1. Introduction
The morphology of the residual limb is a main determining factor for prosthetic socket fit, comfort, and long-term user results for lower-limb amputees. Although modern prosthetic components have enjoyed great technological advancements, the socket, being the primary interface between residuum and device, continues to be a chief source of trouble. Discomfort, trauma to the soft tissues, and instability remain frequent, much of it a function of the art of socket creation based more on clinician experience than on quantitative biomechanical data [
1,
2]. This is reflected in patient-reported outcomes, where socket discomfort is consistently cited as a primary challenge, driving the need for designs that enhance comfort and reduce skin problems [
3,
4]. To this is added the uncertainty in limb volume and shape from fluid shifts, changes in position, and day-long mechanical loading, all of which can be detrimental to socket stability and satisfaction [
5,
6,
7].
To optimize limb–socket coupling and reduce motion artifacts such as pistoning, vacuum-assisted suspension systems (VASS) are increasingly used in practice. The systems establish negative pressure to enhance contact and stabilize the limb. While randomized trials and clinical studies have demonstrated benefits of VASS in terms of suspension, proprioception, and functional outcomes such as walking distance, the underlying biomechanical effects—particularly vacuum-induced morphological changes—are not yet quantified sufficiently [
8,
9]. Without extensive computer analysis and imaging, vacuum pressure effects on limb shape and resultant socket fit are largely theoretical [
10]. The mechanisms by which vacuum is thought to act on residual limb shape include direct soft tissue compression, repositioning interstitial and vascular fluid, and minimal musculoskeletal posture changes within the socket. Such processes, separately or in combination, can alter both the external form and internal stress pattern of the residual limb when under suspension and consequently influence socket fit and load transmission while walking.
More advanced assessment of prosthetic fit and internal limb loading has been made possible with new computational modeling methodologies. Sengeh et al. [
1] pioneered the development of subject-specific, multi-material viscoelastic representations of MRI and in vivo mechanical testing, forming the foundation for data-driven, patient-specific socket design. FEA has also been widely utilized to examine the influence of changes in geometry and material on stress distributions within the limb–socket interface, with implications regarding soft tissue health and mechanical safety [
2,
11,
12].
Along with these computational techniques come advances in morphological assessment tools. Squibb et al. [
6] demonstrated that high-resolution laser scanning can identify fine, clinically relevant shape and volume changes in transtibial residual limbs. Future advances in the field of materials science, such as the application of density-graded lattices, aim to minimize pressure concentrations and enhance comfort [
13]. Simultaneous improvements in real-time pressure sensing and targeted manufacturing protocols have also aimed to increase reproducibility, decrease interface stress peaks, and promote personalization [
14,
15,
16].
Contemporary clinical assessments use complaint and rudimentary functional testing, both of which offer little insight into subsurface tissue behavior [
17,
18,
19,
20]. Interface pressure mapping, while common, assesses only external load distribution and is unable to determine internal states of strain—a primary predictor of deep tissue injury [
21,
22,
23,
24]. Strain-based measurements are progressively recognized to be crucial to the assessment of load-induced tissue trauma, including ischemia, reperfusion injury, and lymphatic compromise [
25,
26,
27].
For the quantification of internal mechanical response, FEA remains the superior tool, with high-resolution mapping of the internal fields of stress and strain under computer-controlled loading simulation [
28,
29]. Yet its clinical application is constrained by demanding setup procedures, limited patient-specific tissue data, and computer-intensive needs. Experimental techniques like digital image correlation (DIC) and digital volume correlation (DVC) have also been proposed to quantify strain but are of limited application. For instance, 3D-DIC is constrained to evaluate surface deformation in unloaded states [
30], while DVC with limb analogs has been questioned on anatomical and material realism grounds [
31].
Despite such methodological advancements, most computational and imaging studies are founded on residual limb geometries collected in uncontrolled or non-weight-bearing states. The validity of finite element simulations and subsequent socket design is therefore fundamentally linked to the fidelity of the underlying input geometry. Any limb deformation during scanning—such as that induced by vacuum compression, wrapping, or other external pressures—can produce anatomical representations that differ from the actual in-socket form during functional loading. This mismatch can propagate systematic error through the design and validation process. Previous studies using MRI, CT, and in-socket pressure measurements have demonstrated that the morphology of the residual limb is highly sensitive to loading conditions and external compression [
32,
33], and more recent work has reinforced that interface pressure patterns are altered by suspension method and tissue stress distribution. Likewise, finite element analyses consistently confirm that socket fit, stress transfer, and pressure distribution are strongly geometry-dependent [
34], with subject-specific modeling now emerging as a dominant approach for improving predictive accuracy. However, no prior work has systematically evaluated vacuum-induced morphological changes in vivo using high-resolution imaging, nor has symmetry/asymmetry analysis been applied to isolate these effects in a patient-specific context.
To bridge this gap, the present study introduces a case-based analysis of vacuum-induced morphological change in a bilateral knee-disarticulation (KD) amputee [
35]. High-resolution CT imaging was used to capture both limbs under standardized conditions: one subjected to vacuum compression and the contralateral limb untreated as a natural control. Through 3D surface reconstruction, volumetric assessment, symmetry/asymmetry comparison, and finite element analysis, we quantitatively evaluate the impact of vacuum-induced morphology on external limb shape and internal stress transfer. These findings highlight the importance of geometry-aware modeling in prosthetic socket design and demonstrate how vacuum application can be leveraged to improve conformity, comfort, and mechanical safety.
2. Materials and Methods
2.1. Clinical Case
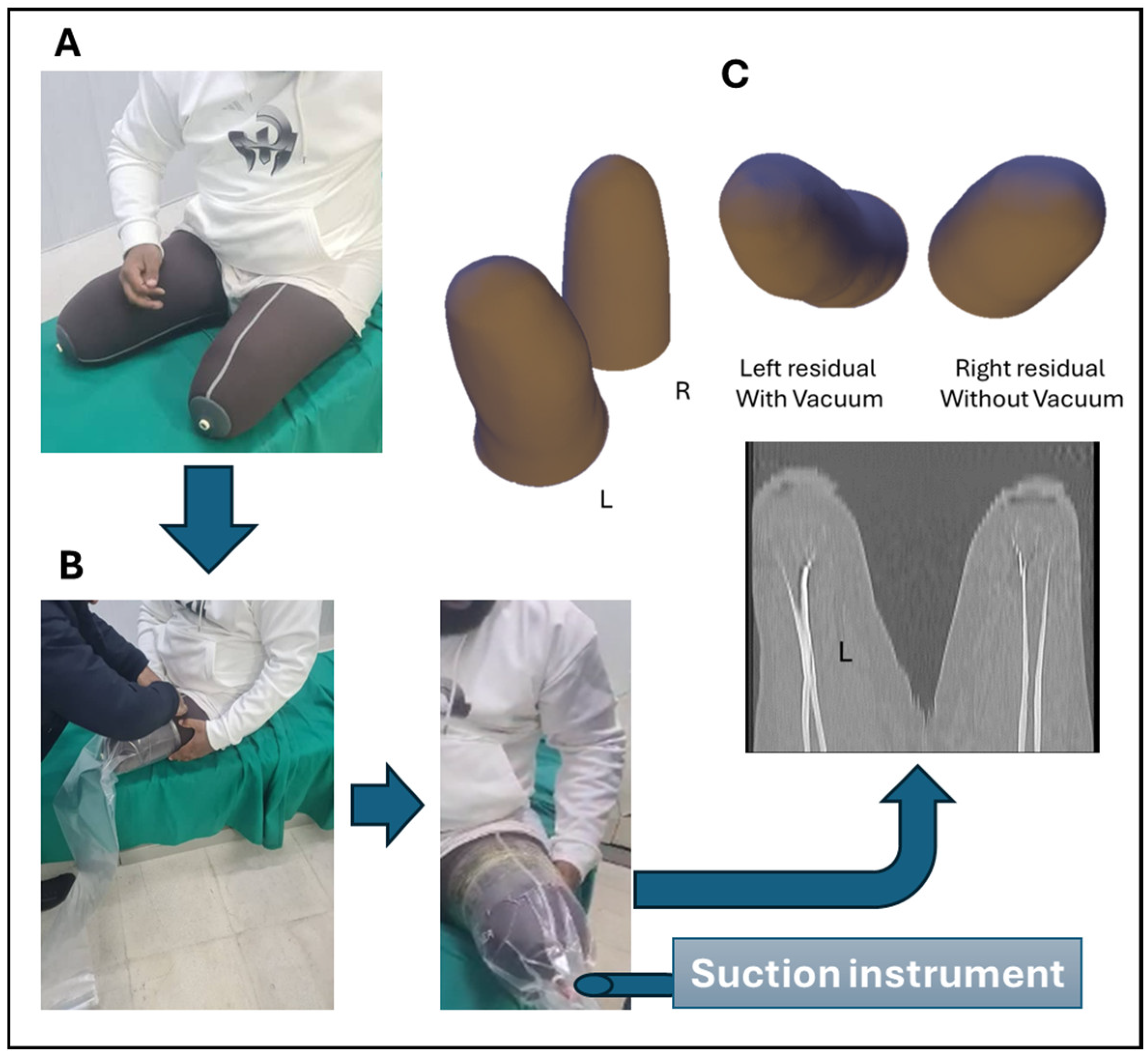
A male patient in his early 40s with a history of bilateral KD amputation secondary to traumatic injury (
Figure 1A) was recruited through the Syrian Centre for Technological Solutions (Tartus, Syria). The amputations were performed ten years prior with successful primary closure and no postoperative complications. The patient presented with long-standing socket-related discomfort, intermittent volume fluctuations in the residual limbs, and soft tissue asymmetries that hindered consistent prosthetic fit and mobility.
Clinical assessment revealed stable surgical sites and preserved proximal sensation, with no evidence of phantom limb pain. The right residual limb demonstrated a more tapered profile suggestive of chronic soft tissue contraction, while the left showed a broader distal contour, likely due to variable fluid retention. Over the course of rehabilitation, the patient had undergone multiple prosthetic socket modifications and functional gait training.
2.2. Medical Images Acquisition Under Vacuum and 3D Model Reconstruction
High-resolution 3D imaging of both residual limbs was performed using CT under standardized conditions. To evaluate the morphological impact of vacuum-induced soft tissue compression, the left limb was scanned while enclosed in a transparent, vacuum-sealed polymer sleeve. This technique was designed to replicate the mechanical compression typically exerted on the residual limb during prosthetic socket loading, thereby enhancing the physiological relevance of the acquired geometry (
Figure 1B). For the vacuum condition, the left residual limb was enclosed in a transparent polymer sleeve and subjected to a controlled negative pressure of −20 mbar (≈ −2 kPa or −15 mmHg) using a manually operated suction pump. This level was selected as a mild sub-atmospheric pressure sufficient to induce measurable soft tissue deformation without causing discomfort, in line with values reported in preliminary pilot tests and low-range vacuum studies. The pressure was maintained for approximately 3 min prior to and during CT acquisition to allow soft tissue stabilization. Although continuous electronic monitoring was not available, the pressure was manually checked and kept stable throughout the scan.
CT imaging was conducted at Al-Andalus University Hospital using a Toshiba 4-slice scanner (Asteion Super 4, model TSX-021B/4B; Canon Medical Systems, Otawara, Japan), with procedural oversight provided by the radiology department and rehabilitation team in coordination with the Faculty of Biomedical Engineering at Al-Andalus University for Medical Sciences (Kadmous, Tartus, Syria). Acquisition parameters included a slice thickness of 1.0 mm, a bone reconstruction kernel, and an in-plane voxel resolution of 0.5 × 0.5 mm, enabling high-fidelity reconstruction of external limb morphology. The right limb served as an uncompressed control and was scanned under identical parameters to ensure comparative consistency.
Acquired CT datasets were exported in DICOM format and processed using 3D Slicer (version 5.8.1; Slicer Community, 2025, Boston, MA, USA). Segmentation of the external soft tissue boundaries was performed manually and refined to eliminate imaging artifacts and maintain anatomical symmetry across limbs. The segmented outputs were reconstructed into high-resolution 3D surface (
Figure 1C) meshes and exported in STL format for further computational analysis.
All spatial measurements were standardized in millimeters (mm) and volumes in cubic centimeters (cm3). Visualization of surface deviation and overlay mapping was performed using ‘vedo’ for optional post hoc inspection, although quantitative comparisons were based strictly on aligned and independently processed STL models.
Subsequent quantitative analysis was conducted using Python (version 3.11; Python Software Foundation, Wilmington, DE, USA), employing the trimesh library for mesh manipulation. Key morphological descriptors—including total volume, surface area, and bounding box dimensions—were computed for each limb. To assess regional deformation patterns, cross-sectional slicing was performed at ten equidistant intervals along the longitudinal (Z) axis. At each level, intersection contours were extracted and analyzed to compute corresponding cross-sectional areas.
2.3. Finite Element Analysis (FEA)
To evaluate the biomechanical safety and performance of the designed prosthetic socket, a comprehensive FEA was performed. The system simulated consisted of three key components: the residual limb (modeled with inner cortical bone and surrounding soft tissue), and a custom-designed socket made from 3D-printed PLA (polylactic acid). The geometry of the residual limb was reconstructed from CT-derived STL models through a reverse engineering workflow combining Meshmixer and SolidWorks 2024. Following segmentation in 3D Slicer, the STL files were imported into Autodesk Meshmixer (version 3.5, Autodesk Inc., San Rafael, CA, USA), where surface cleaning was performed to remove noise, spikes, and residual artifacts. Small holes were filled, and the mesh was smoothed to ensure watertight geometry suitable for model conversion. The cleaned STL meshes were then imported into SolidWorks and the stitched surfaces were converted into solid bodies, allowing volumetric operations and domain separation. To represent anatomical structures, the distal femur was modeled as a cylindrical cortical bone insert aligned with the anatomical axis, while the surrounding soft tissue envelope was reconstructed by lofting around the external contours and subtracting the inner bone region, as shown in
Figure 2A.
Material parameters for cortical bone (E = 18 GPa, ν = 0.30) were adopted from widely used finite element modeling studies [
36], while tensile failure data of human cortical bone supports a yield strength of approximately 110 MPa [
37]. Soft tissue (muscle) properties—E = 60 kPa and Poisson’s ratio close to 0.49—follow values used in biomechanical finite element modeling of skeletal muscles [
34], and muscle density was approximated as 1050 kg/m
3, consistent with reported values for hydrated soft tissues. For the prosthetic socket, PLA material properties (E = 3.2 GPa, ν = 0.35, yield strength ≈ 50 MPa) were taken from established polymer characterization studies [
38]. A summary of all mechanical properties used in the simulation is presented in
Table 1.
The contact interaction between the femur and muscle tissue was defined as bonded, reflecting the anatomical attachment through surrounding connective tissue. In contrast, the interaction between the muscle and the socket was modeled using a no-penetration contact with a friction coefficient of 0.2 to simulate sliding and pressure transfer at the interface. This value is consistent with previous prosthetic socket modeling studies, which report interface friction coefficients in the range of 0.1–0.3 [
39,
40]. Boundary conditions were applied by fixing the distal end of the femur, while a vertical compressive load of 1000 N—representing approximately 1.2 times the body weight—was applied at the superior region of the socket to mimic hip-joint forces during gait, as shown in
Figure 2B. The geometry was discretized using quadratic tetrahedral solid elements (SOLID186). A global element size of 2.5 mm was applied to the femur and socket, while a finer size of 0.8 mm was applied to the surrounding muscle tissue to capture deformation. Mesh refinement was introduced at the bone–muscle and muscle–socket interfaces to improve accuracy and numerical stability. Special attention was given to refining the mesh at contact regions to improve numerical stability and convergence accuracy, as illustrated in
Figure 2C. A mesh independence study was performed by testing three successive refinements: coarse (3.5 mm for femur/socket, 1.2 mm for muscle), medium (2.5 mm and 0.8 mm, as reported above), and fine (1.5 mm and 0.5 mm). Peak von Mises stresses at the socket–residuum interface varied by less than 2% between the medium and fine meshes, confirming mesh convergence; therefore, the medium mesh was adopted for subsequent analyses as a balance between accuracy and computational efficiency.
The analysis was conducted as a static nonlinear simulation with large displacement enabled. The iterative solver FFEPlus was selected to handle geometric nonlinearity and contact conditions. Automatic time stepping was activated with a maximum of 500 load increments and a minimum step size of 1 × 10−5. Convergence criteria were set to residual values below 0.5% for both force and displacement. To mitigate rigid body motion and convergence instability during early load steps, soft spring stabilization was employed. During preliminary simulation runs, convergence issues occurred at approximately 20% load application. These were resolved through a combination of mesh refinement, reduction in time stepping, and enhanced stabilization settings.
3. Results
This study quantified the morphological and mechanical effects of vacuum-induced compression on a residual limb and its corresponding prosthetic socket. Key findings revealed measurable reductions in limb volume and cross-sectional area under vacuum, alongside changes in stress distribution, displacement patterns, and contact pressure at the limb–socket interface. Finite element analysis further demonstrated how these geometric alterations influence the socket’s mechanical performance, including the factor of safety and load-bearing behavior.
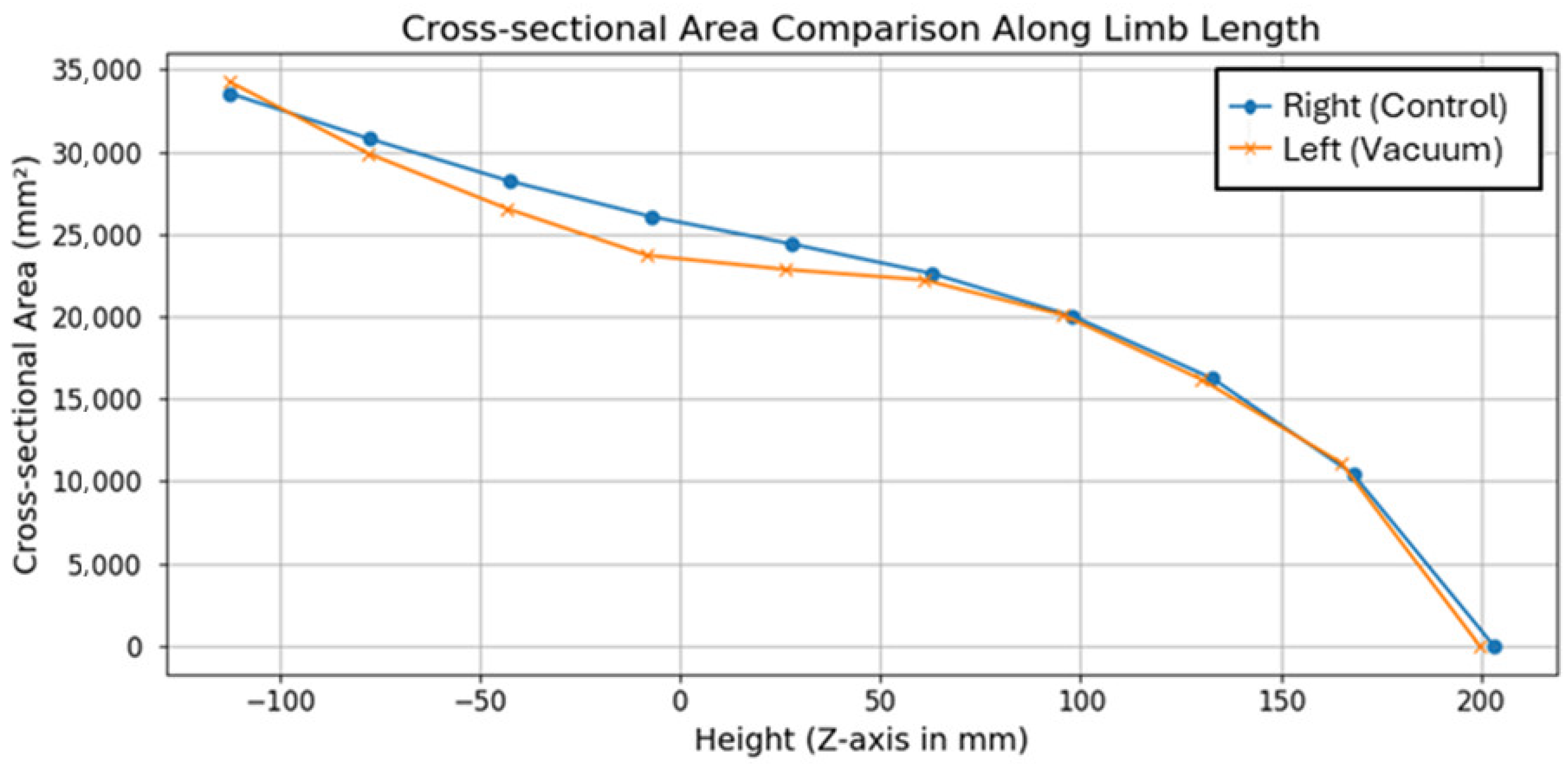
Figure 3 presents the comparison of cross-sectional area along the
Z-axis for both residual limbs. The plot demonstrates a consistent trend of reduced cross-sectional area in the vacuum-compressed limb compared to the untreated control, indicating measurable soft tissue compression. These differences are most pronounced in the medial region of the thigh, where vacuum application resulted in localized reductions of up to 5.3%. Toward the distal end, the cross-sectional areas of the two limbs converge, suggesting minimal vacuum-related deformation in these regions. Notably, the reduction was not uniform but showed direction-dependent asymmetry, particularly along the anteroposterior axis, highlighting the importance of adaptive socket shaping rather than assuming uniform radial compression. This supports the hypothesis that external mechanical pressure induces volumetric and morphological deformation, which is a critical factor for optimizing socket fit. Elastic strain analysis further confirmed that the residual limb geometries recovered their original shapes upon load removal, with maximum elastic strains of 4.7 × 10
−3 mm/mm in the vacuum-compressed limb and 3.1 × 10
−3 mm/mm in the control limb.
Table 2 quantifies overall morphology metrics, including volume, surface area, and bounding box dimensions. The vacuum-compressed limb shows a lower total volume (6595.51 cm
3 vs. 6874.43 cm
3) and slightly reduced surface area. Interestingly, while the compressed limb is overall smaller in volume, its bounding box length is slightly greater in the longitudinal direction, likely reflecting a redistribution of soft tissue rather than uniform shrinkage. These metrics confirm that vacuum-induced compression causes a notable, although localized, alteration in limb geometry. A paired
t-test between area values across anatomical sections showed a statistically significant difference (
p < 0.01), confirming consistent tissue compression.
The finite element simulation provided detailed insights into the stress distribution, strain behavior, and mechanical integrity of the prosthetic socket under physiological load. The anatomical model, composed of cortical bone, soft muscle tissue, and a PLA socket, was evaluated under a static compressive force of 1000 N, simulating a typical hip-joint load during ambulation.
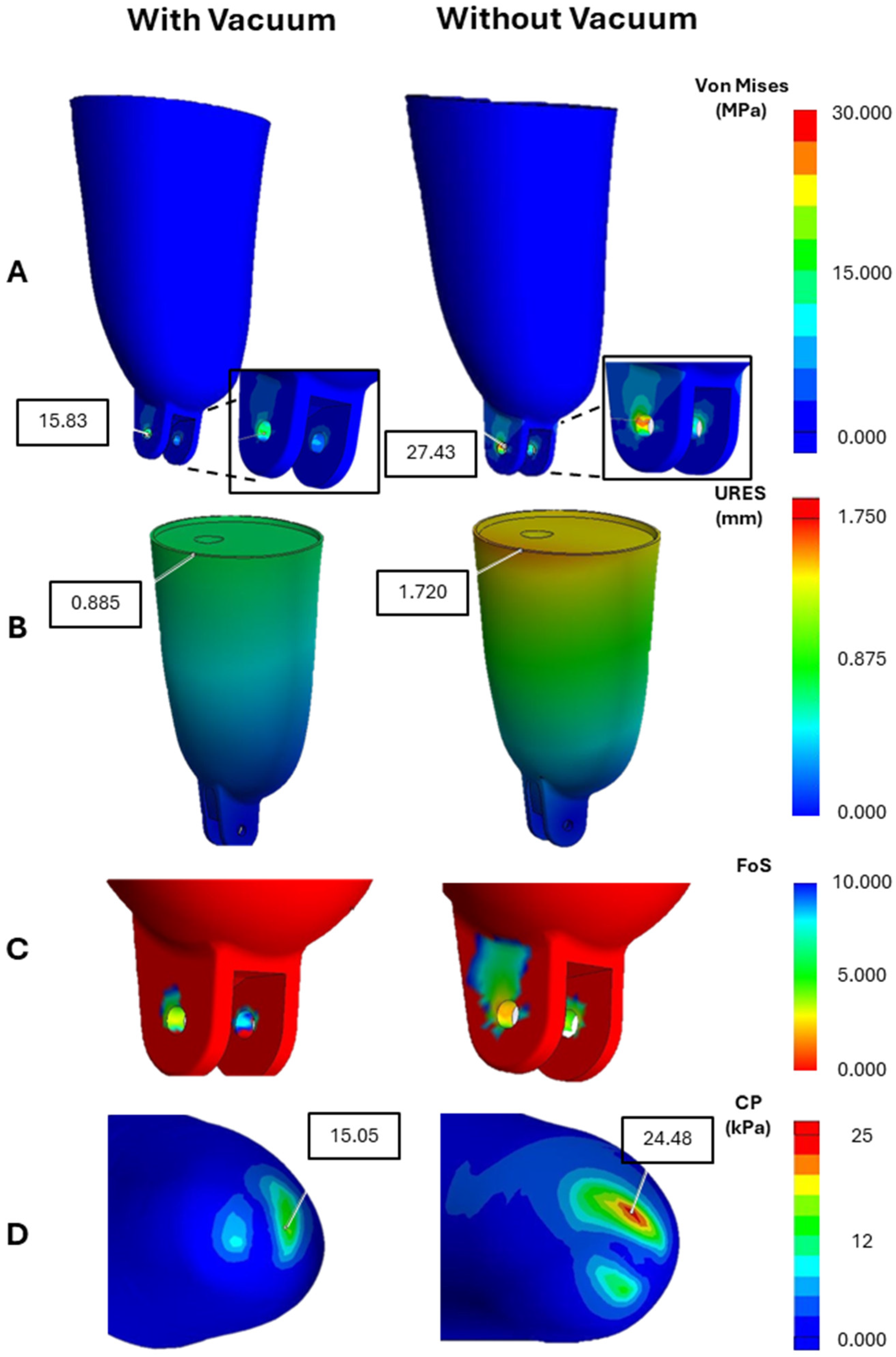
The highest stress concentrations (
Figure 4A) were consistently observed at the distal end and medial–lateral walls of the socket regions subjected to concentrated load transfer during gait. In the non-vacuum model, peak stress reached 27.43 MPa, whereas the vacuum-induced condition exhibited a significantly lower maximum of 15.83 MPa. These values remained well below the yield strength of PLA (~50 MPa), confirming the socket’s mechanical integrity under physiological loading. The reduction in peak stress under vacuum suggests a more favorable load distribution across the socket structure.
Maximum displacement (
Figure 4B) occurred near the socket’s proximal rim, with a peak of 1.72 mm in the non-vacuum model, compared to only 0.885 mm in the vacuum-applied condition. Notably, displacement near the distal fixation zones remained minimal in both cases, indicating effective mechanical constraint and alignment at the attachment interface. The reduced deformation in the vacuum model reflects increased geometric conformity and improved mechanical stability—critical for minimizing motion-induced discomfort.
The minimum factor of safety (FoS) observed in the vacuum condition was approximately 3.0, while the non-vacuum condition approached the lower limit of 2.0 in critical load-bearing zones (
Figure 4C). These findings confirm that the socket remains structurally reliable in both scenarios; however, the vacuum-induced configuration offers a more robust safety margin. This enhanced safety profile supports the use of vacuum-forming techniques in clinical socket fabrication where long-term durability is essential.
Analysis of contact pressure (
Figure 4D) at the limb–socket interface demonstrated a clear improvement in load distribution when vacuum was applied. The non-vacuum condition exhibited localized high-pressure zones with a peak interface pressure of 24.48 kPa, whereas the vacuum-induced model showed a lower and more evenly distributed peak of 15.05 kPa. This pressure homogenization reduces the risk of focal tissue loading, thereby minimizing the potential for skin breakdown, pain, and interface instability. The improved load transfer under vacuum may also contribute to enhanced user comfort and prosthesis control during ambulation.
The comparative analysis (
Figure 5) between prosthetic sockets with and without vacuum-induced compression reveals significant differences in their mechanical behavior. Key parameters such as stress distribution, displacement, FoS, and contact pressure all show notable variation depending on whether a vacuum is applied. The vacuum-assisted socket demonstrates lower stress concentrations and reduced displacement, indicating better structural stability. Additionally, it exhibits higher FoS values, reflecting a safer margin against material failure. Contact pressure under vacuum is more evenly distributed, which helps maintain the residual limb’s natural shape and enhances overall comfort.
4. Discussion
This case study provides preliminary evidence that vacuum application induces measurable changes in residual limb form with potential implications for socket design. The diminutions in limb cross-sectional area (up to 5.3% at mid-shank) confirm that relatively modest volume changes may have significant impacts on pressure distribution, as suggested by Squibb et al. [
6]. As this single-patient design is viewed as exploratory, the findings should be strengthened by cohort studies to foster the generalization of the results.
Anisotropic soft tissue displacement under vacuum conditions, with localized anteroposterior axis elongation, validates previous results that tissue deformation is direction-dependent [
1]. This contradicts the assumption of equal radial compression under traditional socket design, suggesting adaptive or region-specific adjustment might be required. Our current model does capture the layered anatomy (e.g., skin, fat, muscle, bone) or anisotropic character of residual limbs, which would deform more complexly under load. The quantitative validity of the stress results and pressure distribution must be studied in further detail.
Mechanically, geometry redistribution of stresses and displacement reduction overall, and consequently improved load transfer uniformity, is suggested. While this concurs with Rodrigues and Da Gama [
16] on the role of interface geometry, quantified simulated interface pressures are still higher than those of many experimental measurements. This discrepancy is typical of our model idealizations, for example, the homogenization of muscle tissue and linear elastic material response. Most importantly, the consecutive measurement of pressure and strain will be necessary to validate the results and prepare for the clinical application.
The factor of safety remained (>2) under both conditions and reflects the structural adequacy of the socket design. This result points to a “protective effect” that would result from the hypothesized under-loading methodology. However, further clinical endpoints (e.g., skin breakdown incidence rate) or patient-reported outcomes such as comfort and pain should be evaluated in the forthcoming studies.
Finally, the vacuum protocol used a manually controlled sleeve with non-standardized pressure measurement and control. This reduces reproducibility and may not reproduce normal clinical systems. Similarly, CT acquisition had standardized parameters but without repeated trials for measurement variability. These, together with the statistical fallacy of analyzing several slices from one limb as distinct samples, illustrate that the current findings should be interpreted as hypothesis-generating rather than definitive.
The bilateral character of this case study allowed symmetry/asymmetry analysis between vacuum-compressed and control limbs, which were not treated. Morphologically, cross-sectional area profiles were more asymmetric in the proximal and mid-thigh regions, where the vacuum-compressed limb showed up to 5.3% decrease from the contralateral control (
Figure 3). As they progressed towards the distal direction, the two limbs approached one another, indicating the recovery of geometric symmetry under vacuum loading. Mechanically, FEA results indicated that the use of vacuum redistributed contact pressures more evenly across the limb–socket interface (
Figure 4D), reducing the localized peaks (2.42 kPa in control vs. 1.28 kPa for vacuum) and establishing a more symmetrical pressure field. This double observation suggests that while vacuum creates local morphological asymmetry at the tissue level, it also ensures pressure symmetry at the socket interface with the overall effect of increasing load transfer and comfort during walking.
As a patient-specific case study with bilateral residual limbs, the generalizability of these findings is limited and requires validation in larger, more diverse amputee cohorts. The bilateral design reduced inter-subject variability, but systematic studies are still needed to test whether the observed morphological changes persist across different suspension systems, pressure levels, and clinical workflows. The current model also simplifies soft tissue as homogeneous and isotropic. Vacuum compression likely increases apparent tissue stiffness, suggesting that future simulations should include condition-specific elastic modulus values and viscoelastic properties.
Dynamic or time-varying analyses are also needed to capture how vacuum influences limb morphology during gait cycles or under long-term loading. In addition, future studies should integrate patient-reported outcomes such as comfort, pain, and gait performance to validate whether the mechanical advantages predicted by FEA translate into meaningful clinical benefits. These steps would help develop socket design methods that incorporate vacuum-induced morphological adjustments, such as double-scan imaging protocols or computational pipelines that are correct for deformation-driven geometry variation.