Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties
Abstract
1. Introduction
2. Materials and Methods
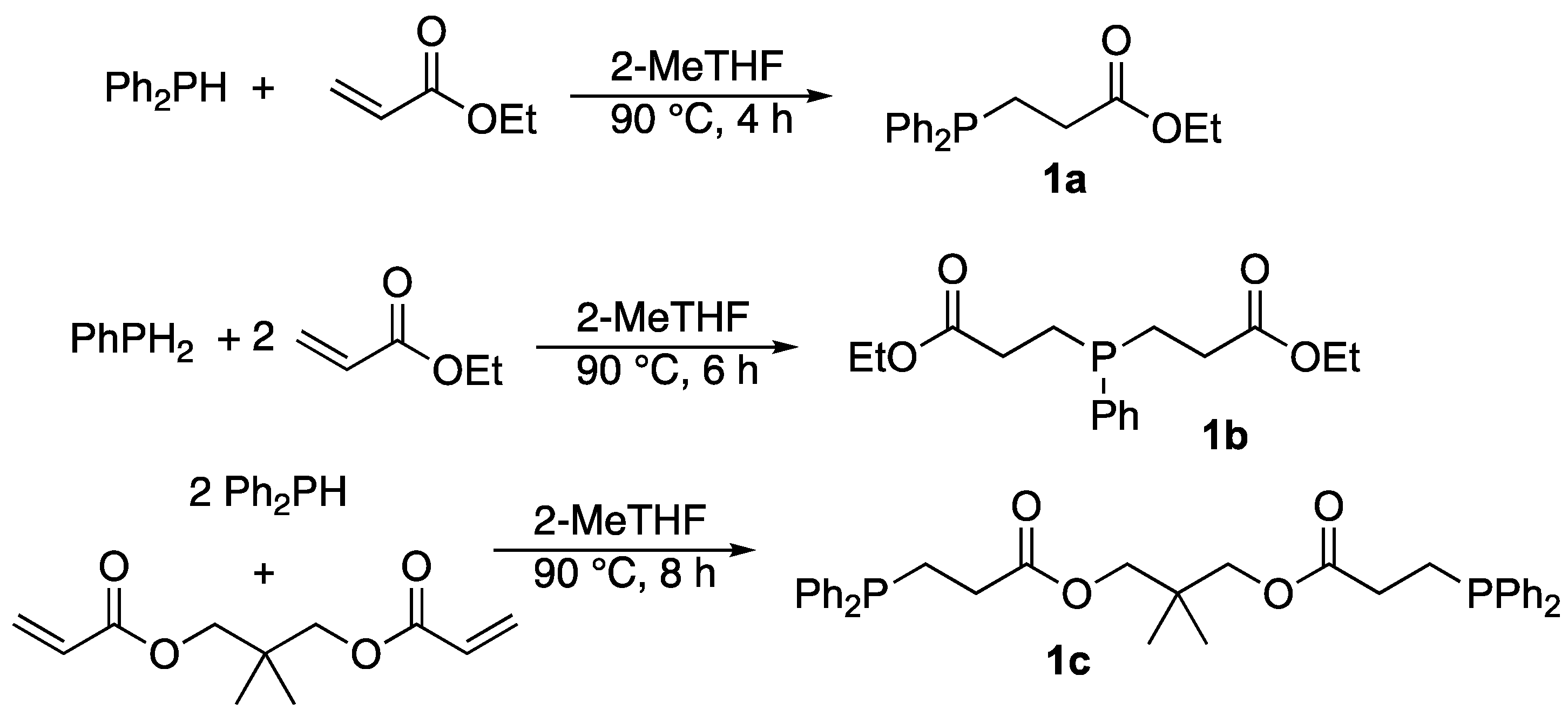
2.1. Synthesis of Ethyl 3-(Diphenylphosphanyl)propanoate 1a
2.2. Synthesis of Diethyl 3,3′-(Phenylphosphanediyl)dipropionate 1b
2.3. Synthesis of 2,2-Dimethylpropane-1,3-diyl Bis(3-(diphenylphosphanyl)propanoate) 1c
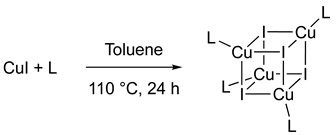
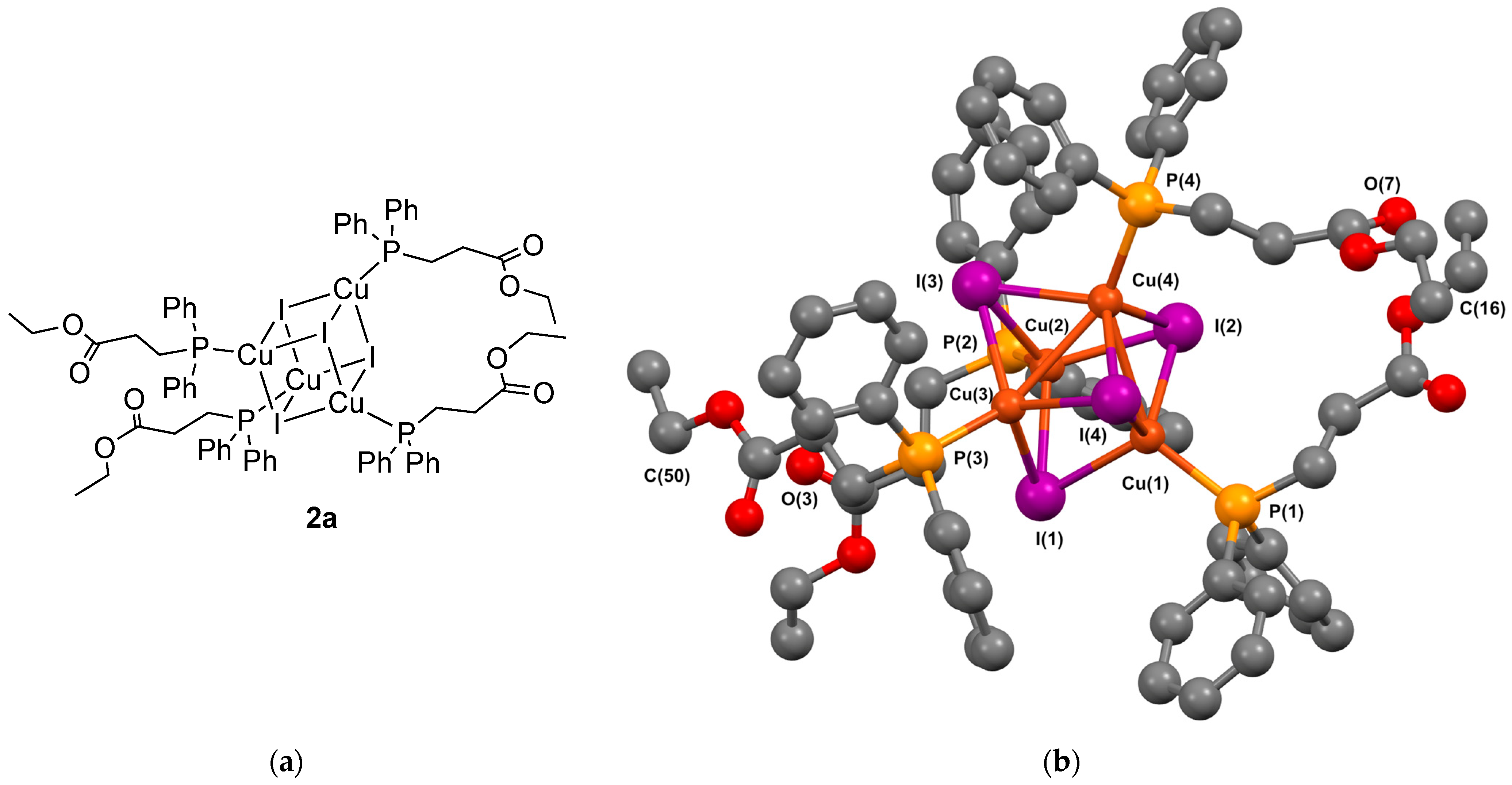
2.4. Synthesis of Cluster [Cu(μ3-I)(1a)]4 2a
2.5. Synthesis of Cluster [Cu(μ3-I)(1b)]4 2b
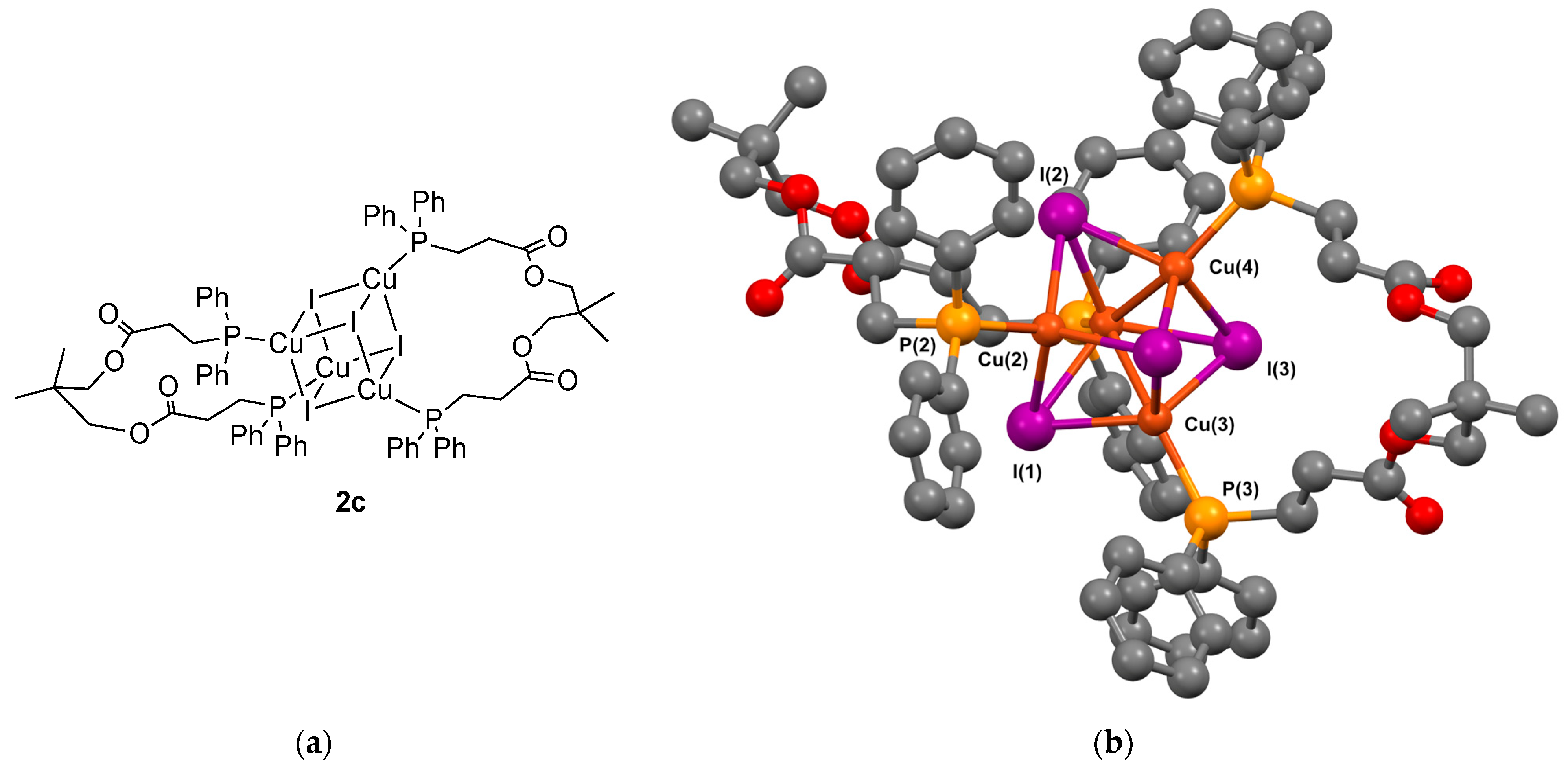
2.6. Synthesis of Cluster [Cu(μ3-I)]4(1c)2 2c
2.7. X-ray Analyses
3. Results
3.1. Synthesis of the Phosphine Ligands 1a–c
3.2. Synthesis and Characterization of the Cubane-like Copper Cluster 2a–c
3.2.1. Synthesis
3.2.2. Crystal Structure of the Cubane Clusters
- Complex 2a
- Complex 2b
- Complex 2c
3.3. Photophysical Characterization
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenger, O.S. Photoactive Complexes with Earth-Abundant Metals. J. Am. Chem. Soc. 2018, 140, 13522–13533. [Google Scholar] [CrossRef]
- Larsen, C.B.; Wenger, O.S. Photoredox Catalysis with Metal Complexes Made from Earth-Abundant Elements. Chem. Eur. J. 2018, 24, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Heinze, K. Photophysics and Photochemistry with Earth-Abundant Metals—Fundamentals and Concepts. Chem. Soc. Rev. 2020, 49, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent Complexes beyond the Platinum Group: The D10 Avenue. Chem. Commun. 2008, 2185–2193. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive Mono- and Polynuclear Cu(i)–Phenanthrolines. A Viable Alternative to Ru(Ii)–Polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Wallesch, M.; Volz, D.; Zink, D.M.; Schepers, U.; Nieger, M.; Baumann, T.; Bräse, S. Bright Coppertunities: Multinuclear CuI Complexes with N-P Ligands and Their Applications. Chem. Eur. J. 2014, 20, 6578–6590. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3648. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-Responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) Complexes—Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Troyano, J.; Zamora, F.; Delgado, S. Copper(I)–Iodide Cluster Structures as Functional and Processable Platform Materials. Chem. Soc. Rev. 2021, 50, 4606–4628. [Google Scholar] [CrossRef] [PubMed]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating Nonradiative Decay in Cu(I) Emitters: >99% Quantum Efficiency and Microsecond Lifetime. Science 2019, 363, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, C.; Han, C.; Yang, H.; Wei, Y.; Xu, H. Balanced Dual Emissions from Tridentate Phosphine-Coordinate Copper(I) Complexes toward Highly Efficient Yellow OLEDs. Adv. Mater. 2016, 28, 5975–5979. [Google Scholar] [CrossRef]
- Shi, S.; Jung, M.C.; Coburn, C.; Tadle, A.; Daniel Sylvinson, M.R.; Djurovich, P.I.; Forrest, S.R.; Thompson, M.E. Highly Efficient Photo- and Electroluminescence from Two-Coordinate Cu(I) Complexes Featuring Nonconventional N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2019, 141, 3576–3588. [Google Scholar] [CrossRef]
- Elie, M.; Sguerra, F.; Di Meo, F.; Weber, M.D.; Marion, R.; Grimault, A.; Lohier, J.-F.; Stallivieri, A.; Brosseau, A.; Pansu, R.B.; et al. Designing NHC–Copper(I) Dipyridylamine Complexes for Blue Light-Emitting Electrochemical Cells. ACS Appl. Mater. Interfaces 2016, 8, 14678–14691. [Google Scholar] [CrossRef] [PubMed]
- Zink, D.M.; Volz, D.; Baumann, T.; Mydlak, M.; Flügge, H.; Friedrichs, J.; Nieger, M.; Bräse, S. Heteroleptic, Dinuclear Copper(I) Complexes for Application in Organic Light-Emitting Diodes. Chem. Mater. 2013, 25, 4471–4486. [Google Scholar] [CrossRef]
- Au, V.K.-M. Organic Light-Emitting Diodes Based on Luminescent Self-Assembled Materials of Copper(I). Energy Fuels 2021, 35, 18982–18999. [Google Scholar] [CrossRef]
- Perruchas, S. Molecular Copper Iodide Clusters: A Distinguishing Family of Mechanochromic Luminescent Compounds. Dalton Trans. 2021, 50, 12031–12044. [Google Scholar] [CrossRef]
- Egly, J.; Bissessar, D.; Achard, T.; Heinrich, B.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. Copper(I) Complexes with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Variety, Photophysics and Effect onto the Optical Properties. Inorg. Chim. Acta 2021, 514, 119971. [Google Scholar] [CrossRef]
- Bissessar, D.; Egly, J.; Achard, T.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. A Stable and Photoreactive Copper-Iodide Cubane Suitable for Direct Post-Functionalization. Eur. J. Inorg. Chem. 2022, 2022, e202200101. [Google Scholar] [CrossRef]
- Wolfsberger, W.; Bank, J.; Werner, H. Synthese Dreizähniger Phosphanliganden RP[(CH2)n]2 (Y = OR′ Oder CO2R′, n = 1 Oder 2) Sowie Einiger Zweizähniger Chiraler Phosphane R2PCH(CH3)CO2Me/Synthesis of Tridentate Phosphine Ligands RP[(CH2)nY]2(Y = OR′ or CO2R′, n = 1 or 2) and Some Bidentate Chiral Phosphines R2PCH(CH3)CO2Me. Z. Nat. B 1995, 50, 1319–1328. [Google Scholar] [CrossRef]
- Kappa CCD Operation Manual; Nonius B.V.: Delft, The Netherlands, 1997.
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXL-97; Universität Göttingen: Göttingen, Germany, 1999. [Google Scholar]
- Bissessar, D.; Egly, J.; Achard, T.; Steffanut, P.; Bellemin-Laponnaz, S. Catalyst-Free Hydrophosphination of Alkenes in Presence of 2-Methyltetrahydrofuran: A Green and Easy Access to a Wide Range of Tertiary Phosphines. RSC Adv. 2019, 9, 27250–27256. [Google Scholar] [CrossRef]
- Huitorel, B.; El Moll, H.; Utrera-Melero, R.; Cordier, M.; Fargues, A.; Garcia, A.; Massuyeau, F.; Martineau-Corcos, C.; Fayon, F.; Rakhmatullin, A.; et al. Evaluation of Ligands Effect on the Photophysical Properties of Copper Iodide Clusters. Inorg. Chem. 2018, 57, 4328–4339. [Google Scholar] [CrossRef]
- Utrera-Melero, R.; Massuyeau, F.; Latouche, C.; Camerel, F.; Perruchas, S. Copper Iodide Clusters Coordinated by Emissive Cyanobiphenyl-Based Ligands. Inorg. Chem. 2022, 61, 4080–4091. [Google Scholar] [CrossRef]
- Kitagawa, H.; Ozawa, Y.; Toriumi, K. Flexibility of Cubane-like Cu4I4 Framework: Temperature Dependence of Molecular Structure and Luminescence Thermochromism of [Cu4I4(PPh3)4] in Two Polymorphic Crystalline States. Chem. Commun. 2010, 46, 6302. [Google Scholar] [CrossRef]
- Benito, Q.; Fargues, A.; Garcia, A.; Maron, S.; Gacoin, T.; Boilot, J.-P.; Perruchas, S.; Camerel, F. Photoactive Hybrid Gelators Based on a Luminescent Inorganic [Cu4I4] Cluster Core. Chem. Eur. J. 2013, 19, 15831–15835. [Google Scholar] [CrossRef]
- Benito, Q.; Maurin, I.; Cheisson, T.; Nocton, G.; Fargues, A.; Garcia, A.; Martineau, C.; Gacoin, T.; Boilot, J.-P.; Perruchas, S. Mechanochromic Luminescence of Copper Iodide Clusters. Chem. Eur. J. 2015, 21, 5892–5897. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) Hybrid Inorganic–Organic Materials with Intriguing Stimuli Responsive and Optoelectronic Properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Perruchas, S.; Le Goff, X.F.; Maron, S.; Maurin, I.; Guillen, F.; Garcia, A.; Gacoin, T.; Boilot, J.-P. Mechanochromic and Thermochromic Luminescence of a Copper Iodide Cluster. J. Am. Chem. Soc. 2010, 132, 10967–10969. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent Copper(I) Complexes with Halogenido-Bridged Dimeric Core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Perruchas, S.; Tard, C.; Le Goff, X.F.; Fargues, A.; Garcia, A.; Kahlal, S.; Saillard, J.-Y.; Gacoin, T.; Boilot, J.-P. Thermochromic Luminescence of Copper Iodide Clusters: The Case of Phosphine Ligands. Inorg. Chem. 2011, 50, 10682–10692. [Google Scholar] [CrossRef] [PubMed]
- Kyle, K.R.; Ryu, C.K.; Ford, P.C.; DiBenedetto, J.A. Photophysical Studies in Solution of the Tetranuclear Copper(I) Clusters Cu4I4L4 (L = Pyridine or Substituted Pyridine). J. Am. Chem. Soc. 1991, 113, 2954–2965. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Wang, Z.; Liu, Z.-M.; Yu, H.-J.; Zhang, J.-H.; Wang, Y.; Mao, R.; Pan, M.; Su, C.-Y. Multiresponsive UV-One-Photon Absorption, Near-Infrared-Two-Photon Absorption, and X/γ-Photoelectric Absorption Luminescence in One [Cu4I4] Compound. Inorg. Chem. 2019, 58, 10736–10742. [Google Scholar] [CrossRef]
- Liu, W.; Fang, Y.; Li, J. Copper Iodide Based Hybrid Phosphors for Energy-Efficient General Lighting Technologies. Adv. Funct. Mater. 2018, 28, 1705593. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Pritchina, E.A.; Berezin, A.S.; Samsonenko, D.G.; Fedin, V.P.; Belogorlova, N.A.; Gritsan, N.P.; Artem’ev, A.V. Beyond Classical Coordination Chemistry: The First Case of a Triply Bridging Phosphine Ligand. Angew. Chem. Int. Ed. 2021, 60, 12577–12584. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Rakhmanova, M.I.; Hei, X.; Samsonenko, D.G.; Stass, D.V.; Bagryanskaya, I.Y.; Ryzhikov, M.R.; Fedin, V.P.; Li, J.; Artem’ev, A.V. A New Subclass of Copper(I) Hybrid Emitters Showing TADF with near-Unity Quantum Yields and a Strong Solvatochromic Effect. Chem. Commun. 2023, 59, 2923–2926. [Google Scholar] [CrossRef]



| Compound | [a] | [a] | ||||
|---|---|---|---|---|---|---|
| [nm] | (x, y) | [μs] | [104s−1] | |||
| 2a | 569 | 0.84 | 0.40, 0.51 | 5.17 | 16.24 | 3.09 |
| 2b | 575 | 0.43 | 0.39, 0.44 | 5.11 | 8.42 | 11.16 |
| 2c | 578 | 0.50 | 0.42, 0.47 | 5.89 | 8.49 | 8.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissessar, D.; Thierry, T.; Egly, J.; Giuso, V.; Achard, T.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties. Symmetry 2023, 15, 1210. https://doi.org/10.3390/sym15061210
Bissessar D, Thierry T, Egly J, Giuso V, Achard T, Steffanut P, Mauro M, Bellemin-Laponnaz S. Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties. Symmetry. 2023; 15(6):1210. https://doi.org/10.3390/sym15061210
Chicago/Turabian StyleBissessar, Damien, Thibault Thierry, Julien Egly, Valerio Giuso, Thierry Achard, Pascal Steffanut, Matteo Mauro, and Stéphane Bellemin-Laponnaz. 2023. "Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties" Symmetry 15, no. 6: 1210. https://doi.org/10.3390/sym15061210
APA StyleBissessar, D., Thierry, T., Egly, J., Giuso, V., Achard, T., Steffanut, P., Mauro, M., & Bellemin-Laponnaz, S. (2023). Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties. Symmetry, 15(6), 1210. https://doi.org/10.3390/sym15061210









