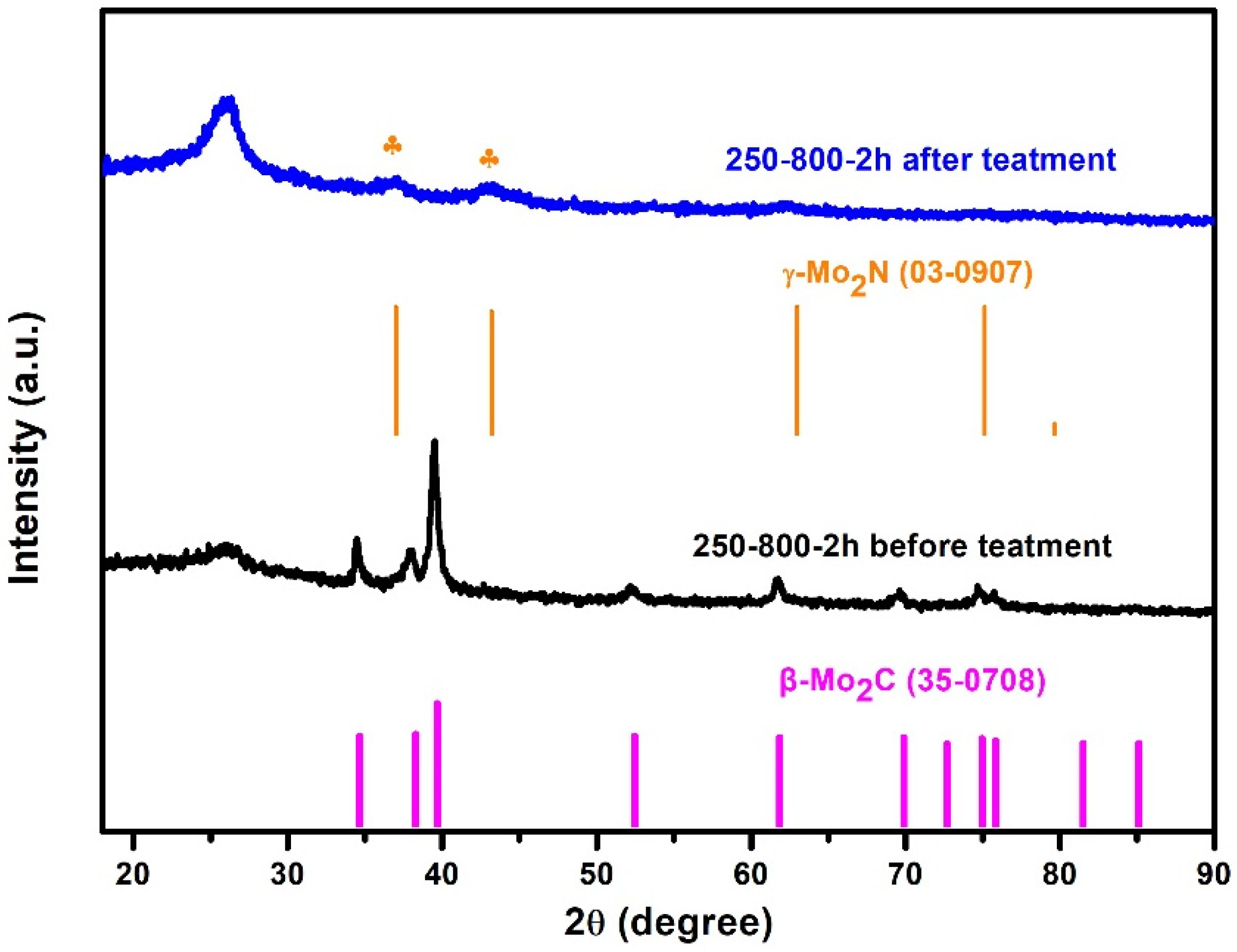
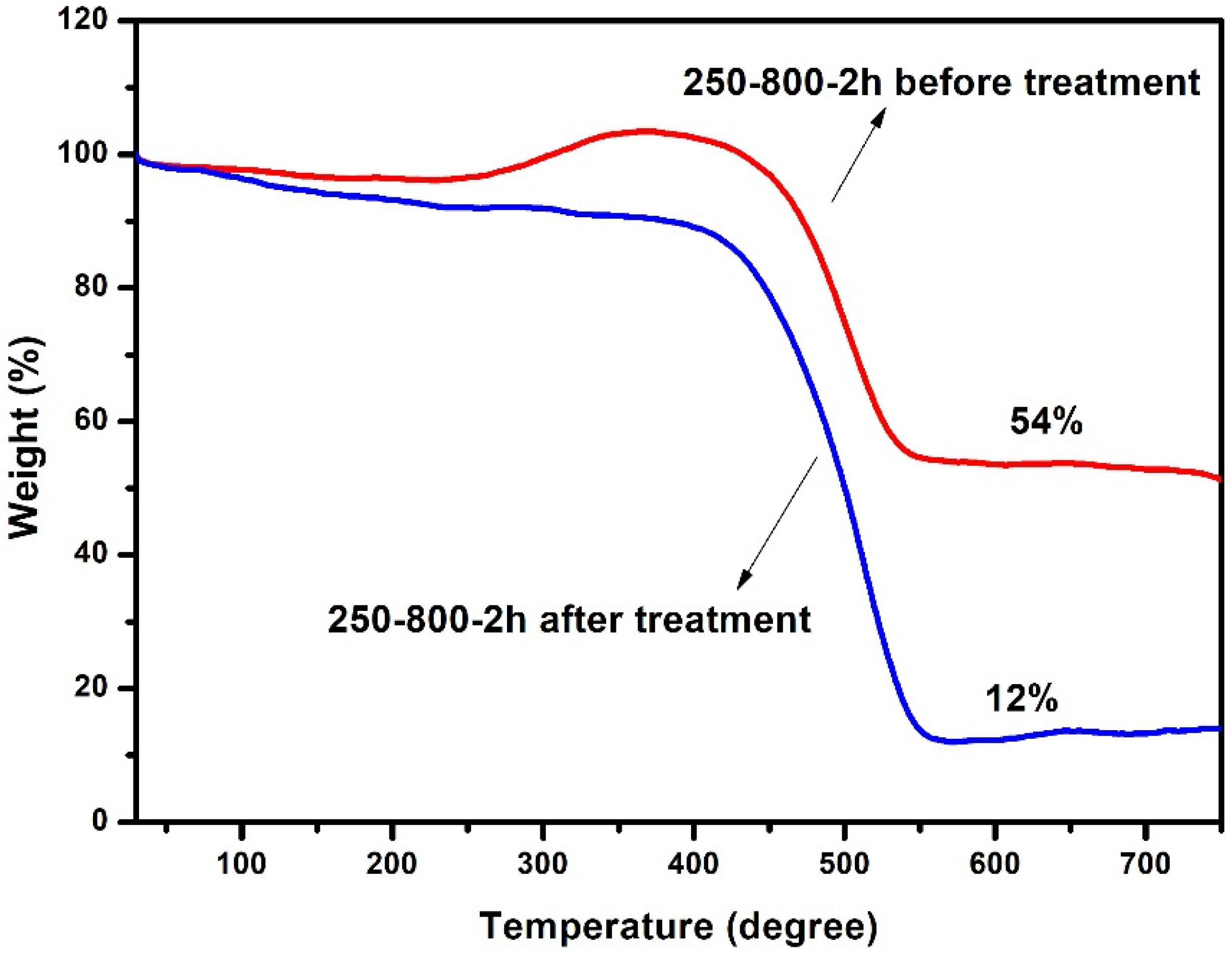
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.H.; Shao, Z.P.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernaecker, C.I.; Weissgarber, T.; Rontzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Jiao, S.L.; Fu, X.W.; Wang, S.Y.; Zhao, Y. Perfecting electrocatalysts via imperfections: Towards the large-scale deployment of water electrolysis technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Chi, J.Q.; Yang, M.; Chai, Y.M.; Yang, Z.; Wang, L.; Dong, B. Design and modulation principles of molybdenum carbide -based materials for green hydrogen evolution. J. Energy Chem. 2020, 48, 398–423. [Google Scholar] [CrossRef]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum carbide-based electrocatalysts for hydrogen evolution reaction. Chem. Eur. J. 2017, 23, 10947–10961. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen-evolution electrocatalyst. Angew. Chem. Int. Ed. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.C.; Li, Q.; Zhang, P.F.; Chen, C.S.; Yan, D.Y.; Mai, Y.Y. Mesoporous Mo2C/carbon hybrid nanotubes synthesized by a dual-template self-assembly approach for an efficient hydrogen production electrocatalyst. Langmuir 2018, 34, 10924–10931. [Google Scholar] [CrossRef]
- Ge, R.Y.; Huo, J.J.; Sun, M.J.; Zhu, M.Y.; Li, Y.; Chou, S.L.; Li, W.X. Surface and Interface Engineering: Molybdenum Carbide-Based Nanomaterials for Electrochemical Energy Conversion. Small 2021, 17, 1903380. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, H.Y.; Pan, Y.P.; Lin, W.Y.; Xu, S.; Sun, Q.Z.; Liu, E.Z.; He, F.; Diao, L.C.; He, C.N.; et al. Graphite Carbon Nanosheet-Coated Cobalt-Doped Molybdenum Carbide Nanoparticles for Efficient Alkaline Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2021, 4, 372–380. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhang, L.; Huang, J.T.; Feng, Z.J.; Xiong, Q.M.; Ye, Z.G.; Chen, Z.; Li, X.B.; Yu, Z.J. Self-supported nickel-doped molybdenum carbide nanoflower clusters on carbon fiber paper for an efficient hydrogen evolution reaction. Nanoscale 2021, 13, 8264–8274. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Li, S.H.; Tan, H.Q.; Khan, S.U.; Ma, Y.Y.; Zang, H.Y.; Wang, Y.H.; Li, Y.G. MoP/Mo2C@C: A new combination of electrocatalysts for highly efficient hydrogen evolution over the entire pH range. ACS Appl. Mater. Interfaces 2017, 9, 16270–16279. [Google Scholar] [CrossRef]
- Xiong, J.; Li, J.; Shi, J.W.; Zhang, X.L.; Suen, N.T.; Liu, Z.; Huang, Y.J.; Xu, G.X.; Cai, W.W.; Lei, X.R.; et al. In situ engineering of double-phase interface in Mo/Mo2C heteronanosheets for boosted hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 341–348. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Sagaltchik, A.; Pachfule, P.; Zhao, C.S.; Thomas, A. Metal-organic precursor-derived mesoporous carbon spheres with homogeneously distributed molybdenum carbide/nitride nanoparticles for efficient hydrogen evolution in alkaline media. Adv. Funct. Mater. 2019, 29, 1807419. [Google Scholar] [CrossRef]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube-graphene hybrid support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef]
- Lee, Y.H.; Brahma, S.; Huang, P.C.; Wang, S.C.; Huang, J.L. Molybdenum carbide (Mo2C) and reduced graphene oxide (rGO) nano-composites as an efficient electrocatalyst for water splitting. Mater. Lett. 2022, 316, 131934. [Google Scholar] [CrossRef]
- He, M.C.; Shi, H.Y.; Wang, P.; Sun, X.D.; Gao, B. Porous molybdenum carbide nanostructures synthesized on carbon cloth by cvd for efficient hydrogen production. Chem. Eur. J. 2019, 25, 16106–16113. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhang, H.; Xu, K.F.; Zhang, Q.L.; Liu, J.H.; He, C.X.; Fan, L.D.; Asefa, T. Unconventional molybdenum carbide phases with high electrocatalytic activity for hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 18030–18038. [Google Scholar] [CrossRef]
- Kang, Q.L.; Li, M.Y.; Wang, Z.R.; Lu, Q.Y.; Gao, F. Agaric-derived N-doped carbon nanorod arrays@nanosheet networks coupled with molybdenum carbide nanoparticles as highly efficient pH-universal hydrogen evolution electrocatalysts. Nanoscale 2020, 12, 5159–5169. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Li, Y.Z.; Xie, J.; Hao, A.Z.; Cao, Y.L. One-pot solution-free construction for hybrids of molybdenum carbide nanoparticles and porous N-doped carbon nanoplates as efficient electrocatalyst of hydrogen evolution. J. Alloys Compd. 2021, 861, 157935. [Google Scholar] [CrossRef]
- Lei, C.S.; Zhou, W.; Feng, Q.G.; Lei, Y.P.; Zhang, Y.; Chen, Y.; Qin, J.Q. Charge engineering of Mo2C@defect-rich N-doped carbon nanosheets for efficient electrocatalytic H2 evolution. Nano-Micro Lett. 2019, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N-Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 29164704. [Google Scholar] [CrossRef]
- Adam, A.; Suliman, M.H.; Awwad, M.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. Controlled growth of small and uniformly dispersed Mo2C on carbon nanotubes as high performance electrocatalyst for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 11797–11807. [Google Scholar] [CrossRef]
- Cheng, Z.; Fu, Q.; Han, Q.; Xiao, Y.; Liang, Y.; Zhao, Y.; Qu, L. A Type of 1 nm molybdenum carbide confined within carbon nanomesh as highly efficient bifunctional electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705967. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Dong, H.; He, X.; Chen, Z. N,P-doped molybdenum carbide nanofibers for efficient hydrogen production. ACS Appl. Mater. Interfaces 2018, 10, 14632–14640. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Q.; Du, S.; Li, J.; Zhang, L.; Lin, K.; Li, H.; Chen, B.; Wu, T.; Chen, D.; et al. One-dimensional porous hybrid structure of Mo2C-CoP encapsulated in N-doped carbon derived from MOF: An efficient electrocatalyst for hydrogen evolution reaction over the entire pH range. ACS Appl. Mater. Interfaces 2018, 10, 42335–42347. [Google Scholar] [CrossRef]
- Jo, H.M.; Kim, Y.; Youn, D.H. One-pot synthesis of molybdenum carbide/N-doped carbon nanotube composite using nitrilotriacetic acid for efficient hydrogen evolution. J. Alloys Compd. 2021, 855, 157420. [Google Scholar] [CrossRef]
- Yang, C.F.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.D.; Li, X.K.; Zhang, Q. Structural transformation of molybdenum carbide with extensive active centers for superior hydrogen evolution. Nano Energy 2022, 98, 107232. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, M.H.; Kim, Y.; Lim, H.K.; Youn, D.H. Facile synthesis of nanostructured molybdenum carbide/nitrogen-doped CNT-RGO composite via a modified urea glass route for efficient hydrogen evolution. J. Alloys Compd. 2019, 805, 113–119. [Google Scholar] [CrossRef]
- Xiong, T.; Jia, J.; Wei, Z.; Zeng, L.; Deng, Y.; Zhou, W.; Chen, S. N-doped carbon-wrapped Mo C heterophase sheets for high-efficiency electrochemical hydrogen production. Chem. Eng. J. 2019, 358, 362–368. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures composed of n-doped carbon nanotubes encapsulating cobalt and beta-Mo2c nanoparticles as bifunctional electrodes for water splitting. Angew. Chem. Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.X.; Cao, J.; Ma, L.; Zhou, K.C.; Yu, Z.M.; Yin, D.F.; Wei, Q.P. Novel three-dimensional Mo2C/carbon nanotubes composites for hydrogen evolution reaction. Mater. Lett. 2020, 277, 128386. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.Y.; Zhang, X.Y.; Pang, G.G.; Li, S.M. Mo2C-embedded biomass-derived honeycomb-like nitrogen-doped carbon nanosheet/graphene aerogel films for highly efficient electrocatalytic hydrogen evolution. New J. Chem. 2020, 44, 1147–1156. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, D.G.; Yu, B.; Hou, W.Q.; Zheng, B.J.; Zhang, W.L.; Chen, Y.F. Scalable synthesis of Mo2C/CNT networks as highly efficient and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 263, 192–200. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.W.; Hu, J.; Niu, S.Q.; Li, Y.Z.; Xu, P. Acid-directed morphology control of molybdenum carbide embedded in a nitrogen doped carbon matrix for enhanced electrocatalytic hydrogen evolution. Inorg. Chem. Front. 2020, 7, 3620–3626. [Google Scholar] [CrossRef]
- Song, Y.J.; Ren, J.T.; Yuan, G.G.; Yao, Y.L.; Liu, X.Y.; Yuan, Z.Y. Facile synthesis of Mo2C nanoparticles on N-doped carbon nanotubes with enhanced electrocatalytic activity for hydrogen evolution and oxygen reduction reactions. J. Energy Chem. 2019, 38, 68–77. [Google Scholar] [CrossRef]
- Wu, S.F.; Chen, M.Y.; Wang, W.W.; Zhou, J.B.; Tang, X.R.; Zhou, D.L.; Liu, C. Molybdenum carbide nanoparticles assembling in diverse heteroatoms doped carbon matrix as efficient hydrogen evolution electrocatalysts in acidic and alkaline medium. Carbon 2021, 171, 385–394. [Google Scholar] [CrossRef]
- Wang, J.H.; Wei, H.F.; Chen, X.; Chen, C.S.; Chen, X.A. Facile preparation of N, P co-doped molybdenum carbide/porous carbon rough microspheres for efficient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 595–604. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Bao, Y.; Huang, T.; Hu, C.; Qiu, H.; Liu, H. Carbon Nanotube Supported Molybdenum Carbide as Robust Electrocatalyst for Efficient Hydrogen Evolution Reaction. Molecules 2023, 28, 192. https://doi.org/10.3390/molecules28010192
Huang Y, Bao Y, Huang T, Hu C, Qiu H, Liu H. Carbon Nanotube Supported Molybdenum Carbide as Robust Electrocatalyst for Efficient Hydrogen Evolution Reaction. Molecules. 2023; 28(1):192. https://doi.org/10.3390/molecules28010192
Chicago/Turabian StyleHuang, Yunjie, Yaqi Bao, Tieqi Huang, Chengzhi Hu, Haiou Qiu, and Hongtao Liu. 2023. "Carbon Nanotube Supported Molybdenum Carbide as Robust Electrocatalyst for Efficient Hydrogen Evolution Reaction" Molecules 28, no. 1: 192. https://doi.org/10.3390/molecules28010192
APA StyleHuang, Y., Bao, Y., Huang, T., Hu, C., Qiu, H., & Liu, H. (2023). Carbon Nanotube Supported Molybdenum Carbide as Robust Electrocatalyst for Efficient Hydrogen Evolution Reaction. Molecules, 28(1), 192. https://doi.org/10.3390/molecules28010192







