Photoelectronic Properties of Chiral Self-Assembled Diphenylalanine Nanotubes: A Computational Study
Abstract
:1. Introduction
2. Computational Details, Models, and Methods
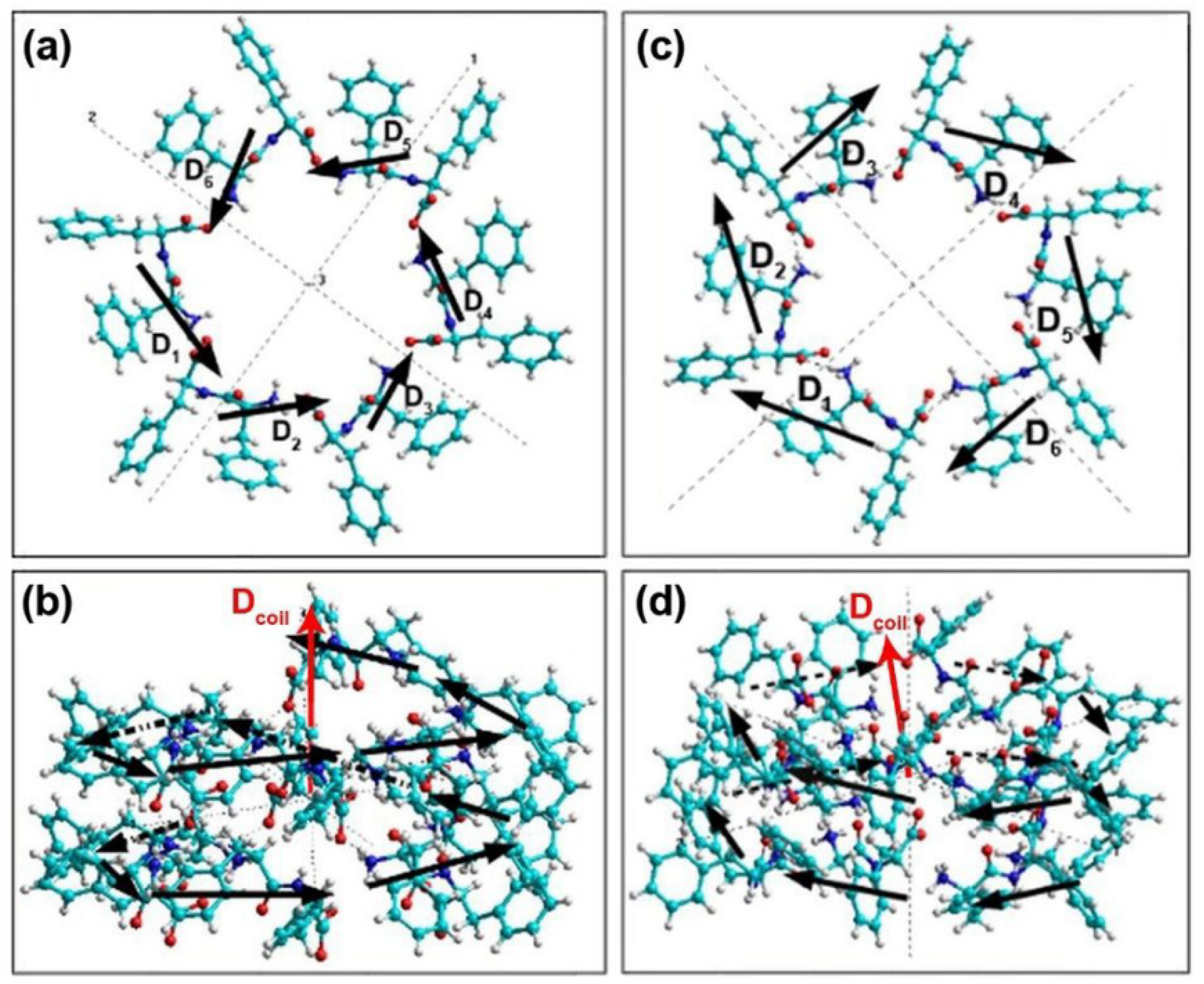
2.1. Basic Models of FF PNTs
2.2. Calculation Methods
3. Results and Discussion
3.1. Electronic Energy Levels without an Electric Field
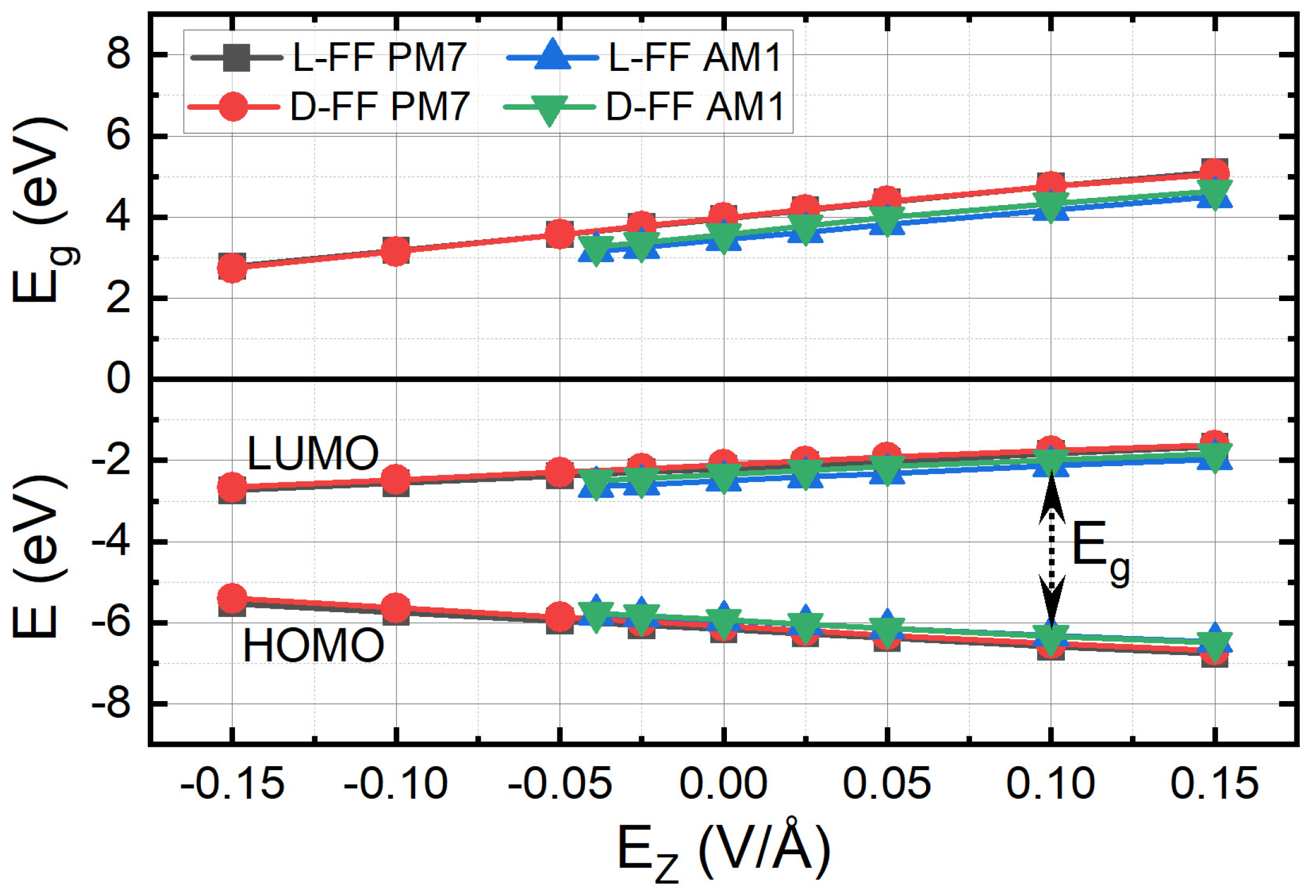
3.2. Influence of the External Electric Field
- (1)
- for the AM1 method: α1 = 7.182 eV/(V/Å) for l-FF and α3 = 7.412 eV/(V/Å) for d-FF;
- (2)
- for the PM7 method: α2 = 7.774 eV/(V/Å) for l-FF and α4 = 7.853 eV/(V/Å) for d-FF.
3.3. Influence of the Intrinsic Electric Field
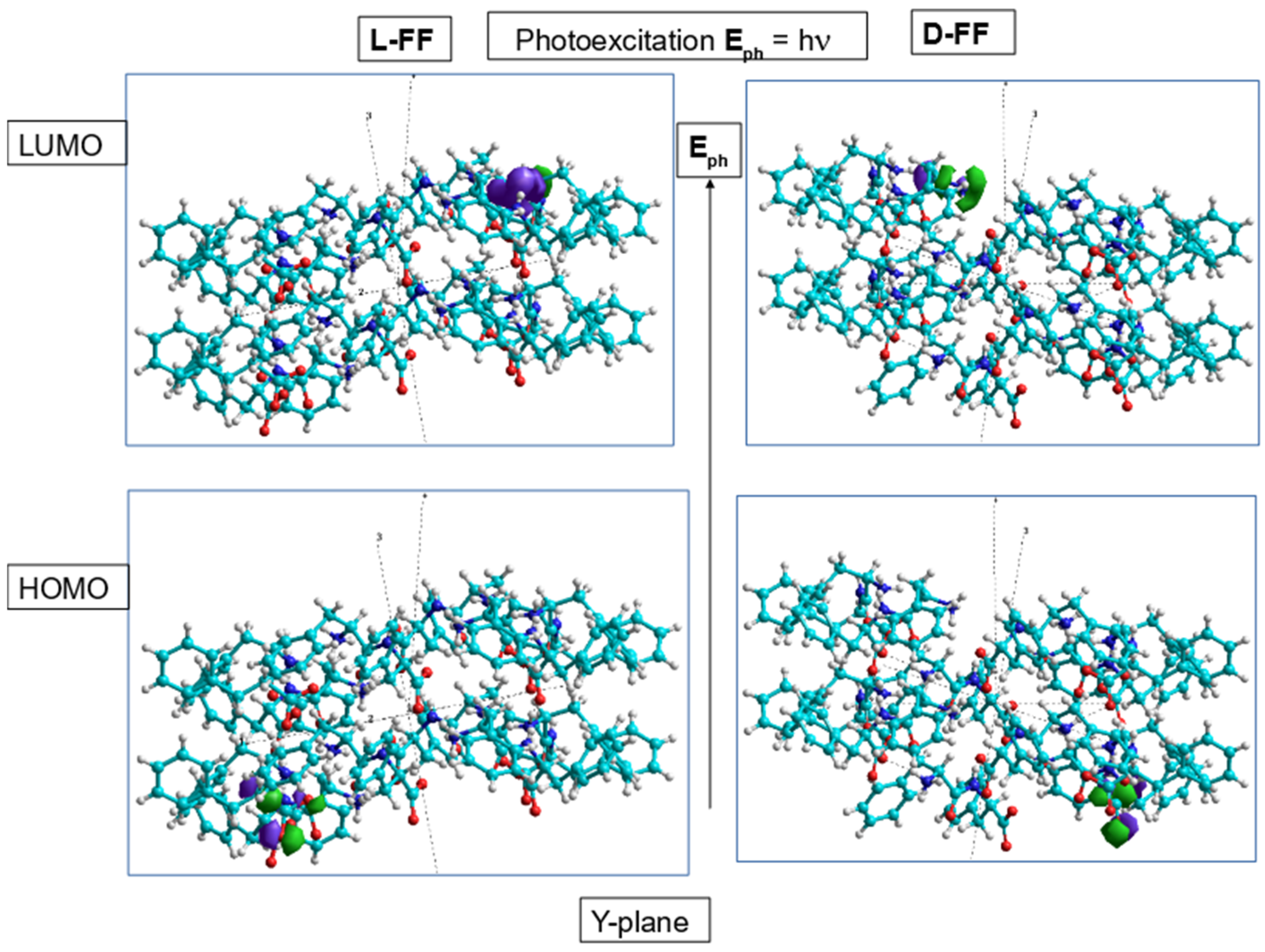
3.4. Exciton Formation and Photoluminescence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sallembien, Q.; Bouteiller, L.; Crassous, J.; Raynal, M. Possible chemical and physical scenarios towards biological homochirality. Chem. Soc. Rev. 2022, 51, 3436–3476. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates and Resolutions; Krieger Publishing Company: Malabar, FL, USA, 1994. [Google Scholar]
- Naaman, R.; Waldeck, D.H. Spintronics and Chirality: Spin Selectivity in Electron Transport Through Chiral Molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef]
- Letokhov, V.S. On difference of energy levels of left and right molecules due to weak interactions. Phys. Lett. A 1975, 53, 275–276. [Google Scholar] [CrossRef]
- Quack, M.; Stohner, J.; Willeke, M. High-Resolution Spectroscopic Studies and Theory of Parity Violation in Chiral Molecules. Ann. Rev. Phys. Chem. 2008, 59, 741–769. [Google Scholar] [CrossRef] [PubMed]
- Vitanov, N.V.; Drewsen, M. Highly Efficient Detection and Separation of Chiral Molecules through Shortcuts to Adiabaticity. Phys. Rev. Lett. 2019, 122, 173202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bischoff, J.; Hernandez-Castillo, A.O.; Sartakov, B.; Meijer, G.; Eibenberger-Arias, S. Quantitative Study of Enantiomer-Specific State Transfer. Phys. Rev. Lett. 2022, 128, 173001. [Google Scholar] [CrossRef]
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406. [Google Scholar] [CrossRef]
- Yuan, C.; Ji, W.; Xing, R.; Li, J.; Gazit, E.; Yan, X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3, 567–588. [Google Scholar] [CrossRef]
- Zelenovskii, P.S.; Romanyuk, K.; Liberato, M.S.; Brandão, P.; Ferreira, F.F.; Kopyl, S.; Mafra, L.M.; Alves, W.A.; Kholkin, A.L. 2D Layered Dipeptide Crystals for Piezoelectric Applications. Adv. Funct. Mater. 2021, 31, 2102524. [Google Scholar] [CrossRef]
- Raymond, D.M.; Nilsson, B.L. Multicomponent peptide assemblies. Chem. Soc. Rev. 2018, 47, 3659. [Google Scholar] [CrossRef]
- Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Arkhipov, S.G.; Vasilev, S.G.; Bystrov, V.S.; Gruzdev, D.A.; Waliczek, M.; Svitlyk, V.; Shur, V.Y.; et al. Chirality-dependent growth of self-assembled diphenylalanine microtubes. Cryst. Growth Des. 2019, 19, 6414–6421. [Google Scholar] [CrossRef]
- Bystrov, V.; Sidorova, A.; Lutsenko, A.; Shpigun, D.; Malyshko, E.; Nuraeva, A.; Zelenovskiy, P.; Kopyl, S.; Kholkin, A. Modeling of Self-Assembled Peptide Nanotubes and Determination of Their Chirality Sign Based on Dipole Moment Calculations. Nanomaterials 2021, 11, 2415. [Google Scholar] [CrossRef] [PubMed]
- Kol, N.; Adler-Abramovich, L.; Barlam, D.; Shneck, R.Z.; Gazit, E.; Rousso, I. Self-Assembled Peptide Nanotubes Are Uniquely Rigid Bioinspired Supramolecular Structures. Nano Lett. 2005, 5, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Zelenovskiy, P.; Kornev, I.; Vasilev, S.; Kholkin, A. On the origin of the great rigidity of self-assembled diphenylalanine nanotubes. Phys. Chem. Chem. Phys. 2016, 18, 29681. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef]
- Akdim, B.; Pachter, R.; Naik, R.R. Self-assembled peptide nanotubes as electronic materials: An evaluation from first-principles calculations. Appl. Phys. Lett. 2005, 106, 183707. [Google Scholar] [CrossRef]
- Amdursky, N.; Molotskii, M.; Aronov, D.; Adler-Abramovich, L.; Gazit, E.; Rosenman, G. Blue luminescence based on quantum confinement at peptide nanotubes. Nano Lett. 2009, 9, 3111–3115. [Google Scholar] [CrossRef]
- Gan, X.; Wu, X.; Zhu, X.; Shen, J. Light-induced ferroelectricity in bioinspired self-assembled diphenylalanine nano-tubes/microtubes. Angew. Chem. Int. Ed. 2013, 52, 2055–2059. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Zhang, J.; Zhu, X.; Chu, P.K. In Situ Thermal Imaging and Absolute Temperature Monitoring by Luminescent Diphenylalanine Nanotubes. Biomacromolecules 2013, 14, 2112–2116. [Google Scholar] [CrossRef]
- Nikitin, T.; Kopyl, S.; Shur, V.Y.; Kopelevich, V.Y.; Kholkin, A.L. Low-temperature photoluminescence in self-assembled diphenylalanine microtubes. Phys. Lett. A 2016, 380, 1658–1662. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhu, R.; Jenkins, K.; Yang, R. Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat. Commun. 2016, 7, 13566. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Kelly, S.; Nguyen, V.; Wu, Y.; Yang, R. Piezoelectric diphenylalanine peptide for greatly improved flexible nanogenerators. Nano Energy 2022, 51, 317–323. [Google Scholar] [CrossRef]
- Vasilev, S.; Zelenovskiy, P.; Vasileva, D.; Nuraeva, A.; Shur, V.Y.; Kholkin, A.L. Piezoelectric properties of diphenylalanine microtubes prepared from the solution. J. Phys. Chem. Solids 2016, 93, 68. [Google Scholar] [CrossRef]
- Carny, O.; Gazit, E. Creating Prebiotic Sanctuary: Self-Assembling Supramolecular Peptide Structures Bind and Stabilize RNA. Orig. Life Evol. Biosph. 2011, 41, 121–132. [Google Scholar] [CrossRef]
- Bystrov, V.; Coutinho, J.; Zelenovskiy, P.; Nuraeva, A.; Kopyl, S.; Zhulyabina, O.; Tverdislov, V. Structures and properties of the self-assembling diphenylalanine peptide nanotubes containing water molecules: Modeling and data analysis. Nanomaterials 2020, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, V.S.; Filippov, S.V. Molecular modelling and computational studies of peptide diphenylalanine nanotubes, containing waters: Structural and interactions analysis. J. Mol. Model. 2022, 28, 81. [Google Scholar] [CrossRef]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chem. Eur. J. 2001, 7, 5153. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.V.; Bdikin, I.; Kopyl, S.; Heredia, A.; Pullar, R.; Kholkin, A. BioFerroelectricity: Diphenylalanine Peptide Nanotubes Computational Modeling and Ferroelectric Properties at the Nanoscale. Ferroelectrics 2012, 440, 3–24. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Coutinho, J.; Zhulyabina, O.A.; Kopyl, S.A.; Zelenovskiy, P.S.; Nuraeva, A.S.; Tverdislov, V.A.; Filippov, S.V.; Kholkin, A.L.; Shur, V.Y. Modeling and physical properties of diphenylalanine peptide nanotubes containing water molecules. Ferroelectrics 2021, 574, 78–91. [Google Scholar] [CrossRef]
- Bystrov, V.S. Photoferroelectricity in di-phenylalanine peptide nanotube. Comput. Condens. Matter 2018, 14, 94–100. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk/ (accessed on 17 December 2022).
- HyperChem. Tools for Molecular Modeling; Release 8.0/01; Hypercube, Inc.: Gainnesville, FL, USA, 2011. [Google Scholar]
- Bystrov, V.S.; Kopyl, S.A.; Zelenovskiy, P.; Zhulyabina, O.A.; Tverdislov, V.A.; Salehli, F.; Ghermani, N.E.; Shur, V.Y.; Kholkin, A.L. Investigation of physical properties of diphenylalanine peptide nanotubes having different chiralities and embedded water molecules. Ferroelectrics 2018, 525, 168–177. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Stewart Computational Chemistry. MOPAC2016 [Electronic Resource], Colorado Springs, CO, USA. 2016. Available online: http://openmopac.net/MOPAC2016.html (accessed on 30 October 2022).
- Fridkin, V.M. Photoferroelectrics; Springer: New York, NY, USA, 1979. [Google Scholar]
- Fridkin, V.M.; Ducharme, S. Ferroelectricity at the Nanoscale; Basic and Applications; Springer: New York, NY, USA; London, UK, 2014. [Google Scholar]
- Bystrov, V.S.; Fridkin, V.M. Two-dimensional ferroelectrics and homogeneous switching. Phys. Usp. 2020, 63, 417–439. [Google Scholar]
- Wang, X.; Wang, P.; Wang, J.; Hu, W.; Zhou, X.; Guo, N.; Huang, H.; Sun, S.; Shen, H.; Lin, T.; et al. Ultrasensitive and Broadband MoS2 Photodetector Driven by Ferroelectrics. Adv. Mater. 2015, 27, 6575–6581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Wang, X.; Chen, Y.; Shao, Q.; Wu, G.; Wang, X.; Lin, T.; Shen, H.; Wang, J.; et al. High-performance β-Ga2O3 thickness dependent solar blind photodetector. Opt. Express 2020, 28, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Handelman, A.; Apter, B.; Turko, N.; Rosenman, G. Linear and nonlinear optical waveguiding in bio-inspired peptide nanotubes. Acta Biomater. 2016, 30, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Handelman, A.; Lavrov, S.; Kudryavtsev, A.; Khatchatouriants, A.; Rosenberg, Y.; Mishina, E.; Rosenman, G. Nonlinear Optical Bioinspired Peptide Nanostructures. Adv. Optical Mater. 2013, 1, 875–884. [Google Scholar] [CrossRef]
- Weyl, H. Symmetry; Princeton University Press: Princeton, NJ, USA, 1952. [Google Scholar]
- Kane, G. Supersymmetry and Beyond: From the Higgs Boson to the New Physics; Basic Books: New York, NY, USA, 2013. [Google Scholar]
- Holmstedt, B.; Frank, H.; Testa, B. (Eds.) Chirality and Biological Activity; Liss: New York, NY, USA, 1990. [Google Scholar]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Tverdislov, V.A. Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 2013, 58, 128–132. [Google Scholar] [CrossRef]


| Type/Method | EHOMO, eV | ELUMO, eV | Eg0, eV | λ, nm |
|---|---|---|---|---|
| d-FF/PM7 | −6.085 | −2.104 | 3.981 | 312 |
| l-FF/PM7 | −6.167 | −2.201 | 3.966 | 313 |
| d-FF/PM6-D3H4 | −6.351 | −2.731 | 3.620 | 343 |
| l-FF/PM6-D3H4 | −6.407 | −2.835 | 3.572 | 348 |
| d-FF/PM3/AM1 | −5.924 | −2.349 | 3.575 | 347 |
| l-FF/PM3/AM1 | −5.941 | −2.4996 | 3.441 | 360 |
| l-FF/DFT [17] | – | – | 4.48 | c.a. 277 |
| d-FF/Opt. abs. [21] | – | – | 4.69 | c.a. 264 |
| l-FF/Opt. abs. [18] | – | – | 3.35–4.13 | 300–370 |
| l-FF/Photolum. [19] | – | – | 4.0–4.13 | 300–310 |
| l-FF/Photolum. [20] | – | – | 4.0 | c.a. 310 |
| Type/ Method | D, Debye | P, C/m2 | EP, GV/m | α, eV/(GV/m) | Eg0, eV | Eg1, eV | Eg2, eV | ΔEex, eV | Δλex, nm |
|---|---|---|---|---|---|---|---|---|---|
| d-FF/PM7 | 146.60 | 0.146 | 4.126 | 0.785 | 3.981 | 5.600 | 4.923 | 0.677 | 30.5 |
| l-FF/PM7 | 146.24 | 0.145 | 4.092 | 0.777 | 3.966 | 5.558 | 4.899 | 0.659 | 30.0 |
| d-FF PM3/AM1 | 137.70 | 0.137 | 3.875 | 0.741 | 3.575 | 5.009 | 4.464 | 0.545 | 30.2 |
| l-FF PM3/AM1 | 140.72 | 0.139 | 3.938 | 0.718 | 3.441 | 4.851 | 4.303 | 0.548 | 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bystrov, V.; Paramonova, E.; Zelenovskii, P.; Kopyl, S.; Shen, H.; Lin, T.; Fridkin, V. Photoelectronic Properties of Chiral Self-Assembled Diphenylalanine Nanotubes: A Computational Study. Symmetry 2023, 15, 504. https://doi.org/10.3390/sym15020504
Bystrov V, Paramonova E, Zelenovskii P, Kopyl S, Shen H, Lin T, Fridkin V. Photoelectronic Properties of Chiral Self-Assembled Diphenylalanine Nanotubes: A Computational Study. Symmetry. 2023; 15(2):504. https://doi.org/10.3390/sym15020504
Chicago/Turabian StyleBystrov, Vladimir, Ekaterina Paramonova, Pavel Zelenovskii, Svitlana Kopyl, Hong Shen, Tie Lin, and Vladimir Fridkin. 2023. "Photoelectronic Properties of Chiral Self-Assembled Diphenylalanine Nanotubes: A Computational Study" Symmetry 15, no. 2: 504. https://doi.org/10.3390/sym15020504
APA StyleBystrov, V., Paramonova, E., Zelenovskii, P., Kopyl, S., Shen, H., Lin, T., & Fridkin, V. (2023). Photoelectronic Properties of Chiral Self-Assembled Diphenylalanine Nanotubes: A Computational Study. Symmetry, 15(2), 504. https://doi.org/10.3390/sym15020504









