Abstract
A three-dimensional (3D) T1-weighted Magnetic Resonance Imaging (MRI) at 7-Tesla system was acquired with a high spatial resolution from fixed brains of male and female ferrets at postnatal days (PDs) 4 to 90, and their age-related sexual difference and laterality were evaluated by MRI-based ex vivo volumetry. The volume of both left and right sides of cerebellar cortex was larger in males than in females on PD 10 and thereafter. When the cerebellar cortex was divided into four transverse domains, i.e., anterior zone (AZ; lobules I–V), central zone (CZ; lobules VI and VII), posterior zone (PZ; lobules VIII–IXa), and nodular zone (NZ; lobules IXb and X), an age-related significantly greater volume in males than in females was detected on either side of all four domains on PD 42 and of the AZ on PD 90, but only on the left side of the PZ on PD 90. Regarding the volume laterality, significant leftward asymmetry was obtained in the CZ and PZ volumes in males, but not in females on PD 90. From asymmetry quotient (AQ) analysis, AQ scores were rightward in the AZ in both sexes already on PD 21, but gradually left-lateralized only in males in the CZ, PZ, and NZ during PDs 42 to 90. The present study suggests that a characteristic counterclockwise torque asymmetry (rostrally right-biased, and caudally left-biased or symmetrical) is acquired in both sexes of ferrets during PDs 42 to 90, although the leftward laterality of the posterior half of the cerebellum was more enhanced in males.
1. Introduction
Morphological and functional asymmetry is reported in brain regions related to preference of hand/paw use [1,2,3], cognition [2,4], emotion [5], and so on. It is well known that large-scale fiber connectivity, such as cerebro-cerebellar connections, is involved in the morphological lateralization [6,7,8]. Hormonal and genetic factors are also considered to implicate asymmetry of brain structures: e.g., a coincidence of the dominant-side (right) of androgen receptor levels [9] and arcuate sulcal length [10] in the frontal lobe of male macaques; a topological correlation of asymmetric pattern of gene expression [11] with earlier emergences of the superior frontal and superior temporal gyri in the right side than in the left side [12]. Furthermore, environmental variables may be involved in the asymmetric morphology of the brain. Monozygotic handedness-discordant twins exhibited the opposite direction of torque asymmetry in the cerebral hemisphere [13].
Some mammalian species, including primates and carnivores, have a distinctive asymmetric feature of the cerebellar morphology, called “torque asymmetry”. The cerebellar torque asymmetry was reportedly clockwise in primates such as humans [13] and chimpanzees [14], but counterclockwise torque asymmetry was observed in carnivores, such as ferrets [15].
The cerebellum exhibits sexual dimorphic aspects of its shape and volume, as well as other brain regions, including the cerebral cortex [10,16], although this has been reported in a limited number of mammalian species. A larger cerebellar volume in men than in women by MRI-based volumetry has been well documented [17,18,19,20,21,22,23]. However, cerebellar volumes did not differ between sexes at a population level in other mammals, such as mice [24] and dogs [25]. Notably, the cerebellar volume was more susceptible to perinatal exposure to polychlorinated biphenyls [26] and schizophrenia [27] in males than in females, while cerebellar morphology and function are altered by chemical and genetic insults, and in human neurodevelopmental disorders, such as autism, schizophrenia, and attention deficit hyperactivity disorder [28].
Our recent Magnetic Resonance Imaging (MRI)-based volumetric analysis revealed that sexual dimorphism of the ferret cerebellum was characterized by increasing the whole volume with an enhancement of counterclockwise torque asymmetry in more males than in females [15]. That report was the first to show sexually-dimorphic asymmetric development of the cerebellum. The present study was aimed to elucidate when sex differences in the volume laterality and the degree of counterclockwise torque asymmetry of the cerebellum were achieved in ferrets during the postnatal development. Volumetric analysis was conducted to measure the cerebellar volume and to evaluate the cerebellar torque asymmetry, based on ex vivo MRI with a high spatial resolution. The cerebellar torque asymmetry was assessed by the difference in asymmetry quotient (AQ) of volumes of four cerebellar transverse domains, which were segmented primarily based on the zebrin II/aldolase C expression pattern [29,30].
2. Materials and Methods
2.1. Samples
Fixed brain samples of male and female ferrets, which had been used in our previous study [31], were used in the present study. Male and female ferrets were purchased at differential postnatal days (PDs) (each of three males at PDs 4, 10, 21, 42, and 90; each of three females at PDs 4, 10, 21, 42, and 90) from SLC (Hamamatsu, Japan). Under a deep anesthetization with an injection of chloral hydrate intraperitoneally at a dosage of 400 µg/g body weight, an intracardiac perfusion with 0.9% NaCl followed by 4% paraformaldehyde (PFA) in a 10 mM phosphate buffer (pH 7.4) was performed in all ferrets. Then, brains were removed by opening the skull, and immersed in the PFA solution until measurement by MRI.
2.2. MRI Measurements
MRI measurements were performed in accordance with our previous study [32]. Three-dimensional (3D) T1-weighted MRI (i.e., short repetition time (TR)/echo time (TE) setting) was acquired using a preclinical 7.0-T MRI system (Magnet; 400 mm inner diameter bore, Kobelco and Jastec, Kobe, Hyogo, Japan) (Console; AVANCE-I, Bruker BioSpin, Ettlingen, Baden-Württemberg, Germany). A birdcage radiofrequency (RF) coil for transmission and reception (70 mm inner diameter, Rapid Biomedical, Rimpar, Germany; or 60-mm inner diameter, Bruker BioSpin) was used with a field of view adequate for the sample dimensions. Slice orientation (transaxial) was precisely adjusted for the cerebral base using pilot-MR images obtained by a gradient-echo sequence. Gradient coils (BGA-12, Bruker BioSpin) were calibrated by a professional technologist within six months, according to Bruker’s calibration manual and software.
3D T1-weighted images covering the entire brain were acquired using rapid acquisition with relaxation enhancement (RARE) sequence, having the following parameters: TR = 300 ms, TE = 9.6 ms (effective TE = 19.2 ms), RARE factor = 4, acquisition matrix = 256 × 256 × 256, number of acquisitions (NEX) = 2, and total scan time = 2 h 43 min 50 s. The field of view (FOV) was adjusted to the size of the samples and ranged from 1.32 × 1.32 × 2.56 to 32 × 32 × 40 mm3. Thus, the voxel resolution was 100–125 × 100–125 × 100–156 µm3. Although a typical short TR/TE parameter setting was used for acquiring the T1-weighted MRI, the strong susceptibility effect at 7 Tesla decreased MRI signals in white matter. An image matrix of the reconstructed data was linearly interpolated two times.
2.3. 3D Volume-Rendered Images
3D T1-weighted MRI images of the entire cerebellum were used for volumetric analysis. Left and right sides of the cerebellum were segmented semi-automatically on the MRI using the “Morpho” tool of the SliceOmatic software package, version 4.3 (TomoVision, Montreal, QC, Canada), based on image contrast, as well as the user’s knowledge of the anatomy. The midline of each side was defined by the position of the cerebral longitudinal fissure. We further segmented four transverse domains of the cerebellar cortex, primarily based on the expression pattern of zebrin II/aldolase C [29,30]: left and right sides of the anterior zone (AZ) (vermal lobules I–V), central zone (CZ) (vermal lobules VI–VII, lobules simplex, and crura I and II of ansiform lobules), posterior zone (PZ) (vermal lobules VIII–IXa, and paramedian lobule), and the nodular zone (NZ) (vermal lobules IXb–X, paraflocculus and flocculus). Boundaries of those domains were defined by major fissures/sulci (Supplemental Figure S1) primarily according to our previous study [15]. During PDs 4 to 10, boundaries of each domain were unclear on MRI images. Therefore, we constructed 3D volume-rendered images of the male and female cerebella from PDs 21 to 90. Segmented images were then analyzed using the 3D-rendering module of the same software. The cerebellar image was rendered in 3D using the surface projection algorithm which best visualized the surface. The 3D-rendered images were then rotated and manipulated in a manner that best visualized the cerebellar morphology by a linear registration method using SliceOmatic software.
2.4. Volumetric Analysis
The volumetric analysis was carried out primarily in accordance with our previous procedure [15,32]. Segmented areas of each cerebellar region were measured using SliceOmatic software. The volumes were calculated by multiplying the combined areas by the slice thickness (156.25 µm), with the total areas of those regions being regarded as the volume of the whole cerebellum.
2.5. Asymmetry Quotient Analysis
For evaluation of asymmetric development of the cerebellum, an asymmetry quotient (AQ) was calculated using the formula ((R − L)/{(R + L) × 0.5}). The sign of the resulting value indicated the direction of asymmetry (positive value = right hemisphere bias; negative value = left hemisphere bias) [33].
2.6. Statistical Analysis
Age-related sexual differences in the brain and cerebellar cortical volumes were evaluated by two-way Analysis of Variance (ANOVA) using sex and age as factors. For evaluation of age- and sex-related changes in the cerebellar volume laterality, three-way repeated measures ANOVA was carried out using postnatal age and sex as intergroup factors, and left/right sides as an intergroup factor. Furthermore, age- and sex-related changes in the volume laterality of cerebellar regions were evaluated by four-way repeated measures ANOVA using postnatal age and sex as intergroup factors, and four cerebellar transverse domains and left/right sides as intergroup factors. Then, Scheffe’s test was carried out, to assess sex and left/right side differences via post hoc testing, when the effects on the interaction of two or more factors by each ANOVA analysis were marked at p < 0.05, and followed by significant simple main effects (p < 0.05).
2.7. Ethics
The experimental procedures in the present study were conducted in accordance with the guidelines of the National Institutes of Health (NIH) for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of Tsukuba International University approved the procedures, and all efforts were made to minimize the number of animals used and their suffering. This study was specifically approved by the Institutional Animal Care and Use Committee of Tsukuba International University.
3. Results
3.1. Developmental Changes in Volume of Entire Cerebellum
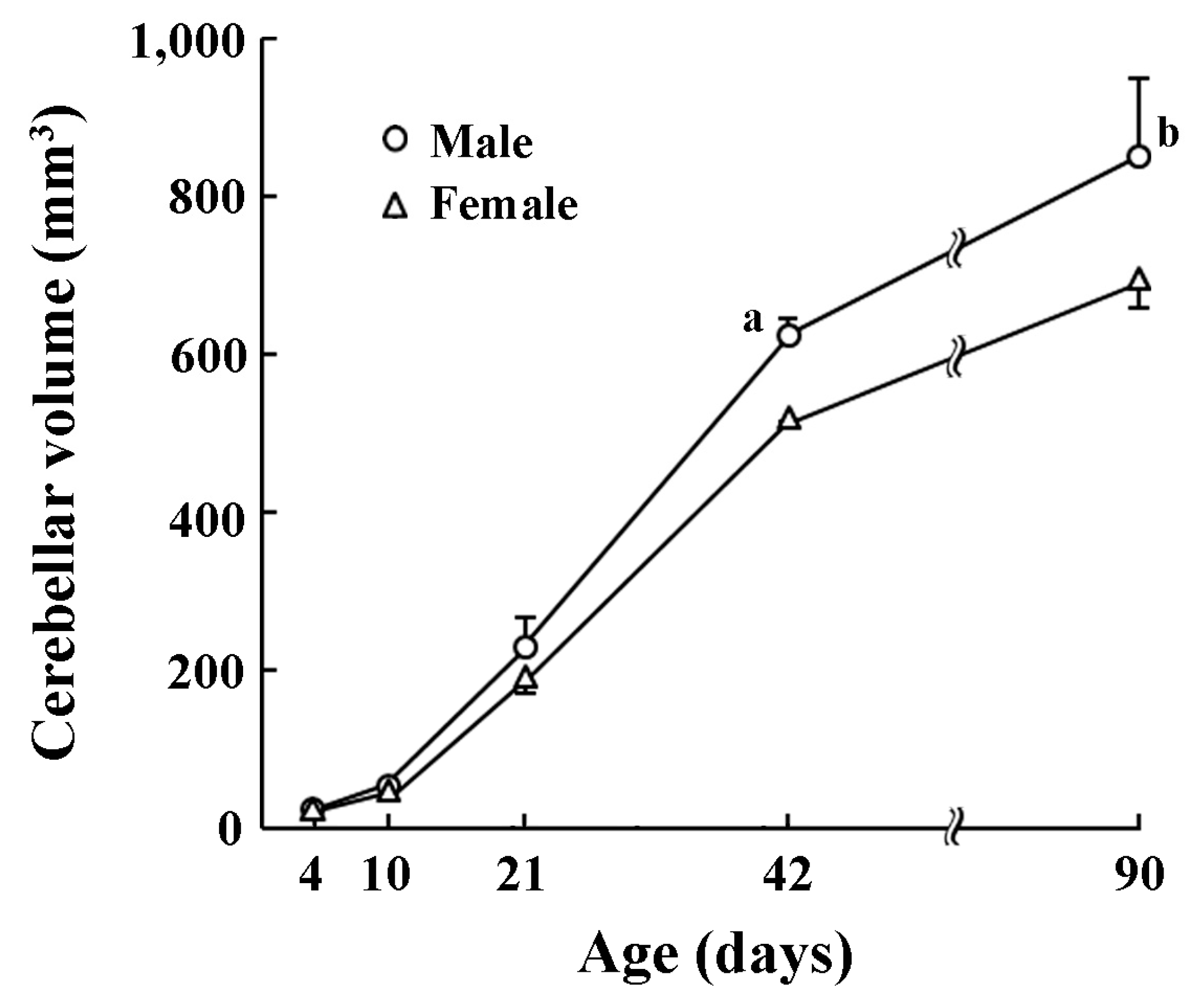
The volume of whole cerebellum increased with postnatal ages (Figure 1). A significant effect on postnatal age × sex interactions was obtained by two-way repeated measures ANOVA (F(4,20) = 5.262, p < 0.05). Cerebellar volumes were statistically greater in males than in females on PDs 21 (p < 0.05), 42 (p < 0.05), and 90 (p < 0.001) by post-hoc testing (Figure 1).
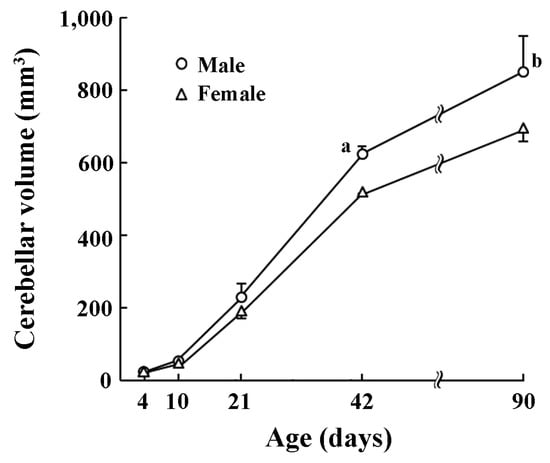
Figure 1.
Changes in cerebellar volume of male and female ferrets during postnatal days 4 to 90. a: p < 0.01, b: p < 0.001 (male vs. female; Scheffe’s test).
The body weight and crown-rump length of male and female ferrets on PDs 4 to 90 are shown in Supplemental Table S1. Age-related sexual difference was obtained in the body weight on PDs 42 and 90, but not in the crown-lump length at any postnatal ages examined. Thus, age-related sexual difference in the cerebellar volume seemed to be independent of body growth.
3.2. Asymmetric Development of Cerebellum
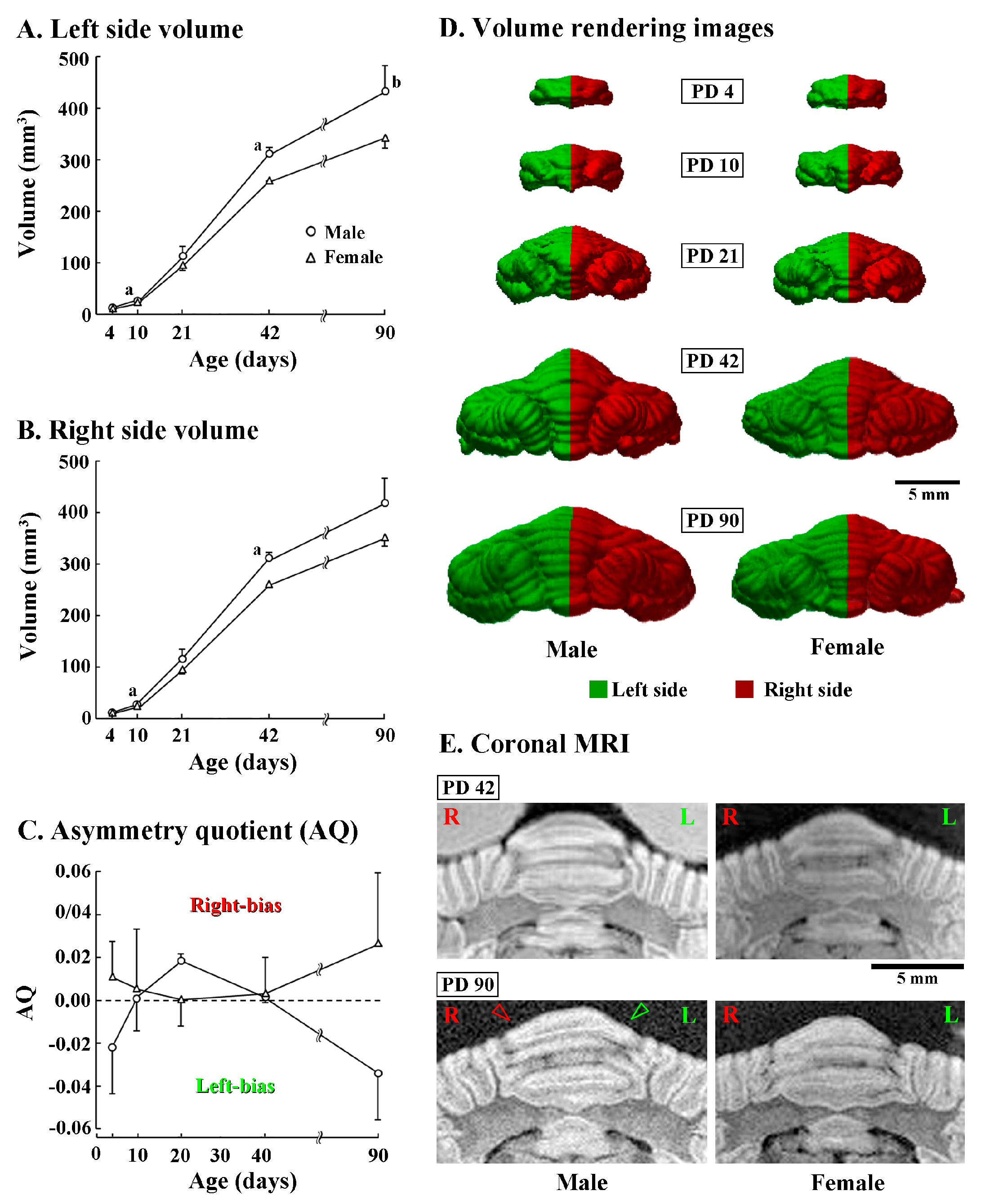
Left and right sides of the cerebellar volume were further compared between sexes during postnatal development. Three-way repeated measures ANOVA revealed a significant effect on interactions among postnatal age, sex and left/right sides [F(4,20) = 7.825, p < 0.05]. A significantly greater volume in males than in females was detected already from PD 10 in both left (p < 0.05) and right (p < 0.05) sides of the cerebellum (Figure 2A,B). Such a trend in sexual difference was observed in both sides of the cerebellum at elder ages, while obtaining a statistical difference in both sides on PD 42 and the right side on PD 90 (Figure 2A,B).
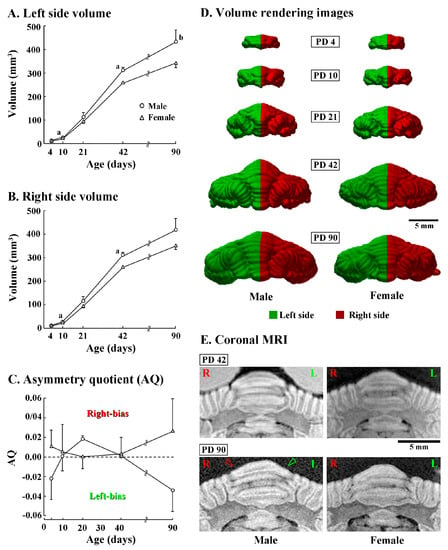
Figure 2.
Changes in volumes, asymmetry quotient, 3D morphology, and coronal Magnetic Resonance Imaging (MRI) images of left/right sides of cerebella of male and female ferrets from postnatal days (PD) 4 to 90. (A) Volume of the left half of the cerebellum; (B) volume of the right half of the cerebellum; and (C) asymmetry quotient (AQ). The AQ was calculated using the formula ((R − L)/{(R + L) × 0.5}). The sign of the resulting value indicated the direction of asymmetry (positive value = right bias; negative value = left bias) [33]; (D) posterior view of 3D volume-rendered images. The cerebellum was divided into right (red) and left (green) sides, whose midline was defined by the position of the cerebral longitudinal fissure; and (E) coronal T1-weighted (i.e., short TR/TE setting) MRI crossing at the level of crown of the lobule VI of vermis. Green and red arrowheads indicate the leftward asymmetry of the lobule VI observable on the coronal MRI image of a male ferret on PD 90. a: p < 0.05, b: p < 0.01 (male vs. female; Scheffe’s test).
Although a significant left/right side difference in the cerebellar volume was not detected in both males and females at any postnatal age by post-hoc testing, AQ scores indicated that the cerebellar volume was leftward in males (AQ = −0.034 ± 0.022) but rightward in females (AQ = 0.027 ± 0.033) on PD 90 (Figure 2C). Such leftward asymmetry in the male cerebellum was apparent in a posterior view of 3D volume rendered images (Figure 2D) and MRI images of posterior vermis at coronal plane (Figure 2E) on PD 90, but obscure on younger ages (Figure 2D,E). In contrast, asymmetry of the cerebellum was unclear in female either by 3D volume rendered images or MRI images at any postnatal age examined (Figure 2D,E).
3.3. Development of Torque Asymmetry of Cerebellar Cortex
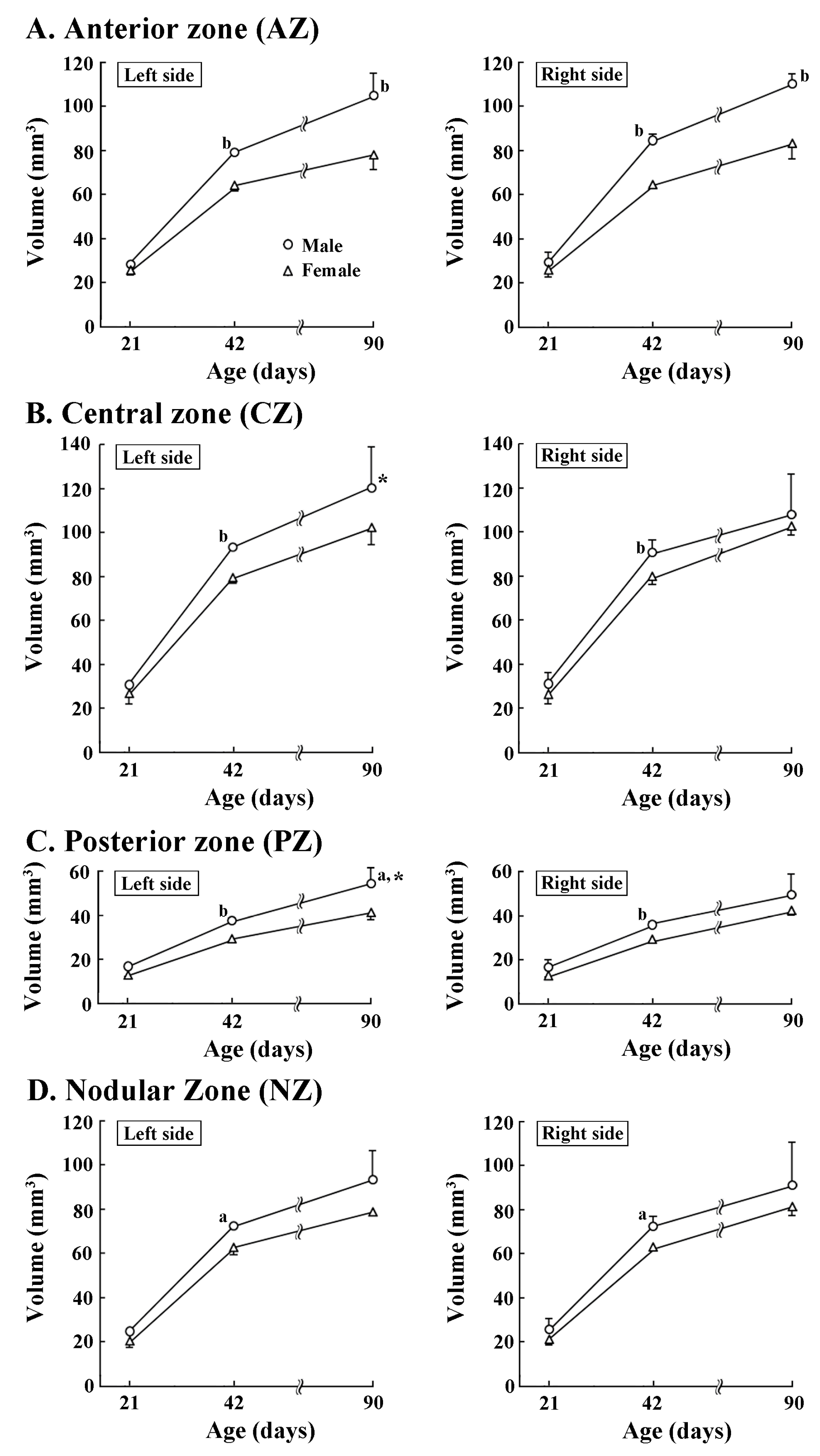
Four transverse domains of the cerebellar cortex were delineated primarily based on the zebrin II/aldolase C expression pattern [29,30]. Their volumes were examined to clarify when regional volume laterality of the cerebellum appeared during postnatal development. Four-way ANOVA revealed significant effects on postnatal age, sex, and left/right side interactions (F(2,12) = 9.8301, p < 0.01), postnatal age, transverse domains and left/right side interactions (F(6,36) = 2.758, p < 0.05), and sex, transverse domains, and left/right sides interactions (F(6,36) = 3.235, p < 0.05), but not on those four interactions. Age-related male-over-female larger volumes were detected in both left and right sides of AZ on PDs 42 and 90 (Figure 3A). However, the AZ volume did not differ between left/right sides in both sexes at any postnatal age examined. In the CZ, PZ, and NZ, significantly greater volumes in males than in females were detected in both sides on PD 42 (Figure 3B–D), but only in the left side of the PZ on PD 90 (Figure 3C). Leftward volume asymmetry was significant in both the CZ and PZ in males on PD 90 (Figure 3C) by enhancing volumes of the left side of those two domains during PDs 42 to 90, when compared to the right side.
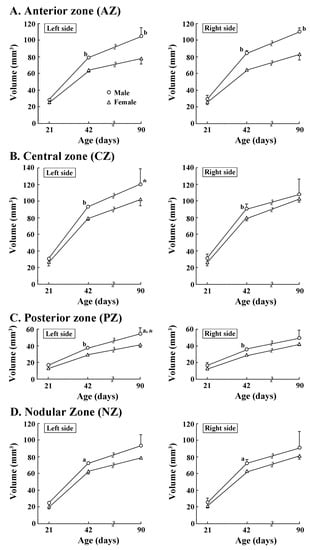
Figure 3.
Changes in volumes of left/right sides of four cerebellar transverse domains of male and female ferrets during postnatal days 21 to 90. (A) Anterior zone; (B) central zone; (C) posterior zone; and (D) nodular zone. Four transverse domains of the cerebellar cortex were defined primarily based on the expression pattern of zebrin II/aldolase C [29,30]. Their boundaries were defined by major fissures/sulci (Supplemental Figure S1) primarily according to our previous study [3]. a: p < 0.05, b: p < 0.001 (male vs. female; Scheffe’s test). *: p < 0.05 (left-side vs. right-side; Scheffe’s test).
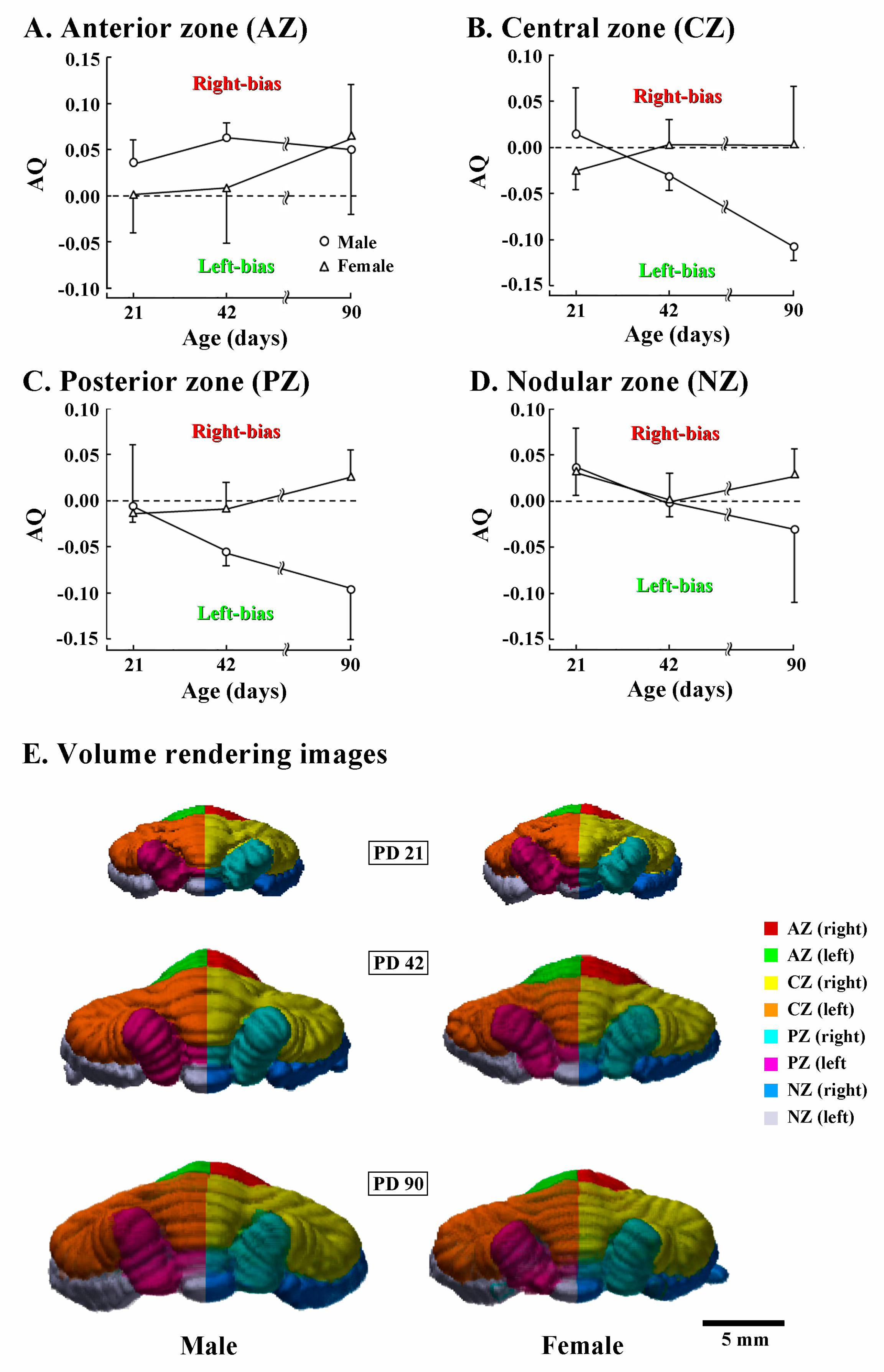
Next, AQ scores of volumes of four transverse cerebellar domains were examined for assessing the asymmetric development of the cerebellar cortex. AQ analysis revealed that the AZ was rightward in males already on PD 21, but symmetrical in females (Figure 4A). In females, AQ scores of AZ were symmetrical on PD 42, and then biased rightward on PD 90 (Figure 4A). In contrast, AQ scores of the CZ and PZ were gradually biased leftward in males, but sustained symmetry in females during PDs 42 to 90 (Figure 4B,C). The leftward bias of the CZ and PZ was clearly shown in 90-day-old males by the leftward curvature of the vermal region of the CZ and PZ from posterior views of the 3D volume-rendering images (Figure 4E). AQ scores of the NZ in both sexes evidenced a slightly rightward bias on PD 21, and symmetrical on PD 42 (Figure 4D). Then, the AQ scores were biased in opposite directions between sexes by PD 90: slightly leftward in males; and slightly rightward in females (Figure 4D). Thus, AQ analysis revealed that the anticlockwise torque asymmetry (rostrally rightward biased, and caudally leftward biased or symmetrical) of the cerebellar cortex was acquired in both sexes during PDs 42 to 90, although the leftward laterality was more enhanced in males than in females.
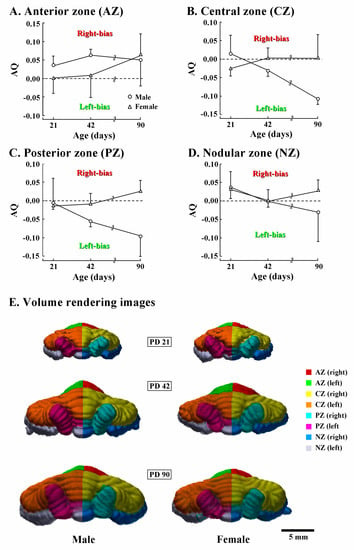
Figure 4.
Changes in asymmetry quotient and 3D morphology of four cerebellar transverse domains of male and female ferrets during PD 21 to 90. (A) Volume of the anterior zone; (B) volume of the central zone; (C) volume of the posterior zone; (D) volume of the nodular zone; and (E) posterior view of 3D volume-rendered images. Four transverse domains of the cerebellar cortex were defined primarily based on the expression pattern of zebrin II/aldolase C [29,30]. Their boundaries were defined by major fissures/sulci (Supplemental Figure S1) primarily according to our previous study [17].
4. Discussion
Torque asymmetry is known as a distinctively characteristic aspect of cerebellar morphology. In the cerebellum, anteriorly left-biased and posterior right-biased absolute volumes of the cerebellum were reported as an intrinsic cerebellar asymmetry [13,34], indicating a clockwise cerebellar torque. A similar clockwise torque pattern was seen in the cerebellum of chimpanzees [14]. In contrast, a counterclockwise cerebellar torque (rostrally leftward biased, and caudally rightward biased or symmetrical) was revealed in the cerebellum of ferrets [15]. The present AQ analysis revealed that the anticlockwise torque asymmetry is archived in both sexes of ferrets during PDs 42 to 90, although the leftward laterality is more enhanced in males. In particular, volumes of the CZ and PZ were left-lateralized in males on PD 42, and thereafter.
The cerebellum is functionally lateralized in relation to large-scale circuits, which made contralateral connections between cerebral association cortices and cerebellar hemispheres [6,7,8]. Such cerebro-cerebellar connections were involved in morphological lateralization of cerebral hemispheres and cerebellum, forming the torque structure. A mirror linkage of cerebral/cerebellar torques (i.e., counterclockwise versus clockwise) was revealed in right-handed humans [13]. In humans, movements of dominant hands in right-handers and visuo-spatial processing activated parietal associated cortices of the right cerebral hemisphere as well as lobules VI and VIII of the left cerebellum [6,35], corresponding to the vermal part of left CZ and PZ in our definition. As mentioned above, the CZ and PZ volumes were left-lateralized in male ferrets on PD 42, and thereafter. However, AQ values of the ferret cerebral cortex calculated on the same brain samples used in the present study were not biased laterally on either side at PDs 42 and 90 (Supplemental Table S2). This predicts that the mirror linkage of cerebral/cerebellar torques was ambiguous in ferrets. Therefore, the counterclockwise cerebellar torque of our ferret model may not have an influence on cerebro-cerebellar connections, and may be prenatal causes, for example, inhibitions of the growth of right cerebral hemisphere and interhemispherical callosal connections by perinatal testosterone [36,37]. On the other hand, circulating levels of androgens, but not estrogens, correlated with an activation of local functional connectivity between the frontal cortex and cerebellum [38]. This evidence showed the possibility that a continuously increasing level of circulating androgens altered morphological lateralization of the cerebellum via an activation of particular cerebro-cerebellar connections. In the present study, the counterclockwise torque asymmetry of the ferret cerebellum was achieved during pubertal to adolescent periods, and was more enhanced in males than in females. Such findings predict the involvement of post-pubertal androgens in the development of cerebellar torque in ferrets. Further studies will be needed to examine longitudinal effects of androgens on the morphological lateralization of the cerebellum, including the torque asymmetry, in post-pubertal ferrets.
In addition to sexual dimorphism of the torque asymmetry, volumes of both sides of the ferret cerebellum were already larger on PD 10 in males than in females, in the present study. In ferrets, the serum/plasma level of testosterone was very low in both sexes during the first three postnatal ages [39], and then began to rise from PD 40 [39,40]. Many investigators have reported a larger cerebellar volume in men than in women by MRI-based volumetry [17,18,19,20,21,22,23]. Given the following evidence, the sexual dimorphism of the cerebellar volume in humans is considered to be altered by a dosage of X-chromosome escape genes, independent of perinatal and/or postpubertal androgens. Normal male subjects (XY) had a smaller volume of precentral gyral gray matter and a larger cerebellar volume than normal female (XX), and as well as Klinefelter subjects (XXY) [41], who showed a low post-pubertal testosterone, albeit sustaining a normal level of perinatal testosterone [42,43]. Furthermore, in transgender male subjects (female-to-male) with prolonged cross-sex hormone therapy, the cerebellar volume was equivalent to normal female subjects, but smaller than normal male subjects [44]. Therefore, the dosage of X-chromosome escape genes, rather than perinatal and/or post-pubertal androgens, may alter the cerebellar growth, resulting in the smaller cerebellar volume in female infants than in male infants.
5. Conclusions
A disturbance of morphological and functional lateralization of the cerebellum is reportedly involved in human neurodevelopmental disorders with cognitive impairments, such as autism [45], schizophrenia [46,47], dyslexia [48], and attention deficit hyperactivity disorder [49]. The direction and extent of the cerebellar torque asymmetry varied depending on species [13,14,34], the hand preference [13], and/or sex [34], independent of genetic factors [13]. The extent of torque asymmetry and the volume laterality of the cerebellum were more enhanced in males than in females in ferrets [15], as well as humans [34]. The present findings will provide advantageous information regarding sex-related morphological specification of the cerebral cortex, and keys to understanding the pathogenesis of neurodevelopmental disorders with gyrification abnormality and gender vulnerability, i.e., schizophrenia and autism.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-8994/9/3/40/s1, Supplemental Figure S1, Supplemental Table S1, Supplemental Table S2.
Acknowledgments
The authors wish to thank Nobuhiro Nitta (Molecular Imaging Center, National Institute of Radiological Sciences, Chiba, Japan) for the MRI measurements. This study was supported by JSPS KAKENHI (15K08144), and partly supported by the Center of Innovation (COI) Program (Japan Science and Technology Agency; JST) for MRI devices and Brain/MINDS (Brain Mapping by Integrated Neurotechnologies for Disease Studies) project by MEXT for imaging technologies.
Author Contributions
Kazuhiko Sawada and Ichio Aoki conceived and designed the experiments; Kazuhiko Sawada performed the experiments and analyzed the data; Kazuhiko Sawada and Ichio Aoki wrote the paper.
Conflicts of Interest
There are no conflicts of interest.
References
- Synder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Hu, D.; Shen, H.; Zhou, Z. Functional asymmetry in the cerebellum: A brief review. Cerebellum 2008, 7, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Amunts, K. Architecture of the cerebral cortex. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 836–895. [Google Scholar]
- Ashwell, K.W.S.; Mai, J.K. Fetal development of the central nervous system. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 31–79. [Google Scholar]
- Alves, N.T.; Fukusima, S.S.; Aznar-Casanova, J.A. Models of brain asymmetry in emotional processing. Psychol. Neurosci. 2008, 1, 63–66. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.A.; Leopold, D.R.; Calhoun, V.D.; Mittal, V.A. Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum. Brain Mapp. 2015, 36, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Sholl, S.A.; Kim, K.L. Androgen receptors are differentially distributed between right and left cerebral hemispheres of the fetal male rhesus monkey. Brain Res. 1990, 516, 122–126. [Google Scholar] [CrossRef]
- Imai, N.; Sawada, K.; Fukunishi, K.; Sakata-Haga, H.; Fukui, Y. Sexual dimorphism of sulcal length asymmetry in cerebrum of adult cynomolgus monkeys (Macaca fascicularis). Congenit. Anom. (Kyoto) 2011, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Collura, R.V.; Ruvolo, M.; Walsh, C.A. Genomic and evolutionary analyses of asymmetrically expressed genes in human fetal left and right cerebral cortex. Cereb. Cortex 2006, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.G.; Dooling, E.C.; Gilles, F.H. Gyral development of the human brain. Ann. Neurol. 1977, 1, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rosch, R.E.; Ronan, L.; Cherkas, L.; Gurd, J.M. Cerebellar asymmetry in a pair of monozygotic handedness-discordant twins. J. Anat. 2010, 217, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Hopkins, W.D. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella). Neuropsychologia 2007, 45, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.S.; Narr, K.L.; Luders, E.; Szeszko, P.R.; Thompson, P.M.; Bilder, R.M.; Toga, A.W. Asymmetries of cortical thickness: Effects of handedness, sex, and schizophrenia. Neuroreport 2007, 18, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Escalona, P.R.; McDonald, W.M.; Doraiswamy, P.M.; Boyko, O.B.; Husain, M.M.; Figiel, G.S.; Laskowitz, D.; Ellinwood, E.H., Jr.; Krishnan, K.R. In vivo stereological assessment of human cerebellar volume: Effects of gender and age. AJNR Am. J. Neuroradiol. 1991, 12, 927–929. [Google Scholar] [PubMed]
- Filipek, P.A.; Richelme, C.; Kennedy, D.N.; Caviness, V.S., Jr. The young adult human brain: An MRI-based morphometric analysis. Cereb. Cortex 1994, 4, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Dupuis, J.H.; Briggs, S.D.; McGavran, C.; Acker, J.D. Differential effects of age and sex on the cerebellar hemispheres and the vermis: A prospective MR study. AJNR Am. J. Neuroradiol. 1998, 19, 65–71. [Google Scholar] [PubMed]
- Rhyu, I.J.; Cho, T.H.; Lee, N.J.; Uhm, C.S.; Kim, H.; Suh, Y.S. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci. Lett. 1999, 270, 149–152. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Williamson, A.; Acker, J.D. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. AJNR Am. J. Neuroradiol. 2001, 22, 1161–1167. [Google Scholar] [PubMed]
- Chung, S.C.; Lee, B.Y.; Tack, G.R.; Lee, S.Y.; Eom, J.S.; Sohn, J.H. Effects of age, gender, and weight on the cerebellar volume of Korean people. Brain Res. 2005, 1042, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Raznahan, A.; Mills, K.L.; Lenroot, R.K. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Koyun, N.; Aydinlioğlu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Nguon, K.; Ladd, B.; Baxter, M.G.; Sajdel-Sulkowska, E.M. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog. Brain Res. 2005, 148, 341–351. [Google Scholar] [PubMed]
- Womer, F.Y.; Tang, Y.; Harms, M.P.; Bai, C.; Chang, M.; Jiang, X.; Wei, S.; Wang, F.; Barch, D.M. Sexual dimorphism of the cerebellar vermis in schizophrenia. Schizophr. Res. 2016, 176, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.L.; McCarthy, M.M. Steroids, sex and the cerebellar cortex: Implications for human disease. Cerebellum 2008, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ozol, K.; Hayden, J.M.; Oberdick, J.; Hawkes, R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 1999, 412, 95–111. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Hawkes, R. Whole-mount immunohistochemistry: A high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002, 50, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Watanabe, M. Development of cerebral sulci and gyri in ferrets (Mustela putorius). Congenit. Anom. (Kyoto) 2012, 52, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. MRI-based morphometric characterizations of sexual dimorphism of the cerebrum of ferrets (Mustela putorius). Neuroimage 2013, 83, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Fan, L.; Tang, Y.; Sun, B.; Gong, G.; Chen, Z.J.; Lin, X.; Yu, T.; Li, Z.; Evans, A.C.; Liu, S. Sexual dimorphism and asymmetry in human cerebellum: An MRI-based morphometric study. Brain Res. 2010, 1353, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Häberling, I.; Corballis, M.C. Cerebellar asymmetry, cortical asymmetry and handedness: Two independent networks. Laterality 2015, 19, 1–18. [Google Scholar]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization: Biological mechanisms, associations, and pathology. 3. A hypothesis and a program for research. Arch. Neurol. Chic. 1985, 42, 634–654. [Google Scholar] [CrossRef]
- Witelson, S.F.; Nowakowski, R.S. Left out axons make men right: A hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia 1991, 29, 327–333. [Google Scholar] [CrossRef]
- Mueller, S.C.; Landré, L.; Wierckx, K.; T’Sjoen, G. A structural MRI study in Transgender persons on cross sex hormone therapy. Neuroendocrinology 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Erskine, M.S.; Baum, M.J. Plasma concentrations of testosterone and dihydrotestosterone during perinatal development in male and female ferrets. Endocrinology 1982, 111, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Kästner, R.; Kästner, D.; Apfelbach, R. Developmental patterns of thyroid hormones and testosterone in ferrets. Horm. Metab. Res. 1987, 19, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Lentini, E.; Kasahara, M.; Arver, S.; Savic, I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex 2013, 23, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.G.; Read, G.; Pan, H.; Fear, C.; Lindenbaum, R.; Crossley, J. Prenatal testosterone levels in XXY and XYY males. Horm. Res. 1994, 42, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Wikstrom, A.M.; Rajpert-DeMeyts, E.; Dunkel, L.; Skakkebaek, N.E.; Juul, A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum. Reprod. Update 2006, 12, 9–48. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Wierckx, K.; Jackson, K.; T’Sjoen, G. Circulating androgens correlate with resting state MRI in transgender men. Psychoneuroendocrinology 2016, 73, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bloss, C.S.; Courchesne, E. MRI neuroanatomy in young girls with autism: A preliminary study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Loeber, R.T.; Cintron, C.M.; Yurgelun-Todd, D.A. Morphometry of individual cerebellar lobules in schizophrenia. Am. J. Psychiatry 2001, 158, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Szeszko, P.R.; Gunning-Dixon, F.; Ashtari, M.; Snyder, P.J.; Lieberman, J.A.; Bilder, R.M. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol. Psychiatry 2003, 53, 450–459. [Google Scholar] [CrossRef]
- Kibby, M.Y.; Fancher, J.B.; Markanen, R.; Hynd, G.W. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J. Child Neurol. 2008, 23, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; Vauss, Y.C.; Snell, J.W.; Lange, N.; et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).