Synthesis and Application for Pb2+ Removal of a Novel Magnetic Biochar Embedded with FexOy Nanoparticles
Abstract
1. Introduction
- (i)
- Develop new biochar composites. To our knowledge, cotton, as one of the abundant, low-cost, and easily accessible raw materials, has not been reported for the synthesis of magnetic composites. Meanwhile, the cellulose content of cotton is almost 100%, much higher than other wood materials. Under the same quality conditions, the yield of biochar prepared from cotton is higher.
- (ii)
- Explore simple synthesis methods for magnetic composites. BC-FexOy was prepared by impregnation calcination using cotton and Fe(NO3)3·9H2O as precursors without the addition of other auxiliary reagents. The process is simple and easy. Characterization shows that FexOy nanoparticles are embedded in BC, indicating the structural stability of the composite.
- (iii)
- Investigate the application of composites. Taking the removal of Pd (II) from wastewater as an example, this study explores the adsorption capacity and recycling characteristics of the composite material, proving that BC-FexOy is an efficient and low-cost new magnetic adsorption composite.
2. Materials and Methods
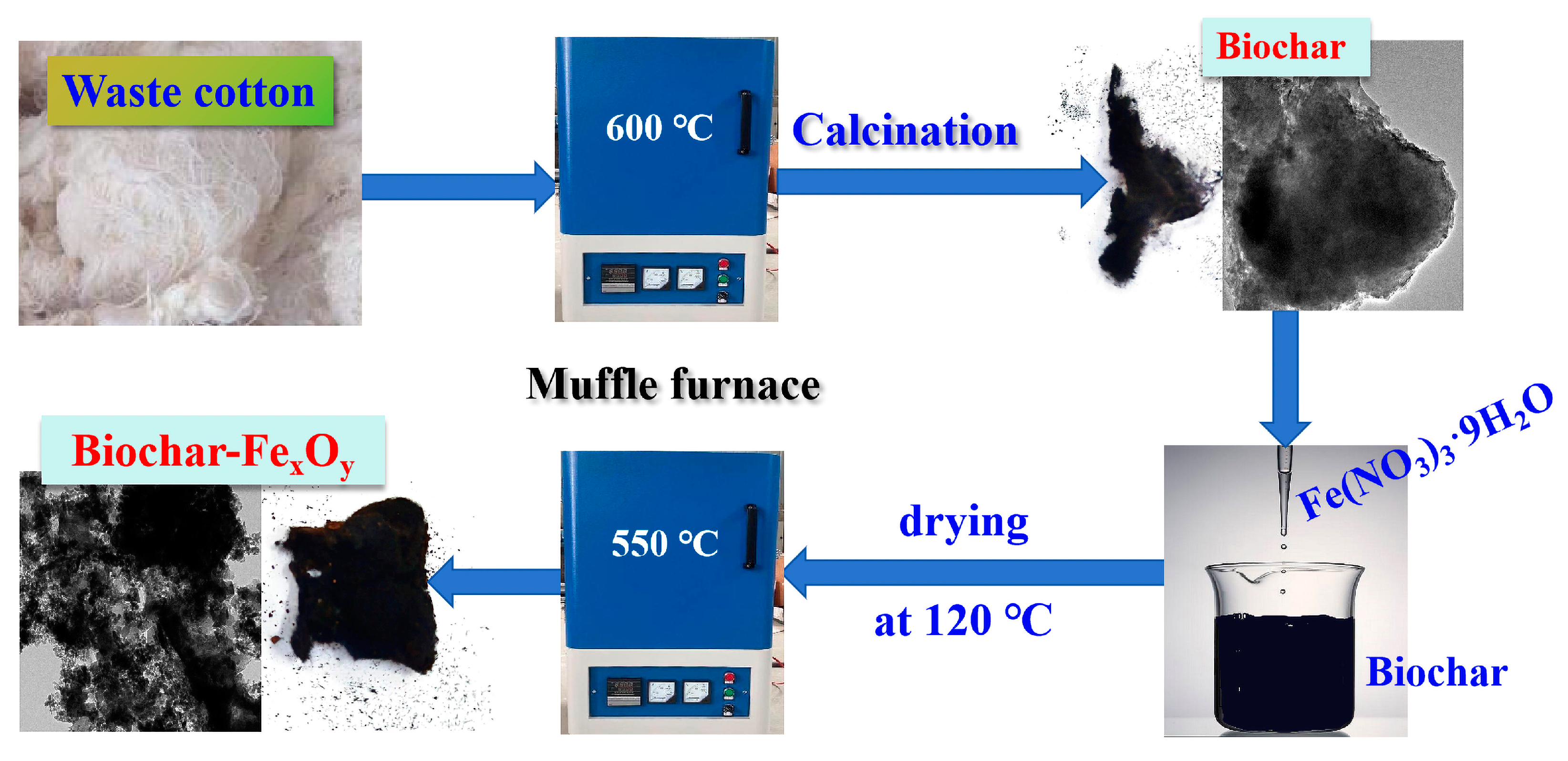
2.1. Synthesis of Materials
2.2. Method of Analysis
2.3. Inquiry into Adsorption Capacity
2.4. Exploring Isothermal Adsorption Kinetics
3. Results and Discussion
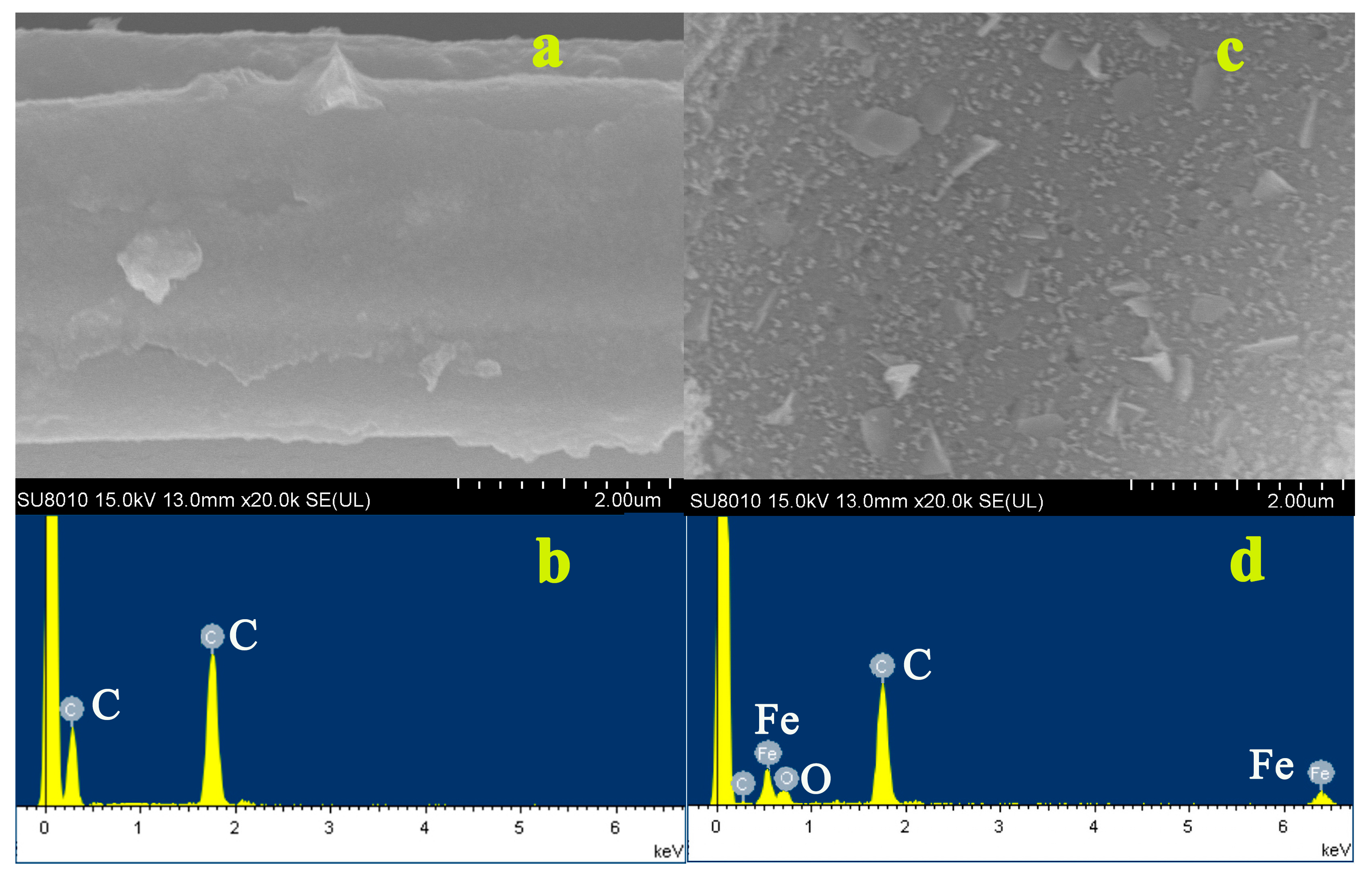
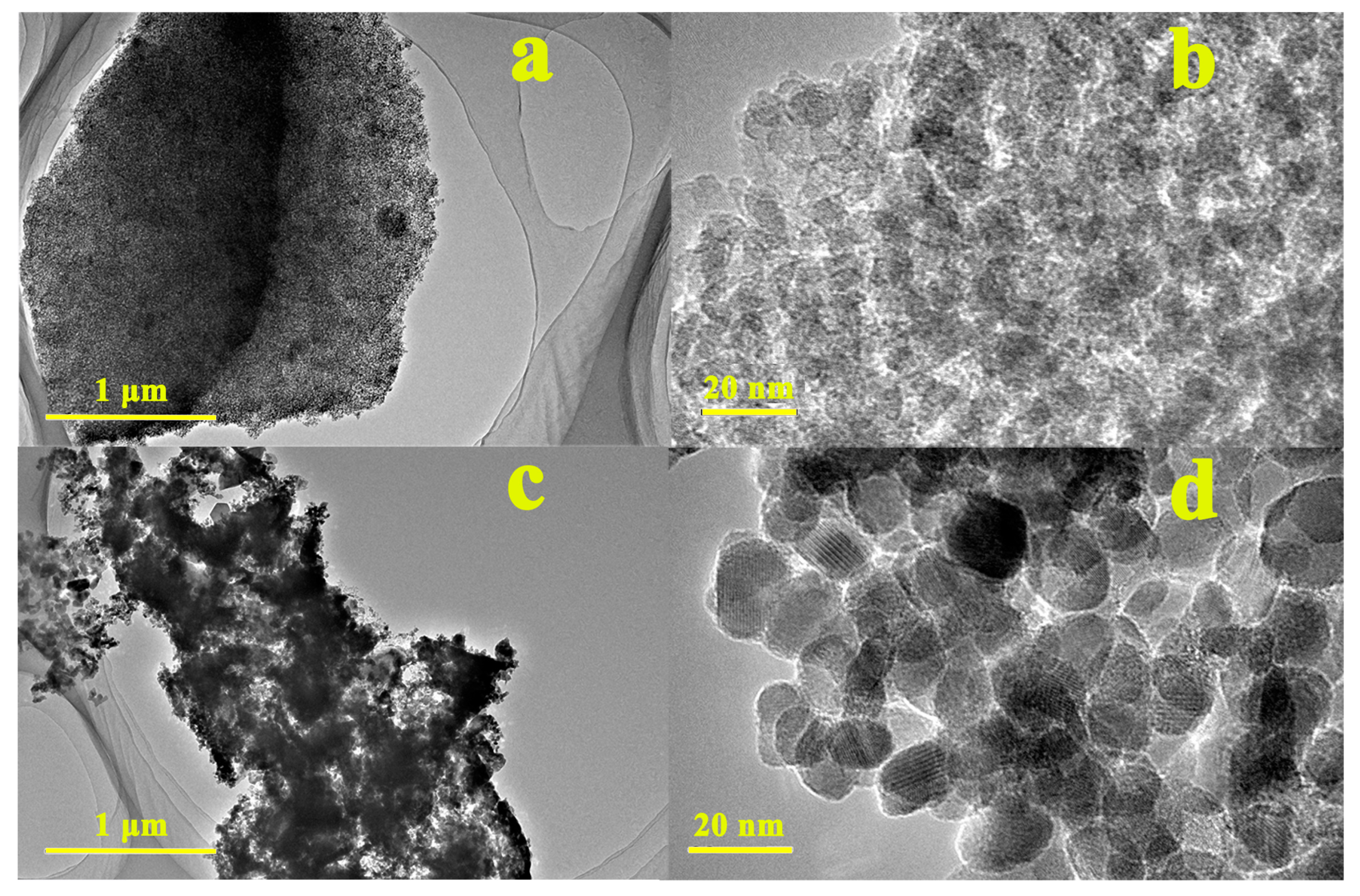
3.1. Characterization of Biochar and Biochar–FexOy
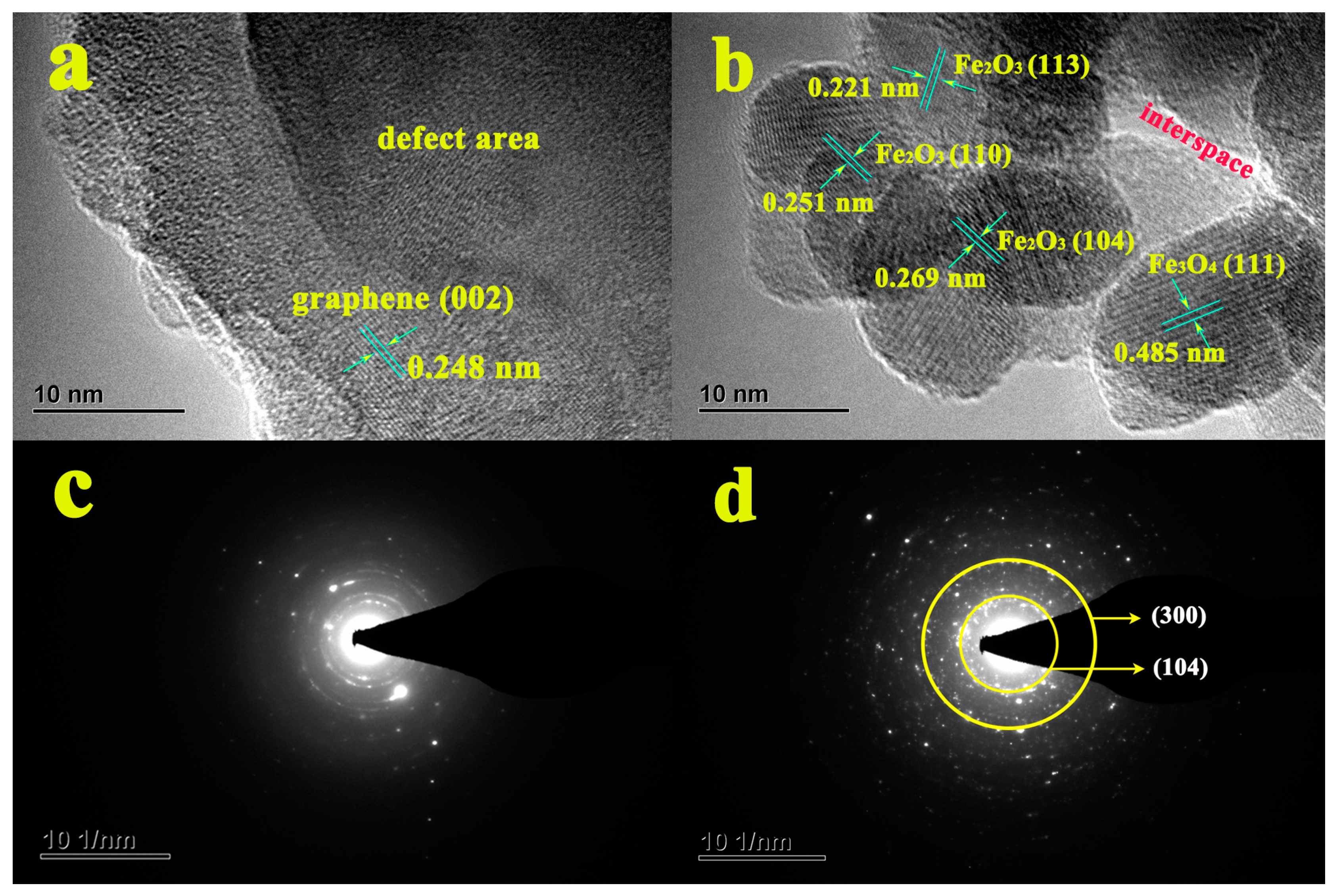
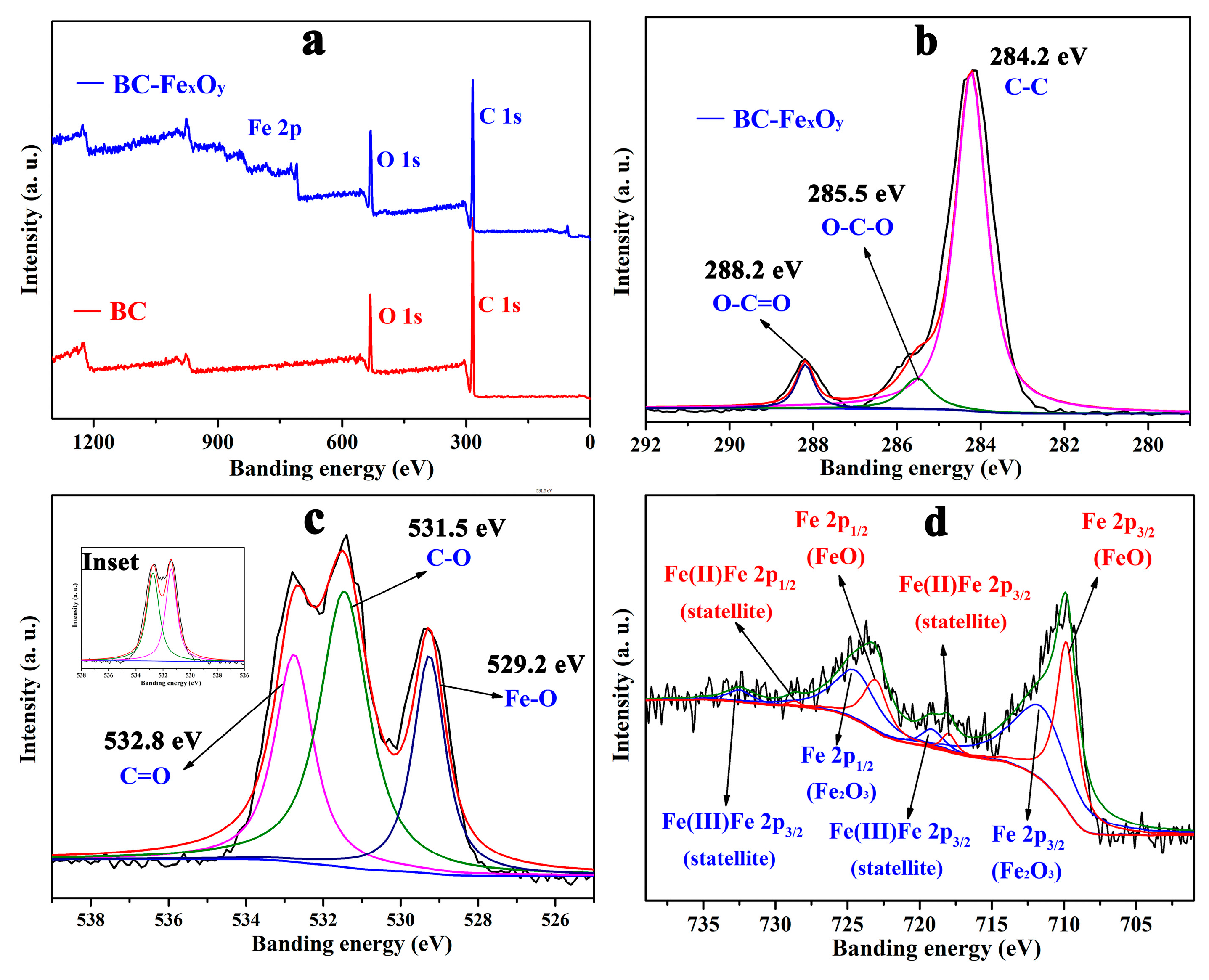
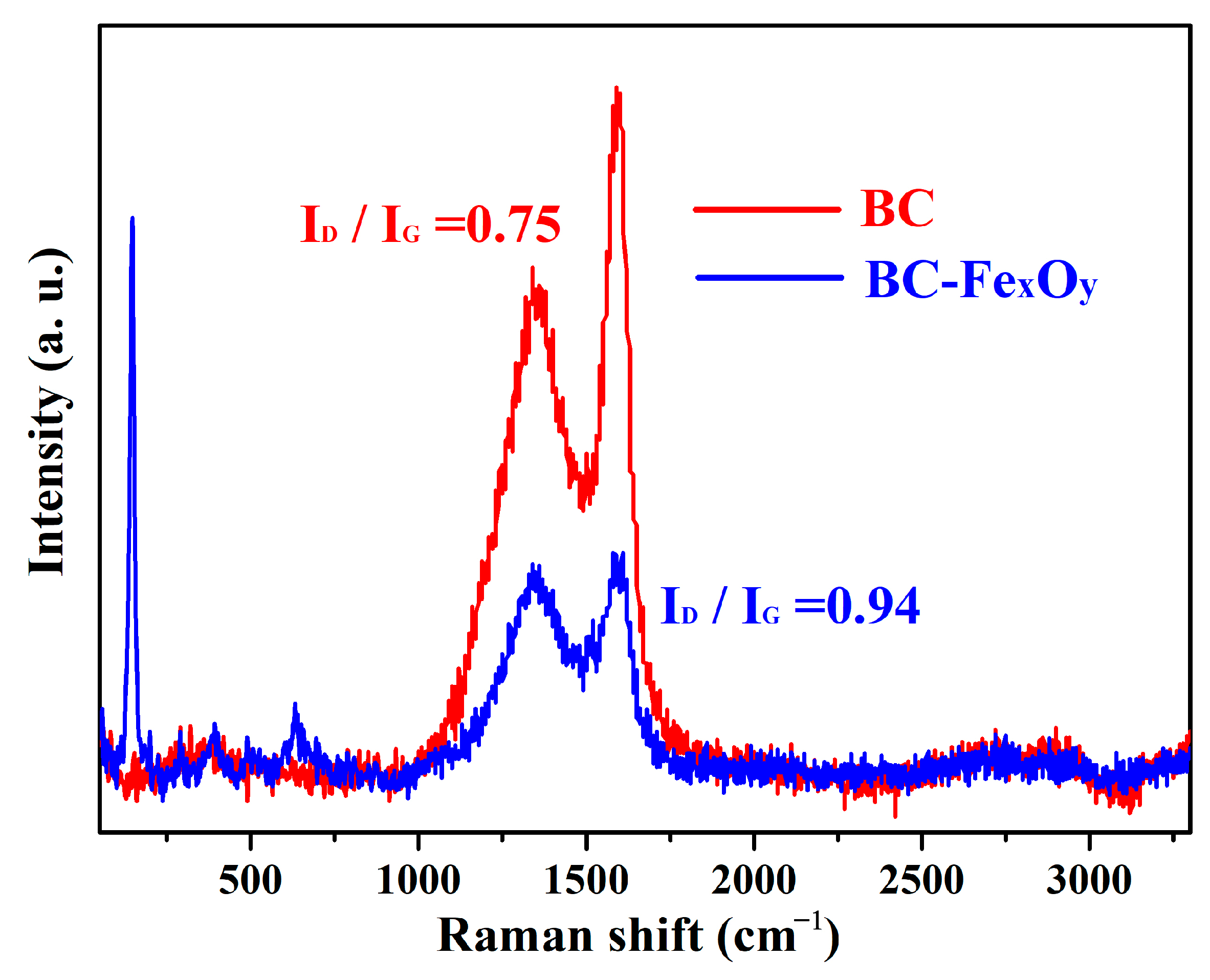
3.2. XRD, XPS, and Raman Analysis
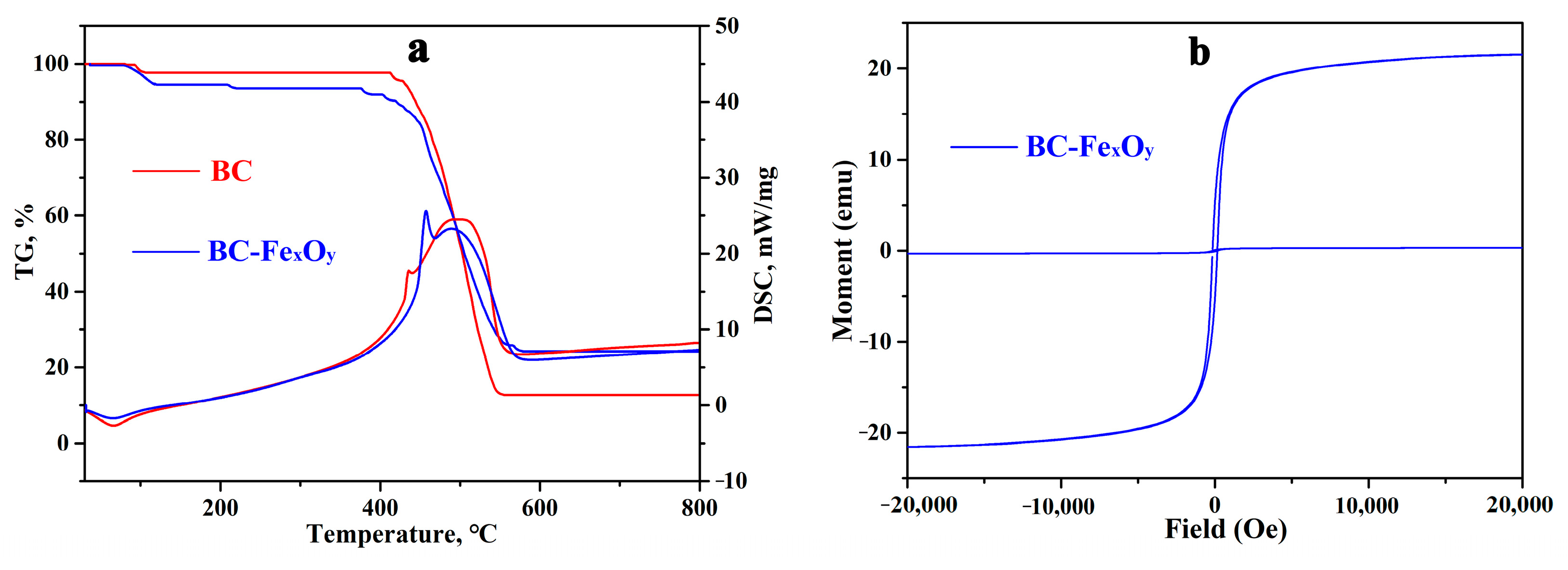
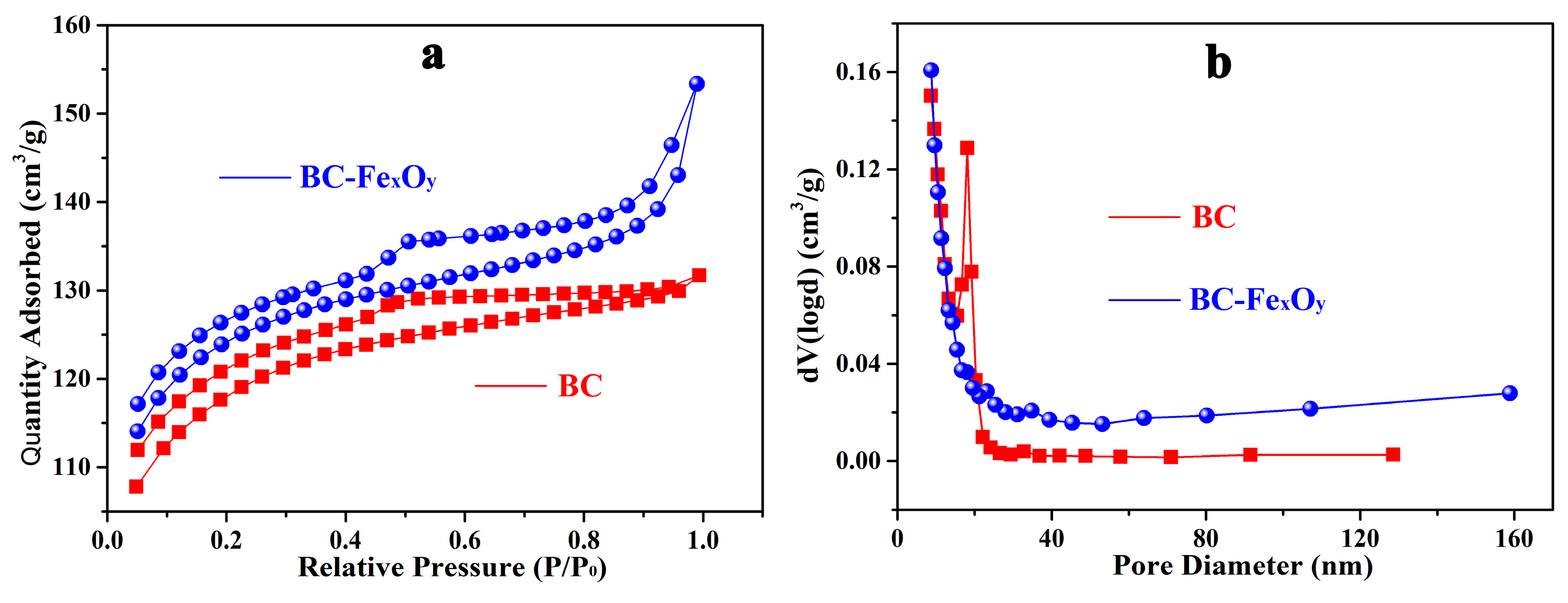
3.3. Physicochemical Properties of BC and BC-FexOy
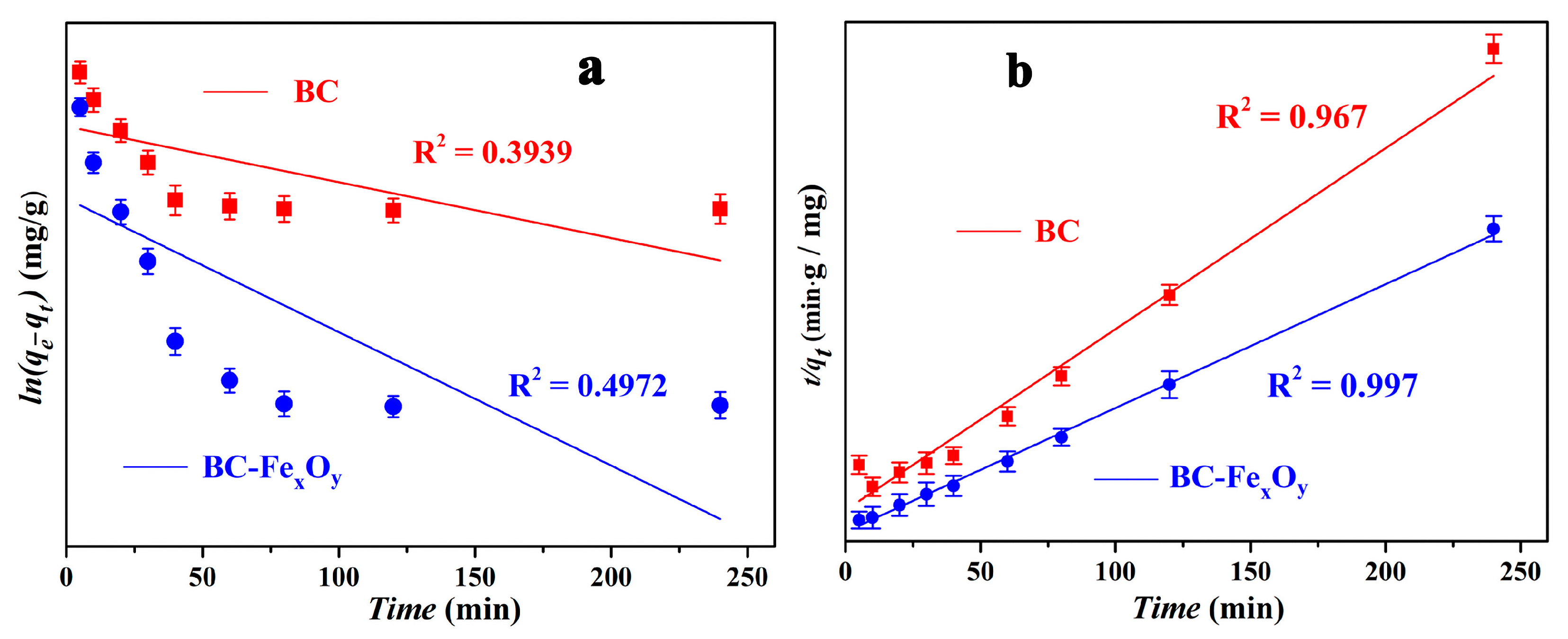
3.4. Exploration of Adsorption Kinetics
3.5. Exploration of Adsorption Isotherm
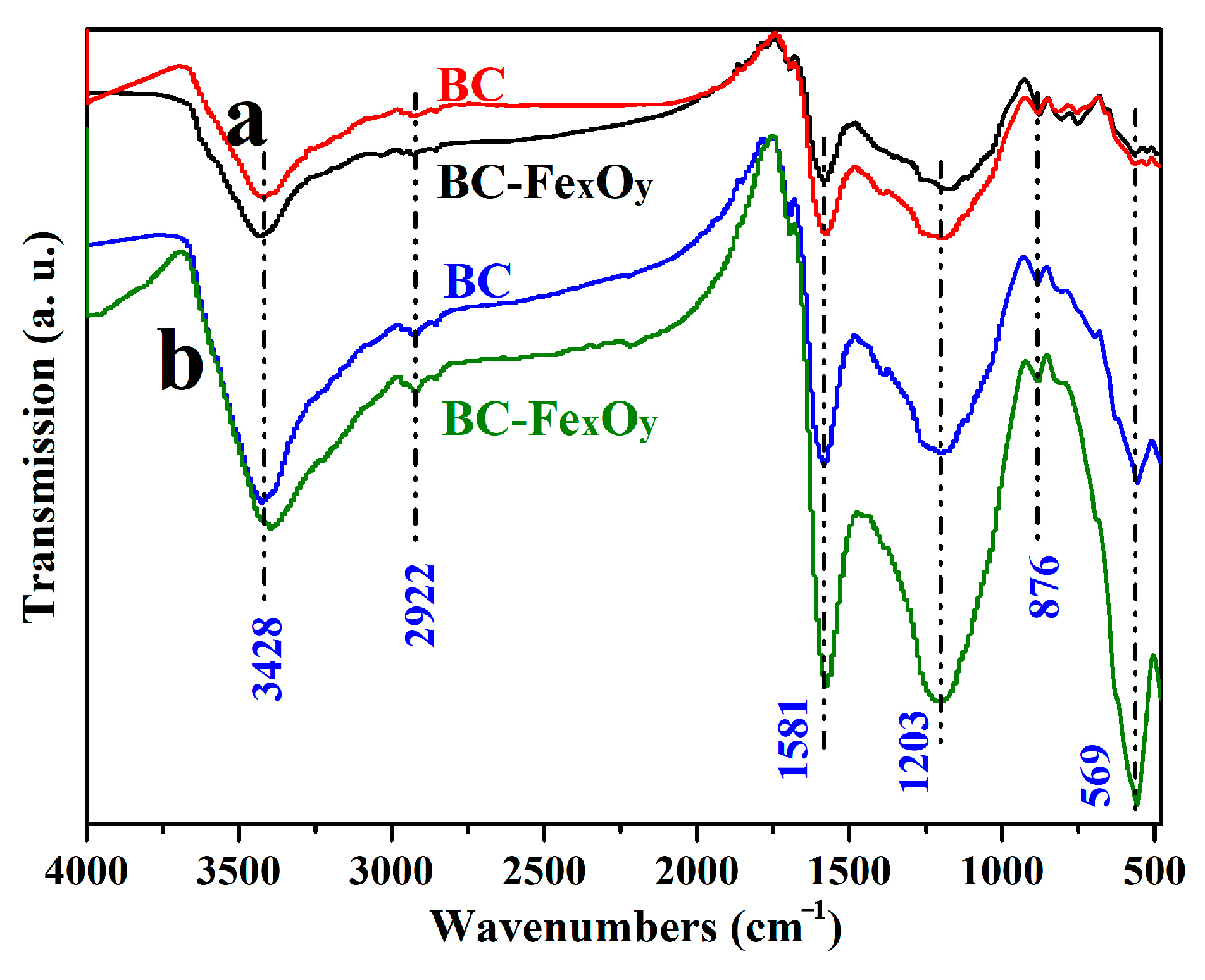
3.6. Characterization of FT-IR Spectroscopy
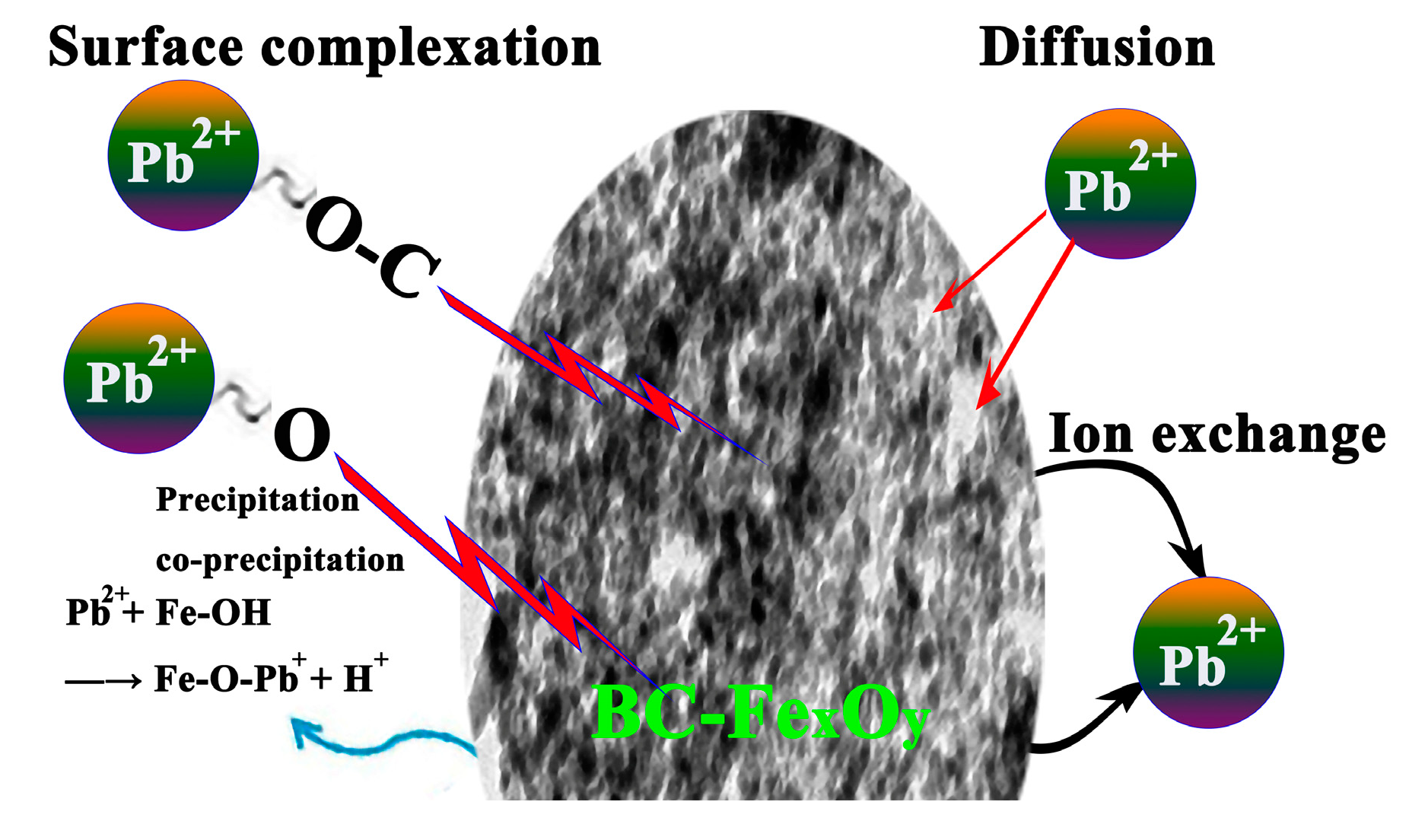
3.7. Absorption Mechanism
- (1)
- During physical adsorption, lead ions diffused on the surface of the composite material. As they move, these lead ions are gradually adsorbed into the micropores of the composite material and effectively fixed. This process utilizes the adsorption capacity of the composite material micropores to achieve the effective removal of lead ions.
- (2)
- The oxygen-containing functional groups that are abundant in biochar play a crucial role. These functional groups have unique chemical properties and can undergo a series of physical and chemical reactions, such as complexation and ion exchange, with heavy metal ions. Specifically, they form stable complexes with heavy metal ions through chelation, effectively binding these ions. Through ion exchange, the ions in the functional groups are replaced with heavy metal ions, further promoting the removal of heavy metal ions. Through the abovementioned mechanism, Pb2+ was effectively removed from wastewater.
- (3)
- The embedding of FexOy nanoparticles into biochar not only increased the specific surface area and porosity of the composite, improving the adsorption efficiency, but also removed Pb2+ through precipitation/co-precipitation by interacting with the hydroxyl functional groups (Fe-OH) on the surface of Fe3O4 [56].
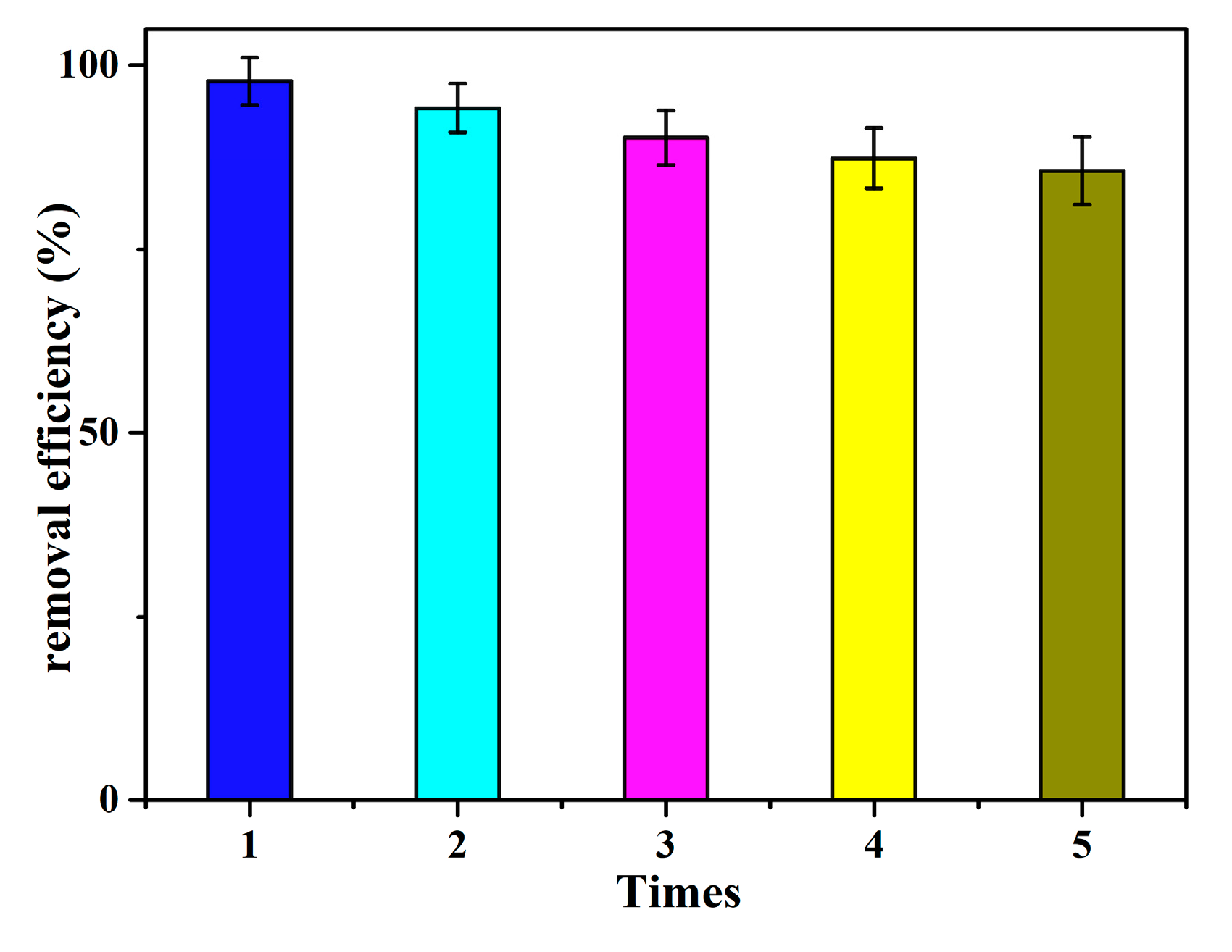
3.8. Reusability Needs to Be Evaluated
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Junuzovic, H.; Selimovic, A.; Begic, S.; Dozic, A.; Cvrk, R.; Ahmetovic, M.; Alibasic, H. Chemical precipitation of cations from aqueous solutions using waste sludge from the solway process as a potential agent. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 329–337. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Sci. Total Environ. 2019, 674, 355–362. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Elimelech, M.; Dykstra, J.E. Tutorial review of reverse osmosis and electrodialysis. J. Membr. Sci. 2022, 647, 120221. [Google Scholar]
- Kumar, A.; Nidheesh, P.V.; Kumar, M.S. Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere 2018, 205, 587–593. [Google Scholar] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar]
- Ezugwu, C.I.; Ujam, O.T.; Ukoha, P.O.; Ukwueze, N.N. Complex formation and extraction studies of N, N-Bis(salicylidene)-3,5-diaminobenzoic Acid on Hg(II) and Ag(I). Chem. Sci. Trans. 2013, 2, 1118–1125. [Google Scholar]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; AlKahtani, A.; Alharthi, F.A.; Aouak, T. Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Neskoromnaya, E.A.; Melezhik, A.V.; Babkin, A.V.; Kulnitskiy, B.A.; Burakov, A.E.; Burakova, I.V.; Tkachev, A.G.; Almalki, A.S.A.; Alsubaie, A. Magnetically active nanocomposite aerogels: Preparation, characterization and application for water treatment. J. Porous Mat. 2022, 29, 5450–5457. [Google Scholar]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar]
- Zhao, P.; Jian, M.; Zhang, Q.; Xu, R.; Liu, R.; Zhang, X.; Liu, H. A new paradigm of ultrathin 2D nanomaterial adsorbents in aqueous media: Graphene and GO, MoS2, MXenes, and 2D MOFs. J. Mater. Chem. A 2019, 7, 16598–16621. [Google Scholar]
- Huang, L.; Liu, R.; Yang, J.; Shuai, Q.; Yuliarto, B.; Kaneti, Y.V.; Yamauchi, Y. Nanoarchitectured porous organic polymers and their environmental applications for removal of toxic metal ions. Chem. Eng. J. 2021, 408, 127991. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Ren, X.; Wang, S.; Lu, L. Inorganic layered ion-exchangers for decontamination of toxic metal ions in aquatic systems. J. Mater. Chem. A. 2017, 5, 19593–19606. [Google Scholar]
- Dong, S.; Xu, W.; Wu, F.; Yan, C.; Li, D.; Jia, H. Fe-modified biochar improving transformation of arsenic form in soil and inhibiting its absorption of plant. Trans. Chin. Soc. Agric. Eng. 2016, 32, 204–212. [Google Scholar]
- Kumar, S.; Masto, R.E.; Ram, L.C.; Sarkar, P.; George, J.; Selvi, V.A. Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 2013, 55, 67–72. [Google Scholar]
- Dinesh, M.; Kumar, H.; Sarswat, A. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis biochars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar]
- Ahmad, S.; Liu, X.; Tang, J.; Zhang, S. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants. Chem. Eng. J. 2022, 431, 133187. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar]
- Li, X.; Qin, Y.; Jia, Y.; Li, Y.; Zhao, Y.; Pan, Y.; Sun, J. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: A review. Chemosphere 2021, 274, 129766. [Google Scholar]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar-based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process Eng. 2021, 41, 101993. [Google Scholar]
- Zhang, J.-Y.; Zhou, H.; Gu, J.-F.; Huang, F.; Yang, W.-J.; Wang, S.-L.; Yuan, T.-Y.; Liao, B.-H. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cdaccumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar]
- Peng, J.; Zhang, Z.Y.; Wang, Z.W.; Zhou, F.; Yu, J.X.; Chi, R.; Xiao, C.Q. Adsorption of Pb2+ in solution by phosphate-solubilizing microbially modified biochar loaded with Fe3O4. J. Taiwan Inst. Chem. E 2024, 156, 105363. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscipl. Toxicol. 2015, 8, 55–64. [Google Scholar]
- Zhang, M.; Jia, F.F.; Dai, M.; Son, S.X. Combined electrosorption and chemisorption of low concentration Pb(II) from aqueous solutions with molybdenum disulfifide as electrode. Appl. Surf. Sci. 2018, 455, 258–266. [Google Scholar]
- Chen, J.; Liao, C.; Guo, X.X.; Hou, S.C.; He, W.D. PAAO cryogels from amidoximated P(acrylic acid-co-acrylonitrile) for the adsorption of lead ion. Eur. Polym. J. 2022, 171, 111192. [Google Scholar]
- Nie, G.Z.; Qiu, S.J.; Wang, X.; Du, Y.; Zhang, Q.R.; Zhang, Y.; Zhang, H.L. A millimeter-sized negatively charged polymer embedded with molybdenum disulfide nanosheets for efficient removal of Pb(II) from aqueous solution. Chin. Chem. Lett. 2021, 32, 2342–2346. [Google Scholar]
- Zhu, Q.L.; Cao, M.; Zhang, X.B.; Tao, K.; Ke, Y.C.; Meng, L. Physicochemical and Infrared Spectroscopic Properties of Gramineae Plants Biochar at Different Pyrolysis Temperatures. Biomass Chem. Eng. 2021, 55, 21–28. [Google Scholar]
- Yao, Y.-Z.; Shi, Y.-J.; Hu, K.-H. Preparation of Molybdenum Disulfide with Different Nanostructures and Its Adsorption Performance for Copper (II) Ion in Water. Nanomaterials 2023, 13, 1194. [Google Scholar]
- Shi, C.J.; Wang, Y.; Zhang, K.; Lichtfouse, E.; Li, C.; Zhang, Y.H. Fe-biochar as a safe and efficient catalyst to activate peracetic acid for the removal of the acid orange dye from water. Chemosphere 2022, 307, 135686. [Google Scholar] [PubMed]
- Ye, Z.; Qie, Y.; Fan, Z.; Liu, Y.; Shi, Z.; Yang, H. Soft magnetic Fe5C2-Fe3C@C as an electrocatalyst for the hydrogen evolution reaction. Dalton T 2019, 48, 4636–4642. [Google Scholar]
- Chen, B.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. 2D sandwich-like carboncoated ultrathin TiO2@defect-rich MoS2 hybrid nanosheets: Synergistic-effectpromoted electrochemical performance for lithium ion batteries. Nano Energy 2016, 26, 541–549. [Google Scholar] [CrossRef]
- Gao, R.; Fu, Q.; Hu, H.; Wang, Q.; Liu, Y.; Zhu, J. Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J. Hazard. Mater. 2019, 371, 191–197. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Zhao, J.; Liu, H.; Zhang, X.; Zhang, H.; Zhu, H.; Peng, Y.; Wang, B. Nitrogendoped graphene oxide aerogel anchored with spinel CoFe2O4 nanoparticles for rapid degradation of tetracycline. Sep. Purif. Technol. 2020, 241, 116690. [Google Scholar] [CrossRef]
- Cheng, P.; Li, T.; Yu, H.; Zhi, L.; Liu, Z.H.; Lei, Z. Biomass-derived carbon fiber aerogel as binder-free electrode for high-rate supercapacitor. J. Phys. Chem. C 2016, 120, 2079–2086. [Google Scholar] [CrossRef]
- Qin, Y.; Li, X.; Wang, L.; Luo, J.; Li, Y.; Yao, C.; Xiao, Z.; Zhai, S.; An, Q. Valuable cobalt/biochar with enriched surface oxygen-containing groups prepared from bio-waste shrimp shell for efficient peroxymonosulfate activation. Sep. Purif. Technol. 2022, 281, 119901. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties ofiron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Lai, L.; Xie, Q.; Fang, W.K.; Xing, M.C.; Wu, D.Y. Removal and recycle of phosphor from water using magnetic core /shell structured Fe3O4@SiO2 nanoparticles functionalized with hydrous aluminum oxide. Environ. Sci. 2016, 37, 1444–1450. [Google Scholar]
- Wang, Y.; Yu, S.; Yuan, H.W.; Zhang, L. Constructing N,S co-doped network biochar confined CoFe2O4 magnetic nanoparticles adsorbent: Insights into the synergistic and competitive adsorption of Pb2+ and ciprofloxacin. Environ. Pollut. 2024, 343, 123178. [Google Scholar] [CrossRef]
- Hu, X.Y.; Chen, Y.J.; Zhang, S.H.; Wang, X.Q.; Li, C.C.; Guo, X. Cd removal from aqueous solution using magnetic biochar derived from maize straw and its recycle. Trans. Chin. Soc. Agric. Eng. 2018, 34, 208–218. [Google Scholar]
- Guo, W.; Ai, Y.; Men, B.; Wang, S. Adsorption of phenanthrene and pyrene by biochar produced from the excess sludge: Experimental studies and theoretical analysis. Int. J. Environ. Sci. Technol. 2017, 14, 1889–1896. [Google Scholar]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Adsorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis loaded on biochars derived from different stock materials. Ecotoxicol. Environ. Saf. 2018, 148, 285–292. [Google Scholar]
- Pei, H.N.; Wang, J.; Yang, Q.F.; Yang, W.X.; Hu, N.; Suo, Y.R.; Zhang, D.H.; Li, Z.H.; Wang, J.L. Interfacial growth of nitrogen-doped carbon with multi-functional groups on the MoS2 skeleton for efficient Pb(II) removal. Sci. Total Environ. 2018, 632, 912–920. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total. Environ. 2020, 746, 141037. [Google Scholar]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar]
- Dewage, N.B.; Fowler, R.E.; Pittman, C.U.; Mohan, D.; Mlsna, T. Lead (Pb2+) sorptive removal using chitosan-modified biochar: Batch and fixed-bed studies. RSC Adv. 2018, 8, 25368–25377. [Google Scholar]
- Li, Y.C.; Yin, H.; Cai, Y.H.; Luo, H.Y.; Yan, C.Y.; Dang, Z. Regulating the exposed crystal facets of α-Fe2O3 to promote Fe2O3-modified biochar performance in heavy metals adsorption. Chemosphere 2023, 311, 136976. [Google Scholar]
- Hamadeen, H.M.; Elkhatib, E.A.; Moharem, M.L. Optimization and mechanisms of rapid adsorptive removal of chromium (VI) from wastewater using industrial waste derived nanoparticles. Sci. Rep. 2022, 12, 14174. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Aboukila, E. Ecofriendly nanoparticles derived from water industry byproducts for effective removal of Cu (II) from wastewater: Adsorption isotherms and kinetics. Inorg. Chem. Commun. 2022, 146, 110062. [Google Scholar]
- Liu, X.; Lai, D.; Wang, Y. Performance of Pb(II) removal by an activated carbon supported nanoscale zero-valent iron composite at ultralow iron content. J. Hazard. Mater. 2019, 361, 37–48. [Google Scholar]
- Jin, M.L.J.H.; Deng, C.; Wang, S. Preparation and characterization of nanoparticles containing Fe3O4 cores in biochar. J. Agro-Environ. Sci. 2018, 37, 592–597. [Google Scholar]
- Dong, X.W.; He, L.Z.; Hu, H.; Liu, N.; Gao, S.; Piao, Y.X. Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem. Eng. J. 2018, 352, 371–379. [Google Scholar]
- Feng, Z.; Chen, N.; Feng, C.; Gao, Y. Mechanisms of Cr (VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@LSBC) using mass-balance and functional group expressions. Colloid Surface A 2018, 551, 17–24. [Google Scholar]
- Zhao, Y.; Zhang, R.; Liu, H.; Li, M.; Chen, T.; Chen, D.; Zou, X.; Frost, R.L. Green preparation of magnetic biochar for the effective accumulation of Pb (II): Performance and mechanism. Chem. Eng. J. 2019, 375, 122011. [Google Scholar]
- Wei, S.X.; Li, C.H.; Ma, W.Q.; Zhang, L.Y.; Li, J.M.; Mao, A.Q.; He, C.; Li, M.H.; Zhu, W.C. Study on the removal mechanism of Zn(II) and Pb(II) by magnetic flake nZVI-Fe3O4. Chin. J. Process Eng. 2024, 24, 346–359. (In Chinese) [Google Scholar]
- Kataria, N.; Garg, V.K. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 208, 818–828. [Google Scholar]

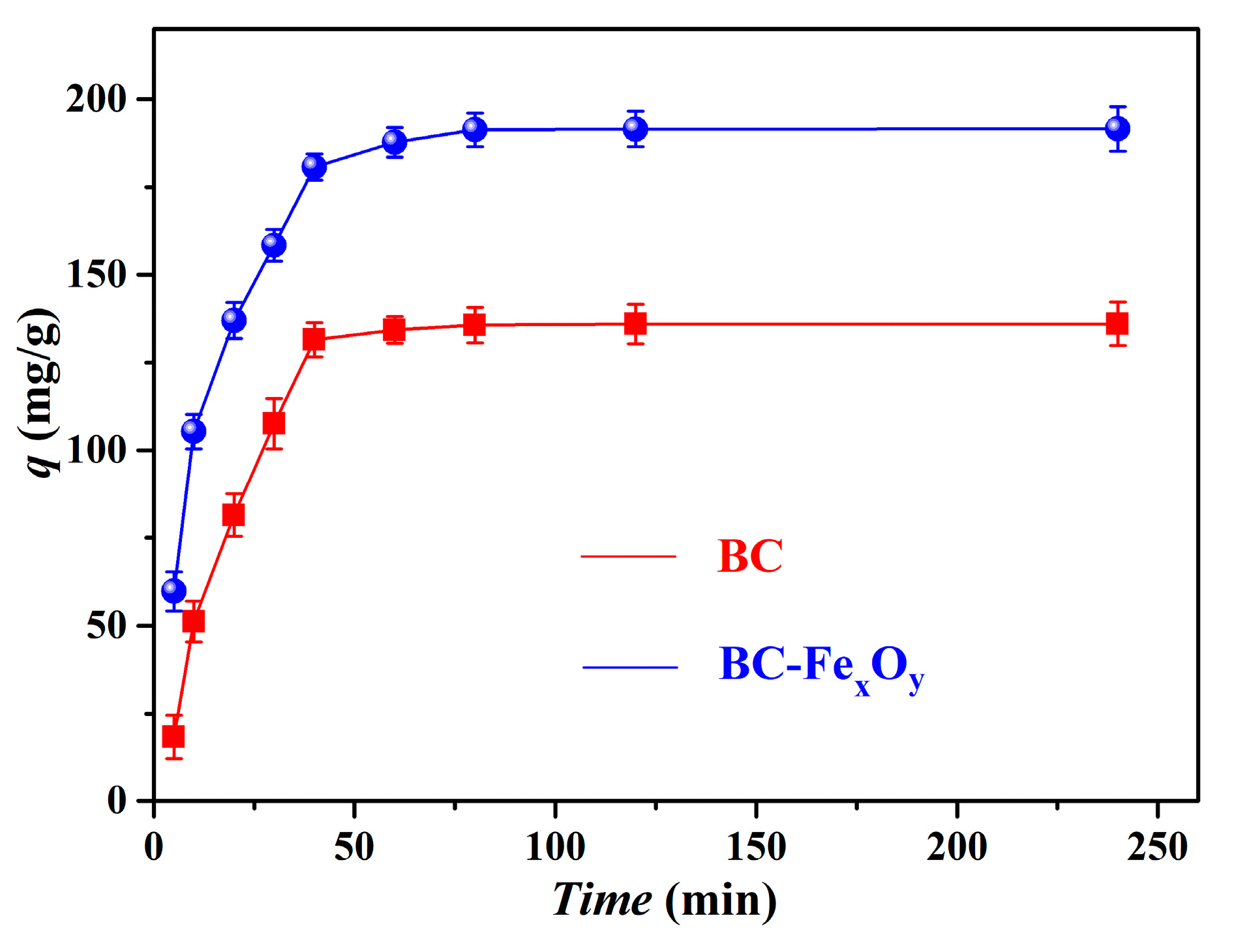

| Materials | Magnetization Strength (emu/g) | Specific Surface (m2/g) | Pore Volume (m3/g) |
|---|---|---|---|
| BC | - | 219 | 0.126 |
| BC-FexOy | 21.6 | 326 | 0.232 |
| Pseudo-First-Order | Intercept | Slope | Statistics | ||
|---|---|---|---|---|---|
| Value | Standard Error | Value | Standard Error | Adj. R2 | |
| BC-FexOy | 4.39251 | 0.27467 | −0.00919 | 0.00308 | 0.4972 |
| BC | 4.88914 | 0.13414 | −0.00384 | 0.00154 | 0.39394 |
| Pseudo-Second-Order | Intercept | Slope | Statistics | ||
|---|---|---|---|---|---|
| Value | Standard Error | Value | Standard Error | Adj. R2 | |
| BC-FexOy | 0.01116 | 0.00242 | 0.00148 | 2.8696 × 10−5 | 0.99699 |
| BC | 0.03695 | 0.01103 | 0.00216 | 1.408 × 10−4 | 0.96704 |
| Materials | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | qm (mg/g) | R2 | n | KF | R2 | |
| Biochar | 0.57 | 149.8 | 0.9935 | 2.72 | 52.29 | 0.6266 |
| Biochar–FexOy | 0.69 | 252.7 | 0.9963 | 2.89 | 112.3 | 0.9063 |
| Biochar Materials | Adsorption Capacity (mg/g) | Refs. |
|---|---|---|
| Microbial modification of iron-carrying biochar | 113.70 | [22] |
| Alginate-modified rice husk biochar | 112.30 | [29] |
| Acid ammonium persulfate oxidize biochar | 135.40 | [44] |
| PSB-modified sludge biochar | 12.17 | [45] |
| H3PO4-modified chicken feather biochar | 24.41 | [46] |
| Chitosan-modified pine biochar | 134.00 | [47] |
| α-Fe2O3-modified biochar (Fe2O3/BC) | 390.60 | [48] |
| Biochar embedded FexOy nanoparticle composite | 252.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Yao, T.; Qian, C. Synthesis and Application for Pb2+ Removal of a Novel Magnetic Biochar Embedded with FexOy Nanoparticles. Symmetry 2025, 17, 516. https://doi.org/10.3390/sym17040516
Yao Y, Yao T, Qian C. Synthesis and Application for Pb2+ Removal of a Novel Magnetic Biochar Embedded with FexOy Nanoparticles. Symmetry. 2025; 17(4):516. https://doi.org/10.3390/sym17040516
Chicago/Turabian StyleYao, Youzhi, Tiancheng Yao, and Cheng Qian. 2025. "Synthesis and Application for Pb2+ Removal of a Novel Magnetic Biochar Embedded with FexOy Nanoparticles" Symmetry 17, no. 4: 516. https://doi.org/10.3390/sym17040516
APA StyleYao, Y., Yao, T., & Qian, C. (2025). Synthesis and Application for Pb2+ Removal of a Novel Magnetic Biochar Embedded with FexOy Nanoparticles. Symmetry, 17(4), 516. https://doi.org/10.3390/sym17040516






