The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid Chromatography Method
3. Results and Discussion
3.1. Effect of the Buffer pH on Vancomycin Elution
3.2. Effect of the pH on the Retention Time of Vancomycin
3.3. Effect of the Mobile Phase on Vancomycin Elution
3.4. Effect of the Stationary Phase on the Vancomycin Retention Time
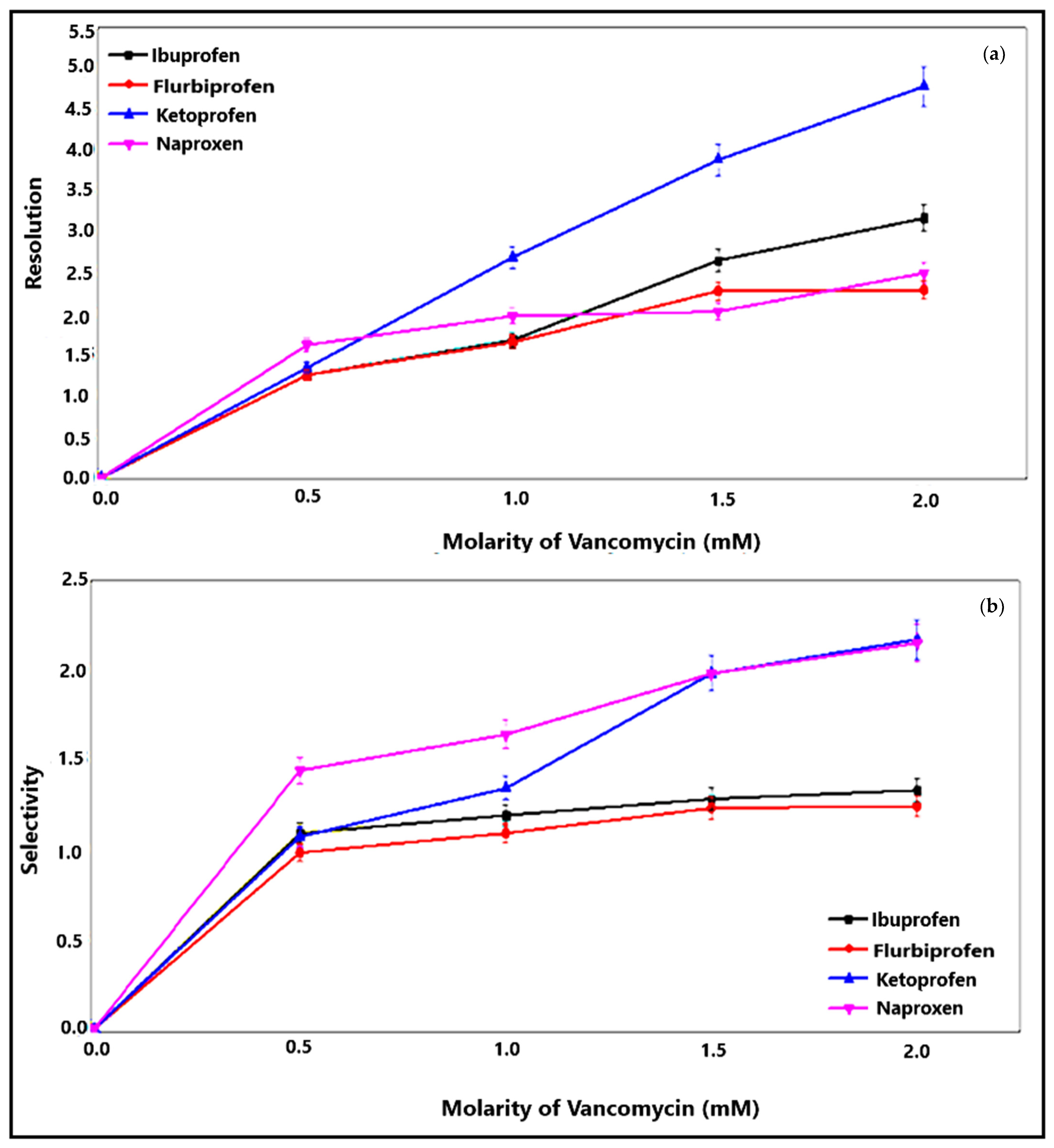
3.5. Enantioselective Separations of Profen NSAIDs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Wu, S.; Zhou, H.; Huang, H.; Zhao, J.; Deng, Y.; Wang, H.; Yang, Y.; Yang, J.; Luo, L. Chiral Pharmaceuticals: Environment Sources, Potential Human Health Impacts, Remediation Technologies and Future Perspective. Environ. Int. 2018, 121, 523–537. [Google Scholar] [CrossRef]
- Maier, N.M.; Franco, P. Wolfgang. Separation of Enantiomers: Needs, Challenges, Perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Björkman, S.; Höglund, P. Clinical Pharmacology of Thalidomide. Eur. J. Clin. Pharmacol. 2001, 57, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Bhushan, R. Methods and Approaches for Determination and Enantioseparation of (RS)-Propranolol. Biomed. Chromatogr. 2019, 33, e4370. [Google Scholar] [CrossRef]
- Fornstedt, T.; Forssén, P.; Samuelsson, J. Chapter 24—Modeling of Preparative Liquid Chromatography. In Liquid Chromatography, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–592. ISBN 978-0-12-805393-5. [Google Scholar]
- Betzenbichler, G.; Huber, L.; Kräh, S.; Morkos, M.-L.K.; Siegle, A.F.; Trapp, O. Chiral Stationary Phases and Applications in Gas Chromatography. Chirality 2022, 34, 732–759. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Zeng, S. Chiral Mobile Phase Additives in HPLC Enantioseparations. In Chiral Separations; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 970, pp. 221–231. ISBN 978-1-62703-262-9. [Google Scholar]
- Lämmerhofer, M. Chiral Recognition by Enantioselective Liquid Chromatography: Mechanisms and Modern Chiral Stationary Phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Mudi, S.Y.; Muhammad, A. Chiral Chromatography and Its Application to the Pharmaceutical Industry: A Review. ChemSearch J. 2011, 2, 8–11. [Google Scholar]
- Yu, L.; Wang, S.; Zeng, S. Chiral Mobile-Phase Additives in HPLC Enantioseparations. In Chiral Separations: Methods and Protocols; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 81–91. ISBN 978-1-4939-9438-0. [Google Scholar]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Deshpande, K.; Pillai, M.; Joshi, V.; Mohanraj, K. Chiral separation of citalopram by reversed phase hplc using sulfated beta cyclodextrin as chiral mobile phase additive. Int. J. Pharm. Pharm. Sci. 2015, 7, 306–310. [Google Scholar]
- Patel, H.B.; Jefferies, T.M. Eluotropic Strength of Solvents: Prediction and Use in Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. A 1987, 389, 21–32. [Google Scholar] [CrossRef]
- Meyer, V.R. Practical High-Performance Liquid Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1-118-68134-7. [Google Scholar]
- Rafferty, J.L.; Zhang, L.; Siepmann, J.I.; Schure, M.R. Retention Mechanism in Reversed-Phase Liquid Chromatography: A Molecular Perspective. Anal. Chem. 2007, 79, 6551–6558. [Google Scholar] [CrossRef]
- Berkecz, R.; Tanács, D.; Péter, A.; Ilisz, I. Enantioselective Liquid Chromatographic Separations Using Macrocyclic Glycopeptide-Based Chiral Selectors. Molecules 2021, 26, 3380. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Papp, L.A.; Szekely-Szentmiklosi, B.; Kelemen, H. The Use of Antibiotics as Chiral Selectors in Capillary Electrophoresis: A Review. Molecules 2022, 27, 3601. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Rundlett, K.L.; Chen, J.-R. Evaluation of the Macrocyclic Antibiotic Vancomycin as a Chiral Selector for Capillary Electrophoresis. Chirality 1994, 6, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Gherdaoui, D.; Bekdouche, H.; Zerkout, S.; Fegas, R.; Righezza, M. Chiral Separation of Ketoprofen on an Achiral NH2 Column by HPLC Using Vancomycin as Chiral Mobile Phase Additive. J. Iran. Chem. Soc. 2016, 13, 2319–2323. [Google Scholar] [CrossRef]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic Amino Acid Building Blocks for Nonribosomal Peptide and Hybrid Polyketide Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.A.; Shapovalova, E.N.; Shpigun, O.A. Separation of β-Blocker and Amino Acid Enantiomers on a Mixed Chiral Sorbent Modified with Macrocyclic Antibiotics Eremomycin and Vancomycin. J. Anal. Chem. 2017, 72, 76–82. [Google Scholar] [CrossRef]
- Qin, F.; Wang, Y.; Wang, L.; Zhao, L.; Pan, L.; Cheng, M.; Li, F. Determination of Trantinterol Enantiomers in Human Plasma by High-Performance Liquid Chromatography–Tandem Mass Spectrometry Using Vancomycin Chiral Stationary Phase and Solid Phase Extraction and Stereoselective Pharmacokinetic Application. Chirality 2015, 27, 327–331. [Google Scholar] [CrossRef]
- Sinha Roy, R.; Yang, P.; Kodali, S.; Xiong, Y.; Kim, R.M.; Griffin, P.R.; Onishi, H.R.; Kohler, J.; Silver, L.L.; Chapman, K. Direct Interaction of a Vancomycin Derivative with Bacterial Enzymes Involved in Cell Wall Biosynthesis. Chem. Biol. 2001, 8, 1095–1106. [Google Scholar] [CrossRef]
- Xiao, L. Enantiomeric Separations Using Macrocyclic Glycopeptide Based Chiral Stationary Phases, an Application and Mechanism Study. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2004. [Google Scholar]
- Riley, C.; Zechinati, B. Separation of Vancomycin and Its Degradation Products 2020. Worldwide Applications WO2020081599A1, 23 April 2020. [Google Scholar]
- Vella, J.; Mifsud, M.; Bartolo, N.S.; Ferrito, V.; Inglott, A.S.; Azzopardi, L.M.; Laferla, G. The Combined Effects of pH and Acetonitrile Composition on the Separation of Two Lincosamide Antibiotics. Asian J. Pharm. Clin. Res. 2014, 7, 96–100. [Google Scholar]
- Djajić, N.; Krmar, J.; Rmandić, M.; Rašević, M.; Otašević, B.; Zečević, M.; Malenović, A.; Protić, A. Modified Aqueous Mobile Phases: A Way to Improve Retention Behavior of Active Pharmaceutical Compounds and Their Impurities in Liquid Chromatography. J. Chromatogr. Open 2022, 2, 100023. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Noszál, B.; Tókés-Kövesdi, M.; Szász, G. Acid-Base Properties and Proton-Speciation of Vancomycin. Int. J. Pharm. 1993, 89, 261–263. [Google Scholar] [CrossRef]
- Dolan, J. Back to Basics: The Role of pH in Retention and Selectivity. LCGC Eur. 2017, 30, 30–33. [Google Scholar]
- Diana, J.; Visky, D.; Roets, E.; Hoogmartens, J. Development and Validation of an Improved Method for the Analysis of Vancomycin by Liquid Chromatography: Selectivity of Reversed-Phase Columns towards Vancomycin Components. J. Chromatogr. A 2003, 996, 115–131. [Google Scholar] [CrossRef]
- Hosotsubo, H. Rapid and Specific Method for the Determination of Vancomycin in Plasma by High-Performance Liquid Chromatography on an Aminopropyl Column. J. Chromatogr. B Biomed. Sci. Appl. 1989, 487, 421–427. [Google Scholar] [CrossRef]
- Horváth, C.; Melander, W.; Molnár, I. Solvophobic Interactions in Liquid Chromatography with Nonpolar Stationary Phases. J. Chromatogr. A 1976, 125, 129–156. [Google Scholar] [CrossRef]
- Valkó, K.; Snyder, L.R.; Glajch, J.L. Retention in Reversed-Phase Liquid Chromatography as a Function of Mobile-Phase Composition. J. Chromatogr. A 1993, 656, 501–520. [Google Scholar] [CrossRef]
- Wu, D.; Lucy, C.A. Study of the Slope of the Linear Relationship between Retention and Mobile Phase Composition (Snyder-Soczewiñski Model) in Normal Phase Liquid Chromatography with Bonded and Charge-Transfer Phases. J. Chromatogr. A 2016, 1475, 31–40. [Google Scholar] [CrossRef]
- Aguilar, M.-I. Reversed-Phase High-Performance Liquid Chromatography. In HPLC of Peptides and Proteins: Methods and Protocols; Aguilar, M.-I., Ed.; Methods in Molecular BiologyTM; Springer: Totowa, NJ, USA, 2004; pp. 9–22. ISBN 978-1-59259-742-0. [Google Scholar]
- Fernandes, C.; Tiritan, M.E.; Cravo, S.; Phyo, Y.; Kijjoa, A.; Silva, A.M.; Cass, Q.B.; Pinto, M.M. New Chiral Stationary Phases Based on Xanthone Derivatives for Liquid Chromatography. Chirality 2017, 29, 430–442. [Google Scholar] [CrossRef]
- Vailaya, A.; Horváth, C. Retention in Reversed-Phase Chromatography: Partition or Adsorption? J. Chromatogr. A 1998, 829, 1–27. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Maia, A.S.; Cass, Q.B.; Tiritan, M.E. Enantioseparation of Chiral Pharmaceuticals in Biomedical and Environmental Analyses by Liquid Chromatography: An Overview. J. Chromatogr. B 2014, 968, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Recognition Mechanisms of Chiral Selectors: An Overview. In Chiral Separations: Methods and Protocols; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 1–33. ISBN 978-1-4939-9438-0. [Google Scholar]
- Grybinik, S.; Bosakova, Z. An Overview of Chiral Separations of Pharmaceutically Active Substances by HPLC (2018–2020). Monatshefte Chem.—Chem. Mon. 2021, 152, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Hellinghausen, G.; Lopez, D.A.; Lee, J.T.; Wang, Y.; Weatherly, C.A.; Portillo, A.E.; Berthod, A.; Armstrong, D.W. Evaluation of the Edman Degradation Product of Vancomycin Bonded to Core-shell Particles as a New HPLC Chiral Stationary Phase. Chirality 2018, 30, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Bouchair, N.; Righezza, M.; Hamdi, A. Reversed-Phase High-Performance Liquid Chromatographic Separation of Some 2-Arylpropionic Acids Using Vancomycin as Chiral Stationary Phase. J. Iran. Chem. Soc. 2015, 12, 921–928. [Google Scholar] [CrossRef]
- Kafková, B.; Bosáková, Z.; Tesárová, E.; Coufal, P.; Messina, A.; Sinibaldi, M. Vancomycin as Chiral Selector for Enantioselective Separation of Selected Profen Nonsteroidal Anti-inflammatory Drugs Incapillary Liquid Chromatography. Chirality Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2006, 18, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Gilmore, A.; Ward, K.; Vowell, C. Vancomycin Molecular Interactions: Antibiotic and Enantioselective Mechanisms. In Chiral Recognition in Separation Methods: Mechanisms and Applications; Berthod, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 223–240. ISBN 978-3-642-12445-7. [Google Scholar]
- Li, Y.; Jin, X.-N.; Cheng, Y.; Ma, X.-F.; Wang, Y. Recent Advances on Chiral Mobile Phase Additives: A Critical Review. J. Anal. Test. 2022, 6, 129–162. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Chiral Separation and Modeling of Baclofen, Bupropion, and Etodolac Profens on Amylose Reversed Phase Chiral Column. Chirality 2017, 29, 386–397. [Google Scholar] [CrossRef]
- De Gauquier, P.; Vanommeslaeghe, K.; Heyden, Y.V.; Mangelings, D. Modelling Approaches for Chiral Chromatography on Polysaccharide-Based and Macrocyclic Antibiotic Chiral Selectors: A Review. Anal. Chim. Acta 2022, 1198, 338861. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Zhang, Y. Chiral Separation of Ketoprofen on an Achiral C8 Column by HPLC Using Norvancomycin as Chiral Mobile Phase Additives. J. Pharm. Biomed. Anal. 2006, 41, 310–314. [Google Scholar] [CrossRef]
- Naghdi, E.; Fakhari, A.R. The Comparison of Some Chiral Selectors for Enantioselective Separation of Naproxen as a Model Drug in Capillary Electrophoresis: Thermodynamic Analysis of the Separation Mechanism. J. Iran. Chem. Soc. 2022, 19, 1701–1709. [Google Scholar] [CrossRef]
- Papp, L.-A.; Krizbai, S.; Dobó, M.; Hancu, G.; Szabó, Z.-I.; Tóth, G. Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode. Molecules 2022, 27, 2986. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nagamatsu, K.; Nishi, H. High-Performance Enantiomer Separation of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) by 3 Μm Reversed-Phase Chiral Columns and Application to the Optical Purity Testing of Naproxen Drug Substances and Its Formulations. Anal. Sci. 2014, 30, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, A.; Heydari, R.; Shamsipur, M. Two Synthetic Methods for Preparation of Chiral Stationary Phases Using Crystalline Degradation Products of Vancomycin: Column Performance for Enantioseparation of Acidic and Basic Drugs. Aaps Pharmscitech 2017, 18, 1855–1862. [Google Scholar] [CrossRef]
- Shahnani, M.; Sefidbakht, Y.; Maghari, S.; Mehdi, A.; Rezadoost, H.; Ghassempour, A. Enantioseparation of Mandelic Acid on Vancomycin Column: Experimental and Docking Study. Chirality 2020, 32, 1289–1298. [Google Scholar] [CrossRef]
- Folprechtová, D.; Kozlov, O.; Armstrong, D.W.; Schmid, M.G.; Kalíková, K.; Tesařová, E. Enantioselective Potential of Teicoplanin-and Vancomycin-Based Superficially Porous Particles-Packed Columns for Supercritical Fluid Chromatography. J. Chromatogr. A 2020, 1612, 460687. [Google Scholar] [CrossRef]
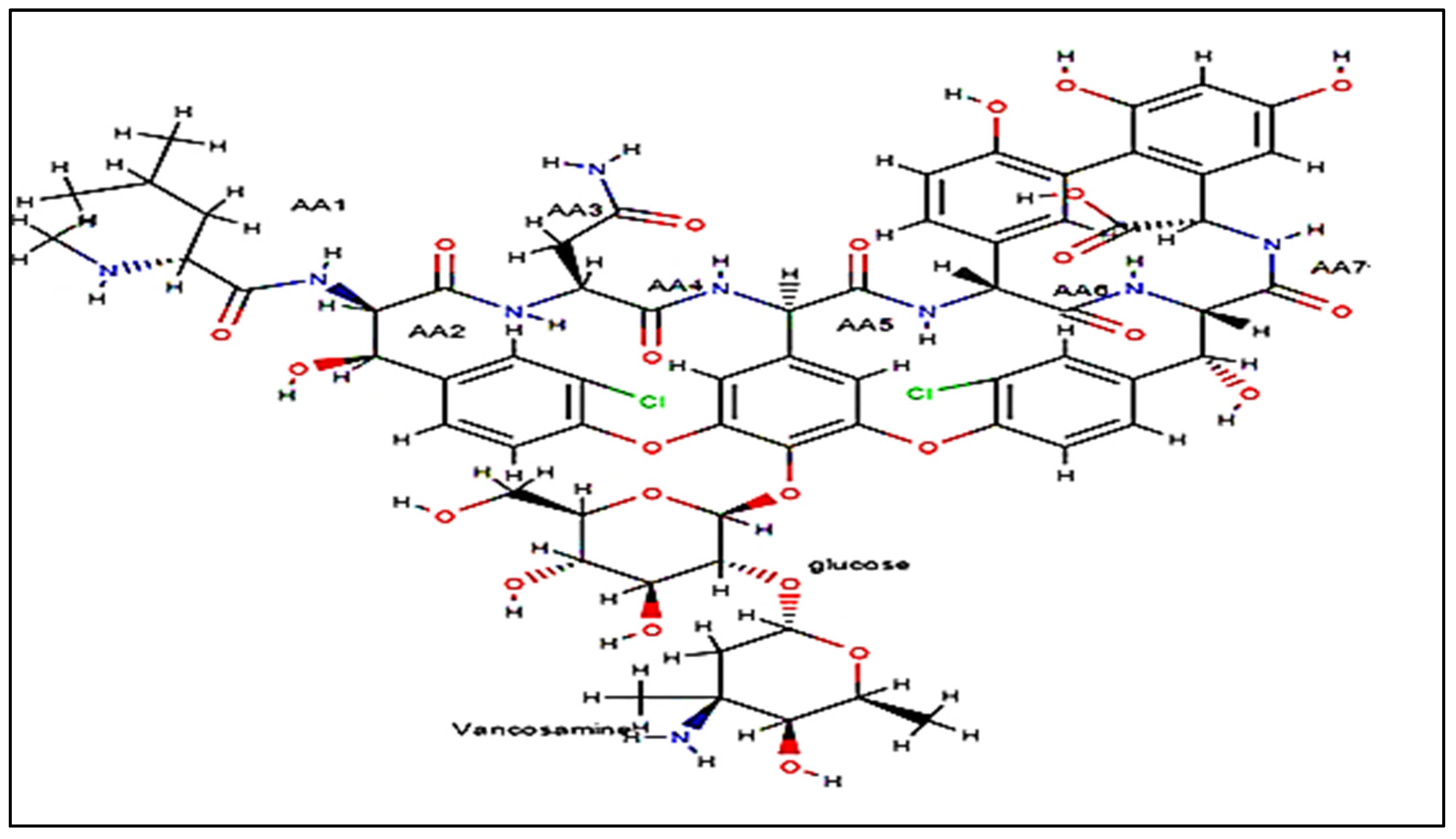

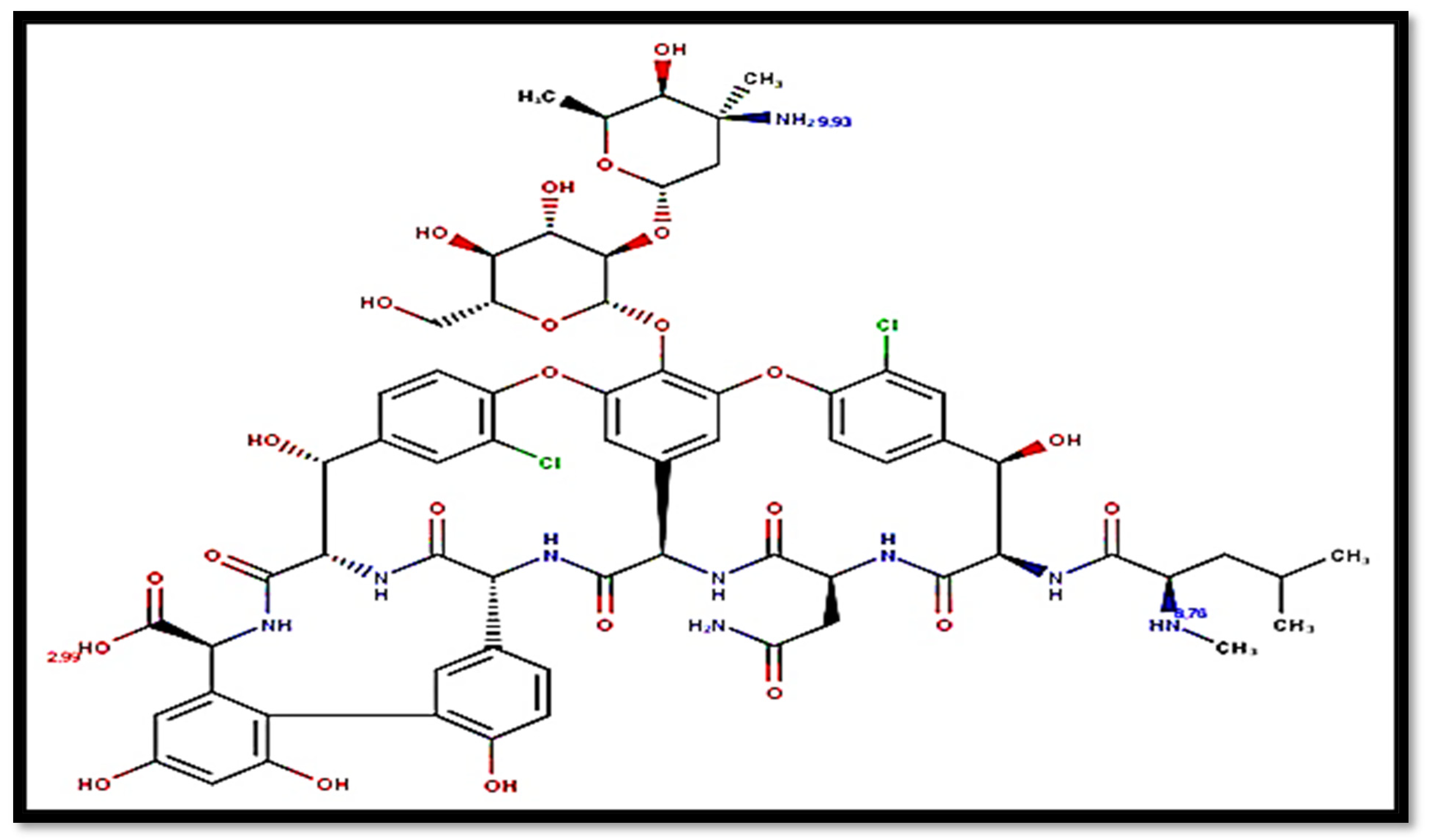
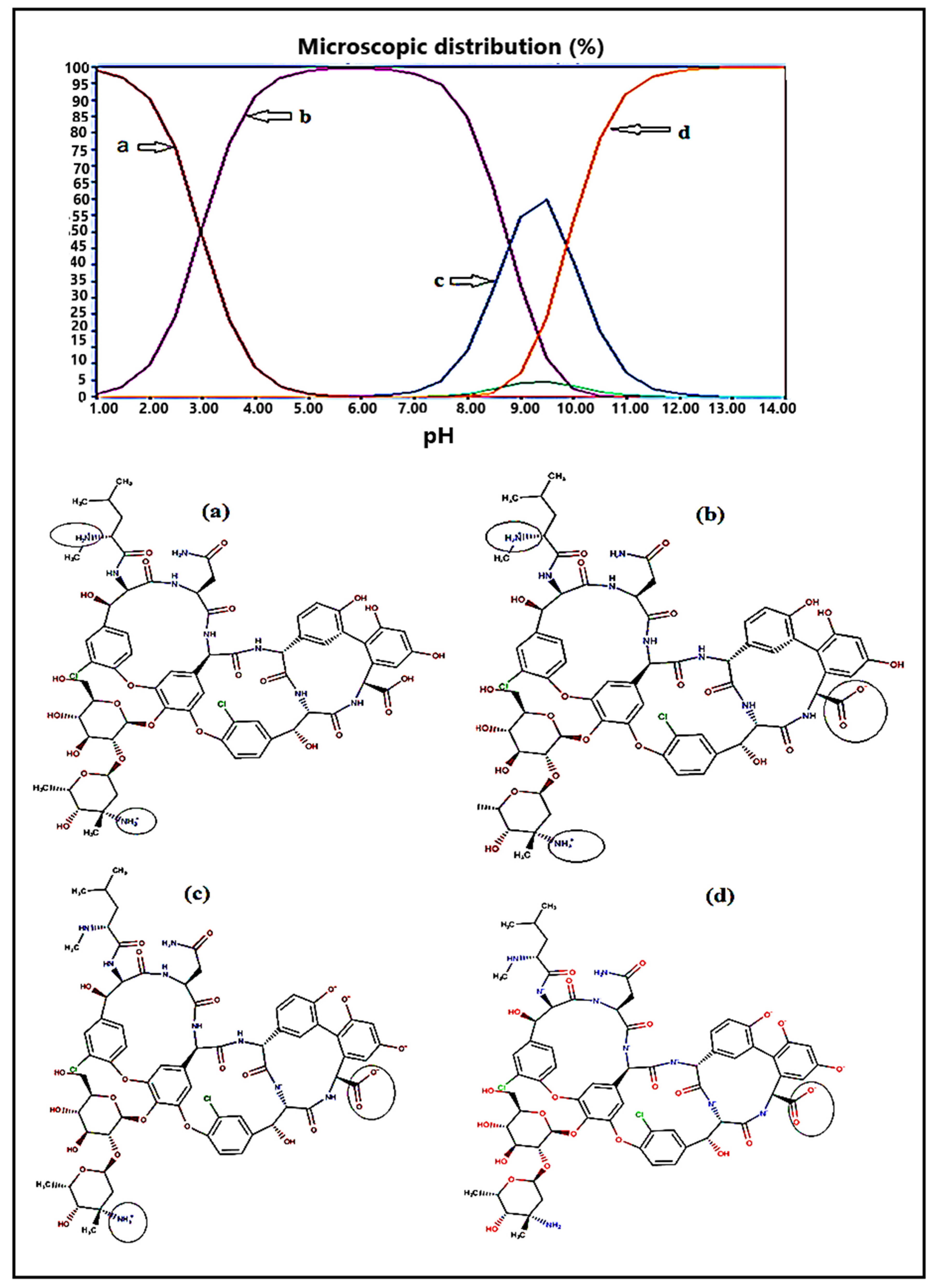



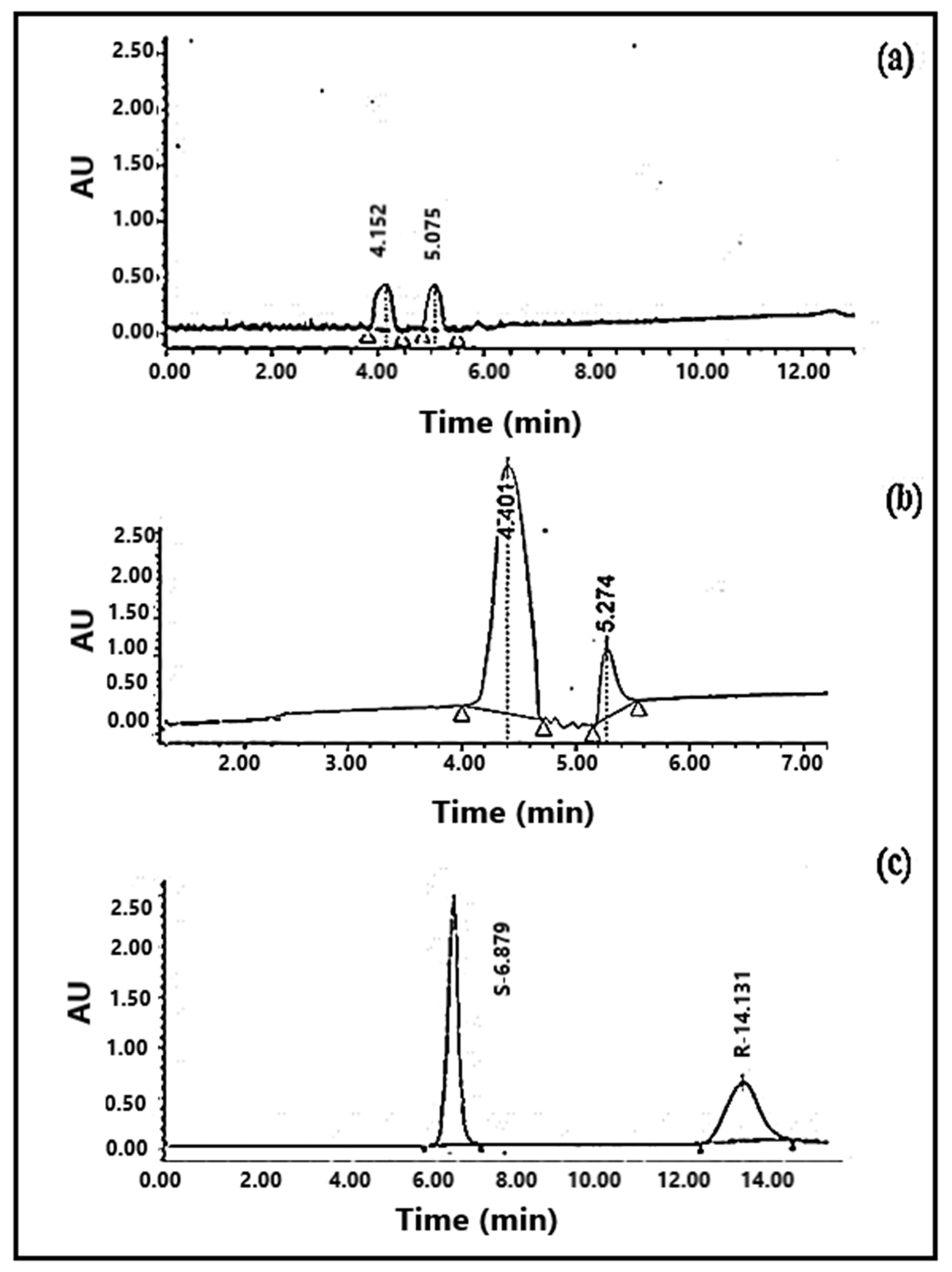
| Mobile Phase Composition | Retention Time in Amino Column (min) | Retention Time in Octadecyl Column (min) |
|---|---|---|
| PBS 0.05 M pH 6.0 | 5.57 | 6.22 |
| PBS 0.05 M pH 6.0/ACN (50/50) | 5.60 | 3.06 |
| PBS 0.05 M pH 6.0/MeOH (50/50) | 7.95 | 4.05 |
| PBS0.05 M pH 6.0/2-Pro (50/50) | 10.70 | 3.82 |
| Profen Drugs | Molarity of Vancomycin (mM) | Retention Time (min) |
|---|---|---|
| Ibuprofen | 0 | 4.420 |
| 0.5 | t1 = 4.530 | |
| t2 = 4.881 | ||
| 1.0 | t1 = 4.325 | |
| t2 = 4.854 | ||
| 1.5 | t1 = 4.216 | |
| t2 = 4.924 | ||
| 2.0 | t1 = 4.152 | |
| t2 = 5.075 | ||
| Flurbiprofen | 0 | 3.854 |
| 0.5 | t1 = 4.215 | |
| t2 = 4.882 | ||
| 1.0 | t1 = 4.621 | |
| t2 = 4.958 | ||
| 1.5 | t1 = 4.401 | |
| t2 = 5.274 | ||
| 2.0 | t1 = 4.406 | |
| t2 = 5.281 | ||
| Ketoprofen | 0 | 4.605 |
| 0.5 | t1 = 5.134 | |
| t2 = 5.477 | ||
| 1.0 | t1 = 5.358 | |
| t2 = 6.874 | ||
| 1.5 | t1 = 6.217 | |
| t2 = 10.584 | ||
| 2.0 | t1 = 6.879 | |
| t2 = 14.130 | ||
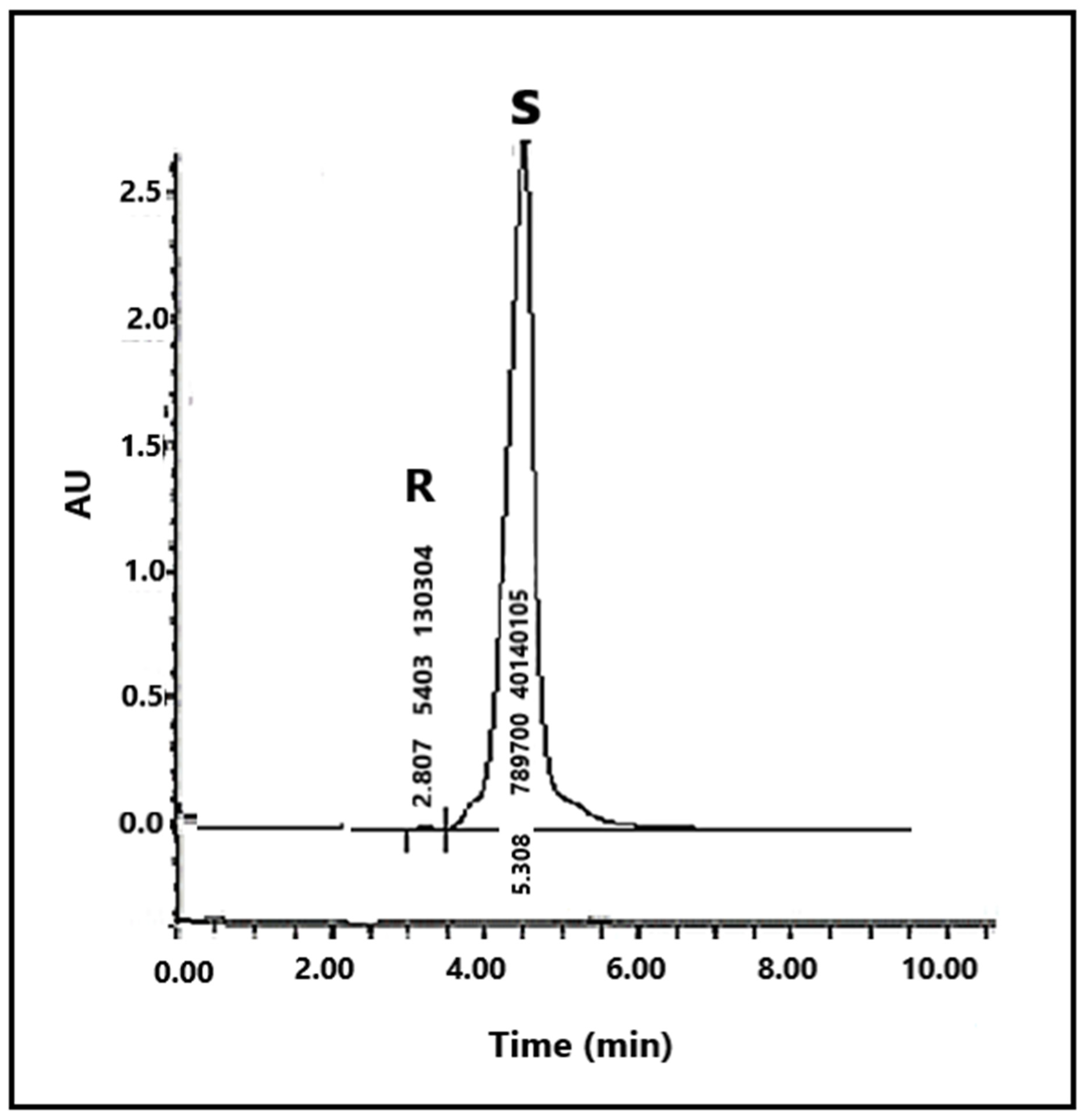
| Naproxen | 0 | 3.080 |
| 0.5 | t1 = 1.211 | |
| t2 = 3.154 | ||
| 1.0 | t1 = 1.325 | |
| t2 = 3.885 | ||
| 1.5 | t1 = 1.889 | |
| t2 = 4.251 | ||
| 2.0 | t1 = 2.867 | |
| t2 = 5.308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherdaoui, D.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Bouazza, F.; Lefnaoui, S.; Zeghdaoui, A.; Amrane, A.; Jaouadi, B.; Zhang, J. The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs. Symmetry 2023, 15, 2154. https://doi.org/10.3390/sym15122154
Gherdaoui D, Yahoum MM, Toumi S, Tahraoui H, Bouazza F, Lefnaoui S, Zeghdaoui A, Amrane A, Jaouadi B, Zhang J. The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs. Symmetry. 2023; 15(12):2154. https://doi.org/10.3390/sym15122154
Chicago/Turabian StyleGherdaoui, Dehbiya, Madiha Melha Yahoum, Selma Toumi, Hichem Tahraoui, Fatma Bouazza, Sonia Lefnaoui, Abdelhamid Zeghdaoui, Abdeltif Amrane, Bassem Jaouadi, and Jie Zhang. 2023. "The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs" Symmetry 15, no. 12: 2154. https://doi.org/10.3390/sym15122154
APA StyleGherdaoui, D., Yahoum, M. M., Toumi, S., Tahraoui, H., Bouazza, F., Lefnaoui, S., Zeghdaoui, A., Amrane, A., Jaouadi, B., & Zhang, J. (2023). The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs. Symmetry, 15(12), 2154. https://doi.org/10.3390/sym15122154








