A Critical Assessment of the Performance of Magnetic and Electronic Indices of Aromaticity
Abstract
:1. Introduction
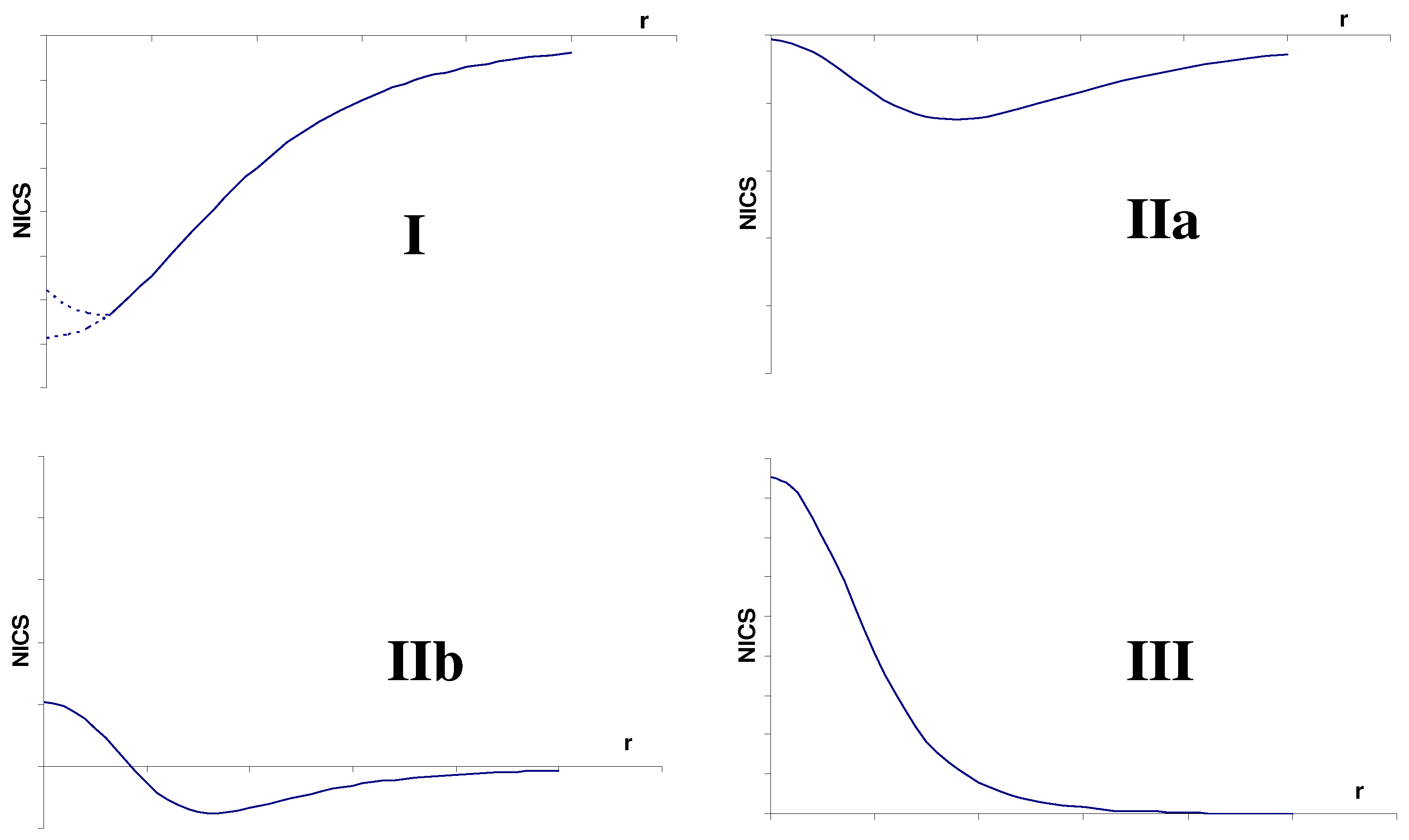
2. Some Reported NICS Drawbacks
3. A Critical Assessment of Aromaticity Indices Using a Test Set
4. NICS Profiles as Aromaticity Criteria in Inorganic Clusters
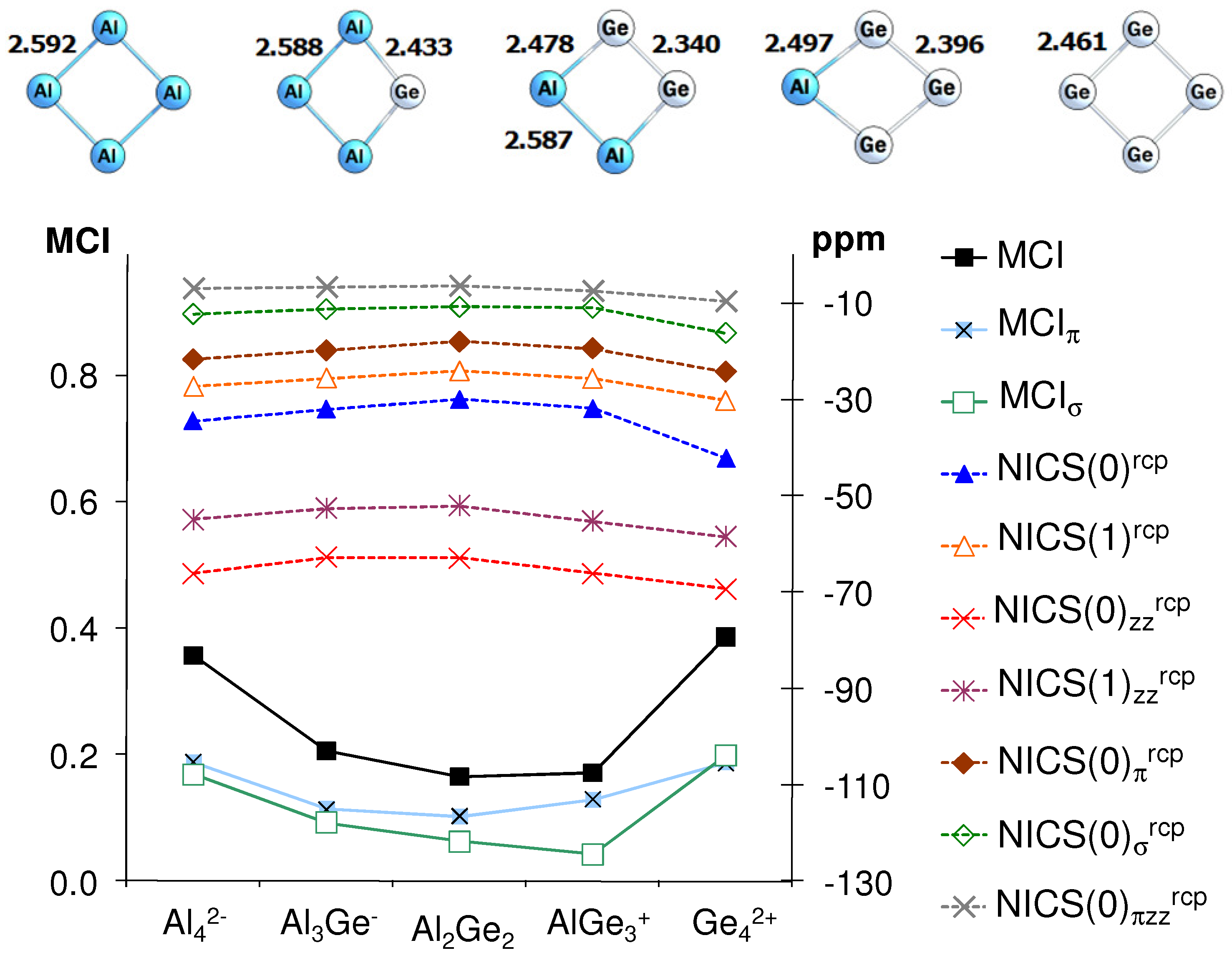
5. NICS vs. MCI Inconsistencies in Inorganic Clusters
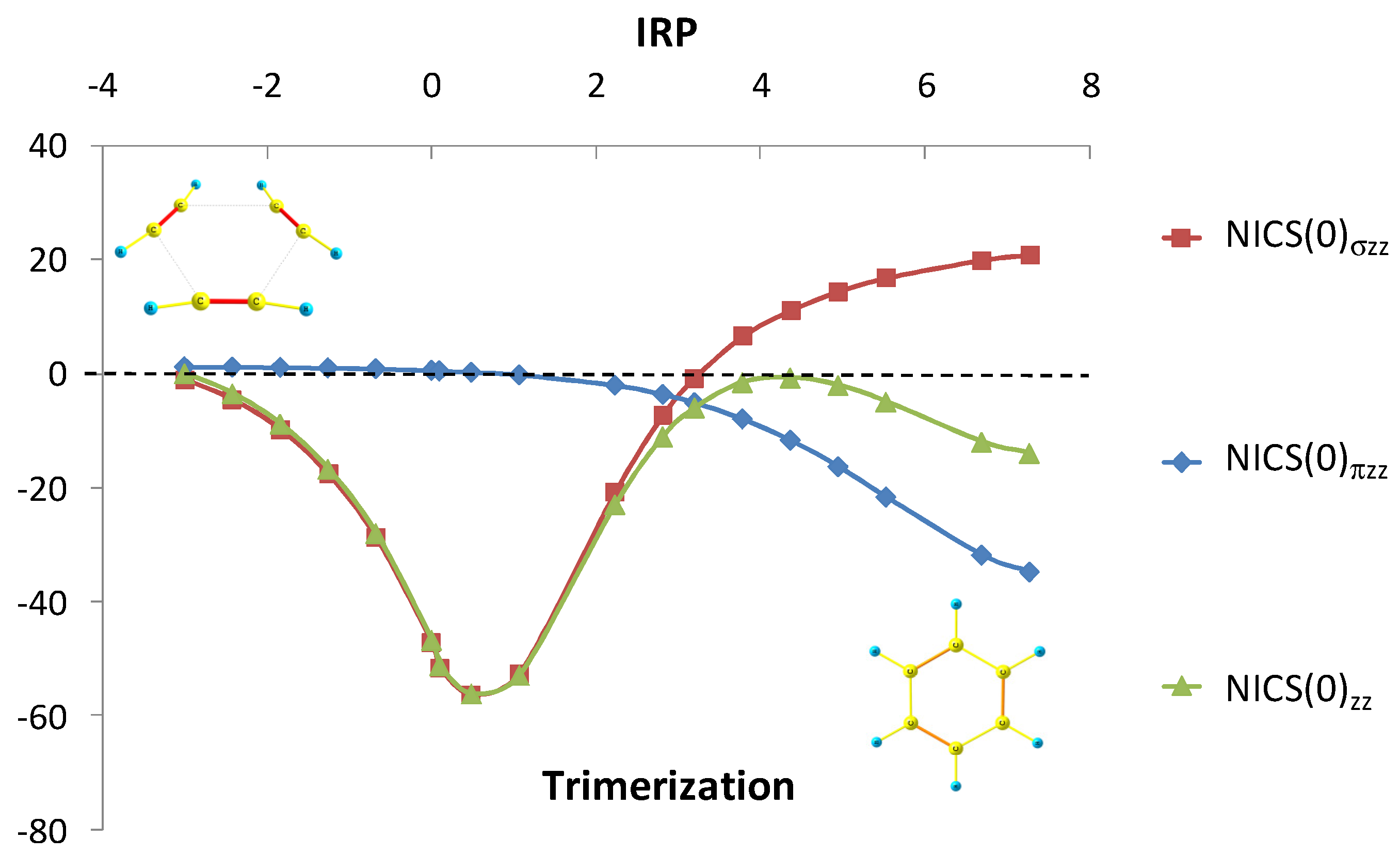
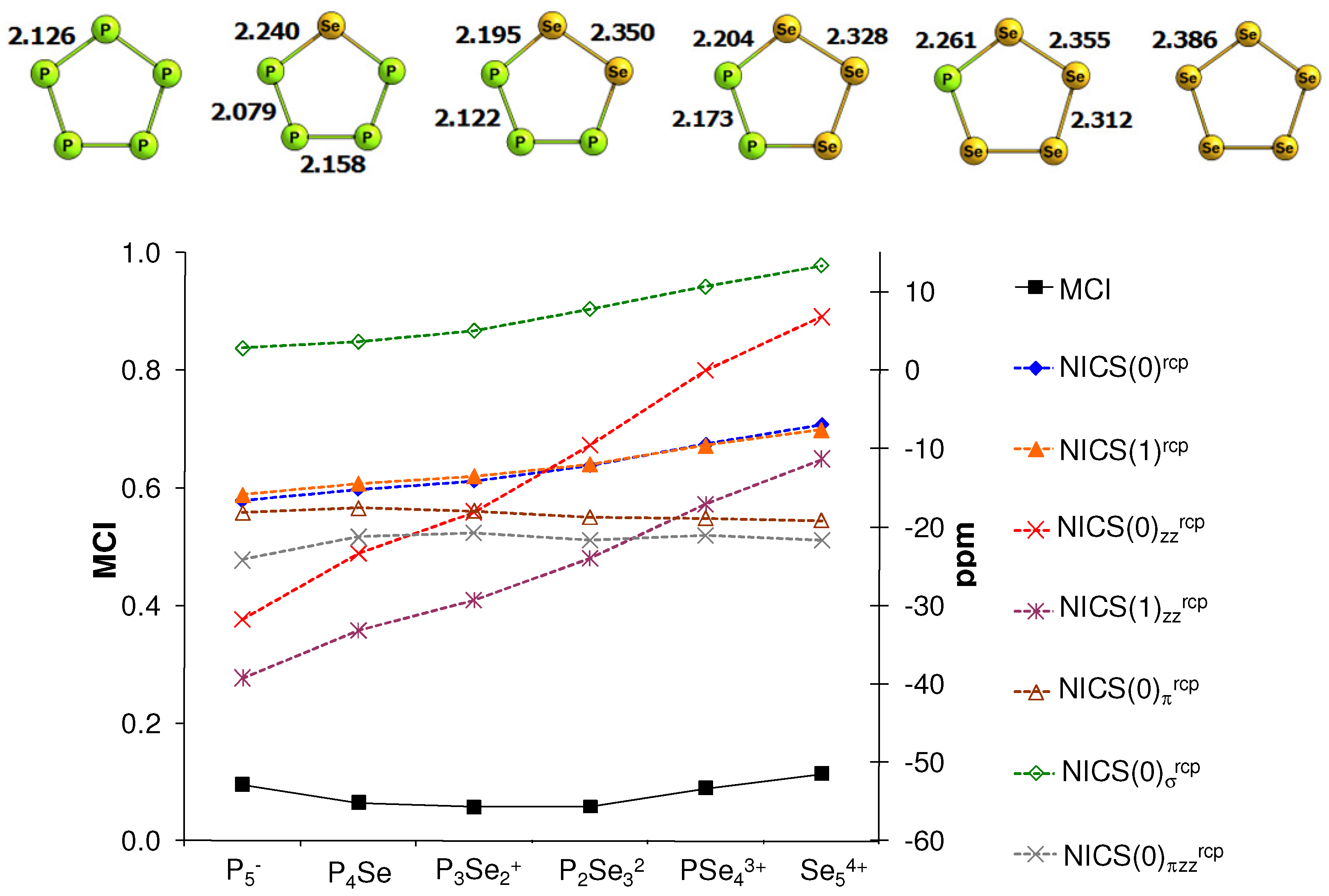
6. NICS and MCI Assessment in a Series of Inorganic Clusters with Predictable Aromatic Trends
7. Conclusions
Acknowledgements
References and Notes
- Schleyer, P.v.R. Introduction: Aromaticity. Chem. Rev. 2001, 101, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.v.R. Introduction: Delocalization–Pi and Sigma. Chem. Rev. 2005, 105, 3433–3435. [Google Scholar] [CrossRef]
- Heilbronner, E. Hückel Molecular Orbitals of Möbius-Type Conformation of Annulenes. Tetrahedron Lett. 1964, 5, 1923–1928. [Google Scholar] [CrossRef]
- Rzepa, H.S. Möbius Aromaticity and Delocalization. Chem. Rev 2005, 105, 3697–3715. [Google Scholar] [CrossRef] [PubMed]
- Bühl, M.; Hirsch, A. Spherical Aromaticity of Fullerenes. Chem. Rev. 2001, 101, 1153–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; King, R. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef] [PubMed]
- Masui, H. Metalloaromaticity. Coord. Chem. Rev. 2001, 219, 957–992. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; McKee, M.L. Aspects of Cyclic Conjugation. Pure Appl. Chem. 1980, 52, 1431–1441. [Google Scholar] [CrossRef]
- Dewar, M.J.S. Chemical Implications of Sigma Conjugation. J. Am. Chem. Soc. 1984, 106, 669–682. [Google Scholar] [CrossRef]
- Cremer, D.; Gauss, J. Theoretical Determination of Molecular Structure and Conformation. 20. Reevaluation of the Strain Energies of Cyclopropane and Cyclobutane-CC and CH Bond Energies, 1, 3 Interactions, and σ-Aromaticity. J. Am. Chem. Soc. 1986, 108, 7467–7477. [Google Scholar] [CrossRef]
- Li, X.; Kuznetsov, A.E.; Zhang, H.-F.; Boldyrev, A.; Wang, L.-S. Observation of All-Metal Aromatic Molecules. Science 2001, 291, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.I.; Wang, L.-S. All-Metal Aromaticity and Antiaromaticity. Chem. Rev. 2005, 105, 3716–3757. [Google Scholar] [CrossRef] [PubMed]
- Tsipis, C.A. DFT Study of "All-Metal" Aromatic Compounds. Coord. Chem. Rev. 2005, 249, 2740–2762. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Averkiev, B.B.; Zhai, H.-J.; Wang, L.-S.; Boldyrev, A.I. Aromaticity and Antiaromaticity in Transition-Metal Systems. Phys. Chem. Chem. Phys. 2008, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhai, H.-J.; Kiran, B.; Wang, L.-S. Observation of d-Orbital Aromaticity. Angew. Chem. Int. Ed. 2005, 44, 7251–7254. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.-J.; Averkiev, B.B.; Zubarev, D.Y.; Wang, L.-S.; Boldyrev, A.I. δ Aromaticity in [Ta3O3]-. Angew. Chem. Int. Ed. 2007, 46, 4277–4280. [Google Scholar] [CrossRef] [PubMed]
- Averkiev, B.B.; Boldyrev, A.I. Hf3 Cluster Is Triply (σ-, π-, and δ-) Aromatic in the Lowest D3h, 1A1 State. J. Phys. Chem. A 2007, 111, 12864–12866. [Google Scholar] [CrossRef]
- Tsipis, A.C.; Kefalidis, C.E.; Tsipis, C.A. The Role of the 5f Orbitals in Bonding, Aromaticity, and Reactivity of Planar Isocyclic and Heterocyclic Uranium Clusters. J. Am. Chem. Soc. 2008, 130, 9144–9155. [Google Scholar] [CrossRef]
- Zhan, C.-G.; Zheng, F.; Dixon, D.A. Electron Affinities of Aln Clusters and Multiple-Fold Aromaticity of the Square Al42- Structure. J. Am. Chem. Soc. 2002, 124, 14795–14803. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Kuznetsov, A.E.; Boldyrev, A.; Wang, L.-S. Multiple Aromaticity and Antiaromaticity in Silicon Clusters. Chem. Phys. Chem. 2004, 5, 1885–1891. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Tian, W.-Q.; Feng, J.-K.; Zhang, G.; Li, W.-Q. Theoretical Study on Structures and Aromaticities of P5- Anion, [Ti(η5-P5)]- and Sandwich Complex [Ti(η5-P5)2]2-. J. Phys. Chem. A 2005, 109, 5645–5655. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.X.; Chen, X.J.; Yuan, Z.S. Theoretical Study in the Aromaticity of the Bimetallic Clusters X2M2 (X = Si, Ge; M = Al, Ga). J. Mol. Struct. (Theochem) 2005, 732, 149–153. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.K. Structural Aspects of Aromaticity. Chem. Rev. 2001, 101, 1385–1419. [Google Scholar] [CrossRef] [PubMed]
- Krygowski, T.M.; Cyrański, M.K.; Czarnocki, Z.; Häfelinger, G.; Katritzky, A.R. Aromaticity: A Theoretical Concept of Immense Practical Importance. Tetrahedron 2000, 56, 1783–1796. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Cyrański, M.K. Energetic Aspects of Cyclic pi-electron Delocalization: Evaluation of the Methods of Estimating Aromatic Stabilization Energies. Chem. Rev. 2005, 105, 3773–3811. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Duran, M.; Solà, M.; Silvi, B. Theoretical Evaluation of Electron Delocalization in Aromatic Molecules by Means of Atoms in Molecules (AIM) and Electron Localization Function (ELF) Topological Approaches. Chem. Rev. 2005, 105, 3911–3947. [Google Scholar] [CrossRef]
- Merino, G.; Vela, A.; Heine, T. Description of Electron Delocalization via the Analysis of Molecular Fields. Chem. Rev. 2005, 105, 3812–3841. [Google Scholar] [CrossRef]
- Poater, J.; García-Cruz, I.; Illas, F.; Solà, M. Discrepancy Between Common Local Aromaticity Measures in a Series of Carbazole Derivatives. Phys. Chem. Chem. Phys. 2004, 6, 314–318. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Barczynski, P.; Musumarra, G.; Pisano, D.; Szafran, M. Aromaticity as a Quantitative Concept. 1. A Statistical Demostration of the Orthogonality of "Classical" and "Magnetic" Aromaticity in Five- and Six- Membered Heterocycles. J. Am. Chem. Soc. 1989, 111, 7–15. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Karelson, M.; Sild, S.; Krygowski, T.M.; Jug, K. Aromaticity as a Quantitative Concept. 7. Aromaticity Reaffirmed as a Multidimensional Characteristic. J. Org. Chem. 1998, 63, 5228–5231. [Google Scholar] [CrossRef]
- Cyrański, M.K.; Krygowski, T.M.; Katritzky, A.R.; Schleyer, P.v.R. To What Extent Can Aromaticity Be Defined Uniquely? J. Org. Chem. 2002, 67, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Jug, K.; Köster, A.M. Aromaticity as a Multi-Dimensional Phenomenon. J. Phys. Org. Chem. 1991, 4, 163–169. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of Aromaticity Basing on the Harmonic Oscillator Model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic studies of Inter- and Intra-Molecular Interactions Reflected in benzenoid Hydrocarbons. Nonequivalence of Indices of Aromaticity. J. Chem. Inform. Comput. Sci. 1993, 33, 70–78. [Google Scholar] [CrossRef]
- Matito, E.; Duran, M.; Solà, M. The Aromatic Fluctuation Index (FLU): A New Aromaticity Index Based on Electron Delocalization. J. Chem. Phys. 2005, 122, 014109. [Google Scholar] [CrossRef] [PubMed]
- Bultinck, P.; Ponec, R.; Van Damme, S. Multicenter Bond Indices as a New Measure of Aromaticity in Polycyclic Aromatic Hydrocarbons. J. Phys. Org. Chem. 2005, 18, 706–718. [Google Scholar] [CrossRef]
- Matta, C.F. Application of the Quantum Theory of Atoms in Molecules to Selected Physico-Chemical and Biophysical Problems: Focus on Correlation with Experiment. J. Comput. Chem. 2003, 24, 453–462. [Google Scholar] [CrossRef]
- Matta, C.F.; Hernández-Trujillo, J. Bonding in Polycyclic Aromatic Hydrocarbons in Terms of the Electron Density and of Electron Delocalization. J. Phys. Chem. A 2003, 107, 7496–7504. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Kuznetsov, A.E. On the Resonance Energy in New All-Metal Aromatic Molecules. Inorg. Chem. 2002, 41, 532–537. [Google Scholar] [CrossRef]
- Hückel, E. Quantentheoretische Beiträge zum Benzolproblem I. Die Elektronenkonfiguration des Benzols und Verwandter Verbindungen. Z. Physik 1931, 70, 104–186. [Google Scholar] [CrossRef]
- Hückel, E. Quanstentheoretische Beiträge zum Benzolproblem II. Quantentheorie der Induzierten Polaritäten. Z. Physik 1931, 72, 310–337. [Google Scholar] [CrossRef]
- Hückel, E. Quantentheoretische Beiträge zum Problem der Aromatischen und Ungesättigten Verbindungen. III. Z. Physik 1932, 76, 628–648. [Google Scholar] [CrossRef]
- Hückel, E. The Theory of Unsaturated and Aromatic Compounds. Z. Elektrochemie 1937, 43, 752–788, 827–849. [Google Scholar]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
- Giambiagi, M.; de Giambiagi, M.S.; dos Santos, C.D.; de Figueiredo, A.P. Multicenter Bond Indices as a Measure of Aromaticity. Phys. Chem. Chem. Phys. 2000, 2, 3381–3392. [Google Scholar] [CrossRef]
- Bultinck, P.; Rafat, M.; Ponec, R.; van Gheluwe, B.; Carbó-Dorca, R.; Popelier, P. Electron Delocalization and Aromaticity in Linear Polyacenes: Atoms in Molecules Multicenter Delocalization Index. J. Phys. Chem. A 2006, 110, 7642–7648. [Google Scholar] [CrossRef] [PubMed]
- Mandado, M.; González-Moa, M.J.; Mosquera, R.A. QTAIM N-Center Delocalization Indices as Descriptors of Aromaticity in Mono and Poly Heterocycles. J. Comput. Chem. 2007, 28, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cioslowski, J.; Matito, E.; Solà, M. Properties of Aromaticity Indices Based on the One-Electron Density Matrix. J. Phys. Chem. A 2007, 111, 6521–6525. [Google Scholar] [CrossRef] [PubMed]
- Mandado, M.; Krishtal, A.; Van Alsenoy, C.; Bultinck, P.; Hermida-Ramón, J.M. Bonding Study in All-Metal Clusters Containing Al4 Units. J. Phys. Chem. A 2007, 111, 11885–11893. [Google Scholar] [CrossRef]
- Roy, D.R.; Bultinck, P.; Subramanian, V.; Chattaraj, P.K. Bonding, Reactivity and Aromaticity in the Light of the Multicenter Indices. J. Mol. Struct. (Theochem) 2008, 854, 35–39. [Google Scholar] [CrossRef]
- Jiménez-Halla, J.O.C.; Matito, E.; Blancafort, L.; Robles, J.; Solà, M. Tuning Aromaticity in Trigonal Alkaline Earth Metal Clusters and Their Alkali Metal Salts. J. Comput. Chem. 2009, 30, 2764–2776. [Google Scholar] [CrossRef] [PubMed]
- Havenith, R.W.A.; Fowler, P.W.; Steiner, E.; Shetty, S.; Kanhere, D.; Pal, S. Aromaticity and Antiaromaticity of LixAl4 Clusters: Ring Current Patterns Versus Electron Counting. Phys. Chem. Chem. Phys. 2004, 6, 285–288. [Google Scholar] [CrossRef]
- Jung, Y.; Heine, T.; Schleyer, P.v.R.; Head-Gordon, M. Aromaticity of Four-Membered-Ring 6π-electron Systems: N2S2 and Li2C4H4. J. Am. Chem. Soc. 2004, 126, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Aihara, J.; Kanno, H.; Ishida, T. Aromaticity of Planar Boron Clusters Confirmed. J. Am. Chem. Soc. 2005, 127, 13324–13330. [Google Scholar] [CrossRef] [PubMed]
- Tsipis, A.C.; Tsipis, C.A. Hydrometal Analogues of Aromatic Hydrocarbons: A New Class of Cyclic Hydrocoppers (I). J. Am. Chem. Soc. 2003, 125, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Tsipis, C.A.; Karagiannis, E.E.; Kladou, P.F.; Tsipis, A.C. Aromatic Gold and Silver Rings: Hydrosilver (I) and Hydrogold (I) Analogues of Aromatic Hydrocarbons. J. Am. Chem. Soc. 2004, 126, 12916–12929. [Google Scholar] [CrossRef]
- Wannere, C.S.; Corminboeuf, C.; Wang, Z.-X.; Wodrich, M.D.; King, R.B.; Schleyer, P.v.R. Evidence for d Orbital Aromaticity in Square Planar Coinage Metal Clusters. J. Am. Chem. Soc. 2005, 127, 5701–5705. [Google Scholar] [CrossRef]
- Corminboeuf, C.; Wannere, C.S.; Roy, D.; King, R.B.; Schleyer, P.v.R. Octahedral and Tetrahedral Coinage Metal Clusters: Is Three-Dimensional d-Orbital Aromaticity Viable? Inorg. Chem. 2006, 45, 214–219. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, Y.F.; Wu, J.I.; Schleyer, P.v.R. Ab Initio Study of the Geometry, Stability, and Aromaticity of the Cyclic S2N3+ Cation Isomers and Their Isoelectronic Analogues. Inorg. Chem. 2009, 48, 6773–6780. [Google Scholar] [CrossRef]
- Chen, Z.; Corminboeuf, C.; Heine, T.; Bohmann, J.; Schleyer, P.v.R. Do All-Metal Antiaromatic Clusters Exist? J. Am. Chem. Soc. 2003, 125, 13930–13931. [Google Scholar] [CrossRef] [PubMed]
- Lazzeretti, P. Ring Currents. In Progress in Nuclear Magnetic Resonance Spectroscopy; Emsley, J.W., Feeney, J., Sutcliffe, L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1–88. [Google Scholar]
- Lazzeretti, P. Assessment of Aromaticity Via Molecular Response Properties. Phys. Chem. Chem. Phys. 2004, 6, 217–223. [Google Scholar] [CrossRef]
- Aihara, J. Nucleus-Independent Chemical Shifts and Local Aromaticities in Large Polycyclic Aromatic Hydrocarbons. Chem. Phys. Lett. 2002, 365, 34–39. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Manoharan, M.; Wang, Z.X.; Kiran, B.; Jiao, H.J.; Puchta, R.; van Eikema Hommes, N.J.R. Dissected Nucleus-Independent Chemical Shift Analysis of π-Aromaticity and Antiaromaticity. Org. Lett. 2001, 3, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Corminboeuf, C.; Heine, T.; Seifert, G.; Schleyer, P.v.R.; Weber, J. Induced Magnetic Fields in Aromatic [n]-Annulenes–Interpretation of NICS Tensor Components. Phys. Chem. Chem. Phys. 2004, 6, 273–276. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Manoharan, M.; Jiao, H.J.; Stahl, F. The Acenes: Is There a Relationship Between Aromatic Stabilization and Reactivity? Org. Lett. 2001, 3, 3643–3646. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Jiao, H.J.; van Eikema Hommes, N.J.R.; Malkin, V.G.; Malkina, O.L. An Evaluation of the Aromaticity of Inorganic Rings: Refined Evidence From Magnetic Properties. J. Am. Chem. Soc. 1997, 119, 12669–12670. [Google Scholar] [CrossRef]
- Corminboeuf, C.; Heine, T.; Weber, J. Evaluation of Aromaticity: A New Dissected NICS Model Based On Canonical Orbitals. Phys. Chem. Chem. Phys. 2003, 5, 246–251. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Weinhold, F. NBO 5.0 Program. Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2001. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Fallah-Bagher-Shaidaei, H.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Which NICS Aromaticity Index for Planar Rings Is Best? Org. Lett. 2006, 8, 863–866. [Google Scholar] [CrossRef]
- Feixas, F.; Matito, E.; Poater, J.; Solà, M. On the Performance of Some Aromaticity Indices: A Critical Assessment Using a Test Set. J. Comput. Chem. 2008, 29, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Jug, K.; Oniciu, D.C. Quantitative Measures of Aromaticity For mono-, Bi-, and Tricyclic Penta- and Hexaatomic Heteroaromatic Ring Systems and Their Interrelationships. Chem. Rev. 2001, 101, 1421–1449. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Fradera, X.; Duran, M.; Solà, M. The Delocalization Index as an Electronic Aromaticity Criterion. Application to a Series of Planar Polycyclic Aromatic Hydrocarbons. Chem. Eur. J. 2003, 9, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Steiner, E.; Fowler, P.W. Ring Currents in Aromatic Hydrocarbons. Int. J. Quant. Chem. 1996, 60, 609–616. [Google Scholar] [CrossRef]
- Steiner, E.; Fowler, P.W.; Havenith, R.W.A. Current Densities of Localized and Delocalized Electrons in Molecules. J. Phys. Chem. A 2002, 106, 7048–7056. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Viglione, R.G.; Zanasi, R. The Local Aromaticity of the Six-Membered Rings in Pyracylene. A Difficult Case for the NICS Indicator of Aromaticity. J. Org. Chem. 2004, 69, 7537–7542. [Google Scholar] [CrossRef] [PubMed]
- Solà, M.; Mestres, J.; Duran, M. Molecular-Size and Pyramidalization–2 Keys for Understanding the Reactivity of Fullerenes. J. Phys. Chem. 1995, 99, 10752–10758. [Google Scholar] [CrossRef]
- Kraakman, P.A.; Valk, J.M.; Niederlander, H.A.G.; Brouwer, D.B.E.; Bickelhaupt, F.M.; Dewolf, W.H.; Bickelhaupt, F.; Stam, C.H. Unusual Reactivity of Small Cyclophanes–Nucleophilic Attack on 11-Chloro[5]Metacyclophane and 8,11-Dichloro[5]Metacyclophane. J. Am. Chem. Soc. 1990, 112, 6638–6646. [Google Scholar] [CrossRef]
- Gready, J.E.; Hambley, T.W.; Kakiuchi, K.; Kobiro, K.; Sternhell, S.; Tansey, C.W.; Tobe, Y. NMR-Studies of Bond Order in Distorted Aromatic Systems. J. Am. Chem. Soc. 1990, 112, 7537–7540. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Bridson, J.N.; Houghton, T.J.; Kennedy, J.W.J.; Mannion, M.R. 1,8-Dioxa[8](2,7)Pyrenophane, a Severely Distorted Polycyclic Aromatic Hydrocarbon. Angew. Chem. Int. Ed. Eng. 1996, 35, 1320–1321. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Bridson, J.N.; Houghton, T.J.; Kennedy, J.W.J.; Mannion, M.R. 1,7-Dioxa[7](2,7)Pyrenophane: The Pyrene Moiety is More Bent Than That of C70. Chem. Eur. J. 1999, 5, 1823–1827. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Bridson, J.N.; Cyranski, M.K.; Kennedy, J.W.J.; Krygowski, T.M.; Mannion, M.R.; Miller, D.O. Nonplanar Aromatic Compounds. 8. Synthesis, Crystal Structures, and Aromaticity Investigation of the 1,n-Dioxa[n]Pyrenophanes. How Does Bending Affect the Cyclic π-Electron Delocalization of the Pyrene System? J. Org. Chem. 2003, 68, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Jenneskens, L.W.; Vaneenige, E.N.; Louwen, J.N. A P-Orbital Axis Vector (POAV) Analysis of Boat-Shaped Benzenes. New J. Chem. 1992, 16, 775–779. [Google Scholar]
- Grimme, S. Theoretical-Study of [4]Paracyclophane and Its Dewar Benzene and Prismane Valence Isomers. J. Am. Chem. Soc. 1992, 114, 10542–10547. [Google Scholar] [CrossRef]
- Dijkstra, F.; van Lenthe, J.H. Aromaticity of Bent Benzene Rings: A VBSCF Study. Int. J. Quant. Chem. 1999, 74, 213–221. [Google Scholar] [CrossRef]
- Zhigalko, M.V.; Shishkin, O.V.; Gorb, L.; Leszczynski, J. Out-of-plane Deformability of Aromatic Systems in Naphthalene, Anthracene and Phenanthrene. J. Mol. Struct. 2004, 693, 153–159. [Google Scholar] [CrossRef]
- Lazzeretti, P.; Malagoni, M.; Zanasi, R. Computational Approach to Molecular Magnetic Properties by Continuous Transformation of the Origin of the Current Density. Chem. Phys. Lett. 1994, 220, 299–304. [Google Scholar] [CrossRef]
- Fowler, P.W.; Steiner, E.; Cadioli, B.; Zanasi, R. Distributed-Gauge Calculations of Current Density Maps, Magnetizabilities, and Shieldings for a Series of Neutral and Dianionic Fused Tetracycles: Pyracylene (C14H8), Acepleiadylene (C16H10), and Dipleiadiene (C18H12). J. Phys. Chem. A 1998, 102, 7297–7302. [Google Scholar] [CrossRef]
- Poater, J.; Bofill, J.M.; Alemany, P.; Solà, M. The Role of Electron Density and Magnetic Couplings on the NICS Profiles of [2.2]Paracyclophane and Related Species. J. Org. Chem. 2006, 71, 1700–1702. [Google Scholar] [CrossRef]
- Caramori, G.F.; Galembeck, S.E.; Laali, K.K. A Computational Study of [2.2]Cyclophanes. J. Org. Chem. 2005, 70, 3242–3250. [Google Scholar] [CrossRef]
- Johnson, C.E.; Bovey, F.A. Calculation of Nuclear Magnetic Resonance Spectra of Aromatic Hydrocarbons. J. Chem. Phys. 1958, 29, 1012–1014. [Google Scholar] [CrossRef]
- Portella, G.; Poater, J.; Bofill, J.M.; Alemany, P.; Solà, M. Local Aromaticity of [n]Acenes, [n]Phenacenes, and [n]Helicenes (n = 1–9). J. Org. Chem. 2005, 70, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Portella, G.; Poater, J.; Bofill, J.M.; Alemany, P.; Solà, M. Erratum of Local Aromaticity of [n]Acenes, [n]Phenacenes, and [n]Helicenes (n = 1–9). J. Org. Chem. 2005, 70, 4560–4560. [Google Scholar] [CrossRef]
- Osuna, S.; Poater, J.; Bofill, J.M.; Alemany, P.; Solà, M. Are Nucleus-Independent (NICS) and 1H NMR Chemical Shifts Good Indicators of Aromaticity in π-Stacked Polyfluorenes? Chem. Phys. Lett. 2006, 428, 191–195. [Google Scholar] [CrossRef]
- Feixas, F.; Jiménez-Halla, J.O.C.; Matito, E.; Poater, J.; Solà, M. Is the Aromaticity of the Benzene Ring in the (η6-C6H6)Cr(CO)3 Complex Larger Than That of the Isolated Benzene Molecule? Pol. J. Chem. 2007, 81, 783–797. [Google Scholar]
- Mitchell, R.H. Measuring Aromaticity by NMR. Chem. Rev. 2001, 101, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.; Brkic, Z.; Berg, D.J.; Barclay, T.M. Effective Aromaticity of Tricarbonylchromiumbenzene, about 25 Enhanced over that of Benzene: Structural Evidence from a Complexed Benzannulene. J. Am. Chem. Soc. 2002, 124, 11983–11988. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.H.; Chen, Y.; Khalifa, N.; Zhou, P. The Synthesis, Aromaticity, and NMR Properties of [14]Annulene Fused Organometallics. Determination of the Effective Bond Localizing Ability ("Relative Aromaticity") and Diamagnetic Anisotropy of Several Organometallic Moieties. J. Am. Chem. Soc. 1998, 120, 1785–1794. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Zhou, P.; Venugopalan, S.; Dingle, T.W. Synthesis of the First Metal-Complexed Benzannulene. An Estimate of the Aromaticity of Tricarbonylchromium-Complexed Benzene Relative to the Aromaticity of Benzene Itself. J. Am. Chem. Soc. 1990, 112, 7812–7813. [Google Scholar] [CrossRef]
- Low, A.A.; Hall, M.B. Benzene Chromium Tricarbonyl Revisited: Theoretical Study of The Structure and Dynamics of (η6-C6H6)Cr(CO)3. Int. J. Quant. Chem. 2000, 77, 152–160. [Google Scholar] [CrossRef]
- Hubig, S.M.; Lindeman, S.V.; Kochi, J.K. Charge Transfer Bonding in Metal-Arene Coordination. Coord. Chem. Rev. 2006, 200-202, 831–873. [Google Scholar] [CrossRef]
- Albright, T.A.; Hofmann, P.; Hoffmann, R.; Lillya, C.P.; Dobosh, P.A. Haptotropic Rearrangements of Polyene-MLn Complexes. 2. Bicyclic Polyene-MCp-M(CO)3 Systems. J. Am. Chem. Soc. 1983, 105, 3396–3411. [Google Scholar] [CrossRef]
- Arrais, A.; Diana, E.; Gervasio, G.; Gobetto, R.; Marabello, D.; Stanghellini, P.L. Synthesis, Structural and Spectroscopic Characterization of Four [(η6-PAH)Cr(CO)3] Complexes (PAH=Pyrene, Perylene, Chrysene, 1,2-Benzanthracene). Eur. J. Inorg. Chem. 2004, 1505–1513. [Google Scholar] [CrossRef]
- Havenith, R.W.A.; Fowler, P.W.; Jenneskens, L.W.; Steiner, E. Trimerization of Ethyne: Growth and Evolution of Ring Currents in the Formation of the Benzene Ring. J. Phys. Chem. A 2003, 107, 1867–1871. [Google Scholar] [CrossRef]
- Santos, J.C.; Polo, V.; Andrés, J. An Electron Localization Function Study of the Trimerization of Acetylene: Reaction Mechanism and Development of Aromaticity. Chem. Phys. Lett. 2005, 406, 393–397. [Google Scholar] [CrossRef]
- Mandado, M.; González-Moa, M.J.; Mosquera, R.A. Characterization of Pericyclic Reactions Using Multicenter Electron Delocalization Analysis. ChemPhysChem 2007, 8, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Schleyer, P.v.R. Aromaticity of Pericyclic Reaction Transition Structures: Magnetic Evidence. J. Phys. Org. Chem. 1998, 11, 655–662. [Google Scholar] [CrossRef]
- Morao, I.; Cossio, F.P. A Simple Ring Current Model For Describing in-plane Aromaticity in Pericyclic Reactions. J. Org. Chem. 1999, 64, 1868–1874. [Google Scholar] [CrossRef]
- Stanger, A. Nucleus-Independent Chemical Shifts (NICS): Distance Dependence and Revised Criteria for Aromaticity and Antiaromaticity. J. Org. Chem. 2006, 71, 883–893. [Google Scholar] [CrossRef]
- Stanger, A. Can Substituted Cyclopentadiene Become Aromatic or Antiaromatic? Chem. Eur. J. 2006, 12, 2745–2751. [Google Scholar] [CrossRef]
- Jiménez-Halla, J.O.C.; Matito, E.; Robles, J.; Solà, M. Nucleus-Independent Chemical Shift (NICS) Profiles in a Series of Monocyclic Planar Inorganic Compounds. J. Organomet. Chem. 2006, 691, 4359–4366. [Google Scholar] [CrossRef]
- Stanger, A. What Is Aromaticity: A Critique of the Concept of Aromaticity–Can It Really Be Defined? Chem. Commun. 2009, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Tsipis, A.C. Efficiency of the NICSzz-scan Curves to Probe the Antiaromaticity of Organic and Inorganic Rings/Cages. Phys. Chem. Chem. Phys. 2009, 11, 8244–8261. [Google Scholar] [CrossRef] [PubMed]
- Tsipis, A.C.; Depastas, I.G.; Karagiannis, E.E.; Tsipis, C.A. Diagnosis of Magnetoresponsive Aromatic and Antiaromatic Zones in Three-Membered Rings of d- and f-Block Elements. J. Comput. Chem. 2010, 31, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Feixas, F.; Matito, E.; Poater, J.; Solà, M. Aromaticity of Distorted Benzene Rings. Exploring the Validity of Different Indicators of Aromaticity. J. Phys. Chem. A 2007, 111, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Feixas, F.; Jiménez-Halla, J.O.C.; Matito, E.; Poater, J.; Solà, M. A Test to Evaluate the Performance of Aromaticity Descriptors in All-Metal and Semimetal Clusters. An Appraisal of Electronic and Magnetic Indicators of Aromaticity. J. Chem. Theory Comput. 2010, 6, 1118–1130. [Google Scholar] [CrossRef]
- Nigam, S.; Majumder, C.; Kulshreshtha, S.K. Structure and Bonding of Tetramer Clusters: Theoretical Understanding of the Aromaticity. J. Mol. Struct. (Theochem) 2005, 755, 187–194. [Google Scholar] [CrossRef]
- Seal, P. Is Nucleus-Independent Chemical Shift Scan a Reliable Aromaticity Index for Planar and Neutral A2B2 Clusters? J. Mol. Struct. (Theochem) 2009, 893, 31–36. [Google Scholar] [CrossRef]
- Jusélius, J.; Straka, M.; Sundholm, D. Magnetic-Shielding Calculations on Al42- and Analogues. A New Family of Aromatic Molecules? J. Phys. Chem. A 2001, 105, 9939–9944. [Google Scholar] [CrossRef]
- Santos, J.C.; Tiznado, W.; Contreras, R.; Fuentealba, P. Sigma-pi Separation of the Electron Localization Function and Aromaticity. J. Chem. Phys. 2004, 120, 1670–1673. [Google Scholar] [CrossRef]
- Fowler, P.W.; Havenith, R.W.A.; Steiner, E. Unconventional Ring Currents in an ‘All-Metal Aromatic’, Al42-. Chem. Phys. Lett. 2001, 342, 85–90. [Google Scholar] [CrossRef]
- Fowler, P.W.; Havenith, R.W.A.; Steiner, E. Ring Current and Electron Delocalization in an All-Metal Cluster, Al42-. Chem. Phys. Lett. 2002, 359, 530–536. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Wang, L.-S.; Kuznetsov, A.E.; Boldyrev, A.I. Probing the Electronic Structure and Aromaticity of Pentapnictogen Cluster Anions Pn5- (Pn = P, As, Sb, and Bi). Using Photoelectron Spectroscopy and ab Initio Calculations. J. Phys. Chem. A 2002, 106, 5600–5606. [Google Scholar] [CrossRef]
- Jin, Q.; Jin, B.; Xu, W.G.; Zhu, W. Aromaticity of Planar P5- Anion in the P5M (M = Li, Na, and K) Clusters. J. Mol. Struct. (Theochem) 2005, 713, 113–117. [Google Scholar] [CrossRef]
- Kraus, F.; Korber, N. The Chemical Bond in Polyphospides: Crystal Structures, the Electron Localization Function, and a New View of Aromaticity in P42- and P5-. Chem. Eur. J. 2005, 11, 5945–5959. [Google Scholar] [CrossRef] [PubMed]
- De Proft, F.; Fowler, P.W.; Havenith, R.W.A.; Schleyer, P.v.R.; Van Lier, G.; Geerlings, P. Ring Currents as Probes of the Aromaticity of Inorganic Monocycles: P5-, As5-, S2N2, S3N3-, S4N3+, S4N42+, S5N5+, S42+ and Se42+. Chem. Eur. J. 2004, 10, 940–950. [Google Scholar] [CrossRef] [PubMed]
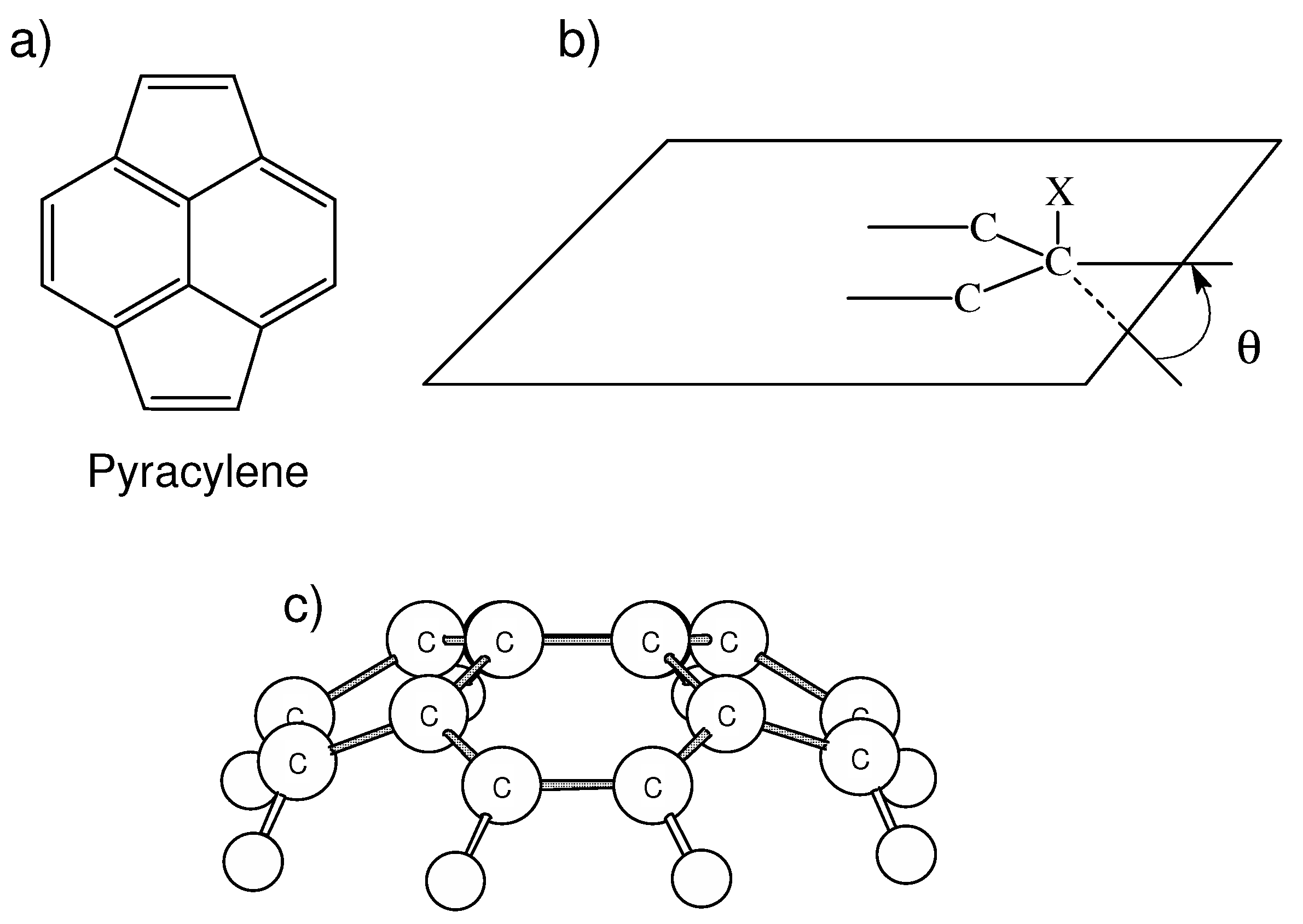

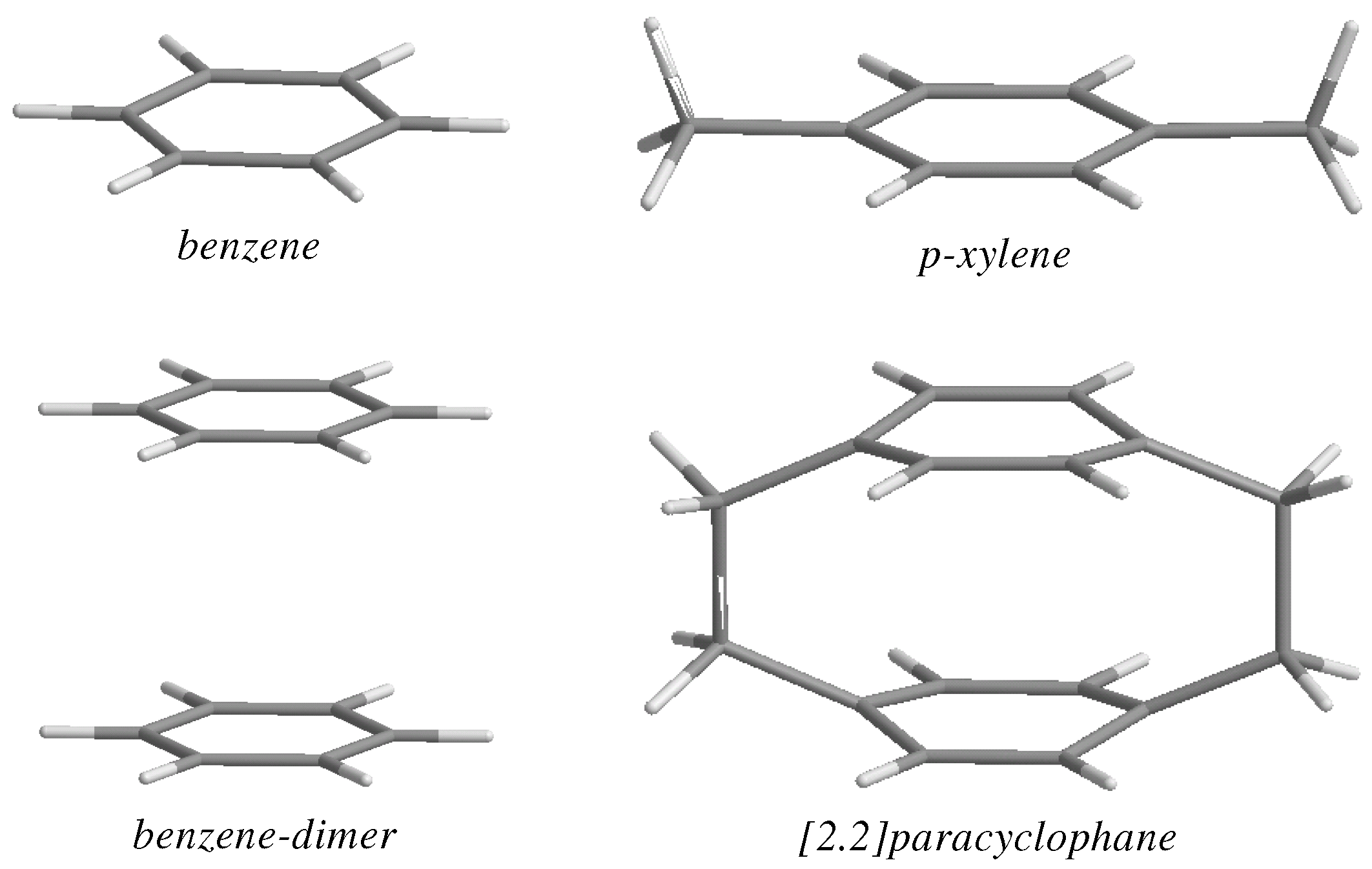

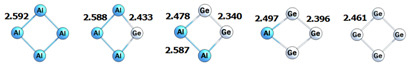
| θ | PDI | HOMA | NICS(0) | NICS(1)in | NICS(1)out |
|---|---|---|---|---|---|
| 0. | 0.0675 | 0.755 | −0.1 | −2.8 | −2.8 |
| 10. | 0.0672 | 0.761 | −0.1 | −3.8 | −2.1 |
| 20. | 0.0666 | 0.771 | −0.6 | −5.5 | −1.6 |
| 30. | 0.0656 | 0.744 | −1.7 | −7.6 | −1.2 |
| 40. | 0.0646 | 0.572 | −3.7 | −9.9 | −0.8 |
| PDI | FLU | MCI | Iring | HOMA | NICS(0) | NICS(1) | NICS(1)zz | NICS(0)π | NICS(0)πzz | |
|---|---|---|---|---|---|---|---|---|---|---|
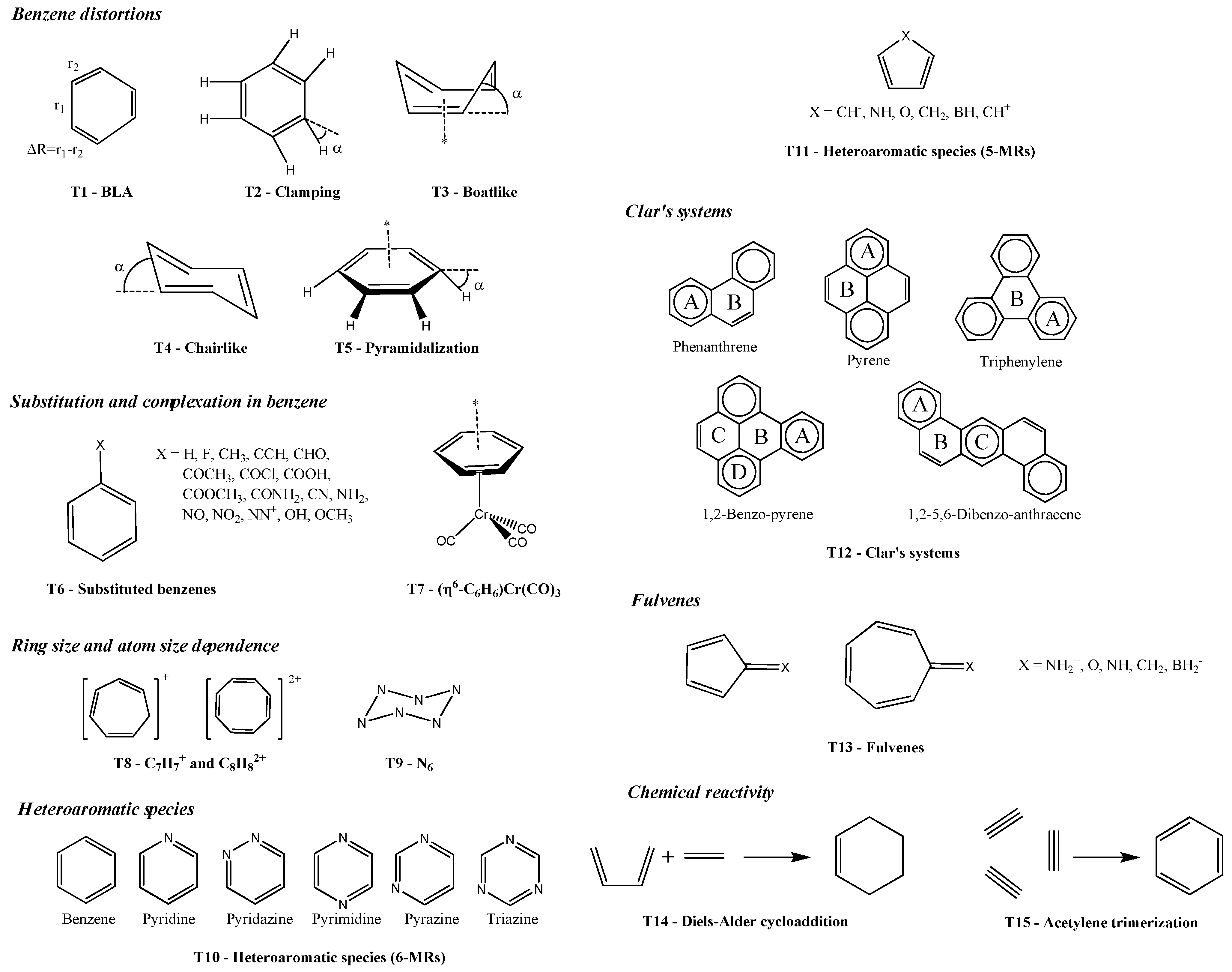
| T1 | Yes | Yes | Yes | Yes | Uncleara | Yes | Yes | Yes | Yes | Yes |
| T2 | Unclearb | Yes | Yes | Yes | Uncleara | Unclearc | Yes | Yes | Unclearc | Yes |
| T3 | Unclearb | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| T4 | Unclearb | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| T5 | Unclearb | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| T6 | Yes | Yes | Yes | Yes | Uncleard | No | No | Yes | Uncleard | Yes |
| T7 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| T8 | N/A | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| T9 | No | Yes | Yes | Yes | Yes | Yes | No | No | N/Ae | N/Ae |
| T10 | No | Unclearf | Unclearg | Unclearg | No | No | No | No | No | Unclearg |
| T11 | N/A | Unclearf | Yes | Yes | Unclearf | Unclearf | Unclearf | Yes | Unclearf | Yes |
| T12 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| T13 | N/A | No | Yes | Unclearf | Unclearf | Yes | Yes | Yes | Yes | Yes |
| T14 | Yes | No | Yes | Yes | No | Unclearh | Unclearh | Yes | Yes | Yes |
| T15 | Yes | No | Yes | Yes | No | Unclearh | Unclearh | Unclearh | No | No |
| Index | Mg32- | NaMg3- | Na2Mg3 |
|---|---|---|---|
| MCI | 0.458 | 0.306 | 0.255 |
| MCIπ | 0.000 | 0.070 | 0.066 |
| NICS(0) | -2.85 | -22.58 | -28.86 |
| NICS(0)ZZ | -12.72 | -17.92 | -21.38 |
| NICS(1) | -4.00 | -20.69b (-15.33)c | -24.07 |
| NICS(1)ZZ | -12.21 | -21.46b (-17.90)c | -24.82 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Solà, M.; Feixas, F.; Jiménez-Halla, J.O.C.; Matito, E.; Poater, J. A Critical Assessment of the Performance of Magnetic and Electronic Indices of Aromaticity. Symmetry 2010, 2, 1156-1179. https://doi.org/10.3390/sym2021156
Solà M, Feixas F, Jiménez-Halla JOC, Matito E, Poater J. A Critical Assessment of the Performance of Magnetic and Electronic Indices of Aromaticity. Symmetry. 2010; 2(2):1156-1179. https://doi.org/10.3390/sym2021156
Chicago/Turabian StyleSolà, Miquel, Ferran Feixas, J. Oscar C. Jiménez-Halla, Eduard Matito, and Jordi Poater. 2010. "A Critical Assessment of the Performance of Magnetic and Electronic Indices of Aromaticity" Symmetry 2, no. 2: 1156-1179. https://doi.org/10.3390/sym2021156
APA StyleSolà, M., Feixas, F., Jiménez-Halla, J. O. C., Matito, E., & Poater, J. (2010). A Critical Assessment of the Performance of Magnetic and Electronic Indices of Aromaticity. Symmetry, 2(2), 1156-1179. https://doi.org/10.3390/sym2021156