Construction of a Symmetrical Bi-Hydroxamate Metal–Organic Framework with Chemical Robustness
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of H4-BDHA
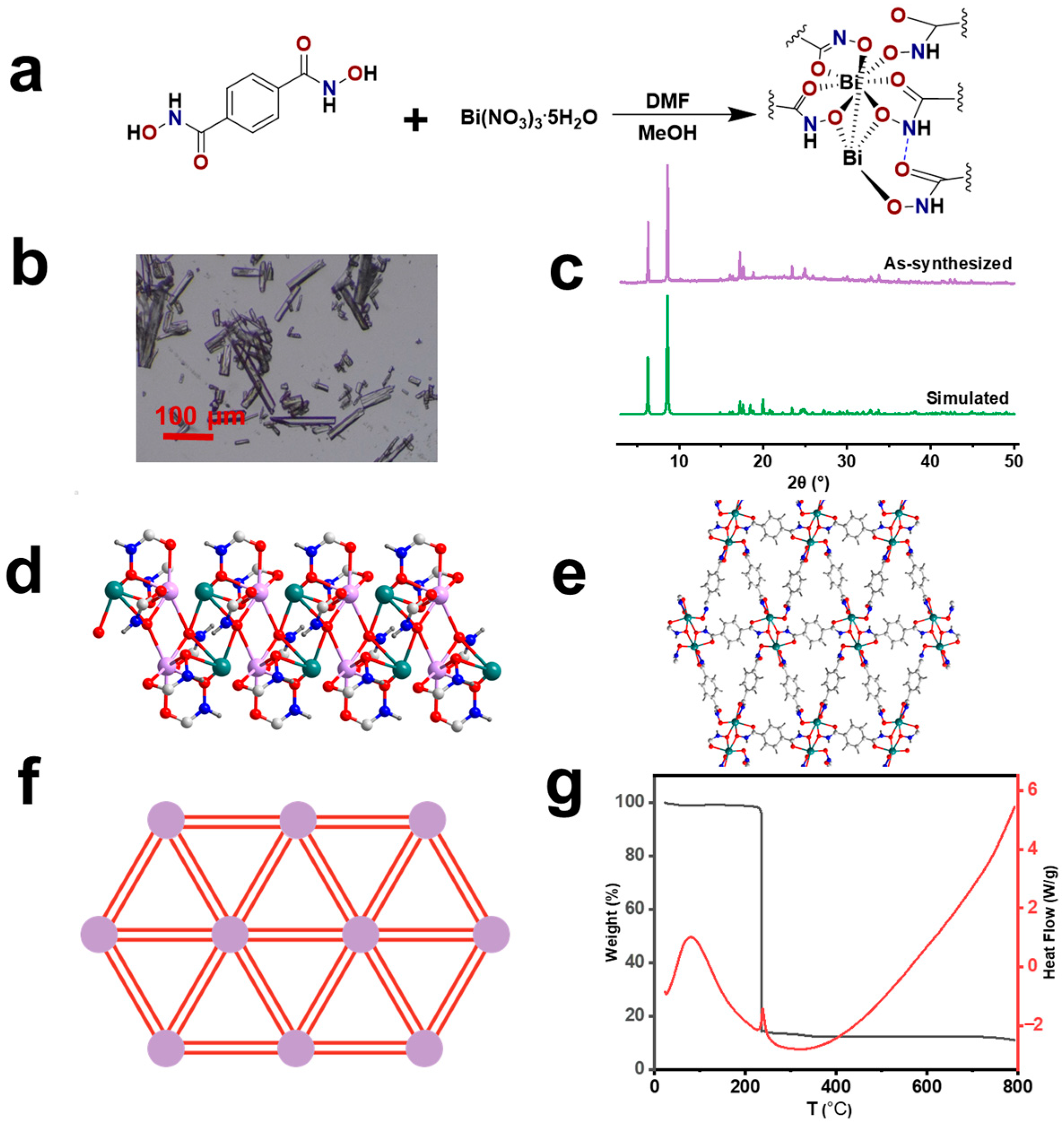
2.2. Synthesis of SUM-91
2.3. Activation of SUM-91
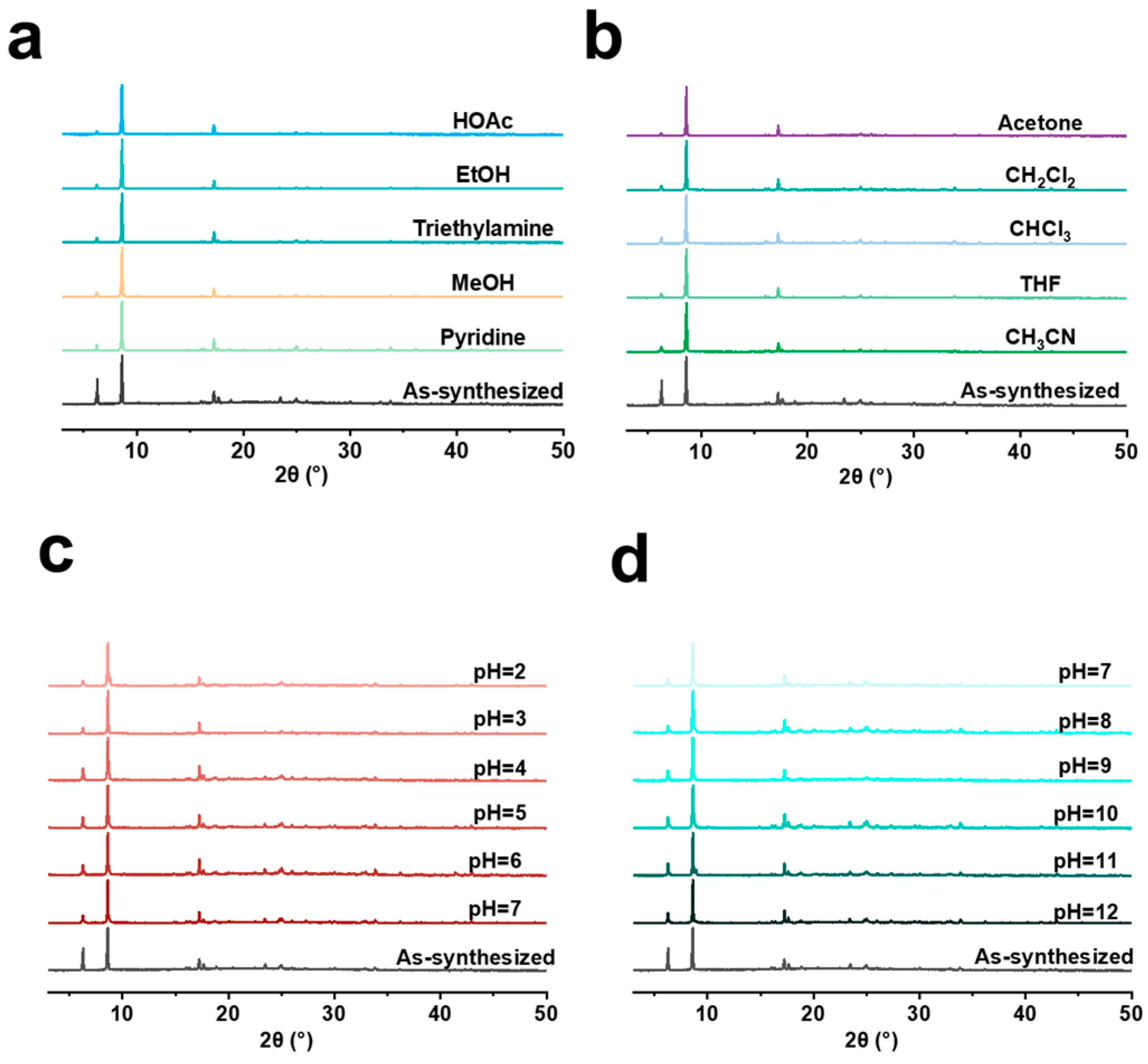
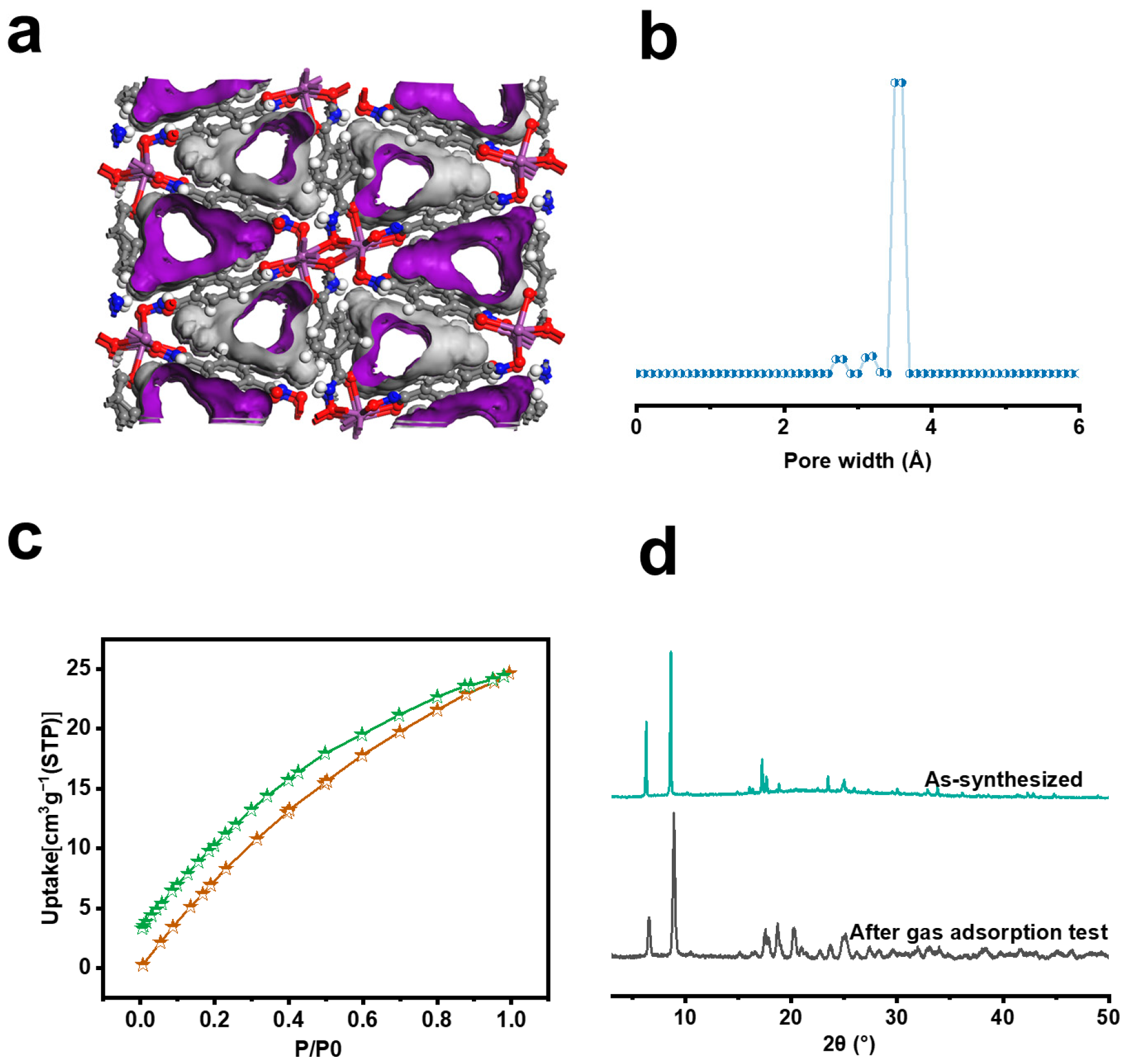
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ejsmont, A.; Andreo, J.; Lanza, A.; Galarda, A.; Macreadie, L.; Wuttke, S.; Canossa, S.; Ploetz, E.; Goscianska, J. Applications of reticular diversity in metal–organic frameworks: An ever-evolving state of the art. Coord. Chem. Rev. 2021, 430, 213655. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ahumada, E.; Díaz-Ramírez, M.L.; Velásquez-Hernández, M.d.J.; Jancik, V.; Ibarra, I.A. Capture of toxic gases in MOFs: SO2, H2S, NH3 and NOx. Chem. Sci. 2021, 12, 6772–6799. [Google Scholar] [CrossRef]
- Jeong, C.; Ansari, M.Z.; Hakeem Anwer, A.; Kim, S.-H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A review on metal-organic frameworks for the removal of hazardous environmental contaminants. Sep. Purif. Technol. 2023, 305, 122416. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Liu, J.; Goetjen, T.A.; Wang, Q.; Knapp, J.G.; Wasson, M.C.; Yang, Y.; Syed, Z.H.; Delferro, M.; Notestein, J.M.; Farha, O.K.; et al. MOF-enabled confinement and related effects for chemical catalyst presentation and utilization. Chem. Soc. Rev. 2022, 51, 1045–1097. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod Packings and Metal-Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc-Adeninate Metal-Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.F.; Howarth, A.J.; Vermeulen, N.A.; Almeida Paz, F.A.; Tomé, J.P.C.; Hupp, J.T.; Farha, O.K. Towards hydroxamic acid linked zirconium metal–organic frameworks. Mater. Chem. Front. 2017, 1, 1194–1199. [Google Scholar] [CrossRef]
- Padial, N.M.; Castells-Gil, J.; Almora-Barrios, N.; Romero-Angel, M.; da Silva, I.; Barawi, M.; García-Sánchez, A.; de la Peña O’Shea, V.A.; Martí-Gastaldo, C. Hydroxamate Titanium–Organic Frameworks and the Effect of Siderophore-Type Linkers over Their Photocatalytic Activity. J. Am. Chem. Soc. 2019, 141, 13124–13133. [Google Scholar] [CrossRef]
- Chiong, J.A.; Zhu, J.; Bailey, J.B.; Kalaj, M.; Subramanian, R.H.; Xu, W.; Cohen, S.M.; Tezcan, F.A. An Exceptionally Stable Metal–Organic Framework Constructed from Chelate-Based Metal–Organic Polyhedra. J. Am. Chem. Soc. 2020, 142, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Berlanga, B.; Castells-Gil, J.; Ganivet, C.R.; Almora-Barrios, N.; González-Platas, J.; Fabelo, O.; Padial, N.M.; Martí-Gastaldo, C. Permanent Porosity in Hydroxamate Titanium–Organic Polyhedra. J. Am. Chem. Soc. 2021, 143, 21195–21199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Samperisi, L.; Kalaj, M.; Chiong, J.A.; Bailey, J.B.; Zhang, Z.; Yu, C.-J.; Sikma, R.E.; Zou, X.; Cohen, S.M.; et al. Metal-hydrogen-pi-bonded organic frameworks. Dalton Trans. 2022, 51, 1927–1935. [Google Scholar] [CrossRef]
- Lai, Q.; Chu, Z.-Q.; Xiao, X.; Dai, D.; Song, T.; Luo, T.-Y.; Tang, W.; Feng, X.; Zhang, Z.; Li, T.; et al. Two-dimensional Zr/Hf-hydroxamate metal–organic frameworks. Chem. Commun. 2022, 58, 3601–3604. [Google Scholar] [CrossRef]
- Feng, X.; Qin, Z.; Lai, Q.; Zhang, Z.; Shao, Z.-W.; Tang, W.; Wu, W.; Dai, Z.; Liu, C. Mixed-matrix membranes based on novel hydroxamate metal–organic frameworks with two-dimensional layers for CO2/N2 separation. Sep. Purif. Technol. 2023, 305, 122476. [Google Scholar] [CrossRef]
- Shao, Z.-W.; Zhang, Z.; Kuang, Y.; Xiong, C.; Yang, J.; Wu, W.; Liu, Y.; Xiong, L.; Duan, X.; Liu, C. Bayesian Optimized Crystallization of a Hydroxamate-Functionalized Covalent Organic Framework for Enhanced Uranyl Uptake. Small 2025, 21, 2411788. [Google Scholar] [CrossRef]
- Sugamata, K.; Zhang, Y.; Amanokura, N.; Shirai, A.; Minoura, M. Alkoxy-Functionalized Hydroxamate/Zinc Metal–Organic Frameworks and the Effects of Substituents and Acid Addition on Their Structures. Inorg. Chem. 2024, 63, 2454–2459. [Google Scholar] [CrossRef]
- Sugamata, K.; Takagi, C.; Awano, K.; Iihama, T.; Minoura, M. Structural analysis of and selective CO2 adsorption in mixed-ligand hydroxamate-based metal–organic frameworks. Dalton Trans. 2020, 49, 9948–9952. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Tang, W.; Xiong, C.; Du, J.; Zhang, Z.; Qiu, Y.; Shao, Z.-W.; Zhou, X.; Liu, C. Construction of robust Cu-MOFs from bifunctional pyridine-hydroxamate linkers for photocatalytic CO2 reduction. Chem. Commun. 2025, 61, 4030–4033. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, N.-N.; Fuhrmann, D.; Merker, S.; Krautscheid, H. A stable lanthanum hydroxamate metal–organic framework with radical character and electrical conductivity. Dalton Trans. 2022, 51, 15946–15953. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, W.; Yang, L.; Song, T.; Xia, Z.; Lai, Q.; Zhou, X.; Xiao, H.; Liu, C. Ce-hydroxamate metal–organic frameworks for photocatalytic H2 generation. Chem. Commun. 2022, 58, 13503–13506. [Google Scholar] [CrossRef]
- Zheng, Z.; Rong, Z.; Iu-Fan Chen, O.; Yaghi, O.M. Metal-Organic Frameworks with Rod Yttrium Secondary Building Units. Isr. J. Chem. 2023, 63, e202300017. [Google Scholar] [CrossRef]
- Xiong, C.; Shao, Z.-W.; Zhang, Z.; Dong, Y.; Xiong, L.; Qiu, Y.; Duan, X.; Liu, Y.; Liu, C. Charge-Tunable Bismuth-Hydroxamate Metal–Organic Frameworks for Photocatalytic Cr(VI) Mitigation. Cryst. Growth Des. 2024, 24, 8688–8694. [Google Scholar] [CrossRef]
- Sugamata, K. Hydroxamate-Based Metal-Organic Frameworks. Chem.—Eur. J. 2025, 31, e202403812. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Z.; Wang, H.; Zeng, G.; Xu, P.; Xiao, R.; Huang, D.; Chen, S.; He, Y.; Zhou, C.; et al. Bismuth-based metal–organic frameworks and their derivatives: Opportunities and challenges. Coord. Chem. Rev. 2021, 439, 213902. [Google Scholar] [CrossRef]
- Keogan, D.M.; Twamley, B.; Fitzgerald-Hughes, D.; Griffith, D.M. Novel class of Bi(iii) hydroxamato complexes: Synthesis, urease inhibitory activity and activity against H. pylori. Dalton Trans. 2016, 45, 11008–11014. [Google Scholar] [CrossRef]
- Keogan, D.M.; Fagan, L.E.; Passos, T.M.; Müller-Bunz, H.; Quilty, B.; Griffith, D.M. Synthesis of polymeric bismuth chlorido hydroxamato complexes; X-ray crystal structure and antibacterial activity of a novel Bi(III) salicylhydroxamato complex. Inorg. Chim. Acta 2017, 460, 134–140. [Google Scholar] [CrossRef]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Tabor, R.F.; Andrews, P.C. Synthesis and structural characterisation of bismuth(iii) hydroxamates and their activity against Helicobacter pylori. Dalton Trans. 2015, 44, 16903–16913. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Mehring, M.; Andrews, P.C. Bismuth(iii) benzohydroxamates: Powerful anti-bacterial activity against Helicobacter pylori and hydrolysis to a unique Bi34 oxido-cluster [Bi34O22(BHA)22(H-BHA)14(DMSO)6]. Chem. Commun. 2014, 50, 15232–15234. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhu, Y.; Li, Z.; Mei, Z.; Shao, Z.-W.; Liu, C. A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture. Symmetry 2024, 16, 1049. [Google Scholar] [CrossRef]
- Willems, T.F.; Rycroft, C.H.; Kazi, M.; Meza, J.C.; Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 2012, 149, 134–141. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Tan, J.-C.; Cheetham, A.K. Heterometallic Inorganic-Organic Frameworks of Sodium−Bismuth Benzenedicarboxylates. Cryst. Growth Des. 2010, 10, 1736–1741. [Google Scholar] [CrossRef]
- Burrows, A.D.; Jurcic, M.; Mahon, M.F.; Pierrat, S.; Roffe, G.W.; Windle, H.J.; Spencer, J. Bismuth coordination networks containing deferiprone: Synthesis, characterisation, stability and antibacterial activity. Dalton Trans. 2015, 44, 13814–13817. [Google Scholar] [CrossRef]
- Wibowo, A.C.; Smith, M.D.; zur Loye, H.-C. New 3D bismuth-oxo coordination polymers containing terephthalate-based ligands: Observation of Bi2O2-layer and Bi4O3-chain motifs. CrystEngComm 2011, 13, 426–429. [Google Scholar] [CrossRef]
- Kan, L.; Li, J.; Luo, X.; Li, G.; Liu, Y. Three novel bismuth-based coordination polymers: Synthesis, structure and luminescent properties. Inorg. Chem. Commun. 2017, 85, 70–73. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Sun, L.X.; Xing, Y.H.; Bai, F.Y. Synthesis and structure of a 3D supramolecular layered Bi-MOF and its application in photocatalytic degradation of dyes. J. Mol. Struct. 2022, 1270, 133895. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Cheetham, A.K. Anionic Metal–Organic Frameworks of Bismuth Benzenedicarboxylates: Synthesis, Structure and Ligand-Sensitized Photoluminescence. Eur. J. Inorg. Chem. 2010, 2010, 3823–3828. [Google Scholar] [CrossRef]
- Xie, W.; Fu, Q.; Chen, G.; Yan, L.; Yang, L.; Yuan, X.; Wen, S.; Ge, L.; Zhang, J.; Zhao, X. Fast diffusion and high C2H2 capture in a 2D MOF with oxygen-riched wide channels for efficient C2H2/CO2 separation. Compos. Part B Eng. 2025, 299, 112414. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bose, S.; Sengupta, D.; Rayder, T.M.; Wang, X.; Kirlikovali, K.O.; Sekizkardes, A.K.; Islamoglu, T.; Farha, O.K. Challenges and opportunities: Metal–organic frameworks for direct air capture. Adv. Funct. Mater. 2024, 34, 2307478. [Google Scholar] [CrossRef]
- Mutyala, S.; Jonnalagadda, M.; Ibrahim, S.M. Effect of modification of UiO-66 for CO2 adsorption and separation of CO2/CH4. J. Mol. Struct. 2021, 1227, 129506. [Google Scholar] [CrossRef]
- Neubertová, V.; Švorčík, V.; Kolská, Z. Amino-modified ZIF-8 for enhanced CO2 capture: Synthesis, characterization and performance evaluation. Microporous Mesoporous Mater. 2024, 366, 112956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Xiong, C.; Shao, Z.-W.; Liu, C. Construction of a Symmetrical Bi-Hydroxamate Metal–Organic Framework with Chemical Robustness. Symmetry 2025, 17, 895. https://doi.org/10.3390/sym17060895
Dong Y, Xiong C, Shao Z-W, Liu C. Construction of a Symmetrical Bi-Hydroxamate Metal–Organic Framework with Chemical Robustness. Symmetry. 2025; 17(6):895. https://doi.org/10.3390/sym17060895
Chicago/Turabian StyleDong, Yue, Chaozhi Xiong, Zhen-Wu Shao, and Chong Liu. 2025. "Construction of a Symmetrical Bi-Hydroxamate Metal–Organic Framework with Chemical Robustness" Symmetry 17, no. 6: 895. https://doi.org/10.3390/sym17060895
APA StyleDong, Y., Xiong, C., Shao, Z.-W., & Liu, C. (2025). Construction of a Symmetrical Bi-Hydroxamate Metal–Organic Framework with Chemical Robustness. Symmetry, 17(6), 895. https://doi.org/10.3390/sym17060895







