Model-Based Regional Control with Anomalous Diffusion of Multi-Drug Combined Cancer Therapy for Volume Predictions
Abstract
1. Introduction
2. Materials and Methods
2.1. A Minimalistic PKPD Model of Lung Tumor Growth
2.2. Regional Anomalous Diffusion
2.3. Predictive Control Strategy for Multi-Drug Therapy Optimization
2.4. Multi-Agent Nash Optimality and Coalition Control
- Players are the participants in a game, in competition against each other. In our context these are the different multi-drug selections and protocols competing for the best patient outcome;
- Actions of a player, denoting here the drug profiles and timeline administered to the patient;
- Information in game theory refers to acquiring knowledge about the game, skills, and forecasting of move effects in finding optimality; in our context, this refers to the knowledge of how the patient responds to the drug profile both past and forecasted in optimum seeking algorithms;
- Strategy refers to the association between a player’s move and the information available at that moment; this is fairly similar in our context denoting the controller’s optimal solution-seeking protocol and can be cooperative or non-cooperative, static or adaptive, etc;
- Utility (or reward) is part of the optimization cost variable and for our case, this is the minimal amount of drug which maximizes the patient outcome, i.e., minimizes a relative ratio between volume growth and cell death rate.
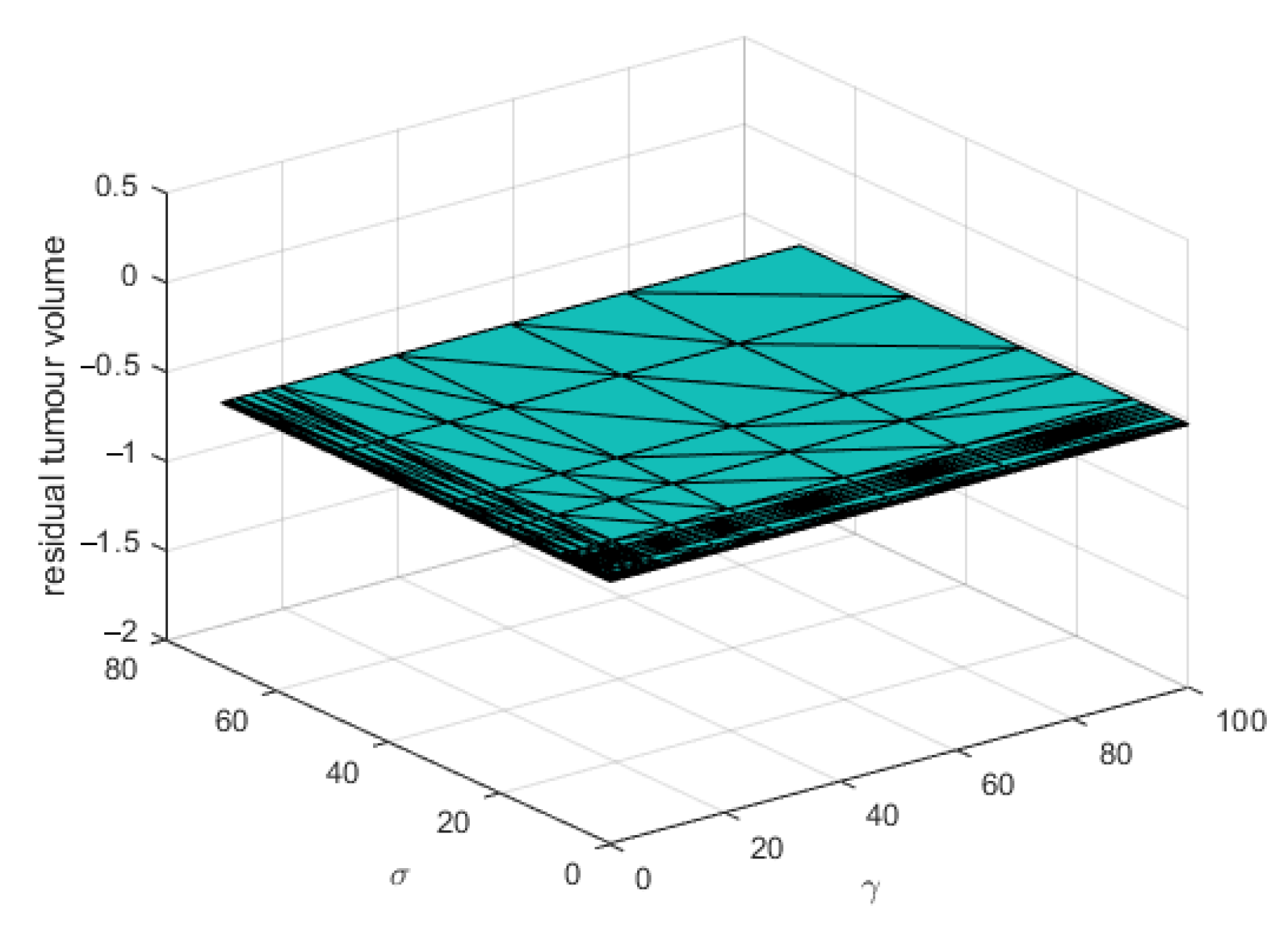
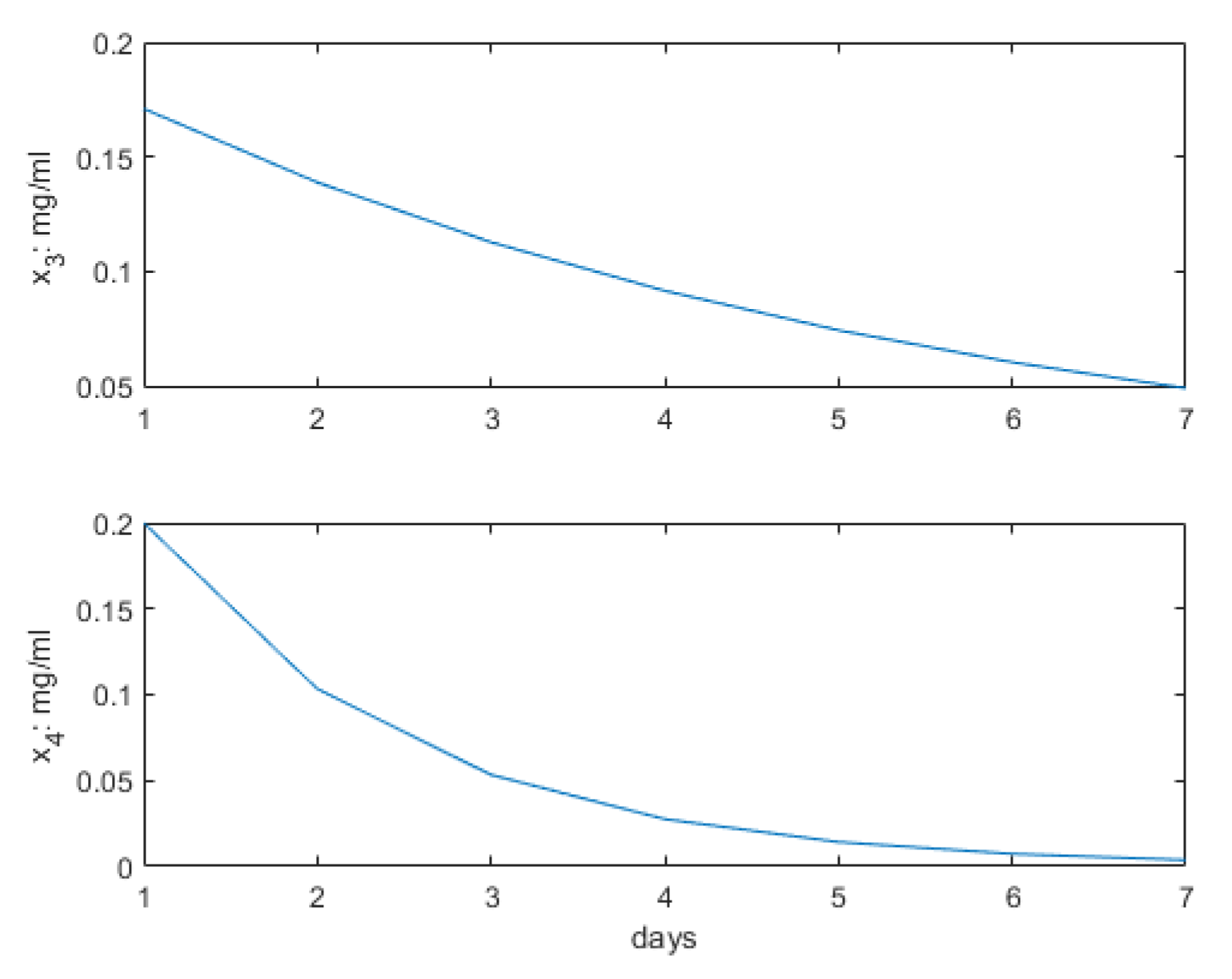
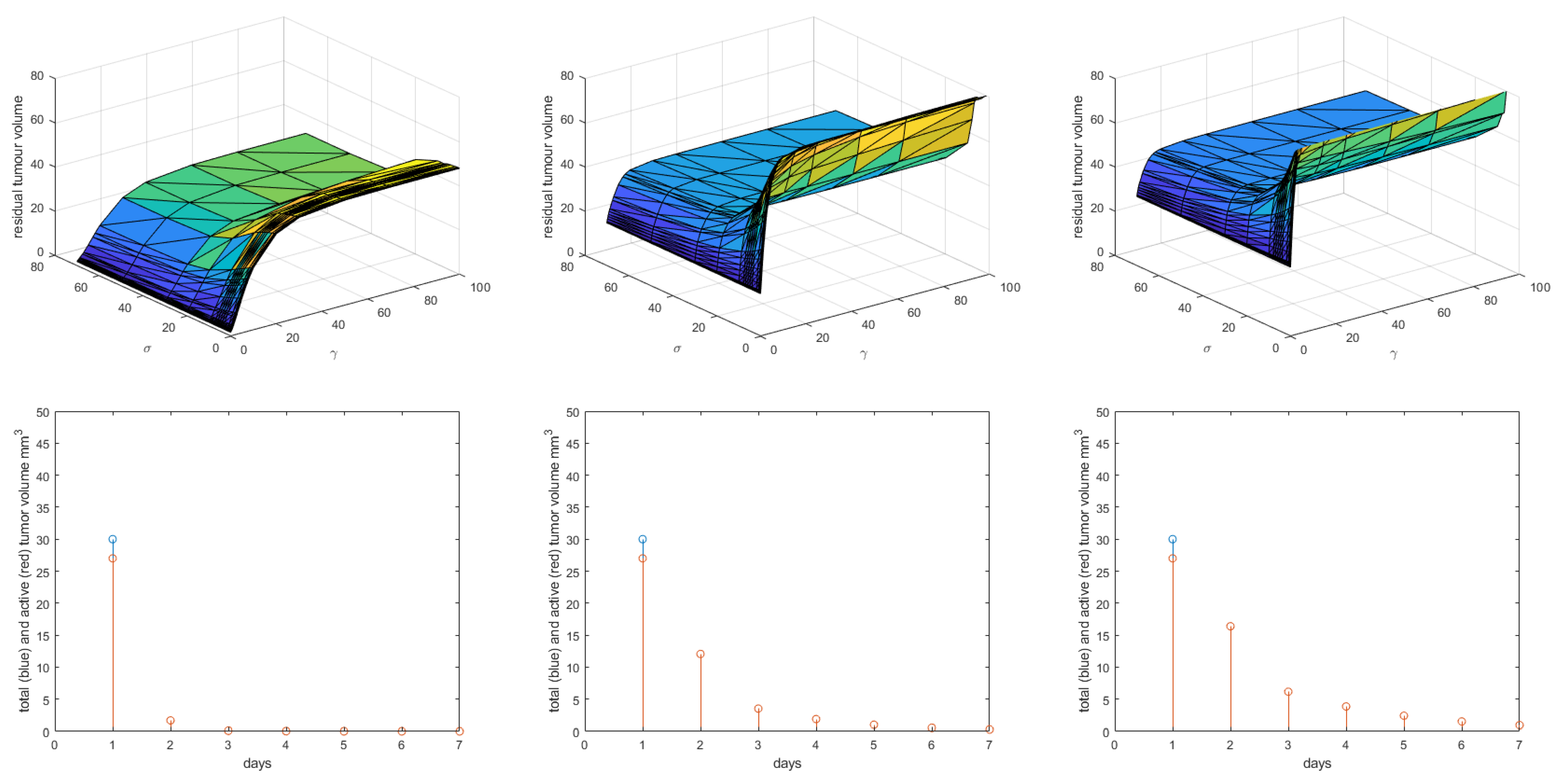
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPC | Model Predictive Control |
| NE | Nash Equilibrium |
| NSCLC | Non-Small Cell Lung Cancer |
| PKPD | Pharmacokinetic-Pharmacodynamic |
| RL | Reinforcement Learning |
| SBRT | Stereotactic Body Radiation Therapy |
References
- Hassan, Z.; Raza, N.; Abdel-Aty, A.-H.; Zakarya, M.; Rahman, R.U.; Yasmeen, A.; Muse, A.H.; Mahmoud, E.E. New fractal soliton solutions and sensitivity visualization for double-chain DNA model. J. Funct. Spaces 2022, 2022, 2297866. [Google Scholar] [CrossRef]
- Amoddeo, A. Indirect contributions to tumor dynamics in the first stage of the avascular phase. Symmetry 2020, 12, 1546. [Google Scholar] [CrossRef]
- Axenie, C.; Bauer, R.; Martínez, M.R. The multiple dimensions of networks in cancer: A perspective. Symmetry 2021, 13, 1559. [Google Scholar] [CrossRef]
- Go, J. Mathematical analysis for the effects of medicine supplies to a solid Tumor. Symmetry 2021, 13, 1988. [Google Scholar] [CrossRef]
- Cacace, F.; Cusimano, V.; Palumbo, P. Optimal impulsive control with application to antiangiogenic tumor therapy. IEEE Trans. Control Syst. Technol. 2020, 28, 106–117. [Google Scholar] [CrossRef]
- Huang, W.; Chen, L.; Kang, L.; Jin, M.; Sun, P.; Xin, X.; Gao, Z.; Bae, Y.H. Nanomedicine-based combination anticancer therapy between nucleic acids and small molecular drugs. Adv. Drug Deliv. Rev. 2017, 115, 82–97. [Google Scholar] [CrossRef]
- Eisenstein, M. New lung-cancer drugs extend survival times. Nature 2020, 587, S10–S12. [Google Scholar] [CrossRef]
- Thomas, D.S.; Cisneros, L.H.; Anderson, A.R.A.; Maley, C.C. In silico investigations of multi-drug adaptive therapy protocols. Cancers 2022, 14, 2699. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poitier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Karachaliou, N.; Pilloto, S.; Lazzari, C.; Bria, E.; De Marini, F.; Rosell, R. Cellular and molecular biology of small cell lung cancer. an overview. Trans. Lung Cancer Res. 2016, 5, 2–15. [Google Scholar]
- Ghita, M.; Billiet, C.; Copot, D.; Verellen, D.; Ionescu, C.M. Parameterisation of respiratory impedance in lung cancer patients from forced oscillation lung function test. IEEE Trans. Biomed. Eng. 2022. [Google Scholar] [CrossRef]
- Ionescu, C.M.; Copot, C.; Verellen, D. Motion compensation for robotic lung tumour radiotherapy in remote locations: A personalised medicine approach. Acta Astronaut. 2017, 132, 59–66. [Google Scholar] [CrossRef]
- Ionescu, C.M. The Human Respiratory System: An Analysis of the Interplay between Anatomy, Structure, Breathing and Fractal Dynamics; Series in BioEngineering; Springer: London, UK, 2013. [Google Scholar]
- Ionescu, C.; Kelly, J.F. Fractional calculus for respiratory mechanics: Power law impedance, viscoelasticity, and tissue heterogeneity. Chaos Solitons Fractals 2017, 102, 433–440. [Google Scholar] [CrossRef]
- Ionescu, C.; Lopes, A.; Copot, D.; Machado, J.A.T.; Bates, J.H.T. The role of fractional calculus in modeling biological phenomena: A review. Commun. Nonlinear Sci. Numer. Simul. 2017, 51, 141–159. [Google Scholar] [CrossRef]
- Drexler, D.A.; Sapi, J.; Kovacs, L. Modeling of tumor growth incorporating the effects of necrosis and the effect of bevacizumab. Complexity 2017, 5985031. [Google Scholar] [CrossRef]
- Sapi, J.; Kovacs, L.; Drexler, D.A.; Kocsis, P.; Gajari, D.; Sapi, Z. Tumor volume estimation and quasi-continuous administration for most effective bevacizumab therapy. PLoS ONE 2015, 10, e0142190. [Google Scholar] [CrossRef]
- Prezzano, K.M.; Ma, S.J.; Hermann, G.M.; Rivers, C.I.; Gomez-Suescun, J.A.; Singh, A.K. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J Clin Oncol 2019, 10, 14–27. [Google Scholar] [CrossRef]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for advanced non-small cell lung cancer: A decade of progress. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e105–e127. [Google Scholar] [CrossRef]
- Ionescu, C.M.; Ghita, M.; Copot, D.; Derom, E.; Verellen, D. A minimal PKPD interaction model for evaluating synergy effects of combined NSCLC therapies. J. Clin. Med. 2020, 9, 1832. [Google Scholar] [CrossRef]
- Ghita, M.; Copot, D.; Billiet, C.; Verellen, D. Ionescu, C.M. Lung cancer dynamics using fractional order impedance modeling on a mimicked lung tumor setup. J. Adv. Res. 2021, 32, 61–71. [Google Scholar] [CrossRef]
- Ghita, M.; Billiet, C.; Copot, D.; Verellen, D.; Ionescu, C.M. Model calibration of pharmacokineticpharmacodynamic lung tumour dynamics for anticancer therapies. J. Clin. Med. 2022, 11(4), 1006. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, A.; Hong, K.-S. Maximum likelihood identification of Wiener-Hammerstein models. Mech. Syst. Signal Process 2003, 41, 54–70. [Google Scholar] [CrossRef]
- Shaikh, A.H.; Barbe, K. Study of Random Forest to Identify Wiener-Hammerstein System. IEEE Trans. Instrum. Meas. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Ionescu, C.M. A computationally efficient Hill curve adaptation strategy during continuous monitoring of dose-effect relation in anaesthesia. Nonlinear Dyn. 2018, 92, 843–852. [Google Scholar] [CrossRef]
- Ghita, M.; Copot, D.; De Keyser, R.; Ionescu, C.M. Pharmaco-impedance modelling for lung cancer therapy with predictive control. In Proceedings of the 9th International Conference on Systems and Control (ICSC), Caen, France, 24–26 November 2021; pp. 423–428. [Google Scholar]
- Ionescu, C.; Cajo Diaz, R.A.; Zhao, S.; Ghita, M.; Ghita, M.; Copot, D. A low computational cost, prioritized, multi-objective optimization procedure for predictive control towards cyber physical systems. IEEE Access 2020, 8, 128152–128166. [Google Scholar] [CrossRef]
- Wang, L. Control System Design and Implementation Using MATLAB; Springer: London, UK, 2009. [Google Scholar]
- Rossiter, J.A. A First Course in Predictive Control, 2nd ed.; CRC Press: Cambridge, UK, 2022. [Google Scholar]
- Li, T.; Peng, G.; Zhy, Q.; Basar, T. The confluence of networks, games and learning a game-theoretic framework for multiagent decision making over network. IEEE Control Syst. Mag. 2022, 42, 35–67. [Google Scholar] [CrossRef]
- Xu, C.; Farman, M.; Hasan, A.; Akgül, A.; Zakarya, M.; Albalawi, W.; Park, C. Lyapunov stability and wave analysis of COVID-19 omicron variant of real data with fractional operator. Alex. Eng. J. 2022, 61, 11787–11802. [Google Scholar] [CrossRef]
- Hu, G.; Pang, Y.; Sun, C.; Hong, Y. Distributed Nash equilibrium seeking: Continuous-time control-theoretic approaches. IEEE Control Syst. Mag. 2022, 42, 68–86. [Google Scholar] [CrossRef]
- Belgioioso, G.; Yi, P.; Grammatico, S.; Pavel, L. Distributed generalized Nash equilibirum seeking: An operator-theoretic perspective. IEEE Control Syst. Mag. 2022, 42, 87–102. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Y.; Chen, Z.; Zhao, M. Data decision and drug therapy based on non-small cell lung cancer in a big data medical system in developing countries. Symmetry 2018, 10, 152. [Google Scholar] [CrossRef]
- Drexler, D.A.; Ferenci, T.; Furedi, A.; Szakacs, G.; Kovacs, L. Experimental data-driven tumor modeling for chemotherapy. IFACPapersOnline 2020, 53, 16245–16250. [Google Scholar] [CrossRef]
- Cacace, F.; Cusimano, V.; Germani, A.; Palumbo, P.; Papi, M. Optimal continuous-discrete linear filter and moment equations for nonlinear diffusions. IEEE Trans. Automat. Contr. 2020, 65, 3961–3976. [Google Scholar] [CrossRef]
- Borri, A.; Palumbo, P.; Papa, F. The stochastic approach for SIR epidemic models: Do they help to increase information from raw data? Symmetry 2022, 14, 2330. [Google Scholar] [CrossRef]
- Lei, J.; Shanbhag, U.V. Stochastic Nash equilibrium problems: Models, analysis, and algorithms. IEEE Control Syst. Mag. 2022, 42, 103–124. [Google Scholar] [CrossRef]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; Freed, B.M.; et al. Characterization of immune responses to anti-PD-1 mono- and combination therapy in hematopoietic humanized mice implanted with tumor xenographs. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef]
- Sculier, J.P.; Paesmans, M.; Bureau, G.; Dabois, G.; Libert, P.; Van Cutsem, G.V.O.; Berchier, M.C.; Ries, F.; Michel, J. Multiple-drug weekly chemotherapy versus standard combination regimen in small-cell lung cancer: A phase III randomized study conducted by the European Lung Cancer Working Party. J. Clin Oncol. 1993, 11, 1858–1865. [Google Scholar] [CrossRef]
- Kovacs, L.; Szeles, A.; Sapi, J.; Drexler, D.A.; Rudas, I.; Harmati, I.; Sapi, Z. Model-based angiogenic inhibition of tumor growth using modern robust control method. Comput. Methods Programs Biomed. 2014, 114, e98–e110. [Google Scholar] [CrossRef]
- Almetwally, E.M.; Jawa, T.M.; Sayed-Ahmed, N.; Park, C.; Zakarya, M.; Dey, S. Analysis of unit-Weibull based on progressive type-II censored with optimal scheme. Alex. Eng. J. 2023, 63, 321–338. [Google Scholar] [CrossRef]
- Khalid, A.; Rehan, A.; Nisar, K.S.; Abdel-Aty, A.-H.; Zakarya, M. Splines solutions of higher-order BVPs that ARISE in consistent magnetized force field. Fractals 2022, 30, 2240043. [Google Scholar] [CrossRef]
- Hahn, J.-O.; Inan, O.T. Physiological closed-loop control in critical care: Opportunities for innovations. Prog. Biomed. Eng. 2022, 4, 033001. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, C.M.; Ghita, M. Model-Based Regional Control with Anomalous Diffusion of Multi-Drug Combined Cancer Therapy for Volume Predictions. Symmetry 2023, 15, 51. https://doi.org/10.3390/sym15010051
Ionescu CM, Ghita M. Model-Based Regional Control with Anomalous Diffusion of Multi-Drug Combined Cancer Therapy for Volume Predictions. Symmetry. 2023; 15(1):51. https://doi.org/10.3390/sym15010051
Chicago/Turabian StyleIonescu, Clara Mihaela, and Maria Ghita. 2023. "Model-Based Regional Control with Anomalous Diffusion of Multi-Drug Combined Cancer Therapy for Volume Predictions" Symmetry 15, no. 1: 51. https://doi.org/10.3390/sym15010051
APA StyleIonescu, C. M., & Ghita, M. (2023). Model-Based Regional Control with Anomalous Diffusion of Multi-Drug Combined Cancer Therapy for Volume Predictions. Symmetry, 15(1), 51. https://doi.org/10.3390/sym15010051







