Fungal Biocatalysis in Stereoselective Oxidation of 2-Phenylethanol
Abstract
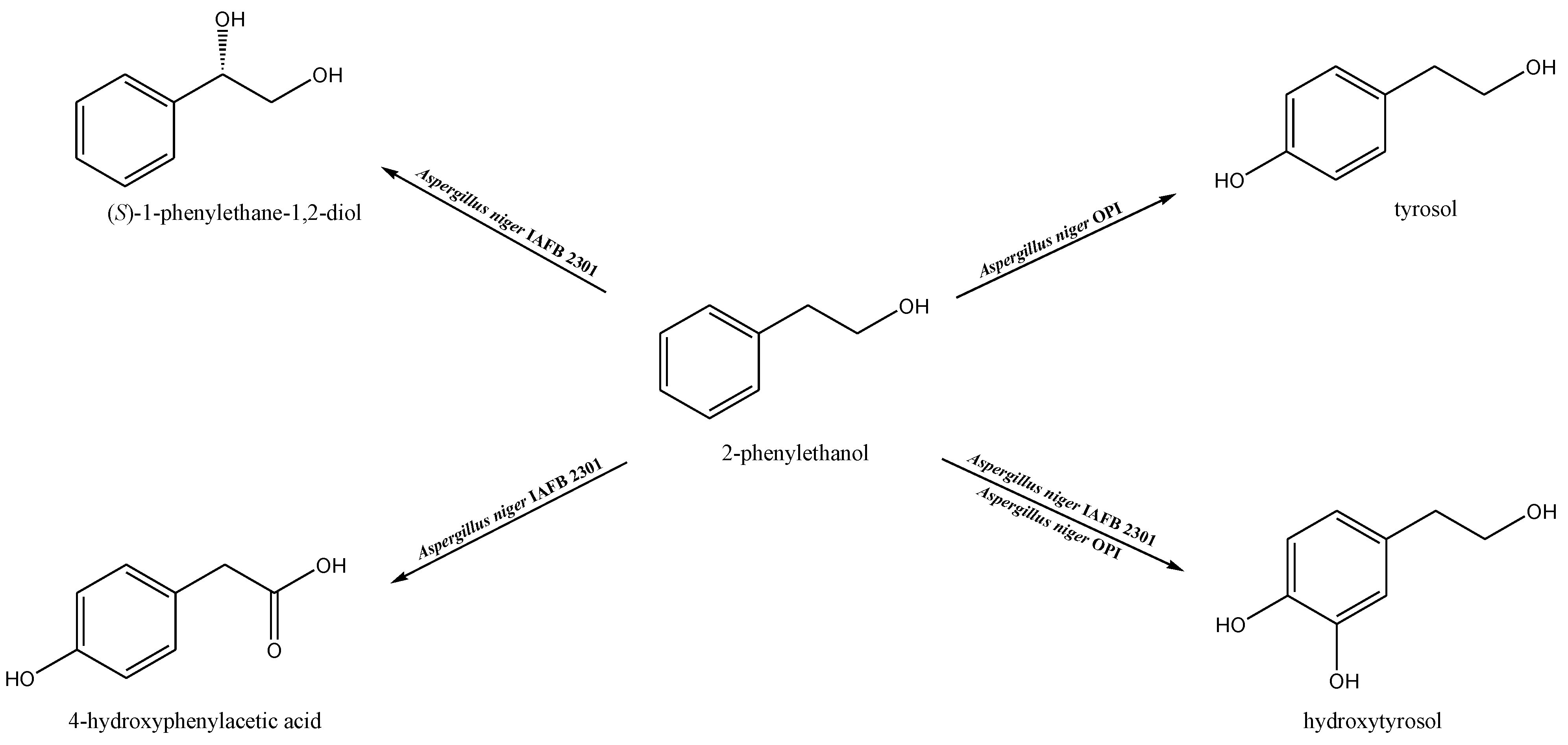
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Cultivation Conditions
2.3.1. Inoculum
2.3.2. Cultivation Conditions—Fresh Biomass of Microorganisms
2.3.3. Immobilization Procedure—Growth of the R. arrhizus and B. brongniartii in the Presence of Polyurethane Foams with Different Porosity
2.3.4. Immobilization of B. bassiana Biomass in Agar-Agar
2.3.5. Immobilization of B. bassiana Biomass in Calcium Alginate
2.3.6. Pre-Incubation of Fresh Biomass of R. arrhizus
2.4. Procedures of Biotransformation
2.5. Semi-Preparative Biotransformation Procedures
2.5.1. Simplified Flow Bioreactor for R. arrhizus
2.5.2. Batch Bioreactor for B. bassiana
2.6. Analytical Methods
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choudhary, M.; Gupta, S.; Dhar, M.K.; Kaul, S. Endophytic Fungi-Mediated Biocatalysis and Biotransformations Paving the Way Toward Green Chemistry. Front. Bioeng. Biotechnol. 2021, 9, 664705. [Google Scholar] [CrossRef] [PubMed]
- Schrewe, M.; Julsing, M.K.; Bühler, B.; Schmid, A. Whole-Cell Biocatalysis for Selective and Productive C–O Functional Group Introduction and Modification. Chem. Soc. Rev. 2013, 42, 6346–6377. [Google Scholar] [CrossRef]
- Madavi, T.B.; Chauhan, S.; Keshri, A.; Alavilli, H.; Choi, K.; Pamidimarri, S.D.V.N. Whole-cell Biocatalysis: Advancements toward the Biosynthesis of Fuels. Biofuels Bioprod. Biorefining 2022, 16, 859–876. [Google Scholar] [CrossRef]
- Schwarz, F.M.; Müller, V. Whole-Cell Biocatalysis for Hydrogen Storage and Syngas Conversion to Formate Using a Thermophilic Acetogen. Biotechnol. Biofuels 2020, 13, 32. [Google Scholar] [CrossRef]
- Raczyńska, A.; Jadczyk, J.; Brzezińska-Rodak, M. Altering the Stereoselectivity of Whole-Cell Biotransformations via the Physicochemical Parameters Impacting the Processes. Catalysts 2021, 11, 781. [Google Scholar] [CrossRef]
- Grogan, G.J.; Holland, H.L. The Biocatalytic Reactions of Beauveria spp. J. Mol. Catal. B Enzym. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Kozłowska, E.; Urbaniak, M.; Hoc, N.; Grzeszczuk, J.; Dymarska, M.; Stępień, Ł.; Pląskowska, E.; Kostrzewa-Susłow, E.; Janeczko, T. Cascade Biotransformation of Dehydroepiandrosterone (DHEA) by Beauveria Species. Sci. Rep. 2018, 8, 13449. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Dmochowska-Gładysz, J.; Bartmańska, A. Transformations of Steroids by Beauveria bassiana. Z. Naturforschung—Sect. C J. Biosci. 2005, 60, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, E.; Matera, A.; Sycz, J.; Kancelista, A.; Kostrzewa-Susłow, E.; Janeczko, T. New 6,19-Oxidoandrostan Derivatives Obtained by Biotransformation in Environmental Filamentous Fungi Cultures. Microb. Cell Fact. 2020, 19, 37. [Google Scholar] [CrossRef]
- Świzdor, A.; Kołek, T.; Panek, A.; Białońska, A. Microbial Baeyer–Villiger Oxidation of Steroidal Ketones Using Beauveria bassiana: Presence of an 11α-Hydroxyl Group Essential to Generation of D-Homo Lactones. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 253–262. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Regioselective O-Glycosylation of Flavonoids by Fungi Beauveria Bassiana, Absidia coerulea and Absidia glauca. Bioorg. Chem. 2019, 93, 102750. [Google Scholar] [CrossRef] [PubMed]
- Perz, M.; Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformation of Flavonoids with -NO2, -CH3 Groups and -Br, -Cl Atoms by Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2023, 24, 9500. [Google Scholar] [CrossRef] [PubMed]
- Łużny, M.; Tronina, T.; Kozłowska, E.; Dymarska, M.; Popłoński, J.; Łyczko, J.; Kostrzewa-Susłow, E.; Janeczko, T. Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2020, 21, 6121. [Google Scholar] [CrossRef]
- Salvi, N.A.; Chattopadhyay, S. Studies on Rhizopus arrhizus Mediated Enantioselective Reduction of Arylalkanones. Tetrahedron 2001, 57, 2833–2839. [Google Scholar] [CrossRef]
- Salvi, N.A.; Patil, P.N.; Udupa, S.R.; Banerji, A. Biotransformations with Rhizopus arrhizus: Preparation of the Enantiomers of 1-Phenylethanol and 1-(Fo-, m- and p-Methoxyphenyl)Ethanols. Tetrahedron Asymmetry 1995, 6, 2287–2290. [Google Scholar] [CrossRef]
- Salokhe, P.R.; Salunkhe, R.S. Rhizopus arrhizus Mediated SAR Studies in Chemoselective Biotransformation of Haloketones at Ambient Temperature. Biocatal. Biotransform. 2022, 40, 219–225. [Google Scholar] [CrossRef]
- Song, K.-N.; Lu, Y.-J.; Chu, C.-J.; Wu, Y.-N.; Huang, H.-L.; Fan, B.-Y.; Chen, G.-T. Biotransformation of Betulonic Acid by the Fungus Rhizopus arrhizus CGMCC 3.868 and Antineuroinflammatory Activity of the Biotransformation Products. J. Nat. Prod. 2021, 84, 2664–2674. [Google Scholar] [CrossRef]
- Holland, H.L. Biotransformations of .DELTA.4-3-Ketosteroids by the Fungus Rhizopus arrhizus. Acc. Chem. Res. 1984, 17, 398–402. [Google Scholar] [CrossRef]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of Bioactive Compounds Extracted from Olive Industry Wastes: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef]
- Heath, R.S.; Ruscoe, R.E.; Turner, N.J. The Beauty of Biocatalysis: Sustainable Synthesis of Ingredients in Cosmetics. Nat. Prod. Rep. 2022, 39, 335–388. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Doyle, E.M.; O’Connor, K.E. Tyrosol to Hydroxytyrosol Biotransformation by Immobilised Cell Extracts of Pseudomonas putida F6. Enzyme Microb. Technol. 2006, 39, 191–196. [Google Scholar] [CrossRef]
- Anissi, J.; Sendide, K.; Ouardaoui, A.; Benlemlih, M.; El Hassouni, M. Production of Hydroxytyrosol from Hydroxylation of Tyrosol by Rhodococcus pyridinivorans 3HYL DSM109178. Biocatal. Biotransformation 2021, 39, 418–428. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. Biotechnological Approaches to Enhance Salidroside, Rosin and Its Derivatives Production in Selected Rhodiola spp. in Vitro Cultures. Phytochem. Rev. 2015, 14, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Han, T. Effects of Salidroside Pretreatment on Expression of Tumor Necrosis Factor-Alpha and Permeability of Blood Brain Barrier in Rat Model of Focal Cerebralischemia-Reperfusion Injury. Asian Pac. J. Trop. Med. 2013, 6, 156–158. [Google Scholar] [CrossRef]
- Zhong, Z.; Han, J.; Zhang, J.; Xiao, Q.; Hu, J.; Chen, L. Pharmacological Activities, Mechanisms of Action, and Safety of Salidroside in the Central Nervous System. Drug Des. Devel. Ther. 2018, 12, 1479–1489. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, Y.; Li, F.-Z.; Ou, X.-Y.; Zeng, Y.-J.; Zong, M.-H.; Lou, W.-Y. Highly Enantioselective Resolution of Racemic 1-Phenyl-1,2-Ethanediol to (S)-1-Phenyl-1,2-Ethanediol by Kurthia gibsonii SC0312 in a Biphasic System. J. Biotechnol. 2020, 308, 21–26. [Google Scholar] [CrossRef]
- Peng, F.; Ou, X.-Y.; Zhao, Y.; Zong, M.-H.; Lou, W.-Y. Highly Selective Resolution of Racemic 1-phenyl-1,2-ethanediol by a Novel Strain Kurthia gibsonii SC 0312. Lett. Appl. Microbiol. 2019, 68, 446–454. [Google Scholar] [CrossRef]
- He, Y.-C.; Ma, C.-L.; Zhang, X.; Li, L.; Xu, J.-H.; Wu, M.-X. Highly Enantioselective Oxidation of Racemic Phenyl-1,2-Ethanediol to Optically Pure (R)-(−)-Mandelic Acid by a Newly Isolated Brevibacterium lutescens CCZU12-1. Appl. Microbiol. Biotechnol. 2013, 97, 7185–7194. [Google Scholar] [CrossRef]
- Trost, B.M.; Belletire, J.L.; Godleski, S.; McDougal, P.G.; Balkovec, J.M.; Baldwin, J.J.; Christy, M.E.; Ponticello, G.S.; Varga, S.L.; Springer, J.P. On the Use of the O-Methylmandelate Ester for Establishment of Absolute Configuration of Secondary Alcohols. J. Org. Chem. 1986, 51, 2370–2374. [Google Scholar] [CrossRef]
- Poterała, M.; Dranka, M.; Borowiecki, P. Chemoenzymatic Preparation of Enantiomerically Enriched (R)-(–)-Mandelic Acid Derivatives: Application in the Synthesis of the Active Agent Pemoline. Eur. J. Org. Chem. 2017, 2017, 2290–2304. [Google Scholar] [CrossRef]
- Lima, J.J.; Aguilar, A.; Sánchez, F.G.; Díaz, A.N. Enantiomeric Fraction of Styrene Glycol as a Biomarker of Occupational Risk Exposure to Styrene. Chemosphere 2017, 168, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Zhang, R.; Zhang, B.; Xiao, R. Improvement of (R)-Carbonyl Reductase-Mediated Biosynthesis of (R)-1-Phenyl-1,2-Ethanediol by a Novel Dual-Cosubstrate-Coupled System for NADH Recycling. Process Biochem. 2012, 47, 1060–1065. [Google Scholar] [CrossRef]
- Nie, Y.; Xu, Y.; Mu, X.Q. Highly Enantioselective Conversion of Racemic 1-Phenyl-1,2-Ethanediol by Stereoinversion Involving a Novel Cofactor-Dependent Oxidoreduction System of Candida parapsilosis CCTCC M203011. Org. Process Res. Dev. 2004, 8, 246–251. [Google Scholar] [CrossRef]
- Pandey, R.K.; Fernandes, R.A.; Kumar, P. An Asymmetric Dihydroxylation Route to Enantiomerically Pure Norfluoxetine and Fluoxetine. Tetrahedron Lett. 2002, 43, 4425–4426. [Google Scholar] [CrossRef]
- Cui, Z.-M.; Zhang, J.-D.; Fan, X.-J.; Zheng, G.-W.; Chang, H.-H.; Wei, W.-L. Highly Efficient Bioreduction of 2-Hydroxyacetophenone to (S)- and (R)-1-Phenyl-1,2-Ethanediol by Two Substrate Tolerance Carbonyl Reductases with Cofactor Regeneration. J. Biotechnol. 2017, 243, 1–9. [Google Scholar] [CrossRef]
- Cao, L.; Lee, J.; Chen, W.; Wood, T.K. Enantioconvergent Production of (R)-1-phenyl-1,2-ethanediol from Styrene Oxide by Combining the Solanum Tuberosum and an Evolved Agrobacterium radiobacter AD1 Epoxide Hydrolases. Biotechnol. Bioeng. 2006, 94, 522–529. [Google Scholar] [CrossRef]
- Peng, F.; Su, H.-H.; Ou, X.-Y.; Ni, Z.-F.; Zong, M.-H.; Lou, W.-Y. Immobilization of Cofactor Self-Sufficient Recombinant Escherichia Coli for Enantioselective Biosynthesis of (R)-1-Phenyl-1,2-Ethanediol. Front. Bioeng. Biotechnol. 2020, 8, 17. [Google Scholar] [CrossRef]
- Peng, F.; Chen, Q.-S.; Li, F.-Z.; Ou, X.-Y.; Zong, M.-H.; Lou, W.-Y. Using Deep Eutectic Solvents to Improve the Biocatalytic Reduction of 2-Hydroxyacetophenone to (R)-1-Phenyl-1,2-Ethanediol by Kurthia gibsonii SC0312. Mol. Catal. 2020, 484, 110773. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Chen, X.; Jia, S.; Wu, Q.; Zhu, D.; Ma, Y. Highly Enantioselective Double Reduction of Phenylglyoxal to (R)-1-Phenyl-1,2-Ethanediol by One NADPH-Dependent Yeast Carbonyl Reductase with a Broad Substrate Profile. Tetrahedron 2013, 69, 3561–3564. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Natural Antioxidants–Properties and Possible Applications. J. Appl. Biotechnol. Bioeng. 2018, 5, 251–258. [Google Scholar] [CrossRef]
- Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Majewska, P.; Żymańczyk-Duda, E. Half-Preparative Scale Synthesis of (S)-1-Phenylethane-1,2-Diol as a Result of 2-Phenylethanol Hydroxylation with Aspergillus niger (IAFB 2301) Assistance. Symmetry 2020, 12, 989. [Google Scholar] [CrossRef]
- Głąb, A.; Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Żymańczyk-Duda, E. Biotransformation of 1- and 2-Phenylethanol to Products of High Value via Redox Reactions. BioTechnologia 2016, 3, 203–210. [Google Scholar] [CrossRef]
- Lubiak-Kozłowska, K.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Olszewski, T.K.; Serafin-Lewańczuk, M.; Żymańczyk-Duda, E. (S)-Thienyl and (R)-Pirydyl Phosphonate Derivatives Synthesized by Stereoselective Resolution of Their Racemic Mixtures With Rhodotorula Mucilaginosa (DSM 70403)—Scaling Approaches. Front. Chem. 2020, 8, 589720. [Google Scholar] [CrossRef] [PubMed]
- Serafin-Lewańczuk, M.; Brzezińska-Rodak, M.; Lubiak-Kozłowska, K.; Majewska, P.; Klimek-Ochab, M.; Olszewski, T.K.; Żymańczyk-Duda, E. Phosphonates Enantiomers Receiving with Fungal Enzymatic Systems. Microb. Cell Fact. 2021, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell Immobilization Strategies for Biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Zingaro, K.A.; Nicolaou, S.A.; Papoutsakis, E.T. Dissecting the Assays to Assess Microbial Tolerance to Toxic Chemicals in Bioprocessing. Trends Biotechnol. 2013, 31, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A Comparative View of Metabolite and Substrate Stress and Tolerance in Microbial Bioprocessing: From Biofuels and Chemicals, to Biocatalysis and Bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ullrich, R. Oxidations Catalyzed by Fungal Peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Stereoselective Benzylic Hydroxylation of Alkylbenzenes and Epoxidation of Styrene Derivatives Catalyzed by the Peroxygenase of Agrocybe aegerita. Green Chem. 2012, 14, 440–446. [Google Scholar] [CrossRef]
- Velasco, B.R.; Gil, G.J.H.; García, P.C.M.; Durango, R.D.L. Production of 2-Phenylethanol in the Biotransformation of Cinnamyl Alcohol by the Plant Pathogenic Fungus Colletotrichum acutatum. Vitae 2010, 17, 272–280. [Google Scholar] [CrossRef]
- Fox, E.M.; Howlett, B.J. Secondary Metabolism: Regulation and Role in Fungal Biology. Curr. Opin. Microbiol. 2008, 11, 481–487. [Google Scholar] [CrossRef]
- Yu, J.-H.; Keller, N. Regulation of Secondary Metabolism in Filamentous Fungi. Annu. Rev. Phytopathol. 2005, 43, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Schmidt-Heydt, M.; Rodríguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of Environmental Stress on Growth, Secondary Metabolite Biosynthetic Gene Clusters and Metabolite Production of Xerotolerant/Xerophilic Fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, K.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Żymańczyk-Duda, E. Biocatalyzed Kinetic Resolution of Racemic Mixtures of Chiral α-Aminophosphonic Acids. J. Mol. Catal. B Enzym. 2013, 91, 32–36. [Google Scholar] [CrossRef]
- Krohn, N.G.; Brown, N.A.; Colabardini, A.C.; Reis, T.; Savoldi, M.; Dinamarco, T.M.; Goldman, M.H.S.; Goldman, G.H. The Aspergillus nidulans ATM Kinase Regulates Mitochondrial Function, Glucose Uptake and the Carbon Starvation Response. G3 Genes|Genomes|Genet. 2014, 4, 49–62. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-17392-9. [Google Scholar]
- Ratledge, C.; Kristiansen, B. Basic Biotechnology; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780511802409. [Google Scholar]
- Bonin, S. Mikroorganizmy Immobilizowane. Agro Przemysł 2008, 6, 20–23. [Google Scholar]
- Hirano, J.; Miyamoto, K.; Ohta, H. Purification and Characterization of Aldehyde Dehydrogenase with a Broad Substrate Specificity Originated from 2-Phenylethanol-Assimilating Brevibacterium sp. KU1309. Appl. Microbiol. Biotechnol. 2007, 76, 357–363. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hirano, J.; Ohta, H. Efficient Oxidation of Alcohols by a 2-Phenylethanol-Degrading Brevibacterium sp. Biotechnol. Lett. 2004, 26, 1385–1388. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Tarafdar, A.; Sirohi, R.; Anoopkumar, A.N.; Kuriakose, L.L.; Awasthi, M.K.; Binod, P.; Varjani, S.; Sindhu, R. Filamentous Fungi for Pharmaceutical Compounds Degradation in the Environment: A Sustainable Approach. Environ. Technol. Innov. 2023, 31, 103182. [Google Scholar] [CrossRef]



| Product | (R)-1-Phenylethane-1,2-diol | (R,S)-1-Phenylethane-1,2-diol | Tyrosol | |
|---|---|---|---|---|
| Biocatalyst | ||||
| B. bassiana DSM 1344 | fresh biomass in flasks | immobilized biomass (agar-agar) | ||
| fresh biomass in bioreactor | ||||
| R. arrhizus DSM 1185 | fresh biomass | fresh biomass | ||
| immobilized biomass (polyurethane foams) | immobilized biomass (polyurethane foams) | |||
| B. brongniartii DSM 6651 | 2-phenylethanol degradation—no desired product formation | |||
| (R)-1-Phenylethane-1,2-diol (e.e. 99.9%) [mg/600 mL] | ||
|---|---|---|
| Strain | Biotransformation Time [Days] | Product [mg] |
| B. bassiana (fresh cells) | 1 | 12.9 (±0.095) |
| 2 | 17.6 (±0.110) | |
| 3 | 28.8 (±0.078) | |
| 4 | 10.0 (±0.130) | |
| 5 | 5.1 (±0.075) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raczyńska, A.; Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Żymańczyk-Duda, E. Fungal Biocatalysis in Stereoselective Oxidation of 2-Phenylethanol. Symmetry 2025, 17, 17. https://doi.org/10.3390/sym17010017
Raczyńska A, Szmigiel-Merena B, Brzezińska-Rodak M, Klimek-Ochab M, Żymańczyk-Duda E. Fungal Biocatalysis in Stereoselective Oxidation of 2-Phenylethanol. Symmetry. 2025; 17(1):17. https://doi.org/10.3390/sym17010017
Chicago/Turabian StyleRaczyńska, Agnieszka, Beata Szmigiel-Merena, Małgorzata Brzezińska-Rodak, Magdalena Klimek-Ochab, and Ewa Żymańczyk-Duda. 2025. "Fungal Biocatalysis in Stereoselective Oxidation of 2-Phenylethanol" Symmetry 17, no. 1: 17. https://doi.org/10.3390/sym17010017
APA StyleRaczyńska, A., Szmigiel-Merena, B., Brzezińska-Rodak, M., Klimek-Ochab, M., & Żymańczyk-Duda, E. (2025). Fungal Biocatalysis in Stereoselective Oxidation of 2-Phenylethanol. Symmetry, 17(1), 17. https://doi.org/10.3390/sym17010017