Solvatochromic Analysis of Triton X-100 in Binary Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectral Measurements
2.3. Theoretical Models
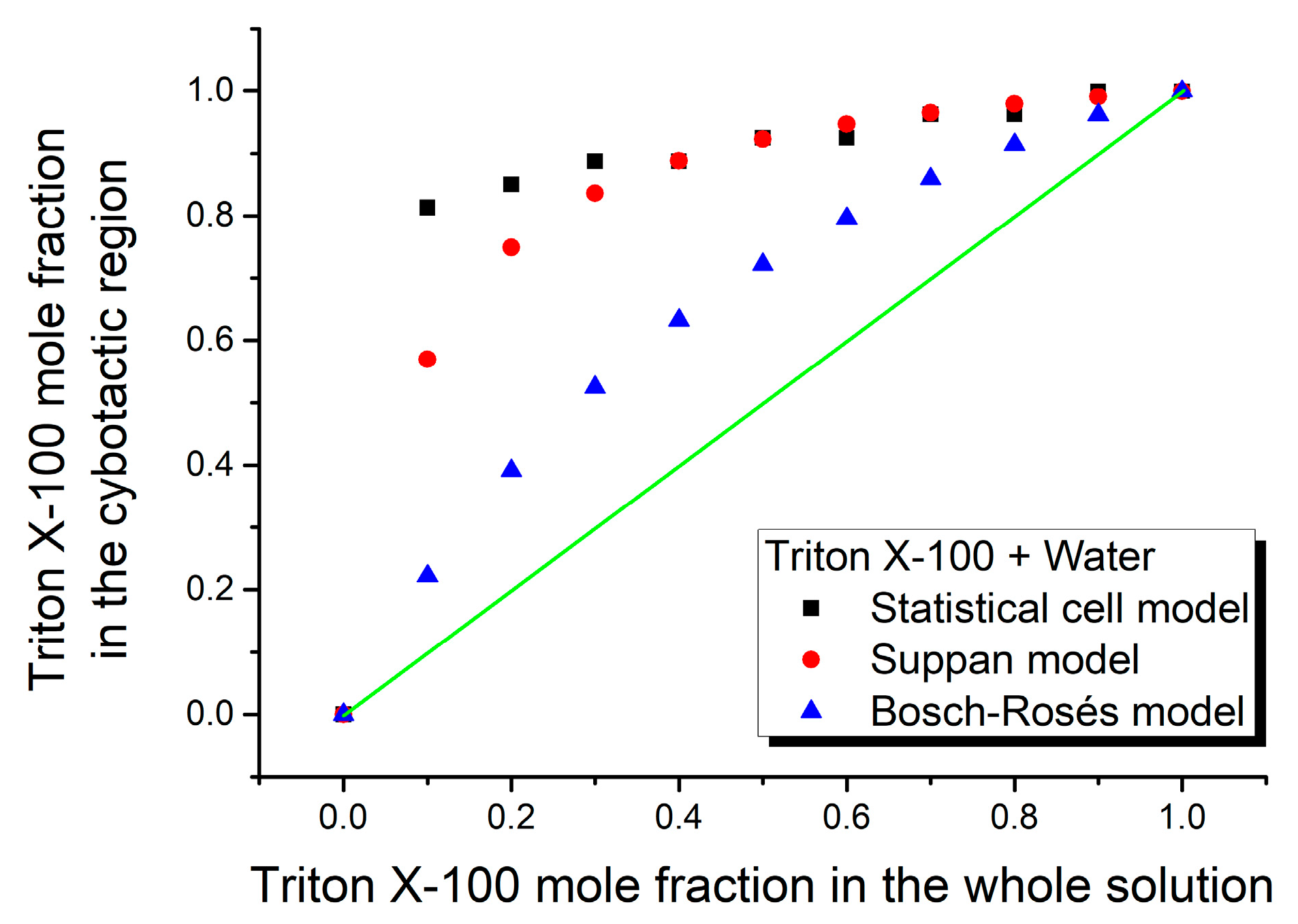
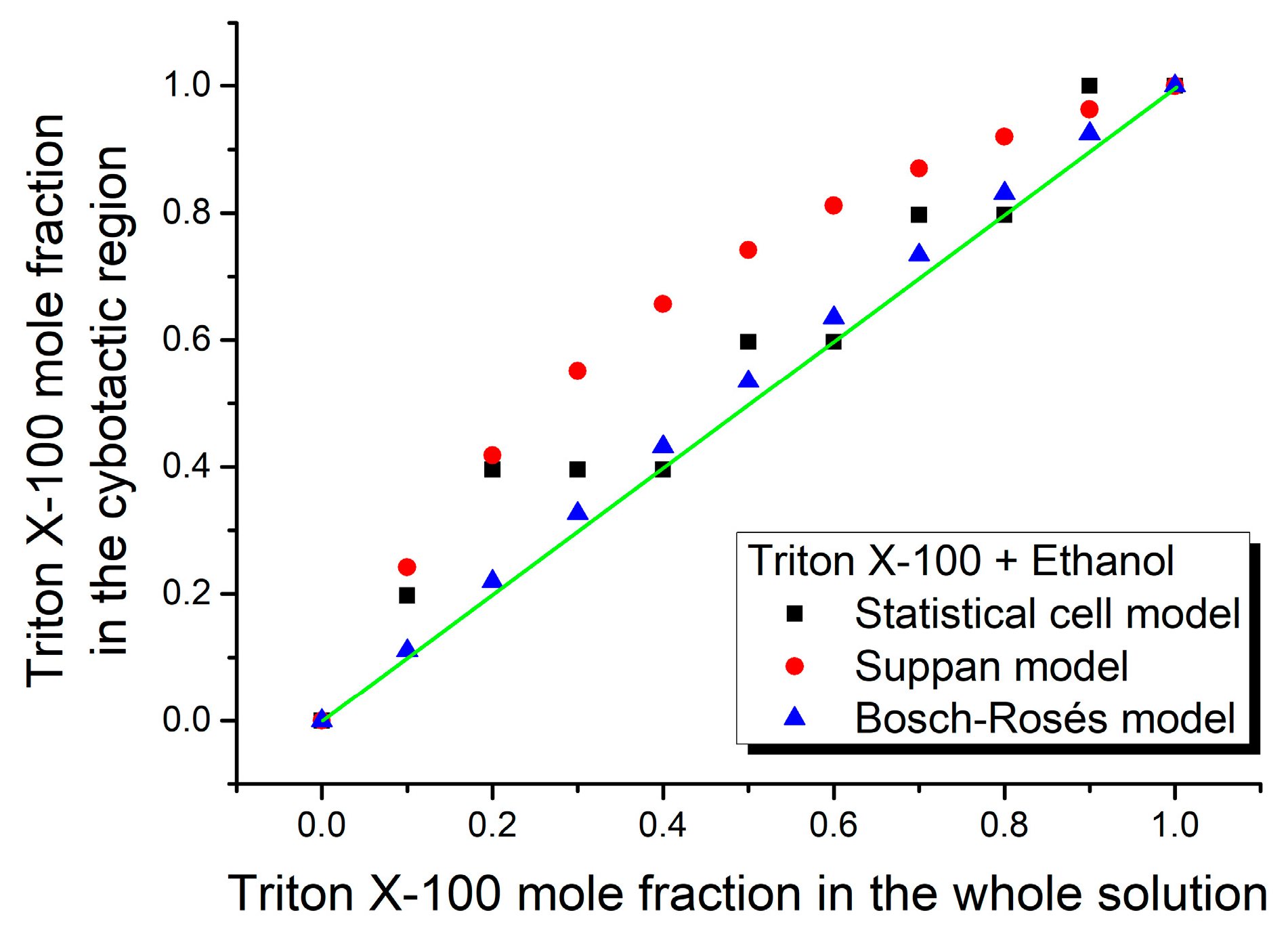
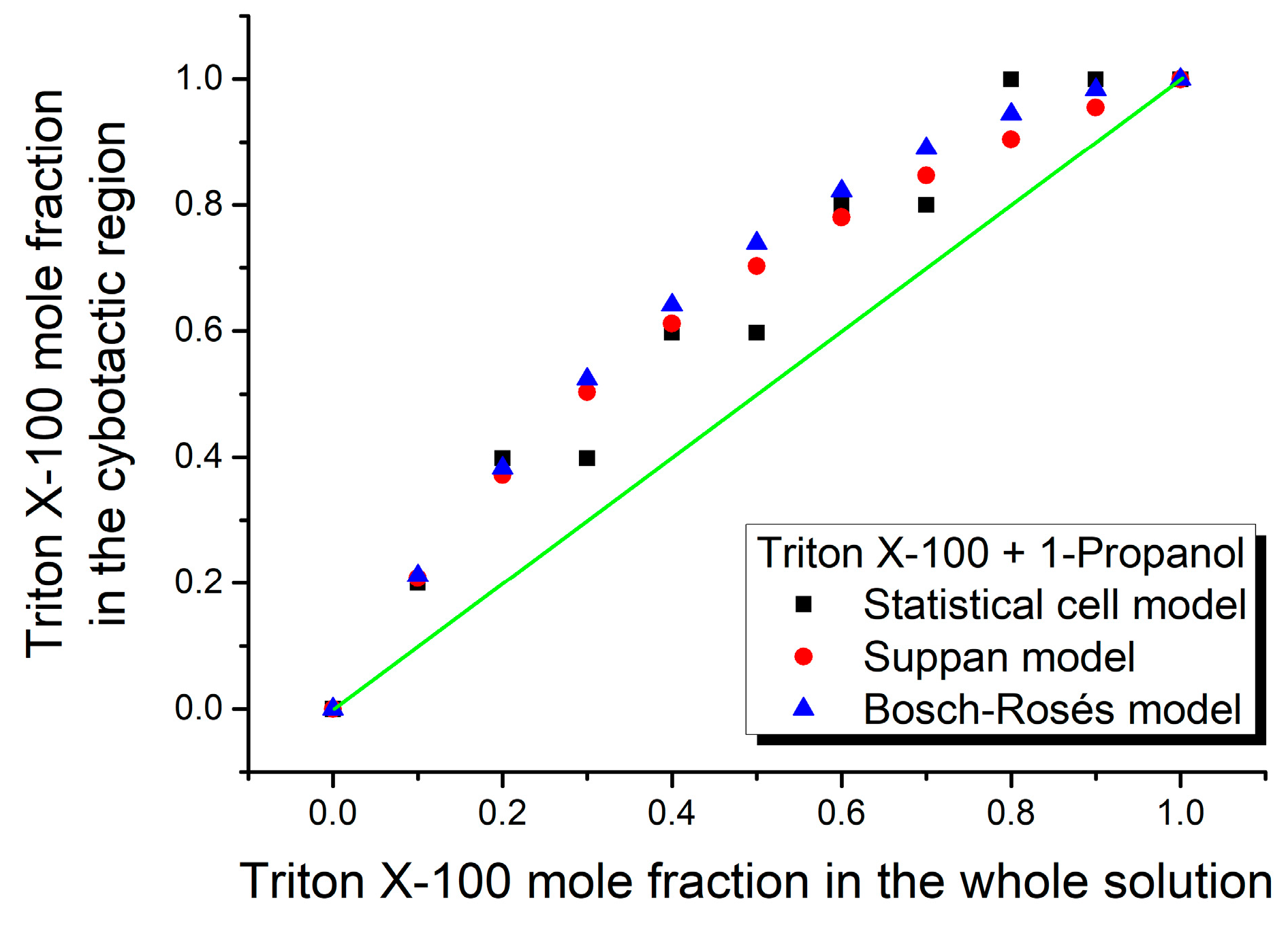
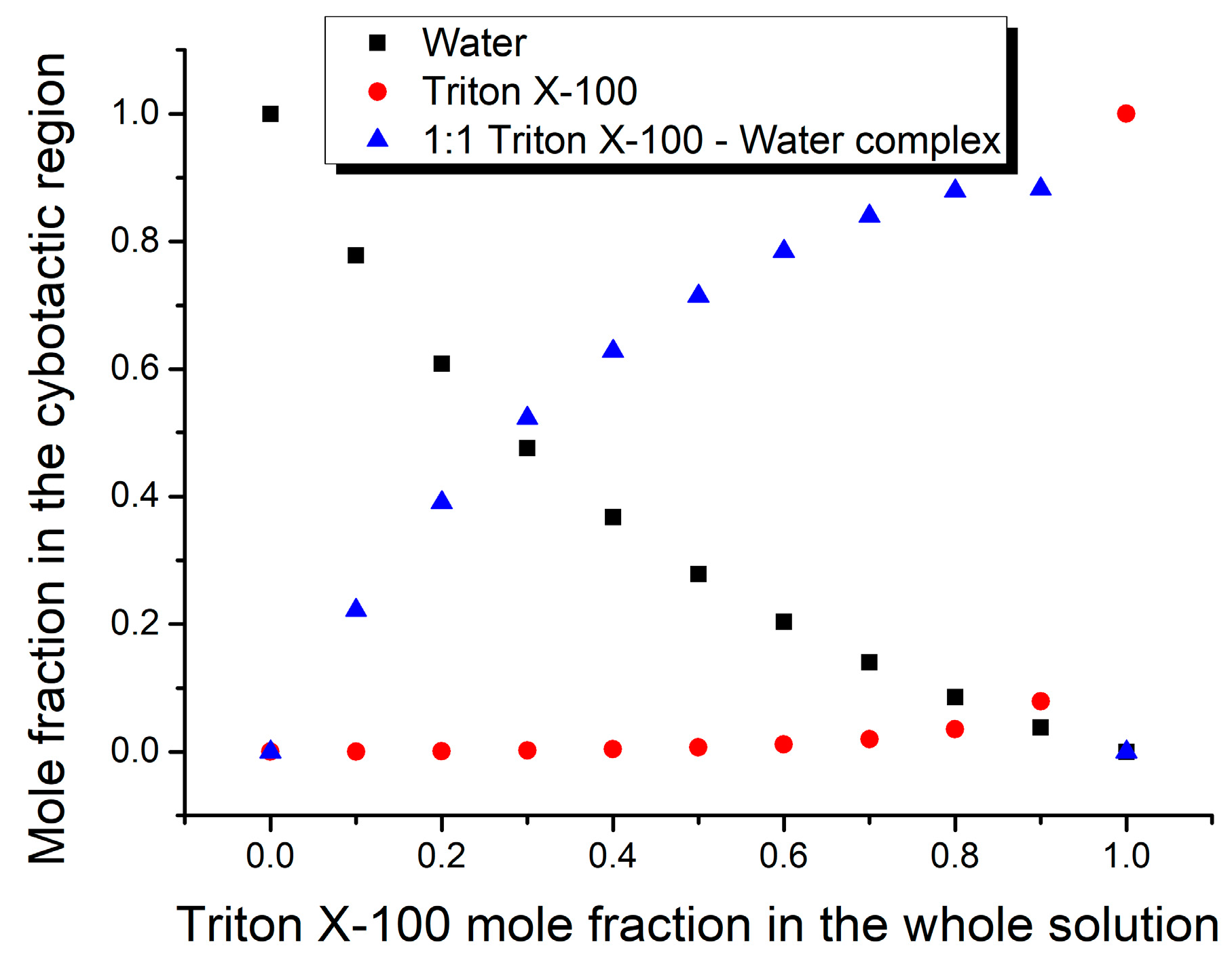
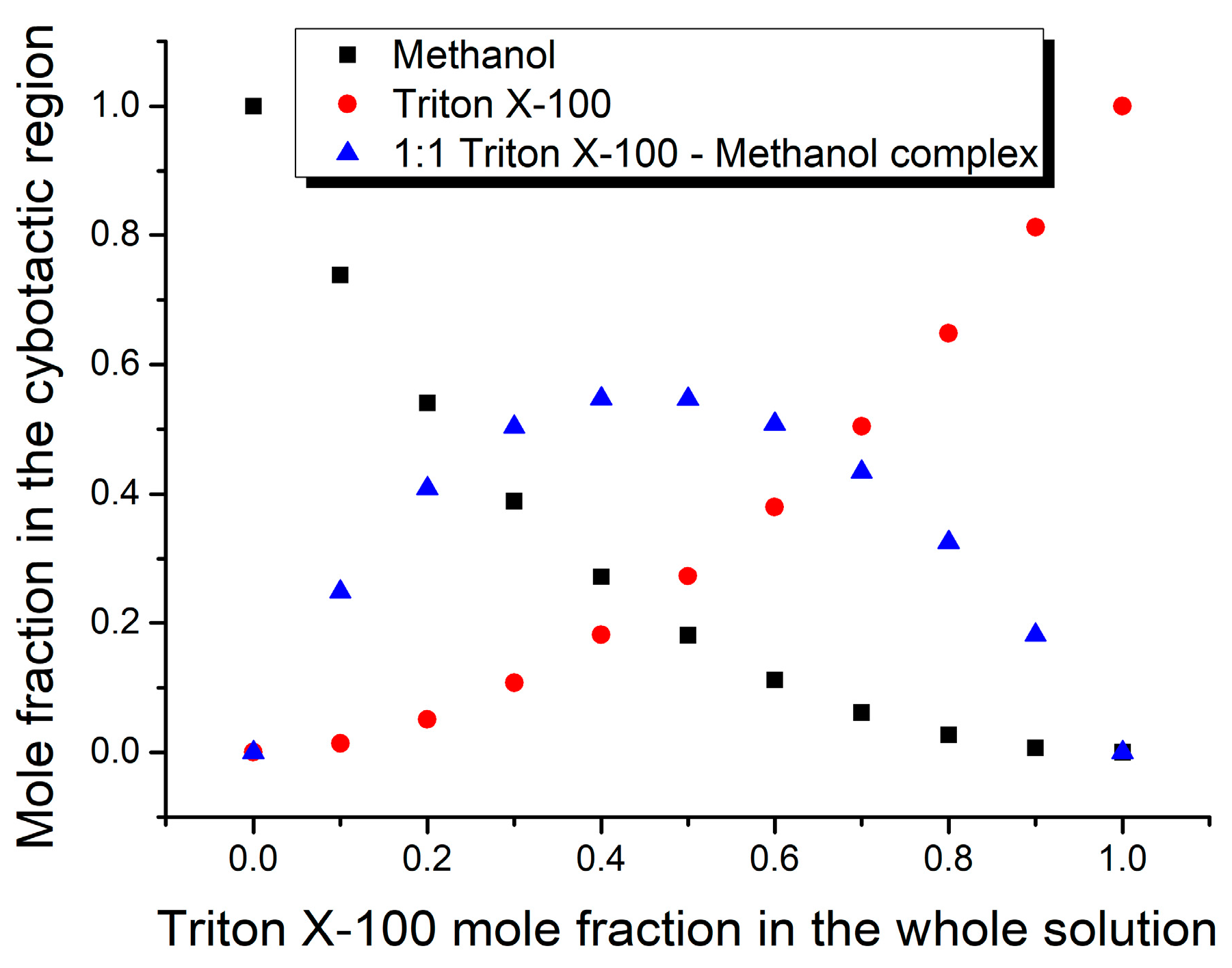
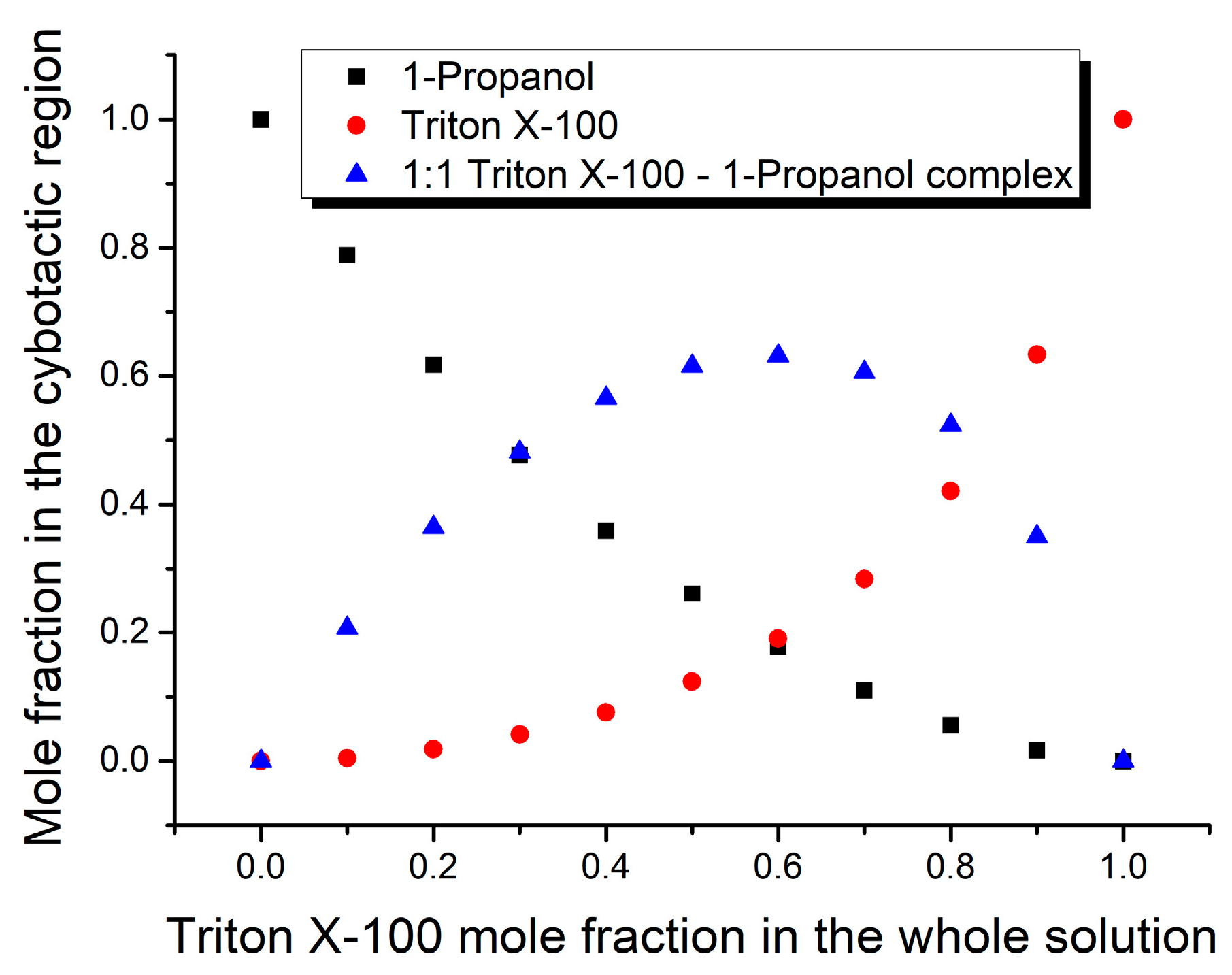
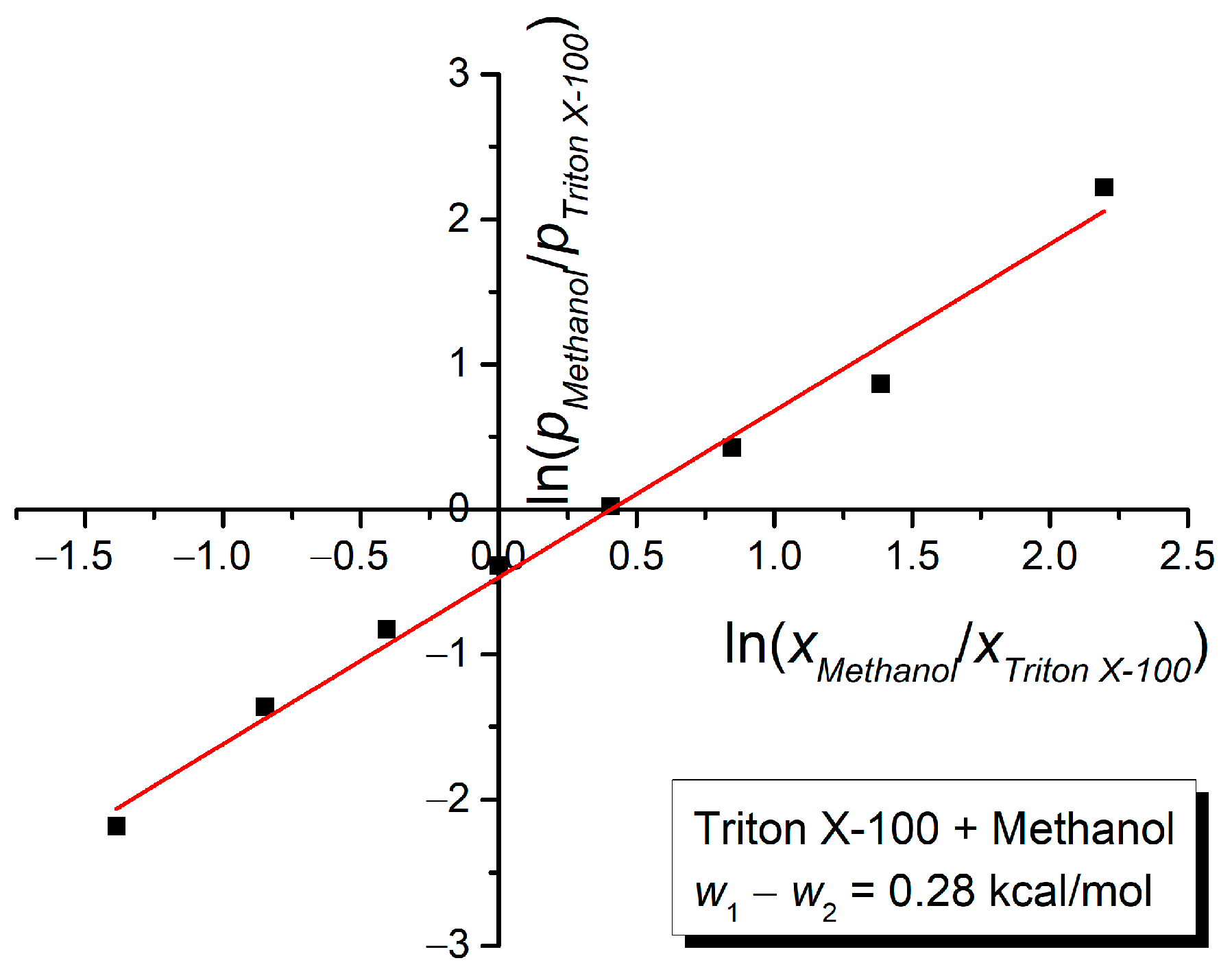
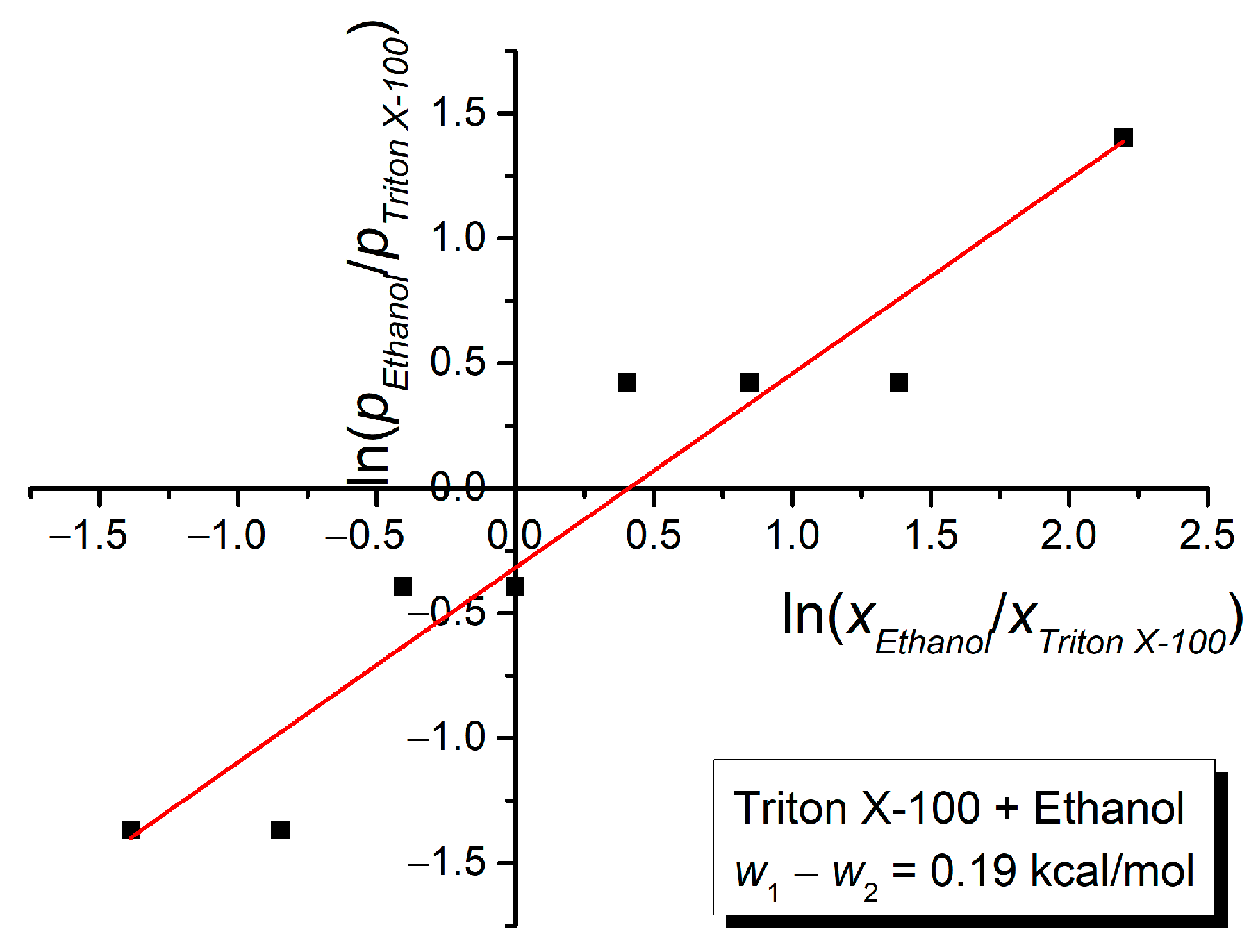
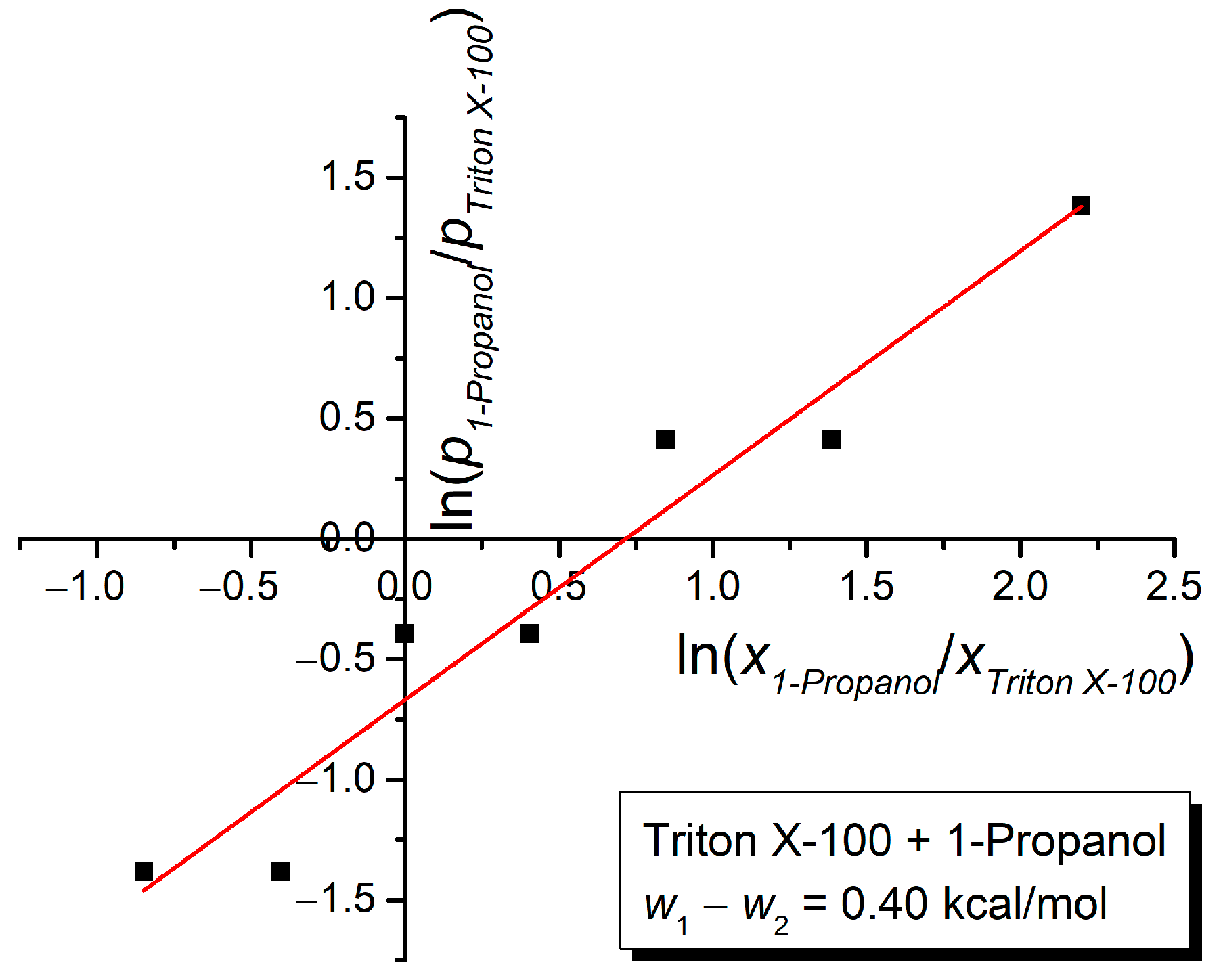
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zana, R. Aqueous surfactant-alcohol systems: A review. Adv. Colloid Interface Sci. 1995, 57, 1–64. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, L.; Xu, J.; Cheng, K.; Wu, B. Two-photon and three photon fluorescence of Triton X-100 in the ultraviolet region. J. Fluoresc. 2021, 31, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Fedyaeva, O.A.; Poshelyuzhnaya, E.G. Optical activity of Triton X-100 micelles in aqueous solutions. Russ. J. Phys. Chem A 2020, 94, 1957–1958. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Wang, K.; Gu, J.; Xiong, Z.; Zhang, W.; Chen, Y. In situ isolation of nuclei or nuclear proteins from adherent cells: A simple, effective method with less cytoplasmic contamination. Biol. Res. 2023, 56, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Su, C.; Hu, S.; Chen, Y.; Shu, Y.; Yue, D.; Zhang, B.; Qi, Z.; Li, S.; Wang, X.; et al. The effect of residual Triton X-100 on structural stability and infection activity of adenovirus particles. Mol. Ther. Methods Clin. Dev. 2020, 19, 35–46. [Google Scholar] [CrossRef]
- Shahraki, S.; Bideskan, A.E.; Aslzare, M.; Tavakkoli, M.; Bahrami, A.R.; Hosseinian, S.; Matin, M.M.; Rad, A.K. Decellularization with Triton X-100 provides a suitable model for human kidney bioengineering using human mesenchymal stem cells. Life Sci. 2022, 295, 120167. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhao, X.; Su, W.; Tang, X. Triton X-100 modified ATP-responsive siRNA delivery agent for antitumor therapy. Mol. Pharm. 2021, 17, 3696–3708. [Google Scholar] [CrossRef]
- Mavani, A.; Ray, D.; Aswal, V.K.; Bhattacharyya, J. Application of drug aggregation to solubilize antimicrobial compound and enhancing its bioavailability. Appl. Biochem. Biotechnol. 2023, 195, 3206–3216. [Google Scholar] [CrossRef]
- Maya, S.A.; Shumon, M.A.H.; Islam, M.R.; Khan, J.M.; Khan, S.A.; Rana, S.; Hoque, M.A.; Rahman, M.M. Investigation of the impact of several diols on the phase behavior and physico-chemical quantities associated with the Triton X-100 and antidiabetic drug mixture. J. Indian Chem. Soc. 2024, 101, 101295. [Google Scholar] [CrossRef]
- Siposova, K.; Sedlak, E.; Kozar, T.; Nemergut, M.; Musatov, A. Dual effect of non-ionic detergent Triton X-100 on insulin amyloid formation. Colloids Surf. B Biointerfaces 2019, 173, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Samir, R.; Amin, H.M. Production of highly immunogenic and safe Triton X-100 produced bacterial ghost vaccine against Shigella flexneri 2b serotype. Gut Pathog. 2023, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Colavita, F.; Quartu, S.; Lalle, E.; Bordi, L.; Lapa, D.; Meschi, S.; Vulcano, A.; Toffoletti, A.; Bordi, E.; Paglia, M.G.; et al. Evaluation of inactivation effect of Triton X-100 on Ebola virus infectivity. J. Clin. Virol. 2017, 86, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.; Rho, J.-E.R.; Yoon, H.-J.; Park, P.S.; Rho, T.-H.D.; Park, J.Y.; Park, L.; Kim, Y.-H.; Lee, J.H. Role of Triton X-100 in chemiluminescent enzyme immunoassays capable of diagnosing genetic disorders. Talanta 2013, 116, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Unver, Y.; Yildiz, S.; Acar, M. Extracellular production of azurin from Pseudomonas aeruginosa in the presence of Triton X-100 or Tween 80. Bioprocess Biosyst. Eng. 2022, 45, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Roy, S.; Pandey, P.; Mohanty, S.; Tandon, R.; Ghosh, S. Macrophage plasticity and differentiation on the decellularized human cornea. J. Mater. Res. 2023, 38, 4625–4640. [Google Scholar] [CrossRef]
- Asgari, F.; Asgari, H.R.; Najafi, M.; Eftekhari, B.S.; Vardiani, M.; Gholipourmalekabadi, M.; Koruji, M. Optimization of decellularized human placental macroporous scaffolds for spermatogonial stem cells homing. J. Mater. Sci. Mater. Med. 2021, 32, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, D.; Zhang, J.; Lin, Q.; Huang, Z. Synthesis of organized hydroxyapatite (HA) using triton X-100. Ceram. Int. 2010, 36, 2441–2447. [Google Scholar] [CrossRef]
- Liu, P.; Luo, Y.; Liu, R.; Fan, W.; Fan, B. Triton X-100 enhanced antibacterial effect of photodynamic therapy against Enterococcus faecalis infection: An in vitro study. Colloids Surf. B Biointerfaces 2024, 240, 113978. [Google Scholar] [CrossRef]
- Muthusamy, P.; Antony, S.A.; Palani, G.; Saravanan, D.; Chithambaram, V. Synergistic in vitro antimicrobial activity of caffeine/AgNPs-triton X-100. Appl. Phys. A 2023, 129, 611. [Google Scholar] [CrossRef]
- Duan, M.; Sun, Q.; Fan, W.; Fan, B. Enhanced antibacterial effect against Enterococcus faecalis by silver ions plus Triton X-100 with low concentrations and cytotoxicity. Braz. J. Microbiol. 2022, 53, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Rajabathar, J.R.; Arunachalam, P.; Al-Lohedan, H.A.; Thankappan, R.; Appaturi, J.N.; Pulingam, T.; Dahan, W.M. Polymer surfactant (Triton-100) assisted low cost method for preparing silver and graphene oxide modified Bi-MnOx nanocomposite for enhanced sensor and anti-microbial health care applications. J. Sol-Gel Sci. Technol. 2021, 97, 638–650. [Google Scholar] [CrossRef]
- Hristozova, A.D.; Simitchiev, K.K.; Kmetov, V.J.; Rosenberg, E. Compatibility of cloud point extraction with gas chromatography: Matrix effects of Triton X-100 on GC-MS and GC-MS/MS analysis of organochlorine and organophosphorus pesticides. Talanta 2024, 269, 125445. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, O.I.; Cherozhuk, T.V.; Kravchenko, O.A.; Baklanov, O.M. Determination of Zn, Mn, and Cd in strata water. J. Water Chem. Technol. 2022, 44, 26–30. [Google Scholar] [CrossRef]
- Yu, Q.; Mou, X.; Guo, L.; Chen, Z.; Lin, R.; Ding, Y. Triton X-100-directed synthesis of carbon nitride and nitrogen-doped carbon for ethylene dichloride dehydrochlorination. Carbon 2022, 196, 110–119. [Google Scholar] [CrossRef]
- Reddy, N.B.; Sundar, C.S.; Rani, C.R.; Rao, K.U.M.; Nayak, S.K.; Reddy, C.S. Triton X-100 catalyzed synthesis of α-aminophosphonates. Arab. J. Chem. 2016, 9, S685–S690. [Google Scholar] [CrossRef]
- Babbar, N.; Sharma, G.; Arya, S.K. Effective degradation of chicken feather waste by keratinase enzyme with Triton X-100 additive. Biocatal. Agric. Biotechnol. 2022, 44, 102447. [Google Scholar] [CrossRef]
- Wang, G.-L.; Din, A.U.; Qiu, Y.-S.; Wang, C.-L.; Wang, D.-H.; Wei, G.-Y. Triton X-100 improves co-production of β-1,3-D-glucan and pullulan by Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 2020, 104, 10685–10696. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.H.T.; Kim, J.; Lee, C.-H.; Ryou, C. Non-ionic detergents Nonidet P-40 and Triton X-100 increase enzymatic activity of plasmin. Biochem. Biophys. Res. Commun. 2019, 512, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zou, C.; Wu, J. Triton X-100 enhances the solubility and secretion ratio of aggregation-prone pullulanase produced in Escherichia coli. Bioresour. Technol. 2015, 194, 137–143. [Google Scholar] [CrossRef]
- Zheng, K.; Xia, W.; Wang, R.; Li, Y.; Zhang, W. Synergistic effect of Triton X-100 and kerosene on the flotation removal of unburned carbon from fly ash. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126668. [Google Scholar] [CrossRef]
- de Oliveira, A.E.; Ahuiar, M.L.; Guerra, V.G. Improved filter media with PVA/citric acid/Triton X-100 nanofibers for filtration of nanoparticles from air. Polym. Bull. 2021, 78, 6387–6408. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, Y.; Qu, J.; Zhang, Y. Remediation of PAHs-contaminated soil by coupling Triton X-100 assisted washing and BiOBr/g-C3N4 photocatalytic process: Insights to the degradation mechanism and soil washing recycling. Chem. Eng. J. 2024, 497, 154285. [Google Scholar] [CrossRef]
- Shen, W.; Xu, J.; Zhu, L. Triton X-100 improves the reactivity and selectivity of sulfidized nanoscale zerovalent iron toward tetrabromobisphenol A: Implications for groundwater and soil remediation. J. Hazard. Mater. 2021, 416, 126119. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Huang, H.; Yi, F.; Wang, M.; Cheng, C.W. Inhibitory effect of calcium carbonate precipitation induced by Triton X-100 and microorganisms on coal dust. Constr. Build. Mater. 2024, 444, 137887. [Google Scholar] [CrossRef]
- Mahdi, W.A.; Hussain, A.; Bukhari, S.I.; Alshehri, S.; Singh, B.; Ali, N. Removal of clarithromycin from aqueous solution using water/Triton X-100/ethanol/olive oil green nanoemulsion method. J. Water Process Eng. 2021, 40, 101973. [Google Scholar] [CrossRef]
- Steevensz, A.; Madur, S.; Feng, W.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Crude soybean hull peroxidase treatment of phenol in synthetic and real wastewater: Enzyme economy enhanced by Triton X-100. Enzyme Microb. Technol. 2014, 55, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Benderrag, A.; Haddou, B.; Daaou, M.; Benkhedja, H.; Bounaceur, B.; Kameche, M. Experimental and modeling studies on Cd (II) ions extraction by emulsion liquid membrane using Triton X-100 as biodegradable surfactant. J. Environ. Chem. Eng. 2019, 7, 103166. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Buddee, S.; Wongnawa, S. Triton X-100 induced cuboid-like BiVO4 microsphere with high photocatalytic performance. J. Environ. Sci. 2017, 55, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, X.; Ai, L.; Fan, H. Triton X-100-assisted synthesis of layered nanosheet-assembled flower-like BiOBr nanostructures with enhanced visible-light photocatalytic degradation of ciprofloxacin. J. Nanopart. Res. 2022, 24, 103. [Google Scholar] [CrossRef]
- Cong, R.P.; Guo, Y.H.; Zhou, J.Q.; Wang, J.W. Effect of Triton X-100 on Shiraia laccase production and its application in triclosan biodegradation. Sustain. Chem. Pharm. 2023, 35, 101209. [Google Scholar] [CrossRef]
- Dave, B.P.; Ghevariya, C.M.; Bhatt, J.K.; Dudhagara, D.R.; Rajpara, R.K. Enhanced biodegradation of total polycyclic aromatic hydrocarbons (TPAHs) by marine halotolerant Achromobacter xylosoxidans using Triton X-100 and β-cyclodextrin—A microcosm approach. Mar. Pollut. Bull. 2014, 79, 123–129. [Google Scholar] [CrossRef]
- Soares, M.E.; Araujo, A.J.; Silva, F.S.; Silva, M.R.A.; Barbosa, N.V. Regeneration of transformer oil using a microemulsion with Triton X-100. Braz. J. Chem. Eng. 2023, 40, 901–911. [Google Scholar] [CrossRef]
- Sardar, R.H.; Bera, A.; Chattopadhyay, S.; Mahato, J.C.; Sarraf, S.; Basu, A.K. Effect of dopants in the HTL layer on photovoltaic properties in hybrid perovskite solar cells. J. Mater. Sci. Mater. Electron. 2023, 34, 2138. [Google Scholar] [CrossRef]
- Rahman, M.F.; Hossain, J.; Kuddus, A.; Moon, M.M.A.; Ismail, A.B.M. Effect of Triton X-100 surfactant on thiol-amine cosolvents assisted facile synthesized CdS thin films on glass substrate by spin coating method. SN Appl. Sci. 2020, 2, 590. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Yoo, B.; Lim, M.K.; Lee, S.Y.; Kim, K.-J. Effect of Triton X-100 in water-added electrolytes on the performance of dye-sensitized solar cells. Electrochim. Acta 2009, 54, 6286–6291. [Google Scholar] [CrossRef]
- Yeoh, C.H.; Chua, C.L.; Woon, K.L. Effects of nanoscale surface modification and triplet energy shielding of a single layer solution processed blue phosphorescent organic light emitting diode by using Triton X-100. Synth. Met. 2013, 172, 44–48. [Google Scholar] [CrossRef]
- Shinde, S.K.; Fulari, V.J.; Kim, D.-Y.; Maile, N.C.; Koli, R.R.; Dhaygude, H.D.; Ghodake, G.S. Chemical synthesis of flower-like hybrid Cu(OH)2/CuO electrode: Application of polyvinyl alcohol and Triton X-100 to enhance supercapacitor performance. Colloids Surf. B Biointerfaces 2017, 156, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, S.M.B.; Wimalasena, I.G.K.J.; Keppetipola, N.M.; Karunarathne, B.C.; Madagedara, A.D.T.; Cojocaru, L.; Uchida, S.; Rajapakse, R.M.G.; Tennakone, K.; Yoshimura, M.; et al. Effect of Triton X-100 surfactant concentration on the wettability of polyethylene-based separators used in supercapacitors. J. Sci. Adv. Mater. Devices 2024, 9, 100801. [Google Scholar] [CrossRef]
- Tevi, T.; Birch, S.W.S.; Thomas, S.W.; Takshi, A. Effect of Triton X-100 on the double layer capacitance and conductivity of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) films. Synth. Met. 2014, 191, 59–65. [Google Scholar] [CrossRef]
- Iyyappan, E.; Wilson, P. Synthesis of nanoscale hydroxyapatite particles using Triton X-100 as an organic modifier. Ceram. Int. 2013, 39, 771–777. [Google Scholar] [CrossRef]
- Kimura, K.; Gibo, M.; Nerome, C.; Kura, T.; Ooshiro, S.; Tamaki, Y. Nanoparticle formation by laser ablation of perylene microcrystals in an aqueous solution of Triton X-100. Chem. Phys. Lett. 2018, 691, 271–275. [Google Scholar] [CrossRef]
- Ramakanth, I.; Kolenčík, M.; Rao, M.S.; Sunil, B.R.; Vijayasree, U.; Durgababu, G.; Devi, S.A.; Šebesta, M.; Siva, T. Tuning the morphology and state of aggregation of fullerene C60 using non-ionic surfactants. Colloid J. 2021, 83, 474–482. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G. Synthesis of Ca(OH)2 nanoparticles with the addition of Triton X-100. Protective treatments on natural stones: Preliminary results. J. Cult. Herit. 2012, 13, 40–46. [Google Scholar] [CrossRef]
- Memon, A.F.; Ameen, S.; Qambrani, N.; Buledi, J.A.; Khand, N.H.; Solangi, A.R.; Taqvi, S.I.H.; Karaman, C.; Karimi, F.; Afsharmanesh, E. An improved electrochemical sensor based on Triton X-100 functionalized SnO2 nanoparticles for ultrasensitive determination of cadmium. Chemosphere 2022, 300, 134634. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, G.; Song, L.; Kuang, G.; Ding, Y.; Bu, S.; Chai, Y.; Fu, Y. Ultra-sensitive electrochemiluminescence monitoring of microRNA via Triton X-100 functionalized carbon dots-based nanocomposites. Sens. Actuators B Chem. 2024, 405, 135314. [Google Scholar] [CrossRef]
- Fonseca, L.P.; Pedrini, L.F.K.; Lima, J.V.M.; Escaliante, L.C.; Santos, S.B.O.; Scalvi, L.V.A. Enhancement of surface properties of sol gel tin dioxide thin films with addition of surfactant in the precursor solution. Appl. Phys. A 2021, 127, 503. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Jiang, H.; Zhang, K.; Chang, K.; Jia, S.; Jiang, W.; Shang, Y.; Lu, W.; Deng, S.; et al. Polypyrrole nanoparticles fabricated via Triton X-100 micelles template approach and their acetone gas sensing property. Appl. Surf. Sci. 2013, 280, 212–218. [Google Scholar] [CrossRef]
- Mukdasai, K.; Mukdasai, S. The fabrication of in situ Triton X-100 on multi-walled carbon nanotubes modified gold electrode for sensitive determination of caffeine. Int. J. Electrochem. Sci. 2018, 13, 58–70. [Google Scholar] [CrossRef]
- Pandey, P.; Somers, A.E.; Hait, S.K.; Forsyth, M.; Ramakumar, S.S.V. Short chain imidazolium ionic liquids: Synthesis and oil miscibility in various base oil by use of surfactant as high performance friction and antiwear lubricant additive. Tribol. Lett. 2021, 69, 95. [Google Scholar] [CrossRef]
- Attia, A.; Abdel-Fatah, H.T.M. Triton X-100 as a non-ionic surfactant for corrosion inhibition of mild steel during acid cleaning. Met. Mater. Int. 2020, 26, 1715–1724. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; El-Housseiny, S.; Abd-El-Fatah, M.A. Improved corrosion resistance of permanganate-phosphate conversion coat on steel surface by surfactants. Sci. Rep. 2023, 13, 15781. [Google Scholar] [CrossRef] [PubMed]
- Rabaeh, K.A.; Basfar, A.A.; Moussa, A.A. Enhancement in sensitivity of nitro blue tetrazolium polyvinyl alcohol film dosimeters by sodium formate and Triton X-100. Radiat. Phys. Chem. 2012, 81, 479–483. [Google Scholar] [CrossRef]
- Maharaj, D.; Mohammed, T.; Mohammed, A.; Addison, L. Enhanced digestion of complex cosmetic matrices for analysis of As, Hg, Cd, Cr, Ni, and Pb using Triton X-100. MethodsX 2021, 8, 101241. [Google Scholar] [CrossRef]
- Robinson, J.E.; Sutton, C.M.; Reid, G.F. Dilute Triton X-100 in water as a reference liquid for hydrometer calibration using Cuckow’s method. Measurement 2014, 57, 132–137. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Principles and applications of solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- Dorohoi, D.O.; Creanga, D.E.; Dimitriu, D.G.; Morosanu, A.C.; Gritco-Todirascu, A.; Mariciuc, G.G.; Puica Melniciuc, N.; Ardelean, E.; Cheptea, C. Computational and spectral means for characterizing the intermolecular interactions in solutions and for estimating excited state dipole moment of solute. Symmetry 2020, 12, 1299. [Google Scholar] [CrossRef]
- Dorohoi, D.O.; Gosav, S.; Morosanu, A.C.; Dimitriu, D.G.; Apreotesei, G.; Gosav, T. Molecular descriptors—Spectral properties relations for characterizing molecular interactions in binary and ternary solutions, excited state dipole moment estimation. Symmetry 2023, 15, 2075. [Google Scholar] [CrossRef]
- Babusca, D.; Benchea, A.C.; Dimitriu, D.G.; Dorohoi, D.O. Solvatochromic characterization of Sudan derivatives in binary and ternary solutions. Anal. Lett. 2016, 49, 2615–2626. [Google Scholar] [CrossRef]
- Dulcescu-Oprea, M.M.; Morosanu, A.C.; Dimitriu, D.G.; Gritco-Todirascu, A.; Dorohoi, D.O.; Cheptea, C. Solvatochromic study of pyridinium-acetyl-benzoyl methylid (PABM) in ternary protic solutions. J. Mol. Struct. 2021, 1227, 129539. [Google Scholar] [CrossRef]
- Dorohoi, D.O.; Dimitriu, D.G.; Morosanu, A.C.; Puica Melniciuc, N.; Hurjui, I.; Miron, M.; Mariciuc, G.G.; Closca, V.; Cheptea, C. Some aryl-1,2,4-triazol-1-ium phenacylids in binary hydroxyl solvent mixtures. Computational and spectral study. Symmetry 2021, 13, 1656. [Google Scholar] [CrossRef]
- Pavel, C.M.; Ambrosi, E.; Dimitriu, D.G.; Dorohoi, D.O. Complex formation and microheterogeneity in water-alcohol binary mixtures investigated by solvatochromic study. Eur. Phys. J. Spec. Top. 2023, 232, 415–425. [Google Scholar] [CrossRef]
- Cheptea, C.; Zara, A.; Ambrosi, E.; Morosanu, A.C.; Diaconu, M.; Miron, M.; Dorohoi, D.O.; Dimitriu, D.G. On the solvatochromism of fluorescein sodium. Symmetry 2024, 16, 673. [Google Scholar] [CrossRef]
- Pop, V.; Dorohoi, D.O.; Delibas, M. Considerations on the statistical model of intermolecular interactions in ternary solutions. An. St. Univ. Al. I. Cuza s. Ib 1986, 32, 79–84. [Google Scholar]
- Suppan, P. Local polarity of solvent mixtures in the field of electronically excited molecules and exciplexes. J. Chem. Soc. Faraday Trans 1 1987, 83, 495–509. [Google Scholar] [CrossRef]
- Bosch, E.; Rosés, M. Relationships between ET polarity and composition in binary solvent mixtures. J. Chem. Soc. Faraday Trans. 1992, 88, 3541–3546. [Google Scholar] [CrossRef]
- Rosés, M.; Ràfols, C.; Ortega, J.; Bosch, E. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 1. A comparison of several preferential solvation models for describing ET(30) polarity of dipolar hydrogen bond acceptor-cosolvent mixtures. J. Chem. Soc. Perkin Trans. 2 1995, 1995, 1607–1615. [Google Scholar] [CrossRef]
- Bosch, E.; Rosés, M.; Herodes, K.; Koppel, I.; Leito, I.; Koppel, I.; Taal, V. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 2. Effect of temperature on the ET(30) polarity parameter of dipolar hydrogen bond acceptor-hydrogen bond donor mixtures. J. Phys. Org. Chem. 1996, 9, 403–410. [Google Scholar] [CrossRef]
- Ortega, J.; Ràfols, C.; Bosch, E.; Rosés, M. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 3. The ET(30) polarity of binary mixtures of hydroxylic solvents. J. Chem. Soc. Perkin Trans. 2 1996, 1996, 1497–1503. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Salari, H.; Fakhraee, M.; Gholami, M.R. Surfactant binary systems: Ab initio calculations, preferential solvation, and investigation of solvatochromic parameters. J. Chem. Eng. Data 2016, 61, 255–263. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Fakhraee, M.; Salari, H.; Gholami, M.R. Probing solvent-solvent and solute-solvent interactions in surfactant binary mixtures: Solvatochromic parameters, preferential solvation, and quantum theory of atoms in molecules analysis. RSC Adv. 2016, 6, 18515. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Taft, R.W. An examination of linear solvation energy relationships. In Progress in Physical Organic Chemistry; Taft, R.W., Ed.; Wiley Interscience: New York, NY, USA, 1981; Volume 13, pp. 485–630. [Google Scholar]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The π* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The α-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The β-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Sator, N.; Pavloff, N.; Couëdel, L. Statistical Physics; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2024; pp. 138–147. [Google Scholar]
- Van, S.-P.; Hammond, G.S. Amine quenching of aromatic fluorescence and fluorescent exciplexes. J. Am. Chem. Soc. 1978, 100, 3895–3902. [Google Scholar] [CrossRef]
- Papadakis, R. Preferential solvation of a highly medium responsive pentacyanoferrate (II) complex in binary solvent mixtures: Understanding the role of dielectric enrichment and the specificity of solute-solvent interactions. J. Phys. Chem. B 2016, 120, 9422–9433. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.N.; Rai, S.; Bhattarai, A.; Sinha, B. Interaction between methyl red and cetyltrimethylammonium bromide under the influence of sodium polystyrene sulphonate in ethanol-water binary solvent systems: A spectrophotometric investigation. Heliyon 2024, 10, e33014. [Google Scholar] [CrossRef] [PubMed]
- Minisy, I.M.; Bober, P.; Šeděnková, I.; Stejskal, J. Methyl red dye in the tuning of polypyrrole conductivity. Polymer 2020, 207, 122854. [Google Scholar] [CrossRef]
- Khadka, B.; Bhattarai, A. UV-Vis studies on interaction between sodium dioctylsulfosuccinate (AOT) and methyl red. Rev. Roum. Chim. 2020, 65, 989–996. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Liu, Q.; Chen, Y.-M.; Liu, Z.-Q.; Xu, C.-W. Determination of acid dissociation constant of methyl red by multi-peaks Gaussian fitting method based on UV-Visible absorption spectrum. Acta Phys. Chim. Sin. 2012, 28, 1030–1036. [Google Scholar] [CrossRef]
- Poblete, T.; Millán, D.; Rezende, M.C. Synergism in the solvation of solvatochromic probes in binary mixtures with ionic liquids. J. Mol. Liq. 2023, 391, 123227. [Google Scholar] [CrossRef]
- Fisher, R.A. On the interpretation of χ2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]


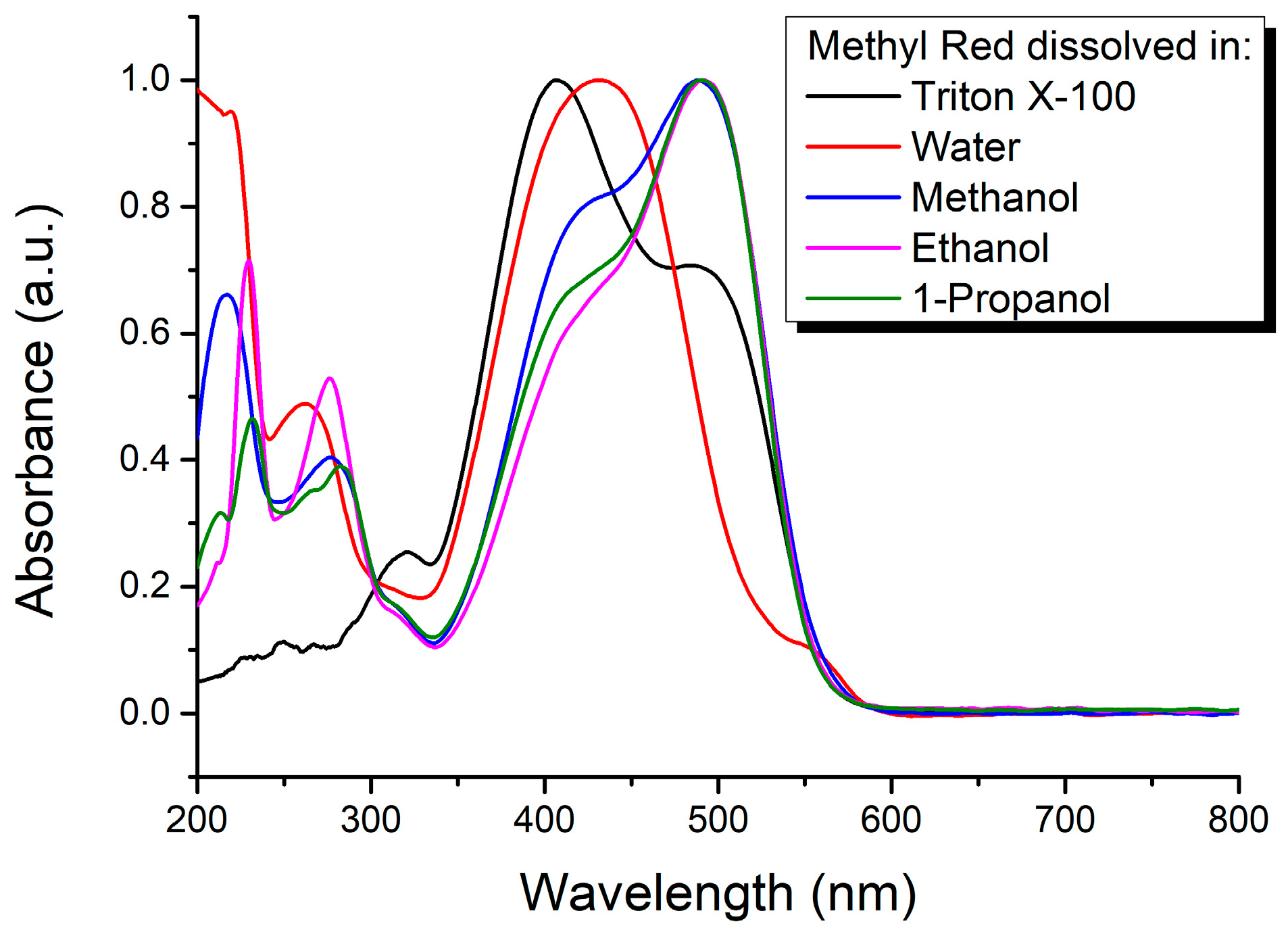



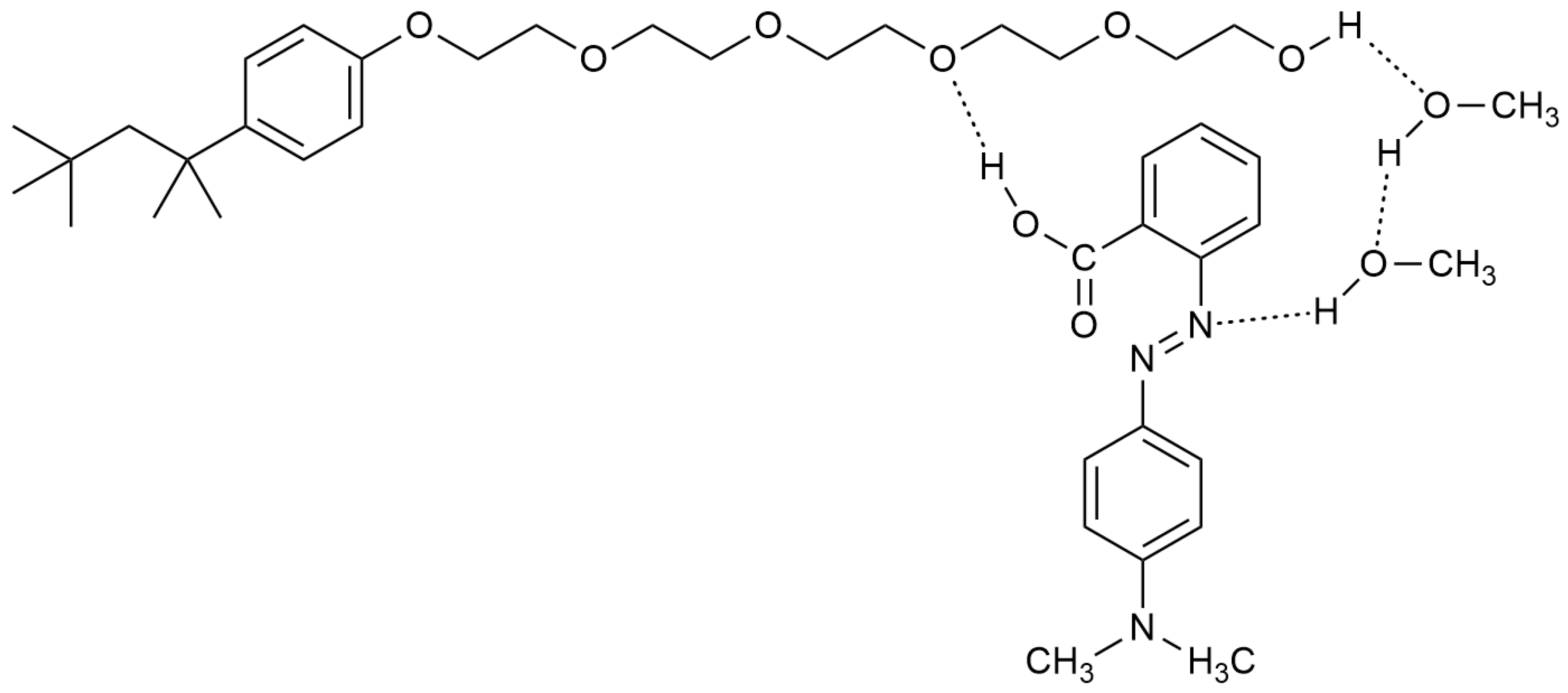


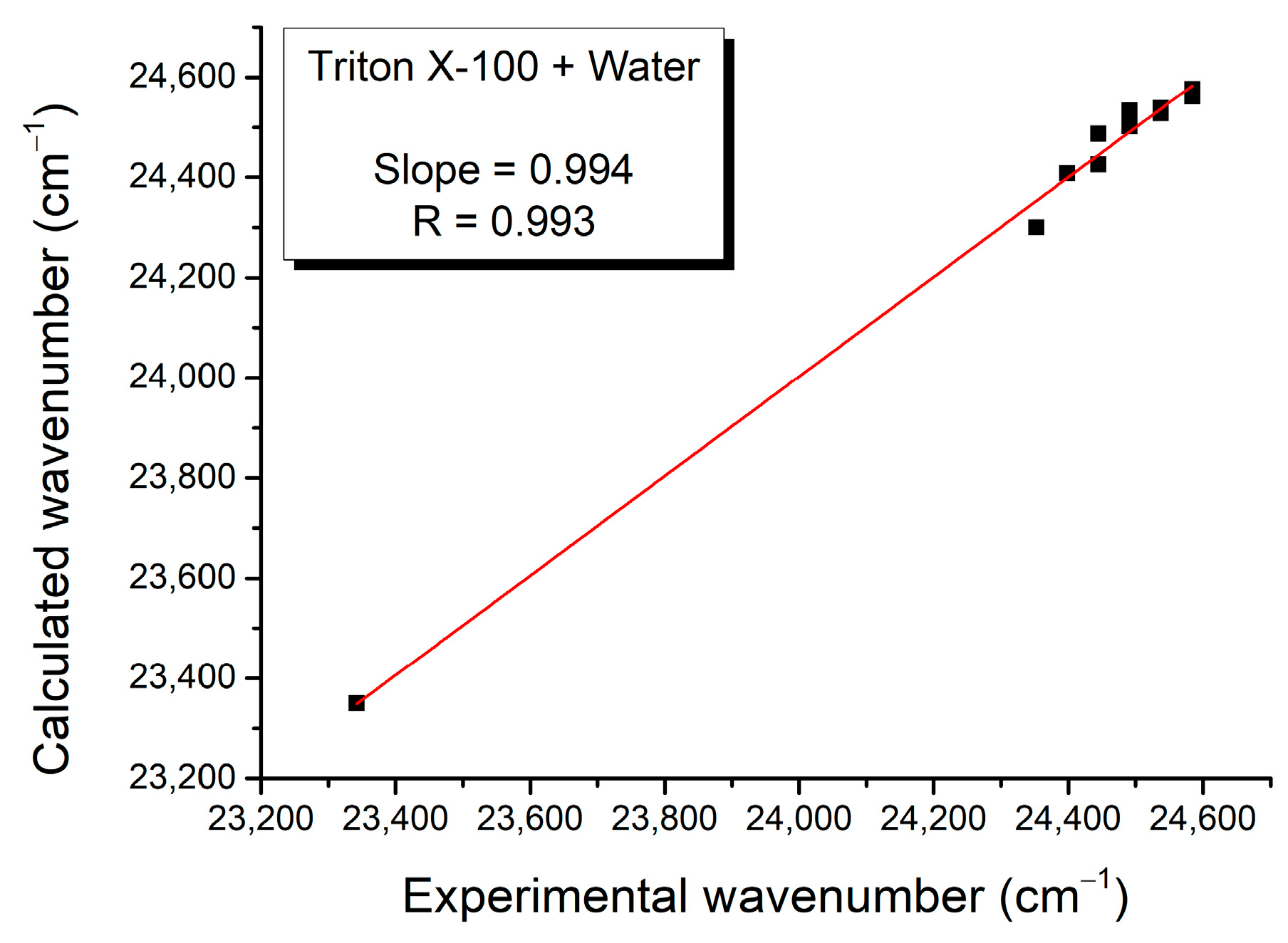
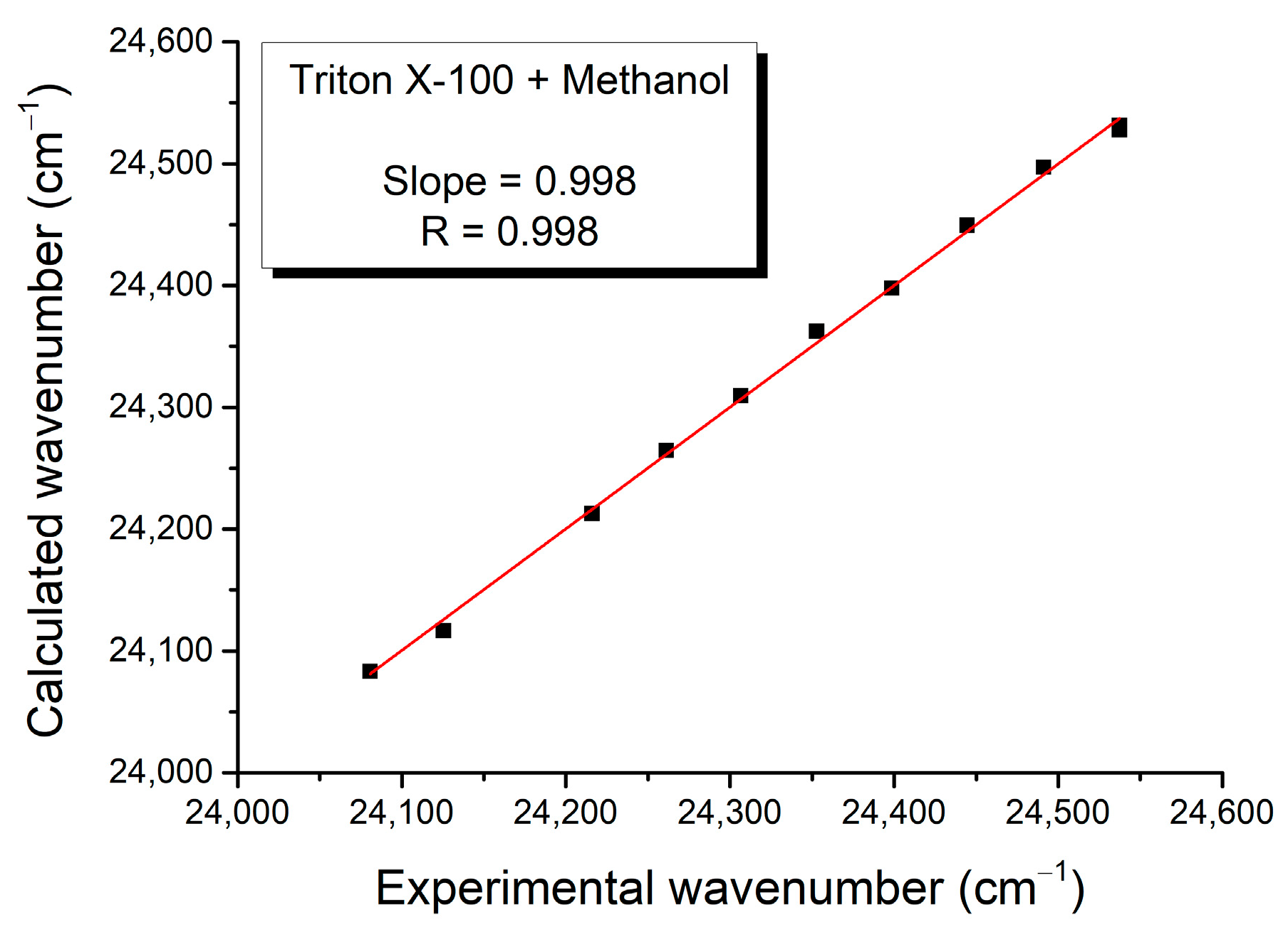
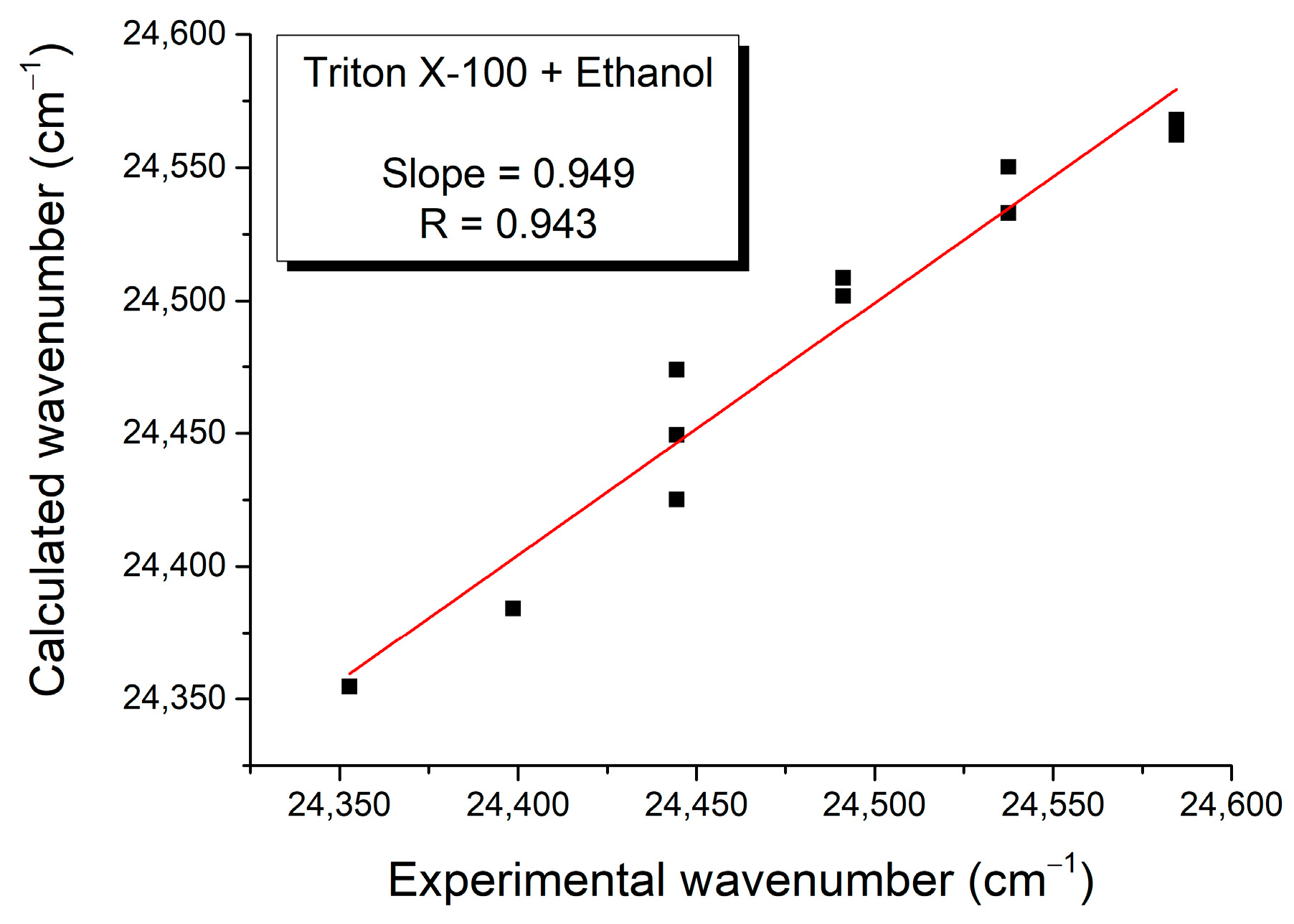
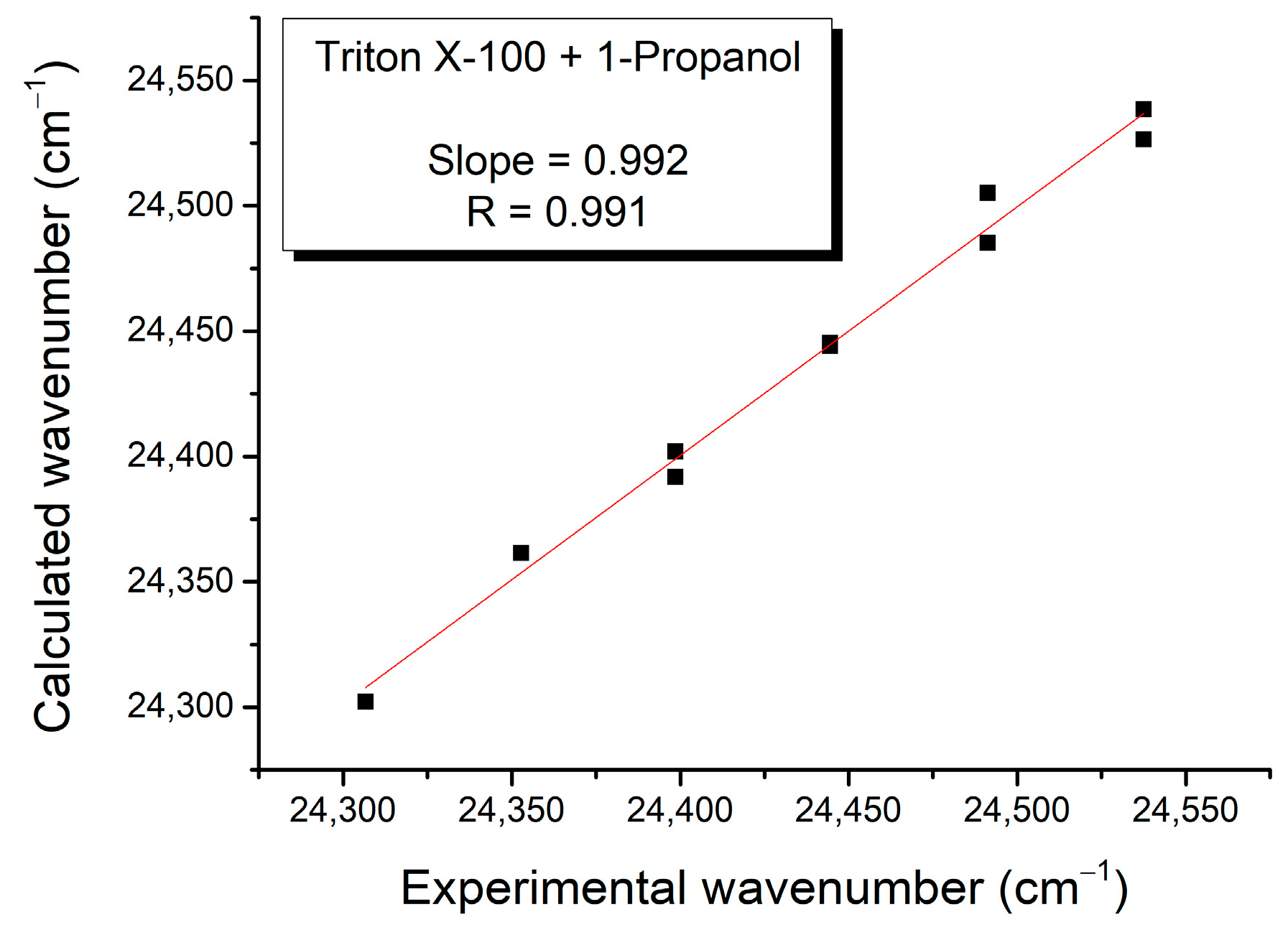



| Mole Fraction of Triton X-100 | π* | β | α | (cm−1) |
|---|---|---|---|---|
| 0.0 | 1.08 | 0.49 | 1.30 | 23,342 |
| 0.1 | 0.88 | 0.63 | 0.59 | 24,353 |
| 0.2 | 0.87 | 0.67 | 0.49 | 24,399 |
| 0.3 | 0.91 | 0.59 | 0.41 | 24,444 |
| 0.4 | 0.88 | 0.62 | 0.39 | 24,444 |
| 0.5 | 0.87 | 0.65 | 0.39 | 24,491 |
| 0.6 | 0.88 | 0.60 | 0.34 | 24,491 |
| 0.7 | 0.87 | 0.62 | 0.35 | 24,537 |
| 0.8 | 0.93 | 0.52 | 0.27 | 24,537 |
| 0.9 | 0.90 | 0.55 | 0.28 | 24,585 |
| 1.0 | 0.87 | 0.57 | 0.31 | 24,585 |
| Mole Fraction of Triton X-100 | π* | β | α | (cm−1) |
|---|---|---|---|---|
| 0.0 | 0.55 | 0.84 | 1.17 | 24,081 |
| 0.1 | 0.80 | 0.65 | 0.83 | 24,125 |
| 0.2 | 0.84 | 0.60 | 0.67 | 24,216 |
| 0.3 | 0.86 | 0.58 | 0.59 | 24,261 |
| 0.4 | 0.88 | 0.59 | 0.54 | 24,307 |
| 0.5 | 0.86 | 0.59 | 0.50 | 24,353 |
| 0.6 | 0.89 | 0.57 | 0.43 | 24,399 |
| 0.7 | 0.88 | 0.55 | 0.37 | 24,444 |
| 0.8 | 0.88 | 0.56 | 0.33 | 24,491 |
| 0.9 | 0.86 | 0.57 | 0.32 | 24,537 |
| 1.0 | 0.87 | 0.57 | 0.31 | 24,585 |
| Mole Fraction of Triton X-100 | π* | β | α | (cm−1) |
|---|---|---|---|---|
| 0.0 | 0.54 | 0.90 | 0.98 | 24,353 |
| 0.1 | 0.82 | 0.62 | 0.66 | 24,399 |
| 0.2 | 0.80 | 0.62 | 0.61 | 24,444 |
| 0.3 | 0.83 | 0.60 | 0.54 | 24,444 |
| 0.4 | 0.87 | 0.56 | 0.46 | 24,444 |
| 0.5 | 0.80 | 0.66 | 0.48 | 24,491 |
| 0.6 | 0.84 | 0.62 | 0.43 | 24,491 |
| 0.7 | 0.88 | 0.57 | 0.35 | 24,537 |
| 0.8 | 0.86 | 0.58 | 0.34 | 24,537 |
| 0.9 | 0.86 | 0.57 | 0.31 | 24,585 |
| 1.0 | 0.87 | 0.57 | 0.31 | 24,585 |
| Mole Fraction of Triton X-100 | π* | β | α | (cm−1) |
|---|---|---|---|---|
| 0.0 | 0.59 | 0.95 | 0.84 | 24,307 |
| 0.1 | 0.71 | 0.72 | 0.66 | 24,353 |
| 0.2 | 0.88 | 0.58 | 0.56 | 24,399 |
| 0.3 | 0.85 | 0.60 | 0.55 | 24,399 |
| 0.4 | 0.86 | 0.58 | 0.47 | 24,444 |
| 0.5 | 0.83 | 0.66 | 0.50 | 24,444 |
| 0.6 | 0.86 | 0.60 | 0.41 | 24,491 |
| 0.7 | 0.85 | 0.61 | 0.38 | 24,491 |
| 0.8 | 0.88 | 0.57 | 0.33 | 24,537 |
| 0.9 | 0.88 | 0.57 | 0.31 | 24,537 |
| 1.0 | 0.87 | 0.57 | 0.31 | 24,585 |
| (cm−1) | C1 | C2 | C3 | Adj. R-Square | F Value |
|---|---|---|---|---|---|
| 29,115 (694) 1 | −5237 (766) | 0.821 | 46.735 | ||
| 22,525 (1105) | 3138 (1860) | 0.156 | 2.846 | ||
| 24,938 (33) | −1194 (60) | 0.975 | 389.796 | ||
| 33,321 (986) | −7625 (668) | −3460 (747) | 0.945 | 87.149 | |
| 26,113 (257) | −1434 (313) | −934 (66) | 0.992 | 639.740 | |
| 24,471 (165) | 746 (260) | −1138 (49) | 0.986 | 355.108 | |
| 27,068 (958) | −2204 (807) | −518 (501) | −833 (118) | 0.992 | 430.510 |
| (cm−1) | C1 | C2 | C3 | Adj. R-Square | F Value |
|---|---|---|---|---|---|
| 23,423 (336) 1 | 1101 (400) | 0.396 | 7.566 | ||
| 25,210 (265) | −1434 (434) | 0.498 | 10.929 | ||
| 24,655 (41) | −569 (68) | 0.873 | 69.116 | ||
| 31,423 (3307) | −4019 (2134) | −6154 (2535) | 0.609 | 8.785 | |
| 25,829 (74) | −1158 (72) | −949 (27) | 0.996 | 1160.524 | |
| 23,910 (53) | 1663 (117) | −1048 (37) | 0.995 | 912.471 | |
| 24,972 (308) | −650 (187) | 764 (270) | −1002 (27) | 0.998 | 1454.548 |
| (cm−1) | C1 | C2 | C3 | Adj. R-Square | F Value |
|---|---|---|---|---|---|
| 24,041 (152) 1 | 542 (186) | 0.429 | 8.511 | ||
| 24,806 (120) | −517 (190) | 0.390 | 7.395 | ||
| 24,654 (26) | −344 (48) | 0.833 | 50.907 | ||
| 22,783 (2103) | 1416 (1469) | 873 (1456) | 0.385 | 4.132 | |
| 25,240 (150) | −569 (145) | −590 (69) | 0.936 | 73.950 | |
| 24,467 (65) | 453 (150) | −536 (73) | 0.912 | 52.958 | |
| 26,028 (780) | −1128 (780) | −511 (497) | −615 (73) | 0.936 | 50.010 |
| (cm−1) | C1 | C2 | C3 | Adj. R-Square | F Value |
|---|---|---|---|---|---|
| 23,890 (146) 1 | 679 (176) | 0.581 | 14.849 | ||
| 24,786 (96) | −529 (149) | 0.538 | 12.643 | ||
| 24,677 (15) | −471 (29) | 0.964 | 271.791 | ||
| 23,664 (1218) | 847 (916) | 138 (737) | 0.530 | 6.646 | |
| 24,905 (83) | −216 (78) | −575 (43) | 0.980 | 241.716 | |
| 24,604 (17) | 208 (43) | −595 (30) | 0.990 | 486.496 | |
| 24,365 (174) | 183 (132) | 339 (103) | −585 (29) | 0.991 | 361.996 |
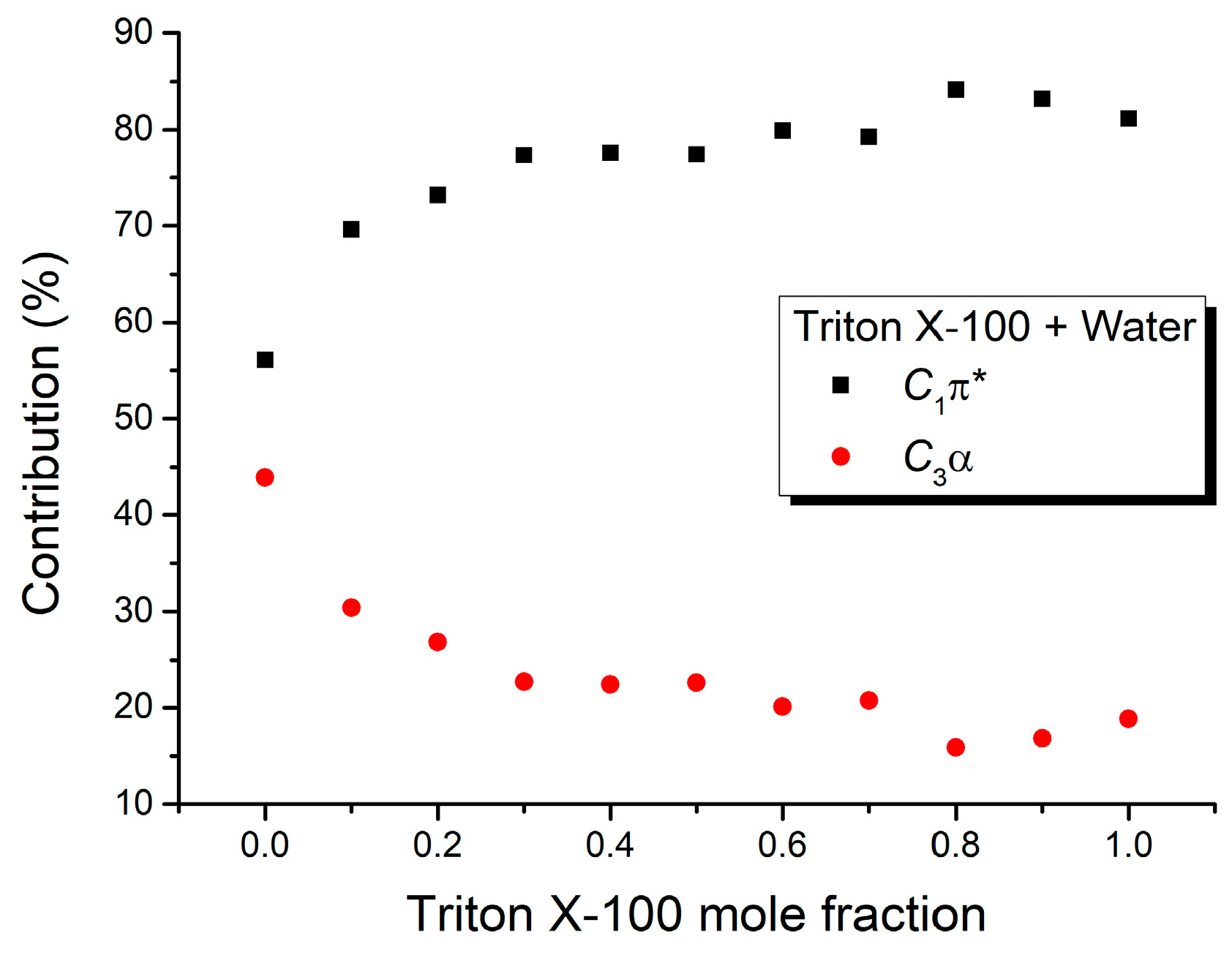
| Mole Fraction of Triton X-100 | C1π* (cm−1) | C1π* (%) | C3α (cm−1) | C3α (%) | (cm−1) |
|---|---|---|---|---|---|
| 0.0 | −1549.17 | 56.07 | −1213.67 | 43.93 | 23,351 |
| 0.1 | −1262.29 | 69.62 | −550.82 | 30.38 | 24,300 |
| 0.2 | −1247.95 | 73.18 | −457.46 | 26.82 | 24,408 |
| 0.3 | −1305.32 | 77.33 | −382.77 | 22.67 | 24,425 |
| 0.4 | −1262.29 | 77.61 | −364.10 | 22.39 | 24,487 |
| 0.5 | −1247.95 | 77.41 | −364.10 | 22.59 | 24,501 |
| 0.6 | −1262.29 | 79.91 | −317.42 | 20.09 | 24,534 |
| 0.7 | −1247.95 | 79.25 | −326.76 | 20.75 | 24,539 |
| 0.8 | −1334.01 | 84.11 | −252.07 | 15.89 | 24,527 |
| 0.9 | −1290.98 | 83.16 | −261.41 | 16.84 | 24,561 |
| 1.0 | −1247.95 | 81.17 | −289.41 | 18.83 | 24,576 |
| Mole Fraction of Triton X-100 | C1π* (cm−1) | C1π* (%) | C2β (cm−1) | C2β (%) | C3α (cm−1) | C3α (%) | (cm−1) |
|---|---|---|---|---|---|---|---|
| 0.0 | −357.47 | 16.46 | 641.44 | 29.54 | −1172.85 | 54.00 | 24,083 |
| 0.1 | −519.95 | 28.13 | 496.35 | 26.85 | −832.02 | 45.02 | 24,117 |
| 0.2 | −545.95 | 32.58 | 458.17 | 27.34 | −671.63 | 40.08 | 24,213 |
| 0.3 | −558.95 | 35.08 | 442.90 | 27.80 | −591.44 | 37.12 | 24,265 |
| 0.4 | −571.94 | 36.57 | 450.54 | 28.81 | −541.32 | 34.62 | 24,309 |
| 0.5 | −558.95 | 37.00 | 450.54 | 29.82 | −501.22 | 33.18 | 24,363 |
| 0.6 | −578.44 | 40.04 | 435.26 | 30.13 | −431.05 | 29.84 | 24,398 |
| 0.7 | −571.94 | 41.97 | 420.00 | 30.82 | −370.90 | 27.22 | 24,449 |
| 0.8 | −571.94 | 42.99 | 427.63 | 32.14 | −330.80 | 24.87 | 24,497 |
| 0.9 | −558.95 | 42.51 | 435.26 | 33.10 | −320.78 | 24.39 | 24,528 |
| 1.0 | −565.45 | 43.12 | 435.26 | 33.19 | −310.76 | 23.70 | 24,531 |
| Mole Fraction of Triton X-100 | C1π* (cm−1) | C1π* (%) | C3α (cm−1) | C3α (%) | (cm−1) |
|---|---|---|---|---|---|
| 0.0 | −307.30 | 34.69 | −578.44 | 65.31 | 24,355 |
| 0.1 | −466.64 | 54.50 | −389.56 | 45.50 | 24,384 |
| 0.2 | −455.25 | 55.84 | −360.05 | 44.16 | 24,425 |
| 0.3 | −472.33 | 59.71 | −318.73 | 40.29 | 24,449 |
| 0.4 | −495.09 | 64.58 | −271.51 | 35.42 | 24,474 |
| 0.5 | −455.25 | 61.64 | −283.32 | 38.36 | 24,502 |
| 0.6 | −478.02 | 65.32 | −253.81 | 34.68 | 24,509 |
| 0.7 | −500.78 | 70.80 | −206.59 | 29.20 | 24,533 |
| 0.8 | −489.40 | 70.92 | −200.68 | 29.08 | 24,550 |
| 0.9 | −489.40 | 72.79 | −182.98 | 27.21 | 24,568 |
| 1.0 | −495.09 | 73.01 | −182.98 | 26.99 | 24,562 |
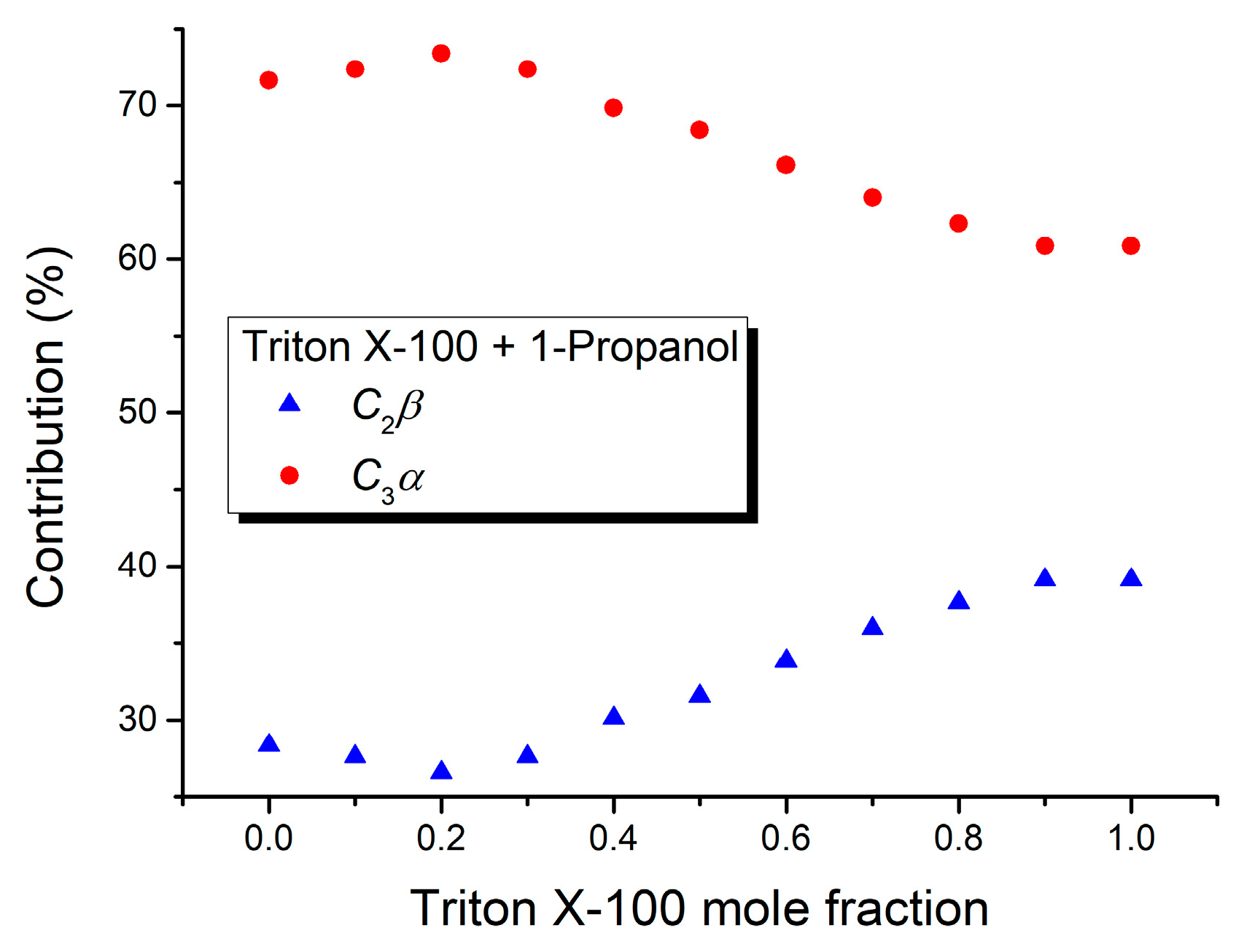
| Mole Fraction of Triton X-100 | C2β (cm−1) | C2β (%) | C3α (cm−1) | C3α (%) | (cm−1) |
|---|---|---|---|---|---|
| 0.0 | 197.61 | 28.34 | −499.57 | 71.66 | 24,302 |
| 0.1 | 149.77 | 27.62 | −392.52 | 72.38 | 24,361 |
| 0.2 | 120.65 | 26.59 | −333.05 | 73.41 | 24,392 |
| 0.3 | 124.81 | 27.62 | −327.10 | 72.38 | 24,402 |
| 0.4 | 120.65 | 30.15 | −279.52 | 69.85 | 24,445 |
| 0.5 | 137.29 | 31.59 | −297.36 | 68.41 | 24,444 |
| 0.6 | 124.81 | 33.86 | −243.84 | 66.14 | 24,485 |
| 0.7 | 126.89 | 35.96 | −226.00 | 64.04 | 24,505 |
| 0.8 | 118.57 | 37.66 | −196.26 | 62.34 | 24,527 |
| 0.9 | 118.57 | 39.14 | −184.36 | 60.86 | 24,538 |
| 1.0 | 118.57 | 39.14 | −184.36 | 60.86 | 24,538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosi, E.; Zara, A.; Dorohoi, D.O.; Dimitriu, D.-G. Solvatochromic Analysis of Triton X-100 in Binary Mixtures. Symmetry 2025, 17, 199. https://doi.org/10.3390/sym17020199
Ambrosi E, Zara A, Dorohoi DO, Dimitriu D-G. Solvatochromic Analysis of Triton X-100 in Binary Mixtures. Symmetry. 2025; 17(2):199. https://doi.org/10.3390/sym17020199
Chicago/Turabian StyleAmbrosi, Ecaterina, Alexandru Zara, Dana Ortansa Dorohoi, and Dan-Gheorghe Dimitriu. 2025. "Solvatochromic Analysis of Triton X-100 in Binary Mixtures" Symmetry 17, no. 2: 199. https://doi.org/10.3390/sym17020199
APA StyleAmbrosi, E., Zara, A., Dorohoi, D. O., & Dimitriu, D.-G. (2025). Solvatochromic Analysis of Triton X-100 in Binary Mixtures. Symmetry, 17(2), 199. https://doi.org/10.3390/sym17020199






