Association Between Gait Lower Limb Intra and Interlimb Coordination and Fear of Falling and Falling History in Older Adults
Abstract
1. Introduction
2. Materials and Methods
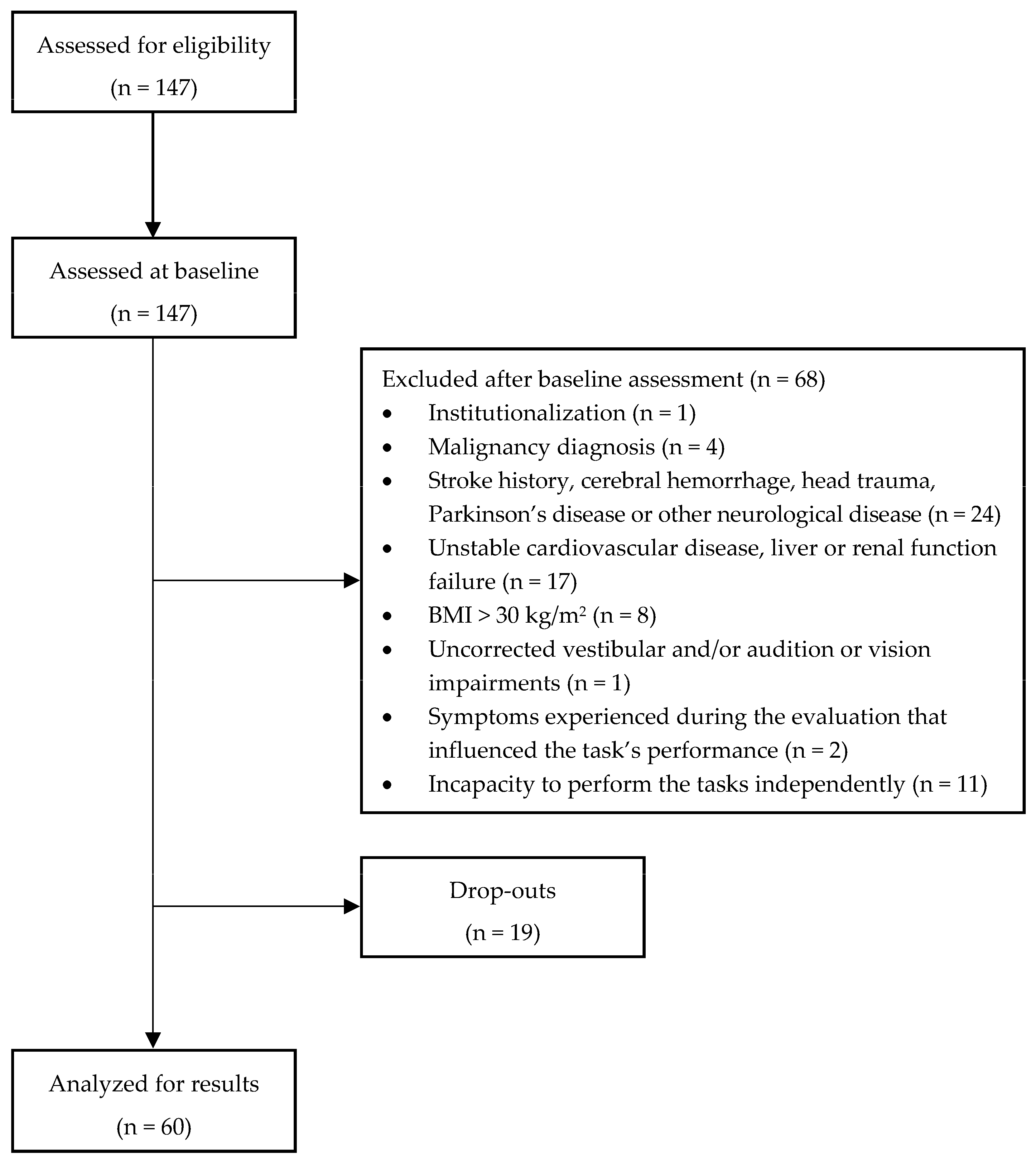
2.1. Study Design, Participants, and Study Criteria
2.2. Instruments and Procedures
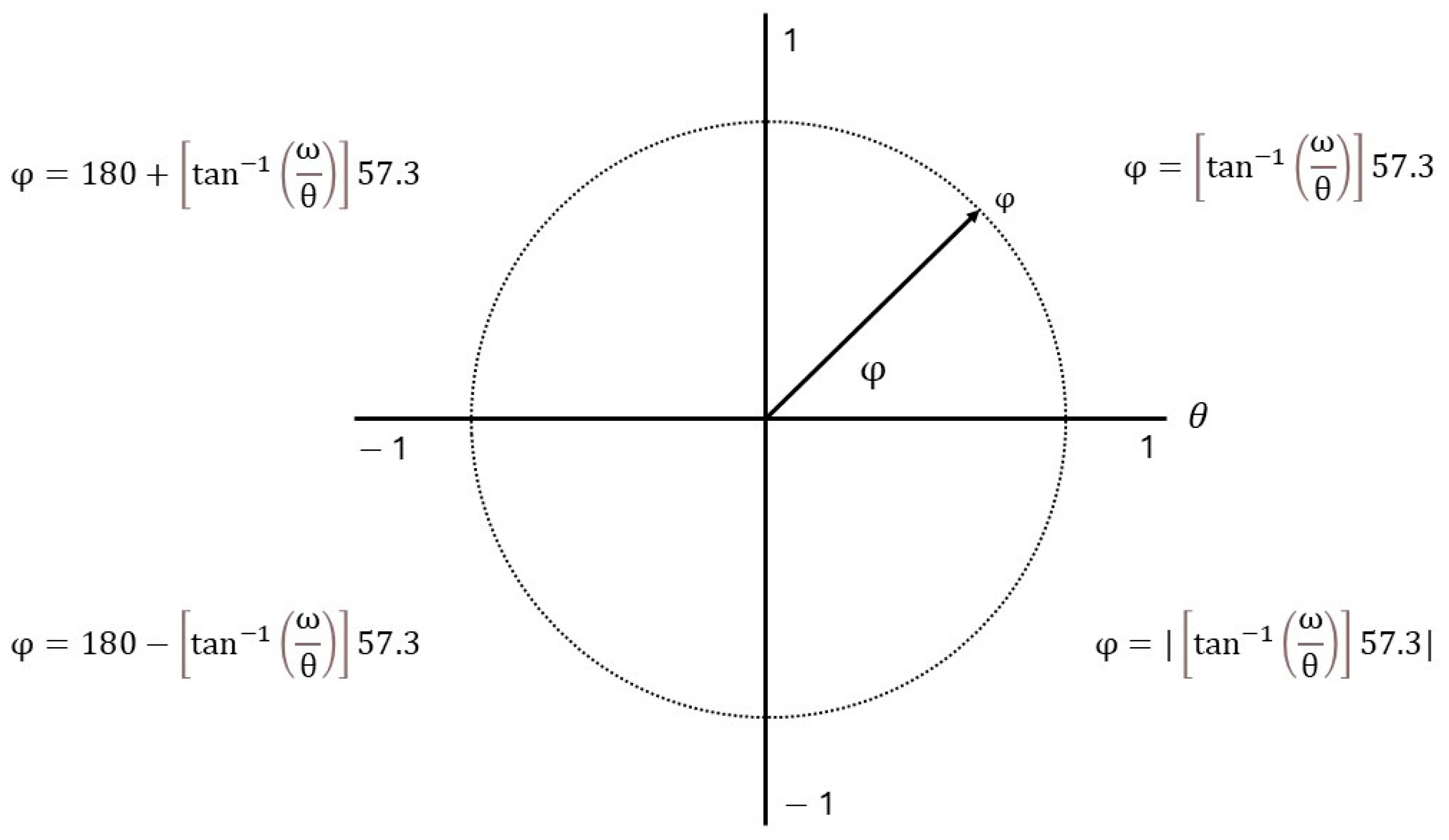
2.3. Data Processing
2.4. Statistical Analysis
3. Results
3.1. Sample Selection and Characterization
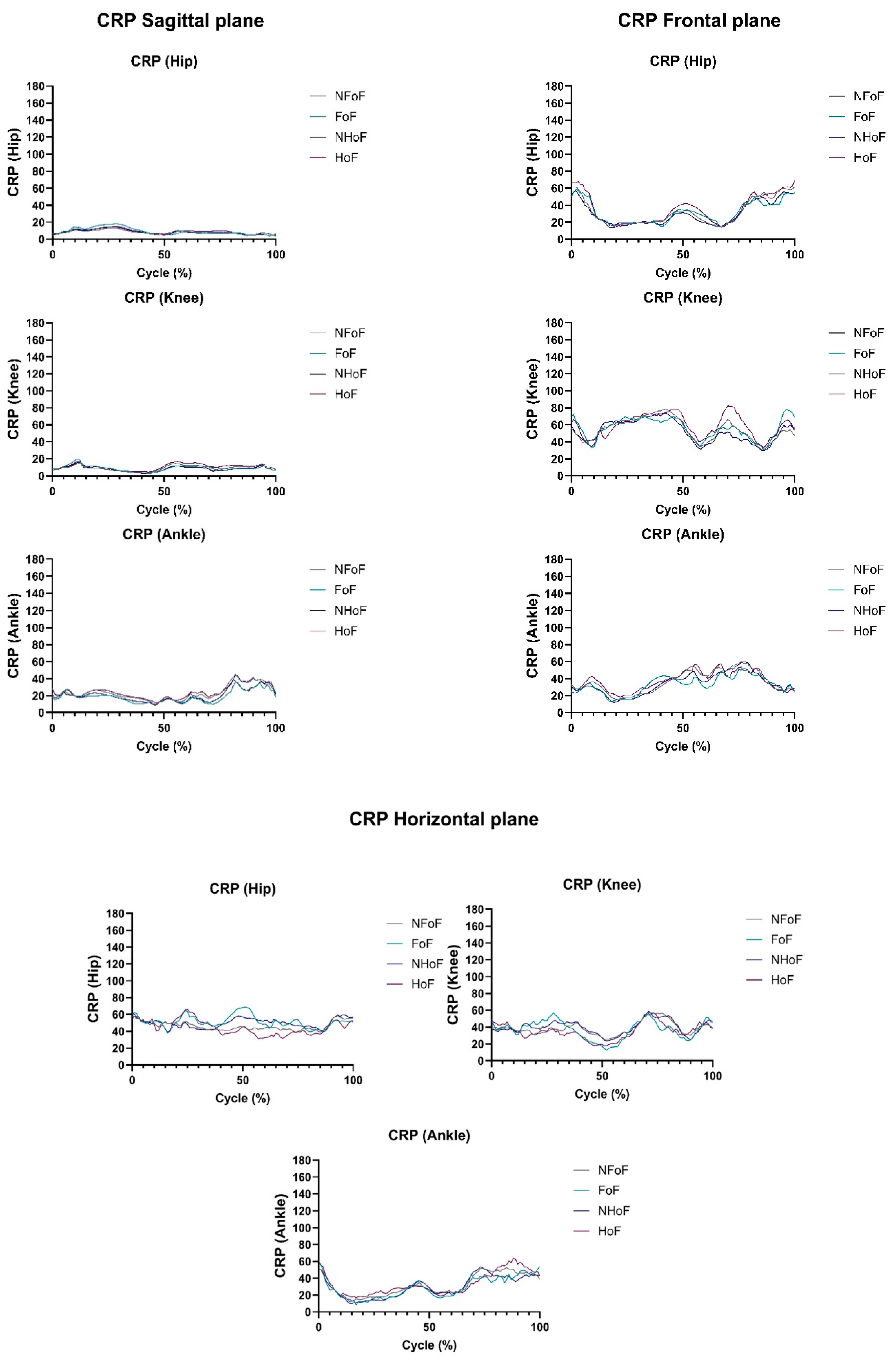
3.2. Intrasegmental Coordination During Gait Measured by CRP
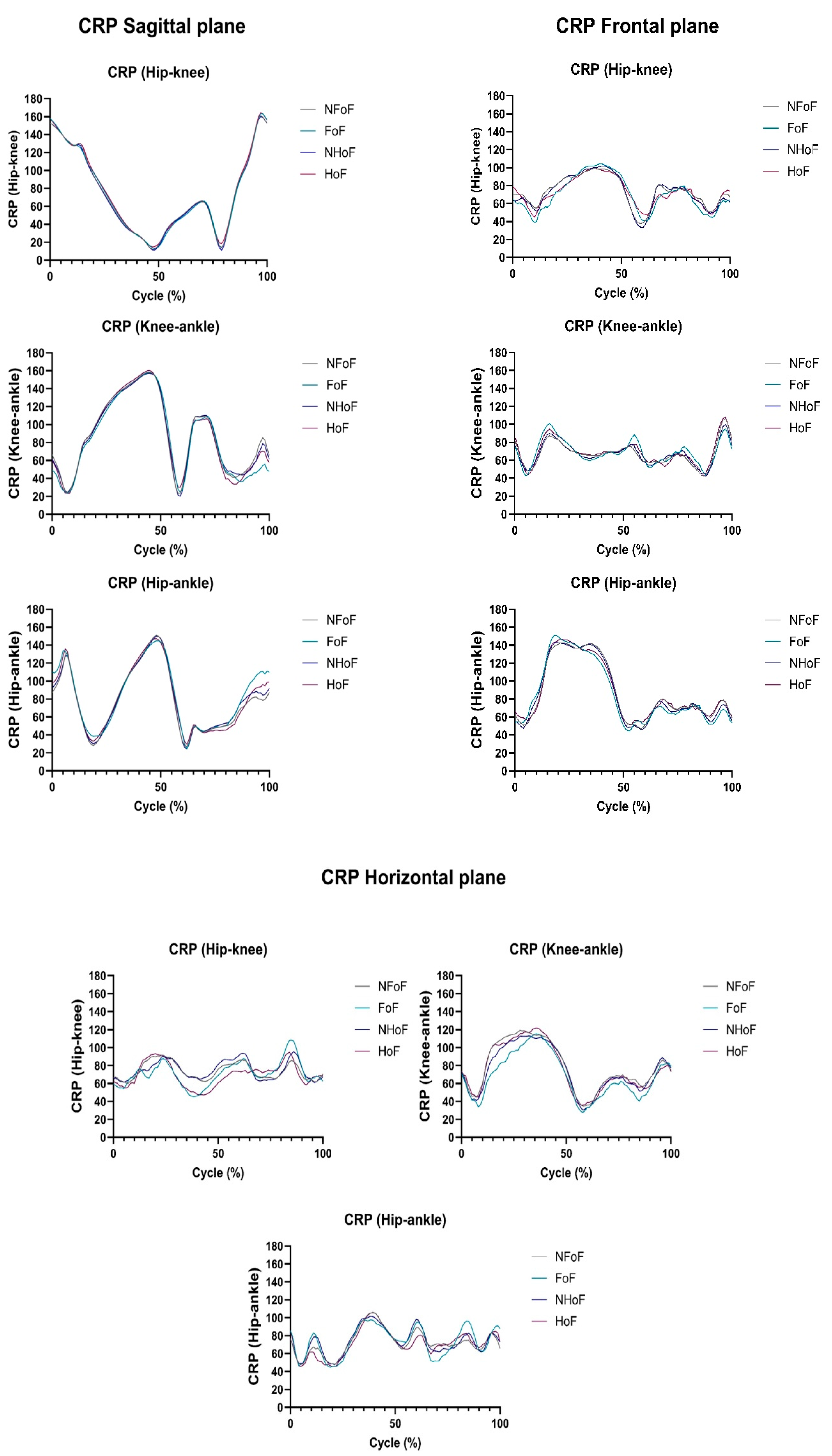
3.3. Intersegmental Coordination During Gait Measured by CRP
4. Discussion
Strengths, Limitations, and Practical Application
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | Continuous Relative Phase |
| HOF | History of Falling |
| FOF | Fear of Falling |
| NHOF | Without History of Falling |
| NFOF | Without Fear of Falling |
| M | Mean |
| CV | Coefficient of Variation |
| SD | Standard Deviation |
| BMI | Body Mass Index |
References
- Noble, J.W.; Prentice, S.D. Intersegmental coordination while walking up inclined surfaces: Age and ramp angle effects. Exp. Brain Res. 2008, 189, 249–255. [Google Scholar] [CrossRef]
- Ogaya, S.; Iwata, A.; Higuchi, Y.; Fuchioka, S. The association between intersegmental coordination in the lower limb and gait speed in elderly females. Gait Posture 2016, 48, 1–5. [Google Scholar] [CrossRef]
- Morfis, P.; Gkaraveli, M. Effects of aging on biomechanical gait parameters in the healthy elderly and the risk of falling. J. Res. Pract. Musculoskelet. Syst. 2021, 5, 59–64. [Google Scholar] [CrossRef]
- Aboutorabi, A.; Arazpour, M.; Bahramizadeh, M.; Hutchins, S.W.; Fadayevatan, R. The effect of aging on gait parameters in able-bodied older subjects: A literature review. Aging Clin. Exp. Res. 2016, 28, 393–405. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Mian, O.S.; Thom, J.M.; Ardigò, L.P.; Narici, M.V.; Minetti, A.E. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. (Oxf. Engl.) 2006, 186, 127–139. [Google Scholar] [CrossRef]
- Schoene, D.; Kiesswetter, E.; Sieber, C.C.; Freiberger, E. Skelettmuskuläre Faktoren, Sarkopenie und Stürze im Alter [Musculoskeletal factors, sarcopenia and falls in old age]. Z. Gerontol. Geriatr. 2019, 52, 37–44. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Falls Prevention in Older Age. 2007. Available online: https://www.who.int/publications/i/item/9789241563536 (accessed on 9 September 2024).
- Gazibara, T.; Kurtagic, I.; Kisic-Tepavcevic, D.; Nurkovic, S.; Kovacevic, N.; Gazibara, T.; Pekmezovic, T. Falls, risk factors and fear of falling among persons older than 65 years of age. Psychogeriatrics 2017, 17, 215–223. [Google Scholar] [CrossRef]
- Chen, W.C.; Li, Y.T.; Tung, T.H.; Chen, C.; Tsai, C.Y. The relationship between falling and fear of falling among community-dwelling elderly. Medicine 2021, 100, e26492. [Google Scholar] [CrossRef]
- Chiu, S.L.; Chou, L.S. Effect of walking speed on inter-joint coordination differs between young and elderly adults. J. Biomech. 2012, 45, 275–280. [Google Scholar] [CrossRef]
- Ippersiel, P.; Robbins, S.M.; Dixon, P.C. Lower-limb coordination and variability during gait: The effects of age and walking surface. Gait Posture 2021, 85, 251–257. [Google Scholar] [CrossRef]
- Hafer, J.F.; Boyer, K.A. Age related differences in segment coordination and its variability during gait. Gait Posture 2018, 62, 92–98. [Google Scholar] [CrossRef]
- d’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Yakovenko, S.; Krouchev, N.; Drew, T. Sequential activation of motor cortical neurons contributes to intralimb coordination during reaching in the cat by modulating muscle synergies. J. Neurophysiol. 2011, 105, 388–409. [Google Scholar] [CrossRef]
- Overduin, S.A.; d’Avella, A.; Roh, J.; Carmena, J.M.; Bizzi, E. Representation of Muscle Synergies in the Primate Brain. J. Neurosci. 2015, 35, 12615–12624. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J. Neurophysiol. 2006, 95, 602–618. [Google Scholar] [CrossRef]
- Israeli-Korn, S.D.; Barliya, A.; Paquette, C.; Franzén, E.; Inzelberg, R.; Horak, F.B.; Flash, T. Intersegmental coordination patterns are differently affected in Parkinson’s disease and cerebellar ataxia. J. Neurophysiol. 2019, 121, 672–689. [Google Scholar] [CrossRef]
- Hacmon, R.R.; Krasovsky, T.; Lamontagne, A.; Levin, M.F. Deficits in intersegmental trunk coordination during walking are related to clinical balance and gait function in chronic stroke. J. Neurol. Phys. Ther. 2012, 36, 173–181. [Google Scholar] [CrossRef]
- Lukšys, D.; Jatužis, D.; Jonaitis, G.; Griškevičius, J. Application of continuous relative phase analysis for differentiation of gait in neurodegenerative disease. Biomed. Signal Process. Control 2021, 67, 102558. [Google Scholar] [CrossRef]
- Salehi, R.; Mofateh, R.; Mehravar, M.; Negahban, H.; Tajali, S.; Monjezi, S. Comparison of the lower limb inter-segmental coordination during walking between healthy controls and people with multiple sclerosis with and without fall history. Mult. Scler. Relat. Disord. 2020, 41, 102053. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.L.; Chou, L.S. Variability in inter-joint coordination during walking of elderly adults and its association with clinical balance measures. Clin. Biomech. 2013, 28, 454–458. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.J.; Kim, K.; Park, Y.H.; Kim, S. The Validity and Reliability of the Mini-Mental State Examination-2 for Detecting Mild Cognitive Impairment and Alzheimer’s Disease in a Korean Population. PLoS ONE 2016, 11, e0163792. [Google Scholar] [CrossRef]
- Santana, I.; Duro, D.; Lemos, R.; Costa, V.; Pereira, M.; Simões, M.R.; Freitas, S. Mini-Mental State Examination: Screening and Diagnosis of Cognitive Decline, Using New Normative Data. Acta Medica Port. 2016, 29, 240–248. [Google Scholar] [CrossRef]
- Lukaski, H.; Johnson, P.; Bolonchuk, W.; Lykken, G. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef]
- Moreira, J.; Cunha, B.; Félix, J.; Santos, R.; Sousa, A.S.P. Kinematic and Kinetic Gait Principal Component Domains in Older Adults With and Without Functional Disability: A Cross-Sectional Study. J. Funct. Morphol. Kinesiol. 2025, 10, 140. [Google Scholar] [CrossRef]
- Zhong, Q.; Ali, N.; Gao, Y.; Wu, H.; Wu, X.; Sun, C.; Ma, J.; Thabane, L.; Xiao, M.; Zhou, Q.; et al. Gait Kinematic and Kinetic Characteristics of Older Adults With Mild Cognitive Impairment and Subjective Cognitive Decline: A Cross-Sectional Study. Front. Aging Neurosci. 2021, 13, 664558. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, W.; Kobayashi, Y.; Inoue, K.; Horie, M.; Yoshihara, K.; Ooie, T. Kinematic characteristics during gait in frail older women identified by principal component analysis. Sci. Rep. 2022, 12, 1676. [Google Scholar] [CrossRef]
- Sinclair, J.; Hebron, J.; Hurst, H.; Taylor, P. The influence of different Cardan sequences on three-dimensional cycling kinematics. Hum. Mov. 2013, 14, 334–339. [Google Scholar] [CrossRef]
- Hamill, J.; van Emmerik, R.E.; Heiderscheit, B.C.; Li, L. A dynamical systems approach to lower extremity running injuries. Clin. Biomech. 1999, 14, 297–308. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Kamali, F.; Razeghi, M.; Haghpanah, S.A. Comparison of the trunk-pelvis and lower extremities sagittal plane inter-segmental coordination and variability during walking in persons with and without chronic low back pain. Hum. Mov. Sci. 2017, 52, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.C.; Steele, C.J.; Nikulin, V.; Villringer, A.; Sehm, B. Kinematic profiles suggest differential control processes involved in bilateral in-phase and anti-phase movements. Sci. Rep. 2019, 9, 3273. [Google Scholar] [CrossRef]
- Ghanavati, T.; Salavati, M.; Karimi, N.; Negahban, H.; Ebrahimi Takamjani, I.; Mehravar, M.; Hessam, M. Intra-limb coordination while walking is affected by cognitive load and walking speed. J. Biomech. 2014, 47, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, U.; Ahmad, I.; García-Luengo, A.V.; Zaman, T.; Al-Noor, N.H.; Kumar, A. Estimation of Coefficient of Variation Using Calibrated Estimators in Double Stratified Random Sampling. Mathematics 2023, 11, 252. [Google Scholar] [CrossRef]
- West, R.M. Best practice in statistics: The use of log transformation. Ann. Clin. Biochem. 2022, 59, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Thiamwong, L.; Xie, R.; Conner, N.E.; Renziehausen, J.M.; Ojo, E.O.; Stout, J.R. Body composition, fear of falling and balance performance in community-dwelling older adults. Transl. Med. Aging 2023, 7, 80–86. [Google Scholar] [CrossRef]
- Priya, S.; Samuel, S.E.; Athira, M.V. Correlation between body mass index and fear of fall on functional mobility in elderly. Int. J. Physiother. Res. 2019, 7, 3257–3261. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Sarquis-Adamson, Y.; Song, H.Y.; Bray, N.W.; Pieruccini-Faria, F.; Speechley, M. Polypharmacy, gait performance, and falls in community-dwelling older adults. Results from the gait and brain study. J. Am. Geriatr. Soc. 2019, 67, 1182–1188. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ishizaki, T.; Masui, Y.; Hori, N.; Inagaki, H.; Ito, K.; Ogawa, M.; Yasumoto, S.; Arai, Y.; Kamide, K.; et al. Effect of number of medications on the risk of falls among community-dwelling older adults: A 3-year follow-up of the SONIC study. Jpn. Geriatr. Soc. 2024, 24, 306–310. [Google Scholar] [CrossRef]
- Sadeghi, H.; Shojaedin, S.S.; Abbasi, A.; Alijanpour, E.; Vieira, M.F.; Svoboda, Z.; Nazarpour, K. Lower-Extremity Intra-Joint Coordination and Its Variability between Fallers and Non-Fallers during Gait. Appl. Sci. 2021, 11, 2840. [Google Scholar] [CrossRef]
- Silva, J.; Atalaia, T.; Abrantes, J.; Aleixo, P. Gait Biomechanical Parameters Related to Falls in the Elderly: A Systematic Review. Biomechanics 2024, 4, 165–218. [Google Scholar] [CrossRef]
- James, E.G.; Leveille, S.G.; Hausdorff, J.M.; Barton, B.; Cote, S.; Karabulut, M.; Conatser, P.; Kennedy, D.N.; Tucker, K.L.; Snih, S.A.; et al. Coordination Impairments Are Associated With Falling Among Older Adults. Exp. Aging Res. 2017, 43, 430–439. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Groups (Mean ± SD) | Between Groups Comparisons (p Value) | |||||
|---|---|---|---|---|---|---|---|
| NFoF (n = 41) | FoF (n = 19) | NHoF (n = 38) | HoF (n = 22) | HoF | FoF | HoF*FoF | |
| Age (y) | 66.68 ± 6.47 | 68.58 ± 5.37 | 66.34 ± 5.11 | 68.91 ± 7.51 | 0.228 | 0.421 | 0.691 |
| Gender (n) | 0.104 b | 0.149 b | ― | ||||
| Men | 18 (43.9%) | 4 (21.1%) | 17 (44.7%) | 5 (22.7%) | |||
| Women | 23 (56.1%) | 15 (78.9%) | 21 (55.3%) | 17 (77.3%) | |||
| Height (m) | 1.62 ± 0.08 | 1.58 ± 0.07 | 1.63 ± 0.08 | 1.57 ± 0.07 | 0.061 | 0.151 | 0.347 |
| Weight (kg) | 65.92 ± 10.56 | 66.54 ± 9.40 | 67.92 ± 10.20 | 63.01 ± 9.45 | 0.114 | 0.557 | 0.557 |
| Body Mass Index (kg/m2) | 25.03 ± 2.84 | 26.67 ± 2.83 | 25.68 ± 3.11 | 25.34 ± 2.59 | 0.496 | 0.037 * | 0.899 |
| Health Condition | 0.597 a | 0.526 a | ― | ||||
| Yes | 31 (75.6%) | 15 (78.9%) | 29 (76.3%) | 17 (77.3%) | |||
| No | 10 (24.4%) | 4 (21.1%) | 9 (23.7%) | 5 (22.7%) | |||
| Number of Health Conditions | 1.39 ± 1.14 | 1.89 ± 1.29 | 1.61 ± 1.26 | 1.45 ± 1.10 | 0.268 | 0.173 | 0.158 |
| Medication | 0.246 a | 0.414 a | ― | ||||
| Yes | 35 (85.4%) | 18 (94.7%) | 332 (84.2%) | 21 (95.5%) | |||
| No | 6 (14.6%) | 1 (5.3%) | 6 (15.8%) | 1 (4.5%) | |||
| Number of Medication | 2.88 ± 2.42 | 3.42 ± 2.36 | 2.42 ± 2.06 | 4.14 ± 2.59 | 0.018 * | 0.720 | 0.672 |
| Number of Falls | 0.44 ± 0.74 | 0.79 ± 1.13 | 0 | 1.50 ± 0.86 | ― | ― | ― |
| History of Falling | ― | 0.264 a | ― | ||||
| Yes | 13 (31.7%) | 9 (47.4%) | 0 | 100% | |||
| No | 28 (68.3%) | 10 (52.6%) | 100% | 0 | |||
| Fear of Falling | 0.104 b | ― | ― | ||||
| Yes | 0 | 100% | 10 (26.3%) | 9 (40.9%) | |||
| No | 100% | 0 | 28 (73.7%) | 13 (59.1%) | |||
| CRP | Plane | Cycle CRP Variable | Groups (Mean ± SD) | Between Groups Comparisons (p Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NFoF (41) | FoF (19) | NHoF (38) | HoF (22) | HoF | FoF | HoF*FoF | ||||
| Hip-Knee | Sagittal | M | L | 73.90 ± 4.61 | 75.04 ± 3.42 | 72.99 ± 4.01 | 76.46 ± 3.87 | 0.004 * | 0.564 | 0.774 |
| R | 74.33 ± 3.89 | 73.01 ± 4.47 | 73.52 ± 3.76 | 74.59 ± 4.62 | 0.261 | 0.201 | 0.937 | |||
| SD | L | 46.91 ± 2.77 | 47.97 ± 3.27 | 46.92 ± 2.57 | 47.81 ± 3.51 | 0.708 | 0.391 | 0.089 | ||
| R | 47.13 ± 2.59 | 47.36 ± 2.56 | 46.85 ± 2.21 | 47.81 ± 3.03 | 0.492 | 0.816 | 0.036 * | |||
| CV | L | 0.64 ± 0.02 | 0.64 ± 0.05 | 0.64 ± 0.04 | 0.62 ± 0.04 | 0.017 * | 0.710 | 0.046 * | ||
| R | 0.64 ± 0.04 | 0.65 ± 0.04 | 0.64 ± 0.04 | 0.64 ± 0.03 | 0.618 | 0.316 | 0.063 | |||
| Frontal | M | L | 66.51 ± 26.54 | 67.89 ± 27.88 | 68.3928.82 | 64.4423.14 | 0.729 | 0.660 | 0.084 | |
| R | 80.21 ± 22.14 | 72.12 ± 24.42 | 76.3124.60 | 79.9819.21 | 0.371 | 0.200 | 0.658 | |||
| SD | L | 44.34 ± 10.22 | 42.73 ± 10.92 | 44.2 ± 11.12 | 43.07 ± 9.17 | 0.746 | 0.796 | 0.159 | ||
| R | 47.99 ± 6.28 | 47.24 ± 12.04 | 47.09 ± 8.70 | 48.91 ± 8.01 | 0.374 | 0.702 | 0.771 | |||
| CV | L | 0.73 ± 0.19 | 0.69 ± 0.18 | 0.72 ± 0.19 | 0.72 ± 0.18 | 0.802 | 0.331 | 0.146 | ||
| R | 0.64 ± 0.17 | 0.68 ± 0.12 | 0.66 ± 0.17 | 0.64 ± 0.14 | 0.491 | 0.332 | 0.997 | |||
| Horizontal | M | L | 76.85 ± 17.39 | 70.38 ± 17.78 | 78.25 ± 18.59 | 68.84 ± 14.33 | 0.052 | 0.252 | 0.482 | |
| R | 71.25 ± 18.88 | 71.93 ± 15.56 | 71.80 ± 19.09 | 70.88 ± 15.61 | 0.626 | 0.990 | 0.352 | |||
| SD | L | 47.91 ± 4.76 | 46.17 ± 4.76 | 48.07 ± 3.83 | 46.14 ± 6.01 | 0.164 | 0.302 | 0.975 | ||
| R | 47.87 ± 5.76 | 47.46 ± 5.64 | 47.04 ± 5.64 | 47.10 ± 5.88 | 0.775 | 0.831 | 0.367 | |||
| CV | L | 0.65 ± 0.13 | 0.69 ± 0.14 | 0.64 ± 0.14 | 0.69 ± 0.11 | 0.216 | 0.325 | 0.381 | ||
| R | 0.69 ± 0.16 | 0.68 ± 0.12 | 0.69 ± 0.16 | 0.68 ± 0.12 | 0.858 | 0.882 | 0.308 | |||
| Knee-Ankle | Sagittal | M | L | 89.54 ± 11.12 | 84.91 ± 10.16 | 88.20 ± 10.93 | 87.86 ± 11.26 | 0.972 | 0.121 | 0.638 |
| R | 86.08 ± 10.69 | 83.85 ± 11.66 | 85.86 ± 9.56 | 84.54 ± 13.24 | 0.538 | 0.419 | 0.336 | |||
| SD | L | 48.90 ± 3.61 | 50.46 ± 4.88 | 48.35 ± 3.76 | 51.20 ± 4.06 | 0.006 * | 0.215 | 0.192 | ||
| R | 48.82 ± 3.83 | 50.64 ± 4.95 | 48.91 ± 4.33 | 50.25 ± 4.08 | 0.124 | 0.088 | 0.048 * | |||
| CV | L | 0.55 ± 0.08 | 0.60 ± 0.09 | 0.56 ± 0.08 | 0.59 ± 0.09 | 0.109 | 0.048 * | 0.246 | ||
| R | 0.58 ± 0.08 | 0.62 ± 0.11 | 0.58 ± 0.08 | 0.61 ± 0.11 | 0.094 | 0.083 | 0.040 * | |||
| Frontal | M | L | 71.15 ± 19.17 | 71.14 ± 22.04 | 68.69 ± 19.12 | 75.40 ± 21.03 | 0.451 | 0.664 | 0.135 | |
| R | 63.61 ± 16.46 | 66.76 ± 15.41 | 65.27 ± 17.74 | 63.47 ± 12.99 | 0.439 | 0.545 | 0.361 | |||
| SD | L | 49.25 ± 7.18 | 46.17 ± 6.68 | 48.32 ± 7.35 | 48.19 ± 6.87 | 0.942 | 0.118 | 0.776 | ||
| R | 46.04 ± 5.78 | 46.90 ± 5.28 | 45.81 ± 5.60 | 47.19 ± 5.61 | 0.496 | 0.728 | 0.724 | |||
| CV | L | 0.72 ± 0.14 | 0.69 ± 0.15 | 0.73 ± 0.14 | 0.67 ± 0.15 | 0.488 | 0.796 | 0.016 * | ||
| R | 0.75 ± 0.14 | 0.73 ± 0.14 | 0.74 ± 0.14 | 0.76 ± 0.13 | 0.267 | 0.563 | 0.348 | |||
| Horizontal | M | L | 77.85 ± 14.50 | 70.05 ± 13.29 | 75.39 ± 13.67 | 75.36 ± 16.13 | 0.455 | 0.087 | 0.129 | |
| R | 78.92 ± 16.67 | 66.31 ± 15.68 | 73.72 ± 18.42 | 77.00 ± 15.27 | 0.124 | 0.009 * | 0.171 | |||
| SD | L | 48.34 ± 6.62 | 45.67 ± 6.08 | 47.81 ± 6.85 | 46.96 ± 6.02 | 0.892 | 0.235 | 0.204 | ||
| R | 48.51 ± 6.94 | 45.20 ± 7.76 | 47.01 ± 8.15 | 48.24 ± 5.66 | 0.091 | 0.143 | 0.030 * | |||
| CV | L | 0.63 ± 0.10 | 0.66 ± 0.08 | 0.64 ± 0.09 | 0.64 ± 0.10 | 0.558 | 0.311 | 0.379 | ||
| R | 0.63 ± 0.08 | 0.70 ± 0.09 | 0.65 ± 0.09 | 0.64 ± 0.09 | 0.298 | 0.004 * | 0.986 | |||
| Hip-Ankle | Sagittal | M | L | 77.03 ± 11.37 | 83.83 ± 9.29 | 78.77 ± 11.46 | 79.89 ± 10.78 | 0.897 | 0.030 * | 0.776 |
| R | 79.48 ± 9.83 | 82.80 ± 9.93 | 80.33 ± 10.16 | 80.88 ± 9.66 | 0.960 | 0.262 | 0.996 | |||
| SD | L | 45.97 ± 3.62 | 45.37 ± 3.08 | 45.55 ± 3.87 | 46.18 ± 2.58 | 0.400 | 0.505 | 0.735 | ||
| R | 44.65 ± 3.34 | 46.36 ± 3.48 | 44.26 ± 3.35 | 46.79 ± 3.06 | 0.004 * | 0.093 | 0.190 | |||
| CV | L | 0.61 ± 0.12 | 0.55 ± 0.09 | 0.59 ± 0.13 | 0.59 ± 0.10 | 0.912 | 0.056 | 0.892 | ||
| R | 0.57 ± 0.10 | 0.57 ± 0.09 | 0.56 ± 0.10 | 0.59 ± 0.09 | 0.249 | 0.859 | 0.609 | |||
| Frontal | M | L | 90.08 ± 15.17 | 85.57 ± 18.17 | 89.85 ± 15.15 | 86.59 ± 17.96 | 0.695 | 0.435 | 0.561 | |
| R | 85.56 ± 15.37 | 84.83 ± 14.16 | 83.19 ± 13.98 | 89.02 ± 15.97 | 0.081 | 0.825 | 0.278 | |||
| SD | L | 51.52 ± 5.14 | 49.80 ± 6.17 | 51.48 ± 5.78 | 50.12 ± 4.97 | 0.410 | 0.304 | 0.710 | ||
| R | 49.77 ± 5.00 | 48.68 ± 3.99 | 49.20 ± 4.36 | 49.82 ± 5.31 | 0.495 | 0.405 | 0.747 | |||
| CV | L | 0.59 ± 0.11 | 0.61 ± 0.15 | 0.59 ± 0.13 | 0.60 ± 0.11 | 0.947 | 0.637 | 0.574 | ||
| R | 0.60 ± 0.11 | 0.59 ± 0.10 | 0.61 ± 0.10 | 0.58 ± 0.11 | 0.219 | 0.753 | 0.331 | |||
| Horizontal | M | L | 70.79 ± 14.16 | 74.48 ± 14.34 | 73.16 ± 14.13 | 69.89 ± 14.43 | 0.193 | 0.383 | 0.253 | |
| R | 74.42 ± 14.70 | 72.71 ± 11.64 | 74.44 ± 13.46 | 72.92 ± 14.46 | 0.798 | 0.732 | 0.828 | |||
| SD | L | 48.08 ± 4.74 | 48.36 ± 4.25 | 48.80 ± 4.64 | 47.09 ± 4.30 | 0.151 | 0.707 | 0.787 | ||
| R | 48.52 ± 4.97 | 47.91 ± 4.27 | 48.56 ± 4.11 | 47.93 ± 5.74 | 0.736 | 0.722 | 0.851 | |||
| CV | L | 0.70 ± 0.11 | 0.67 ± 0.12 | 0.68 ± 0.10 | 0.70 ± 0.11 | 0.336 | 0.448 | 0.185 | ||
| R | 0.67 ± 0.10 | 0.67 ± 0.09 | 0.67 ± 0.10 | 0.67 ± 0.09 | 0.896 | 0.993 | 0.976 | |||
| CRP | Plane | Cycle CRP Variable | Groups (Mean ± SD) | Between Groups Comparisons (p Value) | |||||
|---|---|---|---|---|---|---|---|---|---|
| NFoF (41) | FoF (19) | NHoF (38) | HoF (22) | HoF | FoF | HoF*FoF | |||
| Hip|L and R | Sagittal | M | 8.32 ± 2.97 | 9.37 ± 3.48 | 8.40 ± 2.35 | 9.08 ± 4.23 | 0.898 | 0.345 | 0.497 |
| SD | 6.77 ± 3.17 | 8.05 ± 3.45 | 7.01 ± 2.90 | 7.44 ± 3.93 | 0.752 | 0.168 | 0.611 | ||
| CV | 0.80 ± 0.15 | 0.86 ± 0.17 | 0.82 ± 0.17 | 0.81 ± 0.15 | 0.634 | 0.164 | 0.919 | ||
| Frontal | M | 32.35 ± 12.25 | 31.42 ± 14.73 | 30.17 ± 11.14 | 35.32 ± 15.37 | 0.132 | 0.615 | 0.635 | |
| SD | 30.14 ± 9.67 | 28.58 ± 9.41 | 28.59 ± 9.26 | 31.47 ± 9.94 | 0.227 | 0.518 | 0.735 | ||
| CV | 0.96 ± 0.15 | 0.95 ± 0.16 | 0.97 ± 0.15 | 0.93 ± 0.16 | 0.375 | 0.911 | 0.855 | ||
| Horizontal | M | 46.42 ± 15.37 | 51.50 ± 17.76 | 50.03 ± 17.47 | 44.58 ± 13.37 | 0.146 | 0.208 | 0.760 | |
| SD | 38.62 ± 7.76 | 41.48 ± 9.44 | 39.91 ± 7.98 | 38.87 ± 9.13 | 0.519 | 0.207 | 0.933 | ||
| CV | 0.87 ± 0.16 | 0.85 ± 0.17 | 0.85 ± 0.18 | 0.89 ± 0.11 | 0.303 | 0.452 | 0.977 | ||
| Knee|L and R | Sagittal | M | 9.28 ± 3.76 | 9.04 ± 4.41 | 8.40 ± 2.81 | 10.59 ± 5.16 | 0.046 * | 0.593 | 0.148 |
| SD | 7.33 ± 3.49 | 7.96 ± 4.49 | 6.75 ± 2.08 | 8.86 ± 5.49 | 0.079 | 0.821 | 0.281 | ||
| CV | 0.80 ± 0.12 | 0.88 ± 0.17 | 0.82 ± 0.13 | 0.82 ± 0.16 | 0.639 | 0.064 | 0.403 | ||
| Frontal | M | 54.18 ± 27.05 | 55.84 ± 28.75 | 52.22 ± 29.22 | 58.99 ± 23.85 | 0.347 | 0.865 | 0.256 | |
| SD | 41.02 ± 12.39 | 42.18 ± 10.56 | 39.30 ± 13.37 | 45.00 ± 7.23 | 0.104 | 0.961 | 0.308 | ||
| CV | 0.85 ± 0.23 | 0.87 ± 0.26 | 0.86 ± 0.23 | 0.85 ± 0.24 | 0.864 | 0.596 | 0.281 | ||
| Horizontal | M | 38.52 ± 13.30 | 36.88 ± 14.94 | 39.58 ± 14.33 | 35.27 ± 12.48 | 0.250 | 0.760 | 0.718 | |
| SD | 34.96 ± 9.36 | 30.94 ± 9.94 | 34.85 ± 9.69 | 31.68 ± 9.47 | 0.216 | 0.146 | 0.364 | ||
| CV | 0.93 ± 0.14 | 0.87 ± 0.13 | 0.91 ± 0.13 | 0.92 ± 0.15 | 0.690 | 0.072 | 0.596 | ||
| Ankle|L and R | Sagittal | M | 23.44 ± 12.15 | 18.79 ± 5.71 | 21.46 ± 6.66 | 22.83 ± 15.59 | 0.721 | 0.048 * | 0.450 |
| SD | 21.55 ± 8.35 | 17.13 ± 5.80 | 20.63 ± 6.54 | 19.33 ± 9.86 | 0.407 | 0.038 * | 0.914 | ||
| CV | 0.96 ± 0.22 | 0.92 ± 0.16 | 0.98 ± 0.23 | 0.89 ± 0.14 | 0.362 | 0.872 | 0.198 | ||
| Frontal | M | 36.43 ± 11.67 | 33.02 ± 11.40 | 34.10 ± 11.94 | 37.52 ± 10.90 | 0.241 | 0.220 | 0.921 | |
| SD | 32.03 ± 8.78 | 28.12 ± 9.61 | 30.15 ± 9.83 | 31.91 ± 7.94 | 0.347 | 0.107 | 0.924 | ||
| CV | 0.91 ± 0.17 | 0.87 ± 0.17 | 0.90 ± 0.17 | 0.87 ± 0.15 | 0.688 | 0.523 | 0.648 | ||
| Horizontal | M | 32.10 ± 13.27 | 29.06 ± 8.03 | 29.88 ± 7.62 | 33.30 ± 16.93 | 0.608 | 0.220 | 0.229 | |
| SD | 30.25 ± 8.78 | 29.01 ± 7.20 | 29.53 ± 7.44 | 30.43 ± 9.71 | 0.869 | 0.453 | 0.332 | ||
| CV | 0.97 ± 0.18 | 1.02 ± 0.16 | 1.00 ± 0.15 | 0.96 ± 0.20 | 0.463 | 0.239 | 0.790 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.; Moreira, J.; Sousa, A.S.P. Association Between Gait Lower Limb Intra and Interlimb Coordination and Fear of Falling and Falling History in Older Adults. Symmetry 2025, 17, 818. https://doi.org/10.3390/sym17060818
Castro M, Moreira J, Sousa ASP. Association Between Gait Lower Limb Intra and Interlimb Coordination and Fear of Falling and Falling History in Older Adults. Symmetry. 2025; 17(6):818. https://doi.org/10.3390/sym17060818
Chicago/Turabian StyleCastro, Márcia, Juliana Moreira, and Andreia S. P. Sousa. 2025. "Association Between Gait Lower Limb Intra and Interlimb Coordination and Fear of Falling and Falling History in Older Adults" Symmetry 17, no. 6: 818. https://doi.org/10.3390/sym17060818
APA StyleCastro, M., Moreira, J., & Sousa, A. S. P. (2025). Association Between Gait Lower Limb Intra and Interlimb Coordination and Fear of Falling and Falling History in Older Adults. Symmetry, 17(6), 818. https://doi.org/10.3390/sym17060818








