Symmetry OUT, Asymmetry IN
Abstract
:1. How to Produce a Symmetric Body Plan
1.1. The Bilateral Symmetry of Somite Formation
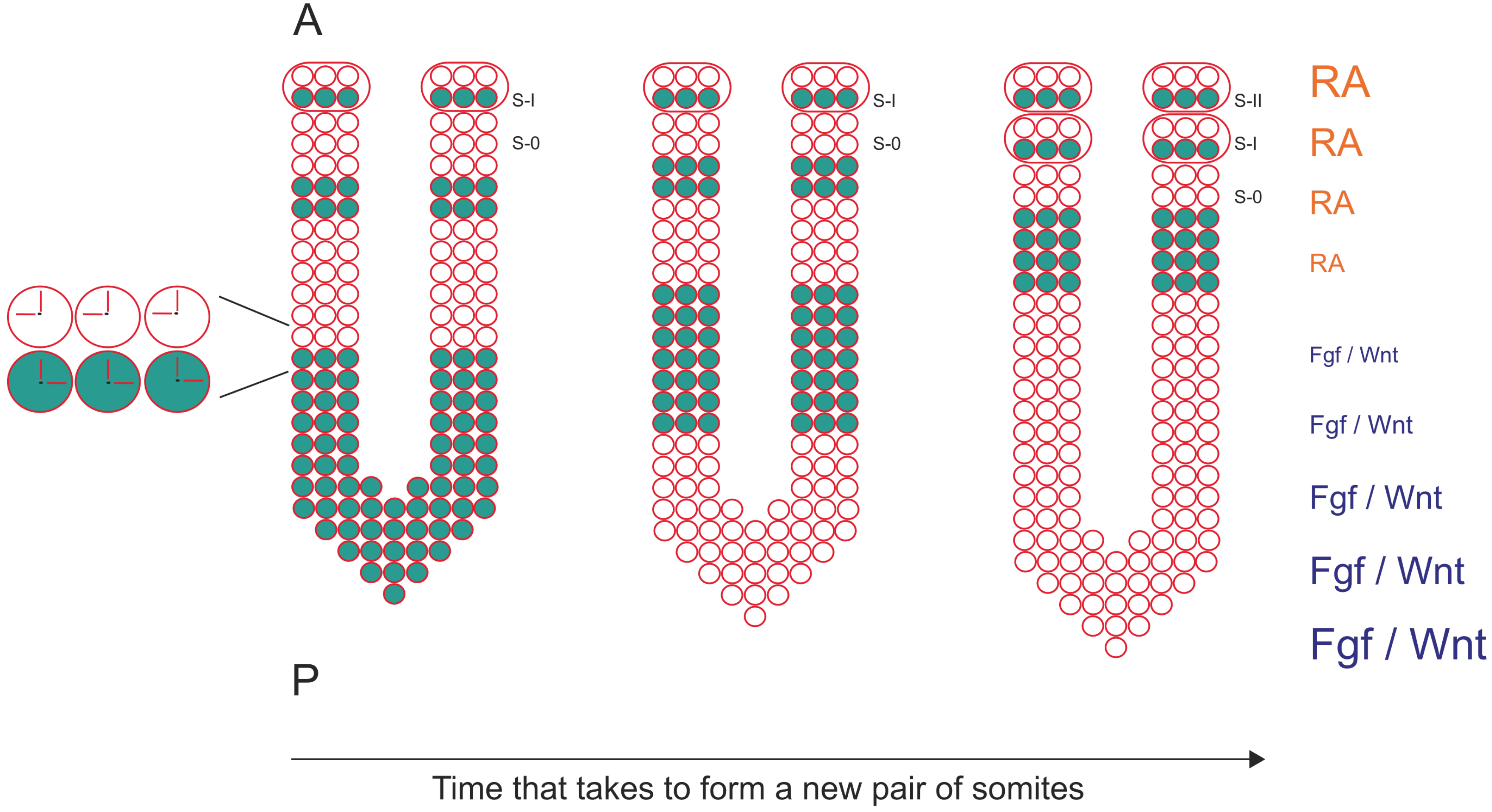
1.2. The Segmentation Clock Sets the Periodicity of Somite Formation
1.3. The Wavefront Sets the Position of Somite Formation
2. Is the Body Plan All about Symmetry?
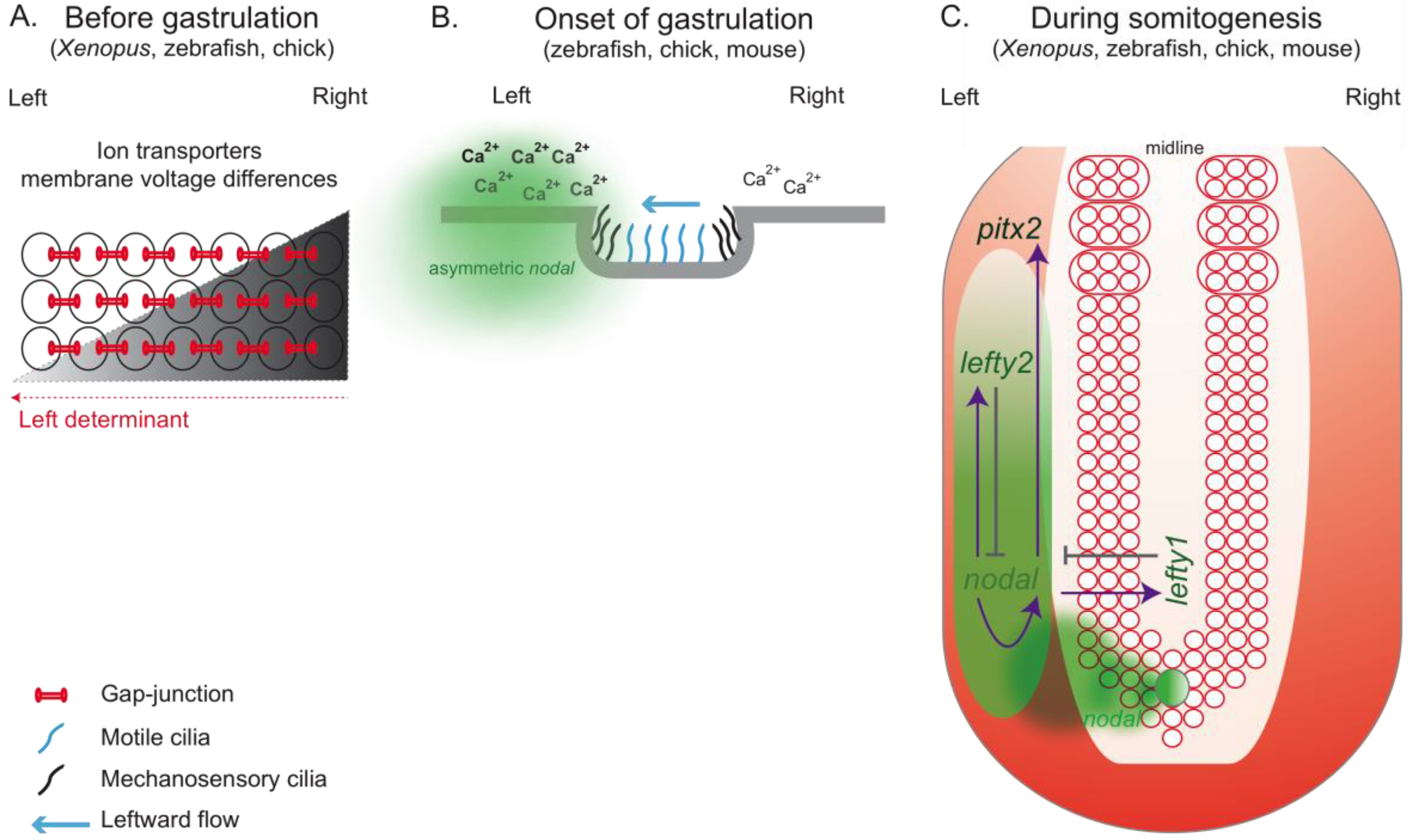
2.1. How Can Cilia Break Symmetry?
2.2. More than Cilia: Other Players in the Scene
3. How Are Symmetric Tissues Protected from LR Asymmetric Signals?
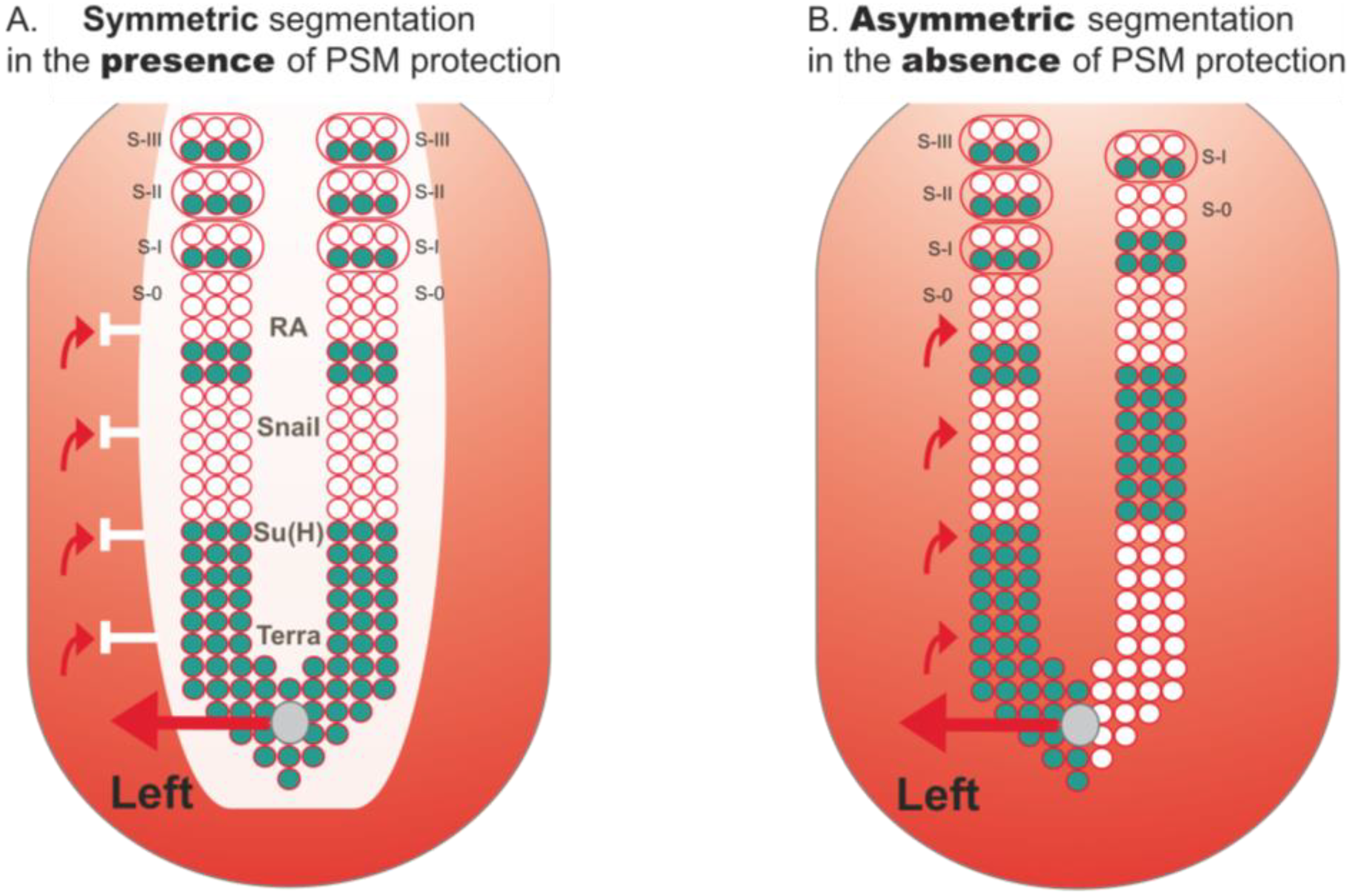
3.1. Retinoic Acid Buffers the PSM from the Influence of LR Signals
3.2. Bridge between LR Patterning and Somitogenesis
4. Human Developmental Disorders Related to the LR Axis
5. Conclusions
Acknowledgements
References and Notes
- Dequéant, M.L.; Pourquié, O. Segmental patterning of the vertebrate embryonic axis. Nat. Rev. Genet. 2008, 9, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Zeeman, E.C. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J. Theor. Biol. 1976, 58, 455–476. [Google Scholar] [CrossRef]
- Palmeirim, I.; Henrique, D.; Ish-Horowicz, D.; Pourquie, O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 1997, 91, 639–648. [Google Scholar] [CrossRef]
- Kageyama, R.; Ohtsuka, T.; Kobayashi, T. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development 2007, 134, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Yoshiura, S.; Ohtsuka, T.; Bessho, Y.; Harada, T.; Yoshikawa, K.; Kageyama, R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 2002, 298, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Oates, A.C.; Ho, R.K. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development 2002, 129, 2929–2946. [Google Scholar] [PubMed]
- Holley, S.A.; Jülich, D.; Rauch, G.J.; Geisler, R.; Nüsslein-Volhard, C. Her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development 2002, 129, 1175–1183. [Google Scholar]
- Dale, J.K.; Maroto, M.; Dequeant, M.L.; Malapert, P.; McGrew, M.; Pourquie, O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature 2003, 421, 275–278. [Google Scholar] [CrossRef]
- Bessho, Y.; Hirata, H.; Masamizu, Y.; Kageyama, R. Periodic repression by the bHLH factor Hes7 is an essential mechanism for the somite segmentation clock. Genes. Dev. 2003, 17, 1451–1456. [Google Scholar] [CrossRef]
- Hirata, H.; Bessho, Y.; Kokubu, H.; Masamizu, Y.; Yamada, S.; Lewis, J.; Kageyama, R. Instability of Hes7 protein is crucial for the somite segmentation clock. Nat. Genet. 2004, 36, 750–754. [Google Scholar] [CrossRef]
- Sieger, D.; Tautz, D.; Gajewski, M. The role of Suppressor of Hairless in Notch mediated signalling during zebrafish somitogenesis. Mech. Dev. 2003, 120, 1083–1094. [Google Scholar] [CrossRef]
- Brend, T.; Holley, S.A. Expression of the oscillating gene her1 is directly regulated by Hairy/Enhancer of Split, T-box, and Suppressor of Hairless proteins in the zebrafish segmentation clock. Dev. Dyn. 2009, 238, 2745–2759. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. Autoinhibition with transcriptional delay: A simple mechanism for the zebrafish somitogenesis oscillator. Curr. Biol. 2003, 13, 1398–1408. [Google Scholar] [CrossRef]
- Conlon, R.A.; Reaume, A.G.; Rossant, J. Notch1 is required for the coordinate segmentation of somites. Development 1995, 121, 1533–1545. [Google Scholar] [PubMed]
- Huppert, S.S.; Ilagan, M.X.; De Strooper, B.; Kopan, R. Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev. Cell. 2005, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, F.J.; Granato, M.; Schach, U.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.P.; Jiang, Y.J.; Kane, D.A.; Kelsh, R.N.; Mullins, M.C.; Odenthal, J.; Warga, R.M.; Allende, M.L.; Weinberg, E.S.; Nüsslein-Volhard, C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development 1996, 123, 153–164. [Google Scholar] [PubMed]
- Jiang, Y.J.; Aerne, B.L.; Smithers, L.; Haddon, C.; Ish-Horowicz, D.; Lewis, J. Notch signalling and the synchronization of the somite segmentation clock. Nature 2000, 408, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Oates, A.C.; Ho, R.K. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development 2002, 129, 2929–2946. [Google Scholar] [PubMed]
- Giudicelli, F.; Ozbudak, E.M.; Wright, G.J.; Lewis, J. Setting the tempo in development: An investigation of the zebrafish somite clock mechanism. PLoS Biol. 2007, 5, e150. [Google Scholar] [CrossRef] [PubMed]
- Jülich, D.; Hwee Lim, C.; Round, J.; Nicolaije, C.; Schroeder, J.; Davies, A.; Geisler, R.; Lewis, J.; Jiang, Y.J.; Holley, S.A. Beamter/deltaC and the role of Notch ligands in the zebrafish somite segmentation, hindbrain neurogenesis and hypochord differentiation. Dev. Biol. 2005, 286, 391–404. [Google Scholar] [CrossRef]
- Ozbudak, E.M.; Lewis, J. Notch signalling synchronizes the zebrafish segmentation clock but is not needed to create somite boundaries. PLoS Genet. 2008, 4, e15. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, K.; Ishimatsu, K.; Yoshimoto, E.; Kondo, S.; Takeda, H. Noise-resistant and synchronized oscillation of the segmentation clock. Nature 2006, 441, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Mara, A.; Schroeder, J.; Chalouni, C.; Holley, S.A. Priming, initiation and synchronization of the segmentation clock by deltaD and deltaC. Nat. Cell Biol. 2007, 9, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Riedel-Kruse, I.H.; Müller, C.; Oates, A.C. Synchrony dynamics during initiation, failure, and rescue of the segmentation clock. Science 2007, 317, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Ferjentsik, Z.; Hayashi, S.; Dale, J.K.; Bessho, Y.; Herreman, A.; De Strooper, B.; del Monte, G.; de la Pompa, J.L.; Maroto, M. Notch is a critical component of the mouse somitogenesis oscillator and is essential for the formation of the somites. PLoS Genet. 2009, 5, e1000662. [Google Scholar] [CrossRef] [PubMed]
- Aulehla, A.; Pourquié, O. Oscillating signaling pathways during embryonic development. Curr. Opin. Cell Biol. 2008, 20, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Feller, J.; Schneider, A.; Schuster-Gossler, K.; Gossler, A. Noncyclic Notch activity in the presomitic mesoderm demonstrates uncoupling of somite compartmentalization and boundary formation. Genes Dev. 2008, 22, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Maroto, M.; Dale, J.K.; Dequéant, M.L.; Petit, A.C.; Pourquié, O. Synchronised cycling gene oscillations in presomitic mesoderm cells require cell-cell contact. Int. J. Dev. Biol. 2005, 49, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Masamizu, Y.; Ohtsuka, T.; Takashima, Y.; Nagahara, H.; Takenaka, Y.; Yoshikawa, K.; Okamura, H.; Kageyama, R. Real-time imaging of the somite segmentation clock: Revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Dequéant, M.L.; Glynn, E.; Gaudenz, K.; Wahl, M.; Chen, J.; Mushegian, A.; Pourquié, O. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 2006, 314, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, J.; McGrew, M.J.; Pourquié, O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 2001, 106, 219–232. [Google Scholar] [CrossRef]
- Aulehla, A.; Wehrle, C.; Brand-Saberi, B.; Kemler, R.; Gossler, A.; Kanzler, B.; Herrmann, B.G. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 2003, 4, 395–406. [Google Scholar] [CrossRef]
- Sawada, A.; Shinya, M.; Jiang, Y.J.; Kawakami, A.; Kuroiwa, A.; Takeda, H. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development 2001, 128, 4873–4880. [Google Scholar] [PubMed]
- Dubrulle, J.; Pourquié, O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 2004, 427, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Delfini, M.C.; Dubrulle, J.; Malapert, P.; Chal, J.; Pourquié, O. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc. Natl. Acad. Sci. USA 2005, 102, 11343–11348. [Google Scholar] [CrossRef] [PubMed]
- Aulehla, A.; Wiegraebe, W.; Baubet, V.; Wahl, M.B.; Deng, C.; Taketo, M.; Lewandoski, M.; Pourquié, O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat. Cell Biol. 2008, 10, 186–193. [Google Scholar] [CrossRef]
- Diez del Corral, R.; Olivera-Martinez, I.; Goriely, A.; Gale, E.; Maden, M.; Storey, K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 2003, 40, 65–79. [Google Scholar] [CrossRef]
- Moreno, T.A.; Kintner, C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev. Cell 2004, 6, 205–218. [Google Scholar] [CrossRef]
- Swindell, E.C.; Thaller, C.; Sockanathan, S.; Petkovich, M.; Jessell, T.M.; Eichele, G. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 1999, 216, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Fliegauf, M.; Benzing, T.; Omran, H. When cilia go bad: Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007, 8, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.M.; Davis, E.E.; Katsanis, N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 2009, 137, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H. Breakthroughs and future challenges in left-right patterning. Dev. Growth Differ. Suppl. 2008, 1, S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Varlet, I.; Robertson, E.J. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 1996, 381, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Kawakami, Y.; Rodríguez-Esteban, C.; Ibañes, M.; Rasskin-Gutman, D.; Rodríguez-León, J.; Büscher, D.; Feijó, J.A.; Izpisúa Belmonte, J.C. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature 2004, 427, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gourronc, F.; Ahmad, N.; Nedza, N.; Eggleston, T.; Rebagliati, M. Nodal activity around Kupffer’s vesicle depends on the T-box transcription factors Notail and Spadetail and on Notch signaling. Dev. Dyn. 2007, 236, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.N.; Martin, G.R. Differences in left-right axis pathways in mouse and chick: Functions of FGF8 and SHH. Science 1999, 285, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Albertson, R.C.; Yelick, P.C. Roles for fgf8 signaling in left-right patterning of the visceral organs and craniofacial skeleton. Dev. Biol. 2005, 283, 310–321. [Google Scholar] [CrossRef]
- Nakaya, M.A.; Biris, K.; Tsukiyama, T.; Jaime, S.; Rawls, J.A.; Yamaguchi, T.P. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 2005, 132, 5425–5436. [Google Scholar] [CrossRef]
- Rodríguez-Esteban, C.; Capdevila, J.; Kawakami, Y.; Izpisúa Belmonte, J.C. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development 2001, 128, 3189–3195. [Google Scholar]
- Afzelius, B.A. A human syndrome caused by immotile cilia. Science 1976, 193, 317–319. [Google Scholar] [CrossRef]
- Afzelius, B.A. The immotile-cilia syndrome: A microtubule-associated defect. CRC Crit. Rev. Biochem. 1985, 19, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Witte, D.P.; Potter, S.S.; Brueckner, M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 1997, 389, 963–966. [Google Scholar] [CrossRef]
- Sulik, K.; Dehart, D.B.; Iangaki, T.; Carson, J.L.; Vrablic, T.; Gesteland, K.; Schoenwolf, G.C. Morphogenesis of the murine node and notochordal plate. Dev. Dyn. 1994, 201, 260–278. [Google Scholar] [CrossRef]
- Singh, G.; Supp, D.M.; Schreiner, C.; McNeish, J.; Merker, H.J.; Copeland, N.G.; Jenkins, N.A.; Potter, S.S.; Scott, W. Legless insertional mutation: Morphological, molecular, and genetic characterization. Genes Dev. 1991, 5, 2245–2255. [Google Scholar] [CrossRef]
- Schreiner, C.M.; Scott, W.J.; Supp, D.M.; Potter, S.S. Correlation of forelimb malformation asymmetries with visceral organ situs in the transgenic mouse insertional mutation, legless. Dev. Biol. 1993, 158, 560–562. [Google Scholar] [CrossRef]
- Lowe, L.A.; Supp, D.M.; Sampath, K.; Yokoyama, T.; Wright, C.V.; Potter, S.S.; Overbeek, P.; Kuehn, M.R. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 1996, 381, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95, 829–837. [Google Scholar] [CrossRef]
- Nonaka, S.; Yoshiba, S.; Watanabe, D.; Ikeuchi, S.; Goto, T.; Marshall, W.F.; Hamada, H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005, 3, e268. [Google Scholar] [CrossRef]
- Nonaka, S.; Shiratori, H.; Saijoh, Y.; Hamada, H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 2002, 418, 96–99. [Google Scholar] [CrossRef]
- Okada, Y.; Nonaka, S.; Tanaka, Y.; Saijoh, Y.; Hamada, H.; Hirokawa, N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell 1999, 4, 459–468. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okada, Y.; Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 2005, 435, 172–177. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef]
- Tabin, C.J.; Vogan, K.J. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Essner, J.J.; Amack, J.D.; Nyholm, M.K.; Harris, E.B.; Yost, H.J. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 2005, 132, 1247–1760. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Zucker, A.G.; Olale, F.; Haycraft, C.J.; Yoder, B.K.; Schier, A.F.; Drummond, I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 2005, 132, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Takashima, S.; Kobayashi, D.; Sumeragi, A.; Shimada, A.; Tsukahara, T.; Yokoi, H.; Narita, T.; Jindo, T.; Kage, T.; Kitagawa, T.; Kimura, T.; Sekimizu, K.; Miyake, A.; Setiamarga, D.; Murakami, R.; Tsuda, S.; Ooki, S.; Kakihara, K.; Naruse, K.; Takeda, H. Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer’s vesicle. Dev. Growth Differ. 2007, 49, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.M.; Amack, J.D.; Peterson, A.G.; Bisgrove, B.W.; Yost, H.J. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 2009, 458, 651–654. [Google Scholar] [CrossRef]
- Okada, Y.; Takeda, S.; Tanaka, Y.; Belmonte, J.C.; Hirokawa, N. Mechanism of nodal flow: A conserved symmetry breaking event in left-right axis determination. Cell 2005, 121, 633–644. [Google Scholar] [CrossRef]
- Schweickert, A.; Weber, T.; Beyer, T.; Vick, P.; Bogusch, S.; Feistel, K.; Blum, M. Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 2007, 17, 60–66. [Google Scholar] [CrossRef]
- Vick, P.; Schweickert, A.; Weber, T.; Eberhardt, M.; Mencl, S.; Shcherbakov, D.; Beyer, T.; Blum, M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev. Biol. 2009, 331, 281–291. [Google Scholar] [CrossRef]
- Essner, J.J.; Vogan, K.J.; Wagner, M.K.; Tabin, C.J.; Yost, H.J.; Brueckner, M. Conserved function for embryonic nodal cilia. Nature 2002, 418, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Männer, J. Does an equivalent of the “ventral node” exist in chick embryos? A scanning electron microscopic study. Anat. Embryol. (Berl) 2001, 203, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Pagán-Westphal, S.M.; Tabin, C.J. The transfer of left-right positional information during chick embryogenesis. Cell 1998, 93, 25–35. [Google Scholar] [CrossRef]
- Gros, J.; Feistel, K.; Viebahn, C.; Blum, M.; Tabin, C.J. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 2009, 324, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Little, C.D.; Rongish, B.J. Rotation of organizer tissue contributes to left-right asymmetry. Anat. Rec. (Hoboken) 2009, 292, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Thorlin, T.; Robinson, K.R.; Nogi, T.; Mercola, M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 2002, 111, 77–89. [Google Scholar] [CrossRef]
- Levin, M.; Mercola, M. Gap junctions are involved in the early generation of left-right asymmetry. Dev. Biol. 1998, 203, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Mercola, M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 1999, 126, 4703–4714. [Google Scholar]
- Kawakami, Y.; Raya, A.; Raya, R.M.; Rodríguez-Esteban, C.; Belmonte, J.C. Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature 2005, 435, 165–171. [Google Scholar] [CrossRef]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef]
- Shu, X.; Huang, J.; Dong, Y.; Choi, J.; Langenbacher, A.; Chen, J.N. Na,K-ATPase alpha2 and Ncx4a regulate zebrafish left-right patterning. Development 2007, 134, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, B.; Latimer, A.J.; Appel, B.; Wente, S.R. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev. Cell 2005, 9, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Hatler, J.M.; Essner, J.J.; Johnson, R.G. A gap junction connexin is required in the vertebrate left-right organizer. Dev. Biol. 2009, 336, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Kema, I.P.; Levin, M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005, 15, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Blakely, R.; Levin, M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev. Neurosci. 2005, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.L. Normal bias in the direction of fetal rotation depends on blastomere composition during early cleavage in the mouse. PLoS One 2010, 5, e9610. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Endo, B.; Abe, M.; Shimizu, M. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature 2009, 462, 790–794. [Google Scholar] [CrossRef]
- Brend, T.; Holley, S.A. Balancing segmentation and laterality during vertebrate development. Semin. Cell Dev. Biol. 2009, 20, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Vermot, J.; Gallego Llamas, J.; Fraulob, V.; Niederreither, K.; Chambon, P.; Dollé, P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 2005, 308, 563–566. [Google Scholar] [CrossRef]
- Lowe, L.A.; Supp, D.M.; Sampath, K.; Yokoyama, T.; Wright, C.V.; Potter, S.S.; Overbeek, P.; Kuehn, M.R. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 1996, 381, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Ahmad, N.; Rebagliati, M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development 2003, 130, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, I.O.; Duester, G. Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat. Cell Biol. 2006, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Vermot, J.; Pourquié, O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 2005, 435, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vilhais-Neto, G.C.; Maruhashi, M.; Smith, K.T.; Vasseur-Cognet, M.; Peterson, A.S.; Workman, J.L.; Pourquié, O. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 2010, 463, 953–957. [Google Scholar] [CrossRef]
- Boettger, T.; Wittler, L.; Kessel, M. FGF8 functions in the specification of the right body side of the chick. Curr. Biol. 1999, 9, 277–280. [Google Scholar] [CrossRef]
- Isaac, A.; Sargent, M.G.; Cooke, J. Control of vertebrate left-right asymmetry by a snail-related zinc finger gene. Science 1997, 275, 1301–1304. [Google Scholar] [CrossRef]
- Sefton, M.; Sánchez, S.; Nieto, M.A. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 1998, 125, 3111–3121. [Google Scholar]
- Morales, A.V.; Acloque, H.; Ocaña, O.H.; de Frutos, C.A.; Gold, V.; Nieto, M.A. Snail genes at the crossroads of symmetric and asymmetric processes in the developing mesoderm. EMBO Rep. 2007, 8, 104–109. [Google Scholar] [CrossRef]
- Fior, R.; Henrique, D. “Notch-Off”: A perspective on the termination of Notch signalling. Int. J. Dev. Biol. 2009, 53, 1379–1384. [Google Scholar] [CrossRef]
- Echeverri, K.; Oates, A.C. Coordination of symmetric cyclic gene expression during somitogenesis by Suppressor of Hairless involves regulation of retinoic acid catabolism. Dev. Biol. 2007, 301, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Saúde, L.; Lourenço, R.; Gonçalves, A.; Palmeirim, I. Terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat. Cell Biol. 2005, 7, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Wang, Y.; Kokubo, H.; Kettlewell, J.R.; Zarkower, D.A.; Johnson, R.L. Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev. Biol. 2006, 290, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.P.; Palmeirim, I.; Bajanca, F. Molecular clocks underlying vertebrate embryo segmentation: A 10-year-old hairy-go-round. Birth Defects Res. C Embryo Today 2007, 81, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Bulman, M.P.; Kusumi, K.; Frayling, T.M.; McKeown, C.; Garrett, C.; Lander, E.S.; Krumlauf, R.; Hattersley, A.T.; Ellard, S.; Turnpenny, P.D. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat. Genet. 2000, 24, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Whittock, N.V.; Sparrow, D.B.; Wouters, M.A.; Sillence, D.; Ellard, S.; Dunwoodie, S.L.; Turnpenny, P.D. Mutated MESP2 causes spondylocostal dysostosis in humans. Am. J. Hum. Genet. 2004, 74, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, D.B.; Chapman, G.; Wouters, M.A.; Whittock, N.V.; Ellard, S.; Fatkin, D.; Turnpenny, P.D.; Kusumi, K.; Sillence, D.; Dunwoodie, S.L. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am. J. Hum. Genet. 2006, 78, 28–37. [Google Scholar] [CrossRef]
- Gruneberg, H. Genetical studies on the skeleton of the mouse: XXIX. PUDGY. Genet. Res. 1961, 2, 384–393. [Google Scholar] [CrossRef]
- Saga, Y.; Hata, N.; Koseki, H.; Taketo, M.M. Mesp2: A novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997, 11, 1827–1839. [Google Scholar] [CrossRef]
- Zhang, N.; Gridley, T. Defects in somite formation in lunatic fringe-deficient mice. Nature 1998, 394, 374–377. [Google Scholar] [CrossRef]
- McDaniell, R.; Warthen, D.M.; Sanchez-Lara, P.A.; Pai, A.; Krantz, I.D.; Piccoli, D.A.; Spinner, N.B. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 2006, 79, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kreiborg, S.; Barr, M.J.; Cohen, M.M.J. Cervical spine in the Apert syndrome. Am. J. Med. Genet. 1992, 43, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lurie, I.W.; Kappetein, A.P.; Loffredo, C.A.; Ferencz, C. Non-cardiac malformations in individuals with outflow tract defects of the heart: The Baltimore-Washington Infant Study (1981–1989). Am. J. Med. Genet. 1995, 59, 76–84. [Google Scholar] [CrossRef]
- Nugent, E.W.; Plauth, W.H.; Edwards, J.E. The Pathology, Pathophysiology, Recognition and Treatment of Congenital Heart Disease; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Sternick, E.B.; Márcio Gerken, L.; Max, R.; Osvaldo Vrandecic, M. Radiofrequency catheter ablation of an accessory pathway in a patient with Wolff-Parkinson-White and Kartagener’s syndrome. Pacing Clin. Electrophysiol. 2004, 27, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Bowers, P.N.; Brueckner, M.; Yost, H.J. The genetics of left-right development and heterotaxia. Semin. Perinatol. 1996, 20, 577–588. [Google Scholar] [CrossRef]
- Kartagener, M.; Stucki, P. Bronchiectasis with situs inversus. Arch. Pediatr. 1962, 79, 193–207. [Google Scholar] [PubMed]
- Kennedy, M.P.; Omran, H.; Leigh, M.W.; Dell, S.; Morgan, L.; Molina, P.L.; Robinson, B.V.; Minnix, S.L.; Olbrich, H.; Severin, T.; Ahrens, P.; Lange, L.; Morillas, H.N.; Noone, P.G.; Zariwala, M.A.; Knowles, M.R. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 2007, 2007. 115, 2814–2821. [Google Scholar] [CrossRef]
- Debrus, S.; Sauer, U.; Gilgenkrantz, S.; Jost, W.; Jesberger, H.J.; Bouvagnet, P. Autosomal recessive lateralization and midline defects: Blastogenesis recessive 1. Am. J. Med. Genet. 1997, 68, 401–404. [Google Scholar] [CrossRef]
- Levin, M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005, 122, 3–25. [Google Scholar] [CrossRef] [PubMed]

© 2010 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lourenço, R.; Saúde, L. Symmetry OUT, Asymmetry IN. Symmetry 2010, 2, 1033-1054. https://doi.org/10.3390/sym2021033
Lourenço R, Saúde L. Symmetry OUT, Asymmetry IN. Symmetry. 2010; 2(2):1033-1054. https://doi.org/10.3390/sym2021033
Chicago/Turabian StyleLourenço, Raquel, and Leonor Saúde. 2010. "Symmetry OUT, Asymmetry IN" Symmetry 2, no. 2: 1033-1054. https://doi.org/10.3390/sym2021033
APA StyleLourenço, R., & Saúde, L. (2010). Symmetry OUT, Asymmetry IN. Symmetry, 2(2), 1033-1054. https://doi.org/10.3390/sym2021033



