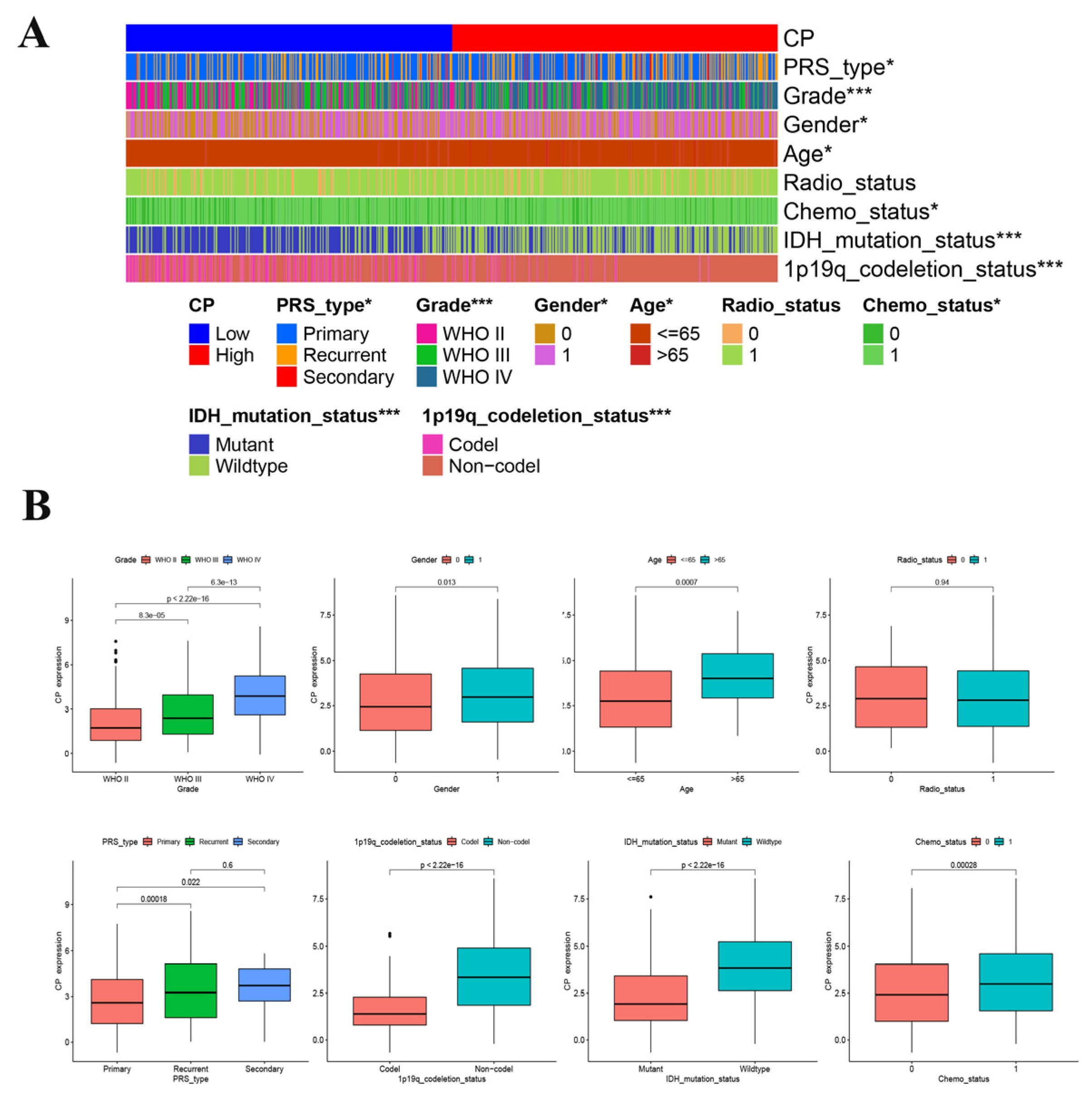
1. Introduction
Graphene is a honeycomb two-dimensional planar carbon nanomaterial composed of only one thick carbon atom, so it has a theoretical specific surface area of more than 2600 m
2/g and can realize the absorption and desorption of various substances [
1]. It can be challenging to synthesize a single layer of graphene, but even graphene with a small number of layers can have a significant specific surface area after ultrasonic treatment. This results in higher adsorption capacity than that of typical adsorption materials. The precursor graphene monoxide surface has many functional groups such as epoxy groups. These functional groups are hydrophilic and can form hydrogen bonds or donor–acceptor complexes with many substances, thus introducing other functional groups or new substances to further enhance water solubility, adsorption capacity and endow graphene with new characteristics [
2]. Although the unique two-dimensional structure of graphene endows it with its own unique properties, it has serious agglomeration. It can be challenging for graphene to fulfill its potential when it consists of dozens or even hundreds of layers [
3]. Currently, scientists and researchers are focusing on creating graphene with only a few layers or even a single layer, ensuring high purity and minimal structural defects. Their synthesis methods are also different. At present, more mature preparation methods are mainly micromechanical stripping, solvent stripping, chemical vapor deposition (CVD), epitaxial growth, and chemical redox [
4]. GO was used to investigate using group absorption, a kinetic absorption model, a model based on isotherms, and the van’t Hoff equation.
To prepare GO, perchlorate is commonly used as an oxidant. It is added to a mixture containing fuming nitric acid and graphite. This process creates graphene oxide. The reaction system is first maintained at 0 °C and then heated to 60–80 °C, and the reaction system is continuously stirred for about 24 h [
5]. The oxidation degree of the product obtained using this method is relatively low. If aiming to obtain graphite oxide with a higher oxidation degree, it may be necessary to extend the reaction time. However, it is essential to note that using perchlorate as an oxidant in the reaction process can pose a certain level of risk [
6]. The method of preparation of GOES using the Brodie method has an advantage in that the oxidation degree can be controlled with the oxidation time. Through the optimization and improvement of this method, the preparation of graphene oxide can be achieved more efficiently. Using perchlorate as an oxidant, graphite powder is acidified with a mixed concentrated sulfuric acid and fuming nitric acid. During the reaction, the temperature of the system is maintained at 0 °C, and the reaction time is about 56 h. The reaction time of this method is relatively long, and the oxidation degree of the product obtained is also very low [
7]. If an attempt is made to improve the oxidation degree by prolonging the reaction time, the carbon layer of the obtained product is seriously damaged. In addition, many toxic gases such as ClO
2 and NO
2 are produced in the reaction process, which also pollutes the environment. Using potassium permanganate instead of perchlorate as an oxidant, graphite was oxidized using a concentrated sulfuric acid and sodium nitrate system. Using permanganate as an oxidant, the safety of the reaction was improved, and products with high oxidation degree and regular structure could be obtained. The toxic gas generated during the reaction process was relatively reduced, causing less environmental pollution [
8]. Hummer’s method is a popular technique for preparing GO. It is known to require an oxidation time of approximately 5 h and has significantly shortened reaction times. Hummer’s method is currently one of the most widely used methods for this purpose. As a new type of carbon material with excellent performance, graphene oxide not only has a high specific surface area, but also has rich oxygen-containing functional groups on its surface, of which –OH, C=O, and COOH functional groups account for more than 60%. The high specific surface area of graphene oxide also makes it an ideal adsorption material, which can be used for treatment of environmental wastewater [
9]. Moreover, compared with other carbon nanomaterials, graphene oxide has good water solubility and rich functional groups, which make its surface easy to chemically modify, and plays an important role in improving the comprehensive properties of materials. In recent years, with an increasingly serious environmental pollution problem, more and more researchers have begun to study and discuss the application of graphene oxide and its composites in environmental wastewater treatment due to its unique structure and good water solubility. The competition between adsorption–desorption of fluorescent agent rhodamine 6G and dopamine on graphene oxide was studied [
10]. It was shown that graphene oxide exhibits fast adsorption performance for both organics due to its hydrophilicity and multiple oxygen-containing functional groups. There are many noncovalent forces between dopamine molecules and graphene oxide layers, including bonding and meta-m forces. However, there is only the m-m force between the rhodamine 6G and graphene oxide layer, and there is no hydrogen bond force. Therefore, in terms of dopamine and rhodamine 6G, graphene oxide has stronger adsorption capacity for the former than the latter.
In this paper, using the large specific surface area of graphene and its high adsorption capacity for aromatic ring-containing pollutants, we prepared graphene/polymer composites by edge-grafting a modified polyaniline macromolecular chain [
11]. Because a polyaniline molecular chain contains many N atoms, it has a good complexing effect on heavy metals. Graphene/polyaniline composites are expected to have good adsorption performance for the heavy metal Pb (II) and have high adsorption performance for the aromatic ring contaminant methylene blue. It is a good adsorbent in wastewater treatment where heavy metals and aromatic pollutants coexist.
3. Results
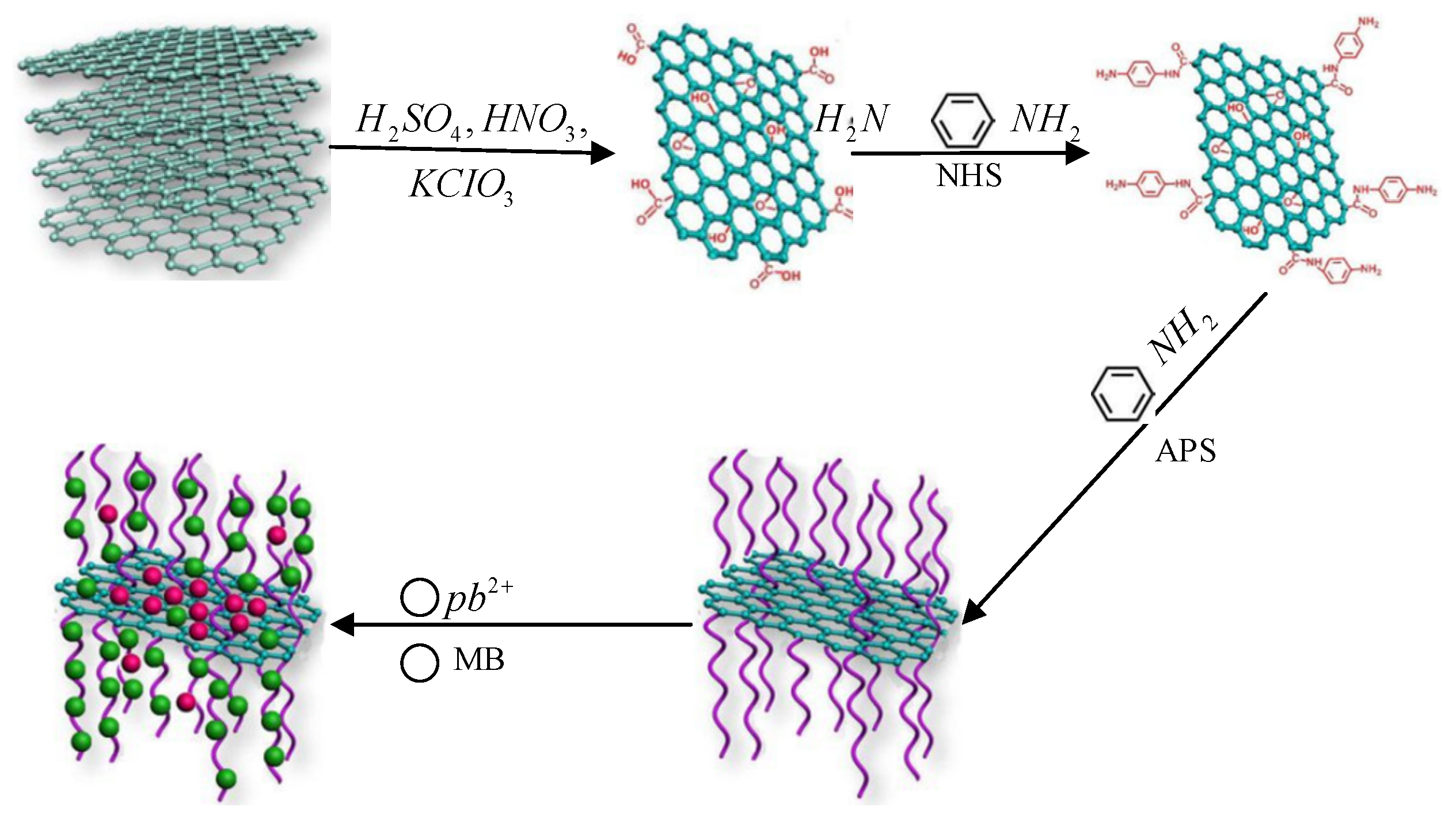
In this study, we used oxygen on a GO surface to attach-NH2 of phenylenediamine as a site and then conducted oxidative graft polymerization of aniline. The authors in [
13] thoroughly reviewed magnetic graphene application. The traditional PANI has a granular structure, and its morphology and performance are not as good as those of rod PANI. The main difference between the two in preparation is that the oxidant of the former is added slowly, while the oxidant of the latter is added quickly. The synthetic route of the product is shown in
Figure 1. An oxidizing agent was added during the polymerization process to prepare GO-PANI nanocomposites.
Figure 1 illustrates the synthesis of the nanocomposites. GO is produced as a starting point for a material with oxygen-containing functional groups. GO surfaces are then modified by attaching amino groups to compounds, such as phenylenediamine (-NH2). The amine-functionalized GO was then oxidatively polymerized with aniline monomers. There are two cases presented, one with slow addition and one with rapid addition of the oxidizing agent. GO-PANI nanocomposite was formed by the growth of polyaniline chains on the GO surfaces. After washing to remove the unreacted components or byproducts, the nanocomposites were dried to stabilize them. Finally, various characterization techniques, such as XRD, were employed to analyze the properties of the GO-PANI nanocomposites.
Figure 1 emphasizes the contrasting approaches for adding oxidizing agents at different rates and the polymerization process.
Figure 2 is an AFM image of GO. It can be seen from the figure that the GO layer is about 0.8 nm, which is consistent with the XRD result of 0.72 nm, indicating that the graphene oxide layer is a single layer.
The composite structure was characterized using FT-IR [
14]. As shown in
Figure 3.
Figure 3 is the infrared spectrum of GO. It can be seen from figure that a wide peak at 3000–3650 cm
−1 is caused by stretching vibration of light functional groups on GO, and absorption peaks at 1725 cm
−1 and 1645 cm
−1 are caused by stretching vibration of carbon oxygen double bonds of carboxyl and pulp on GO lamella [
15]. The absorption peaks at 1385 cm
−1 and 1053 cm
−1 are caused by stretching vibration of carbon oxygen single-bond in carboxyl, epoxy, and alkoxy groups on the GO layer [
16].
Figure 3B shows GO (GO-NH2) modified with p-phenylenediamine. There is no absorption peak near 1725 cm
−1, indicating that the stretching vibration peak of C=O in several bases on the GO sheet layer disappears. At same time, strong absorption peaks at 1645 cm
−1 and 1300 cm
−1 correspond to the stretching vibration of C=O in an amide bond and C–N stretching vibration, respectively, indicating that p-phenylenediamine is grafted to the surface of the GO sheet layer through covalent bonding [
17]. The absorption peak at 1514 cm
−1 is caused by C=C on the benzene ring, and the absorption peak at 775 cm
−1 is caused by the bending vibration outside the N-H bond plane. These characteristic absorption peaks indicate that p-phenylenediamine is successfully grafted onto the GO surface [
18].
Figure 3C shows the infrared spectrum of GO-PANI. The characteristic absorption peaks at 1578 cm
−1 and 1491 cm
−1 are caused by the awakening ring on doped polyaniline and C=C stretching vibration on the benzene ring [
19]. The absorption peak at 1128 cm
−1 is a characteristic absorption peak of wake-up type in doped polyaniline. The absorption peak at 1297 cm
−1 is caused by stretching vibration of C-N on aromatic amine [
20]. The absorption peak at 797 cm
−1 is caused by a C–H out-of-plane bending vibration of the disubstituted benzene ring. The existence of the PANI characteristic absorption peak indicates that PANI grafted successfully onto the GO layer [
21].
Figure 4 is XRD diffraction spectrum of composite. In
Figure 4, at 20 = 12.3°, a narrow and strong absorption peak appears in the GO, and the corresponding layer spacing of the GO is 0.72 nm. This peak belongs to reflection peak of 001 crystal plane, which is related to the preparation method of GO and the number of graphene layers [
22]. The diffraction peak at 20 = 42.0 ° belongs to a graphite-like structure of the graphene 100 crystal plane [
23]. After reaction of p-phenylenediamine with GO, the 001 diffraction peak of graphene oxide lamella moves 20 = 10.2 ° to low angle, and the corresponding layer spacing is 0.87 nm (
Figure 4). The increase in interlayer spacing may be caused by the insertion of p-phenylenediamine and the reaction between layers [
24]. At same time, in the curve of
Figure 4, 20 = 21.3. A new broad peak appears, which is the diffraction peak of the reduced graphene oxide. The distance between the graphene sheets is 0.42 nm. After reaction of p-phenylenediamine with GO, some GO is reduced [
25]. In
Figure 4, characteristic diffraction peaks of PANI appeared at 20 = 15.0°, 20 = 20.1°, and 20 = 25.2°, respectively, corresponding to 011020 of PANI and diffraction peaks of 200 crystal planes. In the GO-PANI composite (
Figure 4), a GO characteristic diffraction peak disappears, and graphene is 20 = 42.0. The characteristic peak of GO-PANI also disappears, indicating that graphene oxide in GO-PANI is in a stripped state, and there is no aggregation structure. Compared with pure PANI, a characteristic diffraction peak of PANI appears in GO-PANI, indicating that the crystallinity of PANI in composite is low.
Figure 5 is Raman spectrum of GO, GO-PANI, and PANI. As shown in
Figure 5A, the G-band at 1598 cm
−1 is caused by sp2 hybrid vibration of carbon atoms in a two-dimensional graphite hexagonal system. The D-band at 1314 cm
−1 is caused by presence of sp3 hybrid carbon atoms due to defects in the graphene. In the GO-PANI composite (
Figure 5B), compared with the GO, two new absorption peaks appeared at 1174 cm
−1 and 1503 cm
−1 due to the introduction of a PANI molecular chain, corresponding to awakening C–H vibration and C=C stretching vibration in the PANI, respectively. It showed that PANI was successfully grafted onto the GO layer.
Figure 6 shows TGA spectra of GO, GO-NH2, GO-PANI, and PANI, respectively. This radar representation illustrates the temperature of thermal weight loss in different groups from Group A to Group F.
Figure 6A shows the weight loss curve of the GO. It can be seen from the curve that the weight loss of GO below 150 °C is about 5 wt. %, which is caused by desorption of water molecules adsorbed on the GO. The weight loss between 150 °C and 600 °C is about 18 wt. %, which is mainly caused by decomposition of oxygen-containing functional groups such as COOH and OH on the GO surface. When p-phenylenediamine is grafted onto the GO surface, weight loss of GO-NH2 is about 34 wt. %, as shown in
Figure 6B.
Figure 6C shows the thermal weight loss curve of PANI. There are two parts of weight loss between 200 °C and 600 °C, which are, respectively, caused by loss of doped acids on the PANI chain and the decomposition of macromolecular chains. When the PANI is grafted onto the GO surface, the initial decomposition temperature of the PANI molecular chain is about 457 °C, which is higher than that of the PANI (412 °C). This shows that the thermal stability of the GO-PANI composite is higher than that of PANI due to the presence of GO lamella.
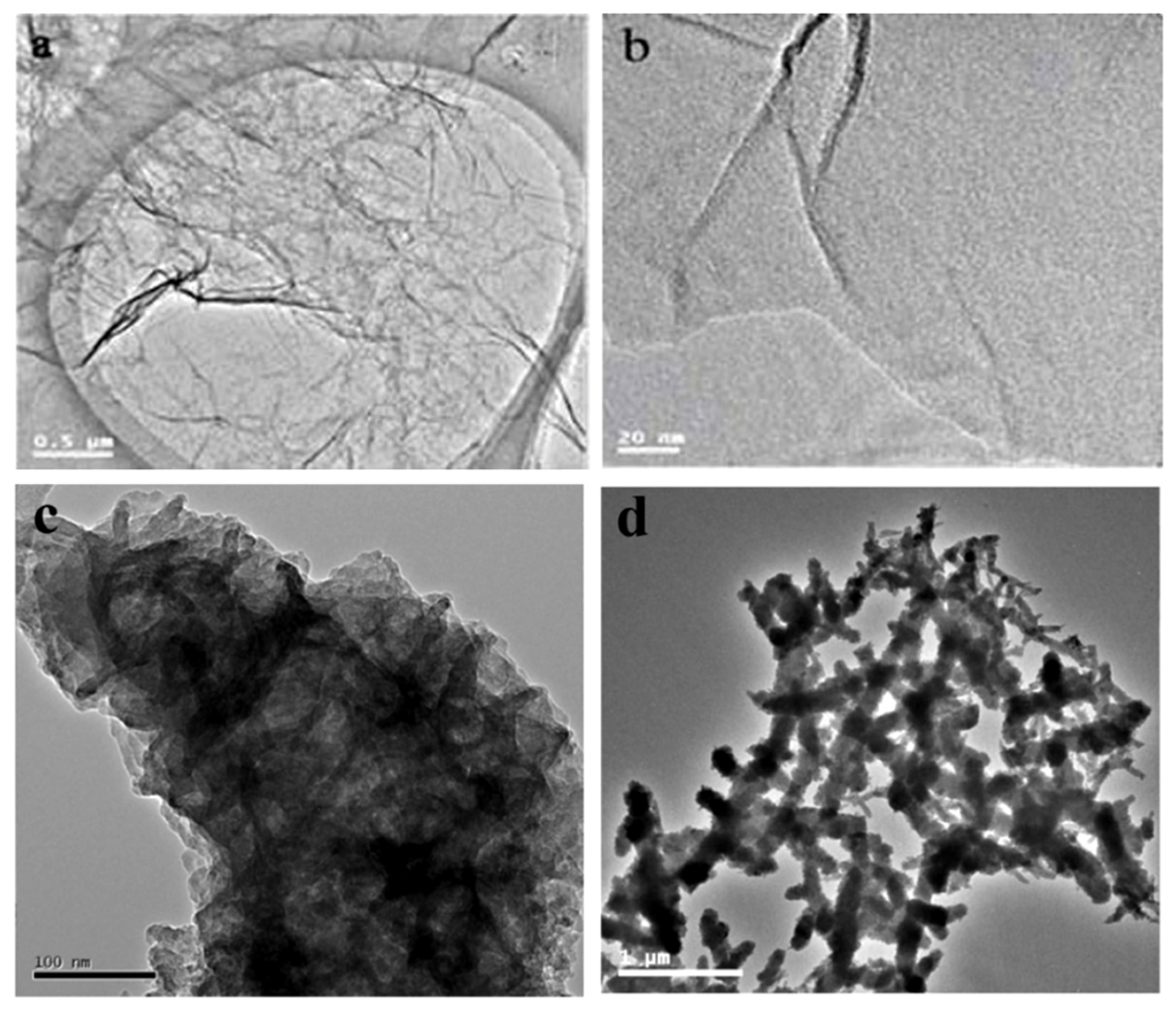
TEM was used to observe the micromorphology of GO, PANI, and GO-PANI, respectively. The specific structure is shown in
Figure 7.
Figure 7a is a TEM diagram of synthesized GO,
Figure 7b is a TEM diagram of high-magnification GO, and large slices of GO with nanometer thickness can be observed.
Figure 7c is a TEM diagram of the GO-PANI composite. Due to II-II interaction, the GO-PANI composite presents a large agglomeration state, and an obvious black rod-like structure can be observed on the surface of the graphene, which is similar to the rod-like structure of pure PANI (
Figure 7d).
In this study, we examined the adsorption behavior of lead ions (Pb (II)) at different concentrations using both PANI and GO-PANI. Using these experimental data, van der Waals equation curves were plotted (ln(qe/Ce) versus 1/T) for both materials. As shown in Equation (1), the van der Waals equation was derived. The linear relationship between ln(qe/Ce) and 1/T for PANI and GO-PANI can be seen in straight lines when plotted against 1/T. The −ΔH/R and ΔS/R represent the slope and intercept of a straight line, respectively. Based on the results, both PANI and GO-PANI absorb lead ions effectively in water treatment applications, especially when the temperature is high. According to these findings, water purification can be achieved with graphene oxide–natural polymer composite materials.
Figure 8 and
Figure 9 are van der Waals equation curves for Pb (II) using GO-PANI and PANI, respectively. The thermodynamic parameters ΔH, ΔS, and ΔG are calculated for PANI and GO-PANI for ΔS at different temperatures. The value of ln (q
e/C
e) can be calculated from van der Waals equation.
R refers to gas constant (8.314 J mol
−1 K
−1), and T refers to absolute temperature (K). A straight line is drawn by ln (q
e/C
e) to 1/T, and the slope and intercept of the line correspond to −ΔH/R and ΔS/R, respectively. The calculated thermodynamic parameters are listed in
Table 4. ΔH is a positive value, indicating that adsorption of PANI and GO-PANI to Pb (II) is an endothermic process. The higher ΔH (ΔH 40 KJ/mol) indicates that adsorption of Pb (II) using PANI and GO-PANI is a chemisorption-dominated process. This result is consistent with quasi-second-order kinetic results. ΔS and ΔH are positive, indicating that PANI and GO-PANI absorb Pb (II), and increased temperature is conducive to the adsorption reaction.
The change in Gibbs free energy caused during adsorption process is calculated according to following formula:
As shown in
Table 4, AG is negative at 288 K, 298 K, and 308 K, indicating that adsorption of Pb (II) using PANI and GO-PANI is a spontaneous adsorption process. The AG of GO-PANI when adsorbing Pb (II) is smaller than that of PANI when adsorbing Pb (II), indicating that GO-PANI absorbs Pb (II) more easily and spontaneously than PANI.
To give full play to excellent adsorption performance of the large layer of graphene oxide on aromatic organic compounds, we grafted polyaniline on edge of graphene in this work and studied adsorption performance of GO-PANI on heavy metal ions and aromatic organic compounds in coexistence system.
Figure 10 shows the co-adsorption experiment of MB (500 mg/L) and Pb (II) (250 mg/L) with 0.6 g/LPANI-GO adsorbent. The experiment shows that GO-PANI has good adsorption capacity for MB and Pb (II) in the coexistence system of MB and Pb (II), and the maximum adsorption capacity is 544 mg/g and 1004 mg/g, respectively. The adsorption capacity of GO-PANI for MB and Pb (II) alone is 734.4 mg/g and 1145 mg/g, and adsorption capacity of GO-PANI for MB and Pb (II) in the co-adsorption system is slightly lower than that in the single adsorption system, which may be the reason why the cationic dyes and metal cations compete in adsorption. When PANI is adsorbent, the maximum adsorption capacity of MB and Pb (II) in the co-adsorption system is 272 mg/g and 301 mg/g, respectively, which is slightly lower than maximum adsorption capacity of polyaniline (401 mg/g and 326 mg/g) for MB and Pb (II). The experimental results show that GOES has good adsorption capacity for MB and Pb (II) in the coexistence system after edge grafting of PANI.
4. Discussion
Water treatment with natural GO polymer composite materials has gained significant interest in recent years. There are many important characteristics associated with the two-dimensional material GO, including its large surface area, powerful adsorption capabilities, and high mechanical properties [
3,
4,
5,
6,
7,
8,
9,
10]. Integrating natural polymers further improves the environmental friendliness of this composite material for the treatment of water. As part of the treatment process, color, organic pollutants, and heavy metals are removed from the water. A substantial surface area and an affinity for adsorption make GO ideal for adsorption. Natural polymers can be added to the GO matrix to increase the adsorption capacity and selectivity of the composite adsorbent [
1,
6]. Natural polymers are ecofriendly, recyclable, and biodegradable since they are nontoxic, renewable, and biodegradable. Several interactions can also occur between these compounds and pollutants, such as electrostatic interactions, hydrogen bonds, π–π interactions, and stacking occurring between the functional groups. Water treatment applications can benefit from natural polymers and GO in combination. This composite material has the ability to effectively purify water while addressing the problems caused by water pollution [
4,
5]. Ultimately, this can assist in supplying communities with clean and safe water.
Numerous dyes have been used to achieve significant economic gains. However, large amounts of dye wastewater are released into environmental water sources, thereby contaminating natural water sources [
11]. The nitration and iodization of benzene, toluene, tea, and other raw materials to form intermediates, followed by the re-ammonization, coupling, and vulcanization of these intermediates, produces an industrial effluent. Due to the variety of dyes, pigments, and intermediates produced, the nature of the wastewater differs. Generally, they can be divided into acidic and alkaline wastewater. Many pollutants, such as sulfonation, nitrification, hydrogenation, reduction, oxidation, and acid (salt) precipitation, are generated during the dye production process. It is estimated that 90% of inorganic raw materials and 10–30% of organic raw materials in dye production are transferred to water, which is characterized by a high pollutant concentration, complex composition, high COD, high chroma, and strong alkalinity, and is one of the key environmental pollutants [
18,
19,
20]. Therefore, effective degradation and treatment of dyes is an essential prerequisite for treating dye wastewater. Adsorbents have become the preferred material for industrial dye wastewater treatment applications because of their unique physical and chemical properties, powerful adsorption capacity, simplicity of preparation, controllable morphology and size, excellent strength, and easy regeneration. Many adsorbents with different controllable sizes, morphologies, and multifunctions have been designed, synthesized, and applied to the adsorption of various dyes. At present, activated carbon, ion exchange resins, and mesoporous molecular sieves are the most common adsorbents, and new nanomaterials are constantly being explored and developed. With the maturity of graphene theory and preparation technology, graphene with a large specific surface area and rich surface oxygen-containing functional groups obtained using new preparation methods has shown powerful adsorption capacity, which has great potential application value in treating organic wastewater and heavy metal ions [
21].
Currently, the preparation technology of graphene is also constantly developing, especially the synthesis technology of graphene oxide as a precursor of graphene, which significantly strengthens the application ability of graphene in various fields [
11,
12,
13,
14,
15]. Carbon atoms in nature have four valence electrons, and each carbon atom in graphene is an SP2 hybrid. They can contribute to the unbonded π electrons. The π electrons and graphene were formed in a two-dimensional vertical plane, forming a conjugate orbit. The π electrons can move freely throughout the graphene plane, providing graphene with good electrical conductivity [
18]. With the rapid development of nanotechnology, materials science, and molecular biology, biomimetic nanofluid systems with asymmetric ion transport properties and environmental adaptations have attracted great interest and have gained more functions. Potential applications in sensing, water purification, energy transmission, and other fields are limited.
This study presents the successful grafting of PANI polymer on the edge of graphene and tests its adsorption properties for MB and Pb2+. The results showed that at 298 K, the maximum adsorption capacity of GO-PANI was 1416 mg/g, 2.3 times that of PANI. The kinetic curves of PANI and GO-PANI adsorption of Pb (II) fit well with the quasi-second-order kinetic model, and the equilibrium adsorption curve conforms to the Langmuir adsorption isotherm. Additionally, the adsorption process was spontaneous, as AG was negative. This study also found that GO-PANI can effectively adsorb both Pb (II) and MB in mixed adsorption systems, with a maximum adsorption capacity for MB and Pb (II) of 544 mg/g and 1004 mg/g, respectively, after 90 min of adsorption. Ultimately, these findings allow for significant applications in sewage treatment. In addition to the presence of characteristic functional groups of both components, FT-IR analysis confirmed the success of graphene oxide–natural polymer composite synthesis. Furthermore, the interactions and bonds between GO and the natural polymer indicate that the two materials have a strong interface, which is likely to improve the adsorption capacity of the composites. Both GO and the natural polymer exhibited diffraction peaks in the composite material, confirming the presence of crystalline phases. Consequently, the natural polymer did not degrade the crystal structure of the composites.
The FT-IR and XRD analyses of GO and the GO-PANI showed that it was successfully synthesized and had a crystalline structure. Based on these findings, it can be concluded that the composite material has desirable properties for adsorbing contaminants, mainly because of the enhanced adsorption capacity of GO and its potential synergistic effects with PANI. FT-IR spectra indicated that specific functional groups might act as adsorption sites for water contaminants. From the XRD results, we better understood the adsorption process and its stability as well as structural changes.
In this study, the FT-IR and XRD characterization results demonstrate that graphene oxide–natural polymer composites can be used in water treatment processes as adsorbent materials, highlighting their potential to tackle water pollution. The composite’s adsorption performance, kinetics, and potential scalability should be further examined in real-world water treatment applications.
In this study, we successfully synthesized GO-PANI nanocomposites by attaching amino groups to GO surfaces and performing oxidative polymerization with aniline monomers. The addition rate of the oxidizing agent affected the morphology and performance of the resulting nanocomposites. Various characterization techniques such as XRD, TEM, and Raman spectroscopy were employed to analyze the properties of the GO-PANI nanocomposites. TEM was used to observe the morphologies of the GO, PANI, and GO-PANI. The results showed that GO-PANI had higher thermal stability and adsorption capacity for lead ions and organic dyes than PANI. The van der Waals equation curves were plotted to calculate the thermodynamic parameters ΔH, ΔS, and ΔG, which indicated that the adsorption of Pb (II) using PANI and GO-PANI was a spontaneous endothermic process. The co-adsorption experiment showed that GO-PANI had good adsorption capacity for MB and Pb (II) in a coexisting system. These findings provide insight into the development of graphene-based materials for environmental applications.