Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
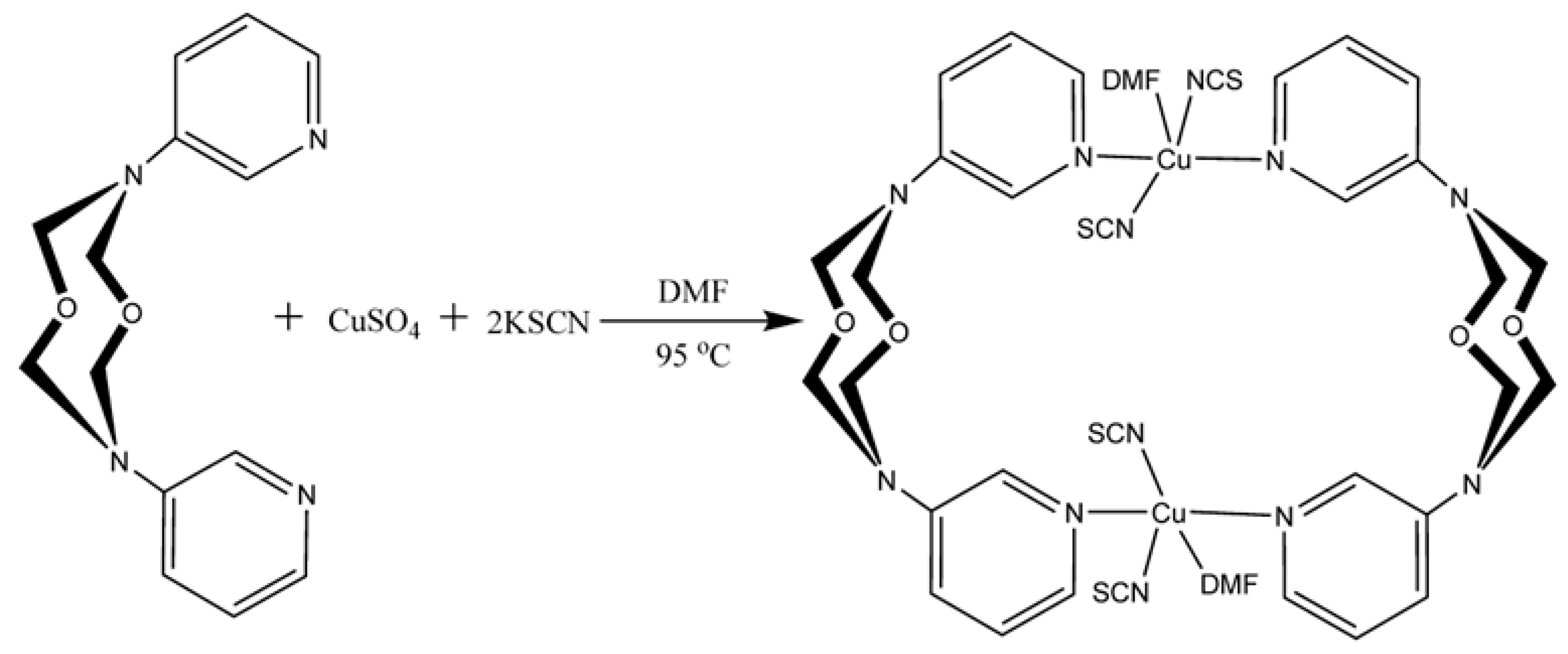
2.2.1. Synthesis of the [Cu(L)(SCN)2(DMF)]2 Complex
2.2.2. Single-Crystal X-Ray Crystallographic Study
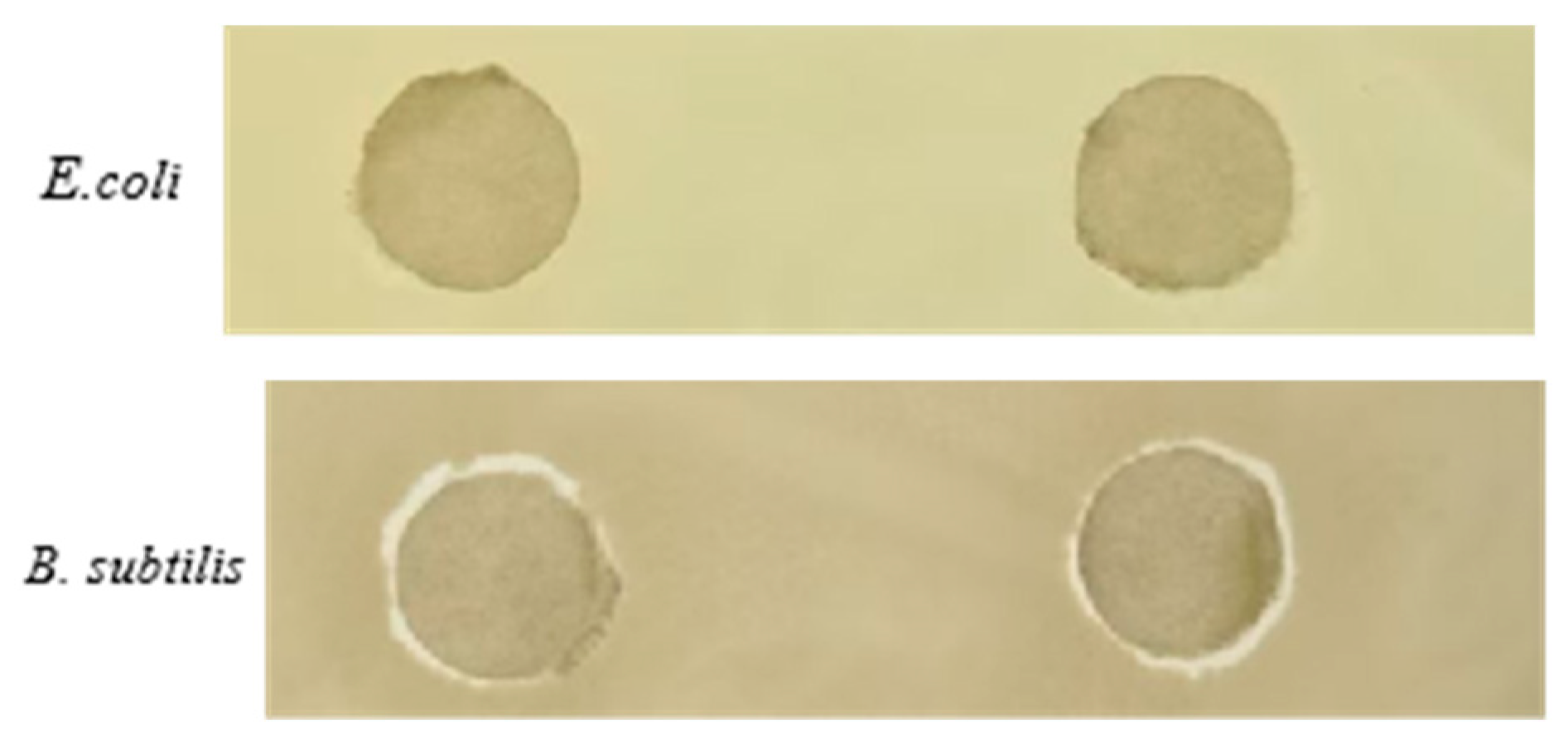
2.2.3. Antibacterial Tests
3. Results and Discussion
3.1. The Structural Analysis
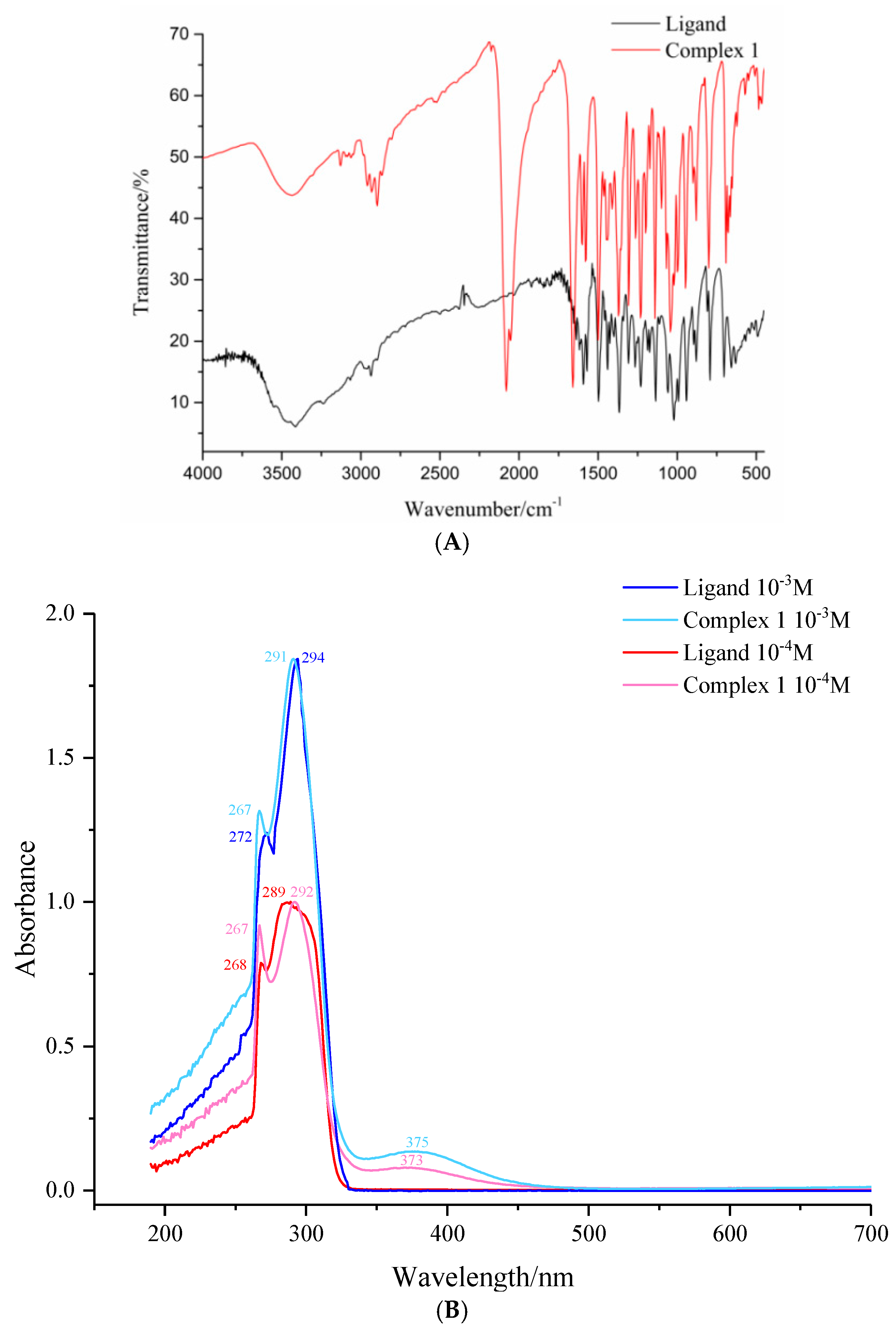
3.2. The IR and UV-Vis Spectra of Complex 1
3.3. DSC-TGA Profiles for Complex 1
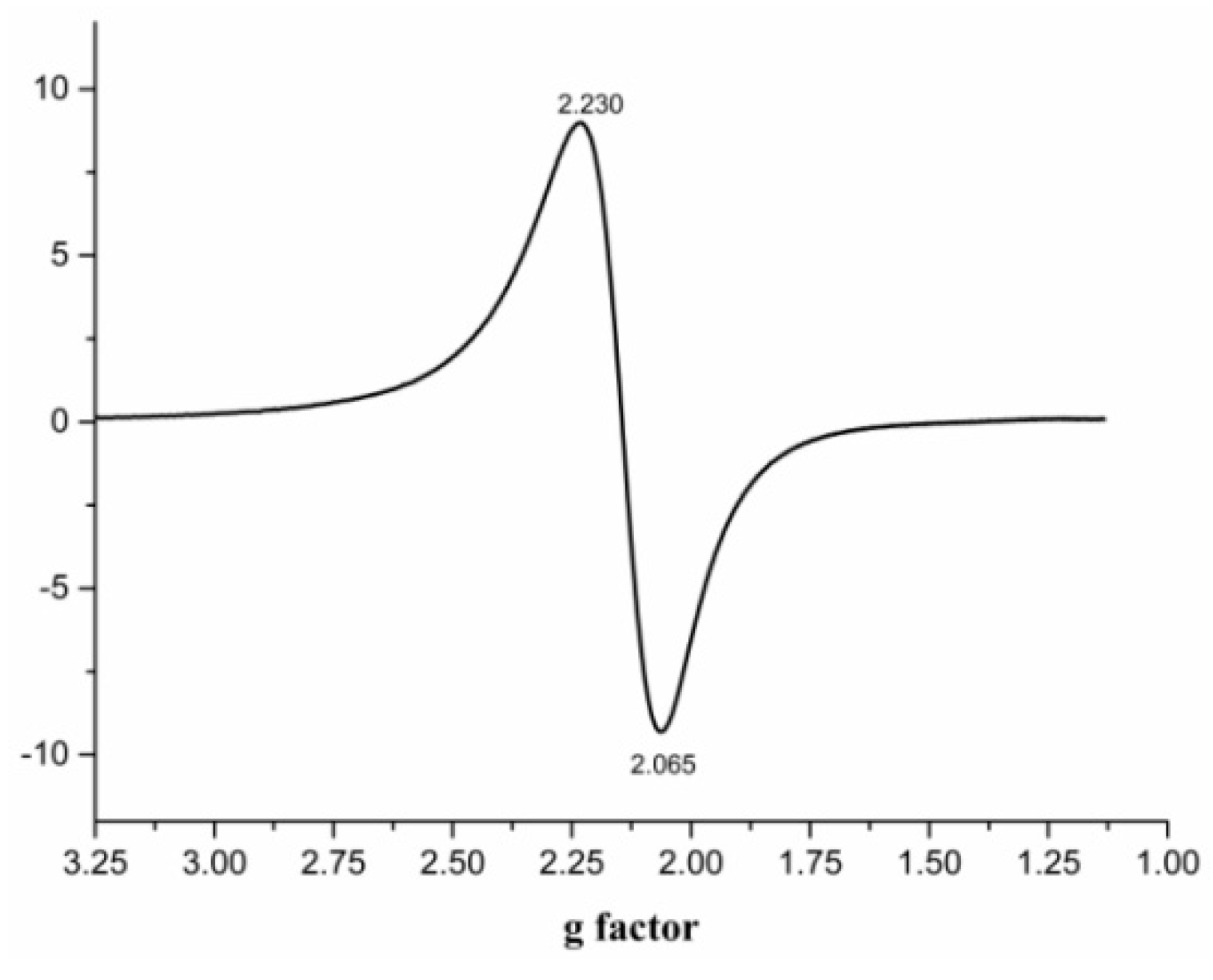
3.4. The EPR Spectra of Complex 1
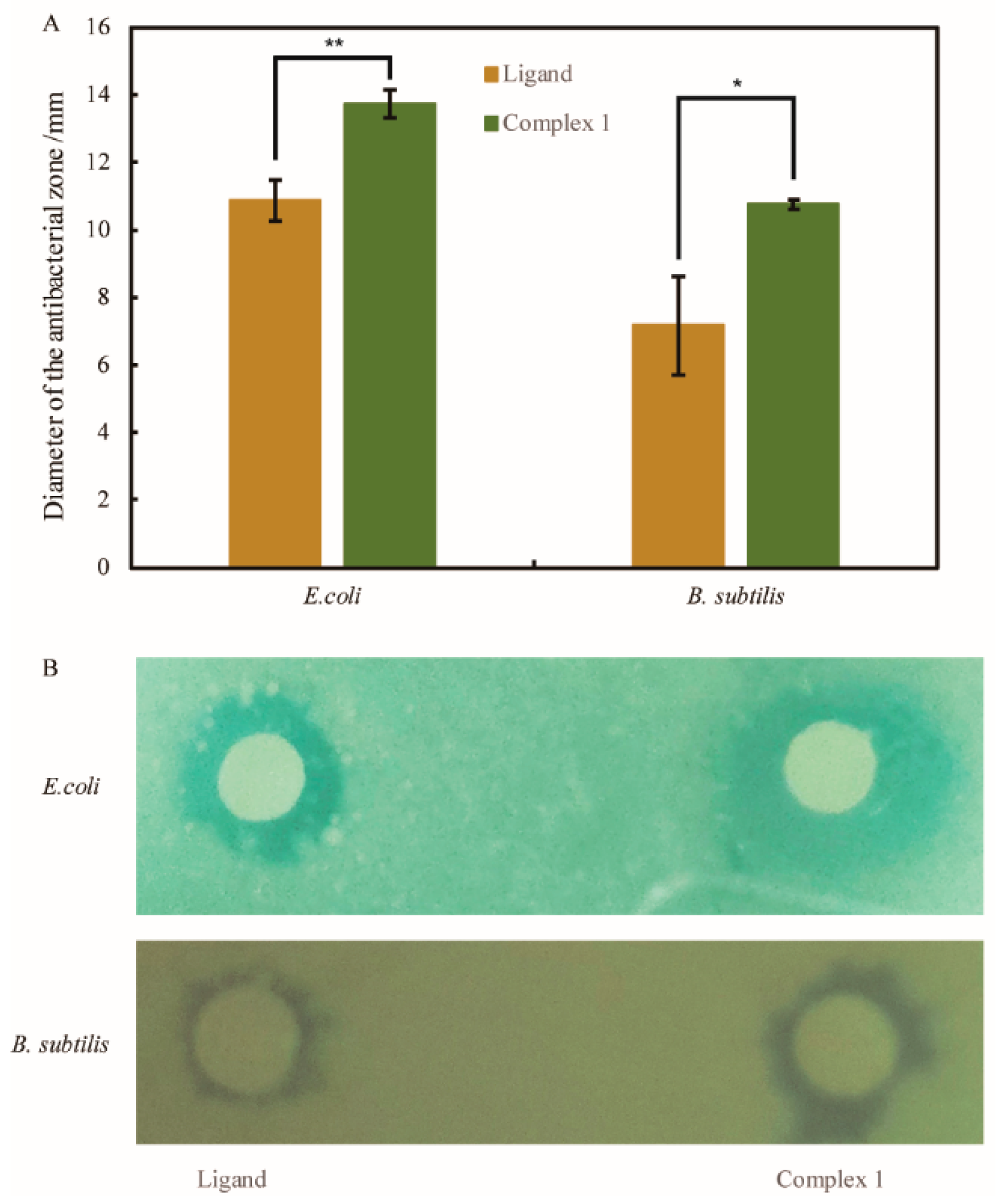
3.5. The Antimicrobial Activities of L and Complex 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wechwithayakhlung, C.; Packwood, D.M.; Harding, D.J.; Pattanasattayavong, P. Structures, bonding, and electronic properties of metal thiocyanates. J. Phys. Chem. Solids 2021, 154, 110085. [Google Scholar] [CrossRef]
- Li, L.-L.; Yuan, R.-X.; Liu, L.-L.; Ren, Z.-G.; Zheng, A.-X.; Cheng, H.-J.; Li, H.-X.; Lang, J.-P. Formation of [CuSCN]n-Based Topological Structures via a Family of Flexible Benzimidazolyl-Based Linkers with Different Spacer Lengths. Cryst. Growth Des. 2010, 10, 1929–1938. [Google Scholar] [CrossRef]
- Pramanik, S.; Mandal, P.C.; Das, K.; Pathak, S.; Mukhopadhyay, S. Synthetic, Structural and Supramolecular Features of a New Dithiocyanato-κ-N, κ-S-Copper(II) Complex: Insights Through Computational Studies. J. Chem. Crystallogr. 2023, 53, 417–430. [Google Scholar] [CrossRef]
- Qin, Y.; Jianfang, Q. Synthesis, Structure and Properties of 3D Copper(I) Pseudohalide Compound Constructed by Conformationally Flexible N-Heterocylic Ligand. J. Synth. Cryst. 2022, 51, 1059–1068. [Google Scholar]
- Han, X.; Shen, K.; Huang, G.; Li, C.; Mao, S.; Shi, X.; Wu, H. Synthesis, structure, electrochemical properties and superoxide radical scavenging activities of two thiocyanate copper(II) complexes with different pyridyl-benzoxazole ligands. J. Mol. Struct. 2018, 1169, 18–24. [Google Scholar] [CrossRef]
- El-bendary, M.M.; Akhdhar, A.; Ali, E.M.M.; Kalantan, A.A.; Davaasuren, B.; Jaremko, M.; Babgi, B.A. Synthesis, structure characterization and antitumor activities of copper and cobalt thiocyanate complexes with 3-acetlpyridine ligand. Polyhedron 2023, 242, 116511. [Google Scholar] [CrossRef]
- Laachir, A.; Rhoufal, F.; Guesmi, S.; Ketatni, E.M.; Saadi, M.; Ammari, L.E.; Mentré, O.; Bentiss, F. Novel Cu coordination polymer self-assembled from 2,5-bis(pyridin-4-yl)-1,3,4-oxadiazole and thiocyanate ions: Synthesis, structural characterization, Hirshfeld surface analysis, thermal and magnetic studies. J. Mol. Struct. 2022, 1269, 133790. [Google Scholar] [CrossRef]
- Laachir, A.; Zine, H.; Guesmi, S.; Mostafa Ketatni, E.; Saadi, M.; El Ammari, L.; Mentré, O.; Bentiss, F. Unusual mixed-valence CuII/CuI coordination polymer based on 2,5-bis(pyridine-2-yl)-1,3,4-thiadiazole and thiocyanate: Synthesis, structural characterization and antimicrobial in vitro activity assessment. Polyhedron 2021, 209, 115494. [Google Scholar] [CrossRef]
- Abalintsina, S.A.; Conde, M.A.; Eni, D.B.; Agwara, M.O. Synthesis, characterization and antimicrobial properties of Cobalt(III), Nickel(III), Copper(II) and Zinc(II) complexes with pyridine and thiocyanate. Sci. Afr. 2022, 18, e01402. [Google Scholar] [CrossRef]
- Gashu, M.; Aragaw, B.A.; Tefera, M.; Abebe, A. Poly(bis(2,2′-bipyridine) hydroxy Copper(II) iodide modified glassy carbon electrode for electrochemical determination of chloroquine in pharmaceuticals and biological samples. Sens. Bio-Sens. Res. 2023, 42, 100598. [Google Scholar] [CrossRef]
- Tsague Chimaine, F.; Mbom, Y.D.; Amah, C.B.Y.; Bekindaka, E.D.; Agwara, M.O. Synthesis, crystal structure, photoluminescent and antimicrobial properties of a thiocyanato-bridged copper(II) coordination polymer. Cogent Chem. 2016, 2, 1253905. [Google Scholar] [CrossRef]
- Li, L.; Dong, S.; He, W.; Wang, H.; Ding, P.; Liu, S.; Qian, W. Synthesis, crystal structures, electrochemistry and thermal stabilities of two copper complexes built by 3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. Polyhedron 2023, 231, 116268. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64 Pt 1, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, X.-M.; Ren, X.-M. Syntheses, crystal structures and magnetic properties of two halogen bridged dinuclear copper(II) complexes [(4,4′-diethylester-2,2′-biquinoline)2Cu2(μ-X)2X2] (X−=Cl−, Br−). Polyhedron 2014, 83, 24–29. [Google Scholar] [CrossRef]
- Li, L. Syntheses, crystal structures and thermal stabilities of two copper complexes based on 3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. J. Struct. Chem. 2022, 63, 1929–1937. [Google Scholar] [CrossRef]
- Qiu, L.X.; Wan, F.; Zhu, B.B.; Sun, Y.Q.; You, Y.; Chen, Y.P. Synthesis, Structure and Spectroscopy Study of a 1D Copper Coordination Polymer Based on a Carboxybenzyl Viologen Ligand and SCN-Anion. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2015, 35, 1340–1344. [Google Scholar] [PubMed]
- Li, H.H.; Chen, Z.R.; Li, J.Q.; Huang, C.C.; Hu, X.L. Crystal and electronic structure of novel organic-inorganic hybrid coordination polymer {[C12H28N2][(Pb 3I8) (DMF)2]•2DMF}n. Acta Chim. Sin.-Chin. Ed. 2005, 63, 697–702. [Google Scholar]
- Dhara, P.K.; Pramanik, S.; Lu, T.-H.; Drew, M.G.B.; Chattopadhyay, P. Copper(II) complexes of new tetradentate NSNO pyridylthioazophenol ligands: Synthesis, spectral characterization and crystal structure. Polyhedron 2004, 23, 2457–2464. [Google Scholar] [CrossRef]
- Karlin, K.D.; Hayes, J.C.; Juen, S.; Hutchinson, J.P.; Zubieta, J. Tetragonal vs. trigonal coordination in copper(II) complexes with tripod ligands: Structures and properties of [Cu(C21H24N4)Cl]PF6 and [Cu(C18H18N4)Cl]PF6. Inorg. Chem. 1982, 21, 4106–4108. [Google Scholar] [CrossRef]


| Complex | L | 1 |
|---|---|---|
| Empirical formula | C14H16N4O2 | C38H46Cu2N14O6S4 |
| Formula weight | 272.31 | 1050.21 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/n | P−1 |
| a (Å) | 8.8945(13) | 10.4637(13) |
| b (Å) | 11.3334(16) | 11.0234(13) |
| c (Å) | 13.575(2) | 11.7951(15) |
| α (°) | 90 | 62.755(2) |
| β (°) | 102.522(3) | 75.035(2) |
| γ (°) | 90 | 76.914(2) |
| V (Å3) | 1335.9(3) | 1 159.2(2) |
| Z | 2 | 1 |
| F(000) | 576 | 540 |
| μ (mm−1) | 0.094 | 1.158 |
| GoF on F2 | 1.071 | 1.080 |
| Final R indices [I > 2σ(I)]: R1, wR2 | R1 = 0.042 9, wR2 = 0.136 6 | R1 = 0.056 1, wR2 = 0.167 6 |
| R indices (all data): R1, wR2 | R1 = 0.056 6, wR2 = 0.158 6 | R1 = 0.078 5, wR2 = 0.205 1 |
| Bond | Length/Å | Bond | Angle (°) |
|---|---|---|---|
| Cu(1)—O(3) | 2.383(5) | O(3)—Cu(1) -N(1) | 92.6(2) |
| Cu(1)—N(1) | 2.030(4) | O(3)—Cu(1) -N(2) | 89.4(2) |
| Cu(1)—N(2) | 2.037(4) | O(3)—Cu(1) -N(5) | 88.2(2) |
| Cu(1)—N(5) | 1.992(4) | O(3)—Cu(1) -N(6) | 99.0(2) |
| Cu(1)—N(6) | 1.971(4) | N(1)—Cu(1) -N(5) | 89.4(1) |
| S(1)—C(15) | 1.611(5) | N(1)—Cu(1) -N(6) | 87.71(1) |
| N(5)—C(15) | 1.162(6) | N(5)—Cu(1) -N(2) | 91.51(1) |
| N(6)—C(16) | 1.146(6) | N(5)—Cu(1) -N(6) | 172.41(2) |
| N(6)—Cu(1) -N(2) | 91.12(1) |
| Microorganism | Antibacterial Circle/mm |
|---|---|
| E. coli | 0 |
| B. subtilis | 5.62 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Wang, Z.; Xia, J.; Wang, H.; Dong, S.; Lou, S.; Ding, P.; Li, L. Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. Symmetry 2025, 17, 791. https://doi.org/10.3390/sym17050791
Qian W, Wang Z, Xia J, Wang H, Dong S, Lou S, Ding P, Li L. Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. Symmetry. 2025; 17(5):791. https://doi.org/10.3390/sym17050791
Chicago/Turabian StyleQian, Wei, Zibo Wang, Jingfeng Xia, Hongxia Wang, Shuling Dong, Shuai Lou, Ping Ding, and Li Li. 2025. "Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane" Symmetry 17, no. 5: 791. https://doi.org/10.3390/sym17050791
APA StyleQian, W., Wang, Z., Xia, J., Wang, H., Dong, S., Lou, S., Ding, P., & Li, L. (2025). Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. Symmetry, 17(5), 791. https://doi.org/10.3390/sym17050791





