Donor-Site Morbidity after Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Stair Climbing Asymmetry and Functional Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Gait Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Zorzi, M.; Del Mistro, A.; Da Mosto, M.C.; Tirelli, G.; Buzzoni, C.; Rugge, M.; Polesel, J.; Guzzinati, S. The evolution of the epidemiological landscape of head and neck cancer in Italy: Is there evidence for an increase in the incidence of potentially HPV-related carcinomas? PLoS ONE 2018, 13, e0192621. [Google Scholar]
- Van Dijk, B.A.C.; Brands, M.T.; Geurts, S.M.E.; Merkx, M.A.W.; Roodenburg, J.L.N. Trends in oral cavity cancer incidence, mortality, survival and treatment in the Netherlands. Int. J. Cancer 2016, 139, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Hennessy, M.; Lehman, E.; Lin, W.; Agudo, A.; Ahrens, W.; Boccia, S.; Brennan, P.; Brenner, H.; Cadoni, G.; et al. Risk factors for head and neck cancer in more and less developed countries: Analysis from the INHANCE consortium. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Al-Sharif, R.M.; Batwa, D.Y.; Alotaibi, T.N.; Alwadai, N.M.; Alsharif, A.H.; Azhar, N.A.; Moulana, A.A.; Alahmadi, S.M.; Althobaiti, M.Y.; Bayyumi, D.F.; et al. Epidemiology and types of oral cancer. Int. J. Community Med. Public Health 2022, 9, 1017–1022. [Google Scholar] [CrossRef]
- Syczewska, M.; Krajewski, R.; Kirwil, M.; Szczerbik, E.; Kalinowska, M. Gait changes in patients after reconstruction of facial bones with fibula and iliac crest free vascularized flaps. Acta Bioeng. Biomech. 2018, 20, 185–190. [Google Scholar]
- Awad, M.E.; Altman, A.; Elrefai, R.; Shipman, P.; Looney, S.; Elsalanty, M. The use of vascularized fibula flap in mandibular reconstruction; A comprehensive systematic review and meta-analysis of the observational studies. J. Cranio-Maxillofac. Surg. 2019, 47, 629–641. [Google Scholar] [CrossRef]
- Baj, A.; Lovecchio, N.; Bolzoni, A.; Mapelli, A.; Giannì, A.B.; Sforza, C. Stair ascent and descent in assessing donor-site morbidity following osteocutaneous free fibula transfer: A preliminary study. J. Oral Maxillofac. Surg. 2015, 73, 184–193. [Google Scholar] [CrossRef]
- Bolzoni, A.; Mapelli, A.; Baj, A.; Sidequersky, F.V.; Giannì, A.B.; Sforza, C. Valutazione tridimensionale dei movimenti mandibolari dopo ricostruzione con lembo libero di fibula. Acta Otorhinolaryngol. Ital. 2015, 35, 371–378. [Google Scholar] [CrossRef]
- Di Giuli, R.; Zago, M.; Beltramini, G.A.; Pallotta, M.L.; Bolzoni, A.; Baj, A.; Giannì, A.B.; Sforza, C. Donor-Site Morbidity After Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Gait Performance. J. Oral Maxillofac. Surg. 2019, 77, 648–657. [Google Scholar] [CrossRef]
- Feuvrier, D.; Sagawa, Y.; Béliard, S.; Pauchot, J.; Decavel, P. Long-term donor-site morbidity after vascularized free fibula flap harvesting: Clinical and gait analysis. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kearns, M.; Ermogenous, P.; Myers, S.; Ghanem, A.M. Osteocutaneous flaps for head and neck reconstruction: A focused evaluation of donor site morbidity and patient reported outcome measures in different reconstruction options. Arch. Plast. Surg. 2018, 45, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.F.; Peng, X.; Samman, N. Donor-site morbidity of free fibula and DCIA flaps. J. Oral Maxillofac. Surg. 2013, 71, 1604–1612. [Google Scholar] [CrossRef]
- Momoh, A.O.; Yu, P.; Skoracki, R.J.; Liu, S.; Feng, L.; Hanasono, M.M. A prospective cohort study of fibula free flap donor-site morbidity in 157 consecutive patients. Plast. Reconstr. Surg. 2011, 128, 714–720. [Google Scholar] [CrossRef]
- Shindo, M.; Fong, B.P.; Funk, G.F.; Karnell, L.H. The fibula osteocutaneous flap in head and neck reconstruction: A critical evaluation of donor site morbidity. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Börner, B.I.; Hasse, A.; Sieg, P. Donor site morbidity after microvascular fibula transfer. Clin. Oral Investig. 2001, 5, 214–219. [Google Scholar] [CrossRef]
- Ling, X.F.; Peng, X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast. Reconstr. Surg. 2012, 129, 657–674. [Google Scholar] [CrossRef]
- Hatchell, A.C.; Schrag, C.H.; Temple-Oberle, C.F.; Matthews, J.L.; McKenzie, C.D.; Matthews, T.W.; Chandarana, S.P.; Dort, J.C.; Baaqeel, R. Patient-reported outcomes after fibula free flap harvest: A pilot study. Plast. Reconstr. Surg. 2021, 148, 1007e–1011e. [Google Scholar] [CrossRef]
- Hadouiri, N.; Decavel, P.; Feuvrier, D.; Parratte, B.; Pauchot, J.; Sagawa, Y. Donor-site morbidity after vascularized free flap fibula: Gait analysis during prolonged walk condition. Ann. Phys. Rehabil. Med. 2016, 59, e119. [Google Scholar] [CrossRef]
- Harris, B.N.; Bewley, A.F. Minimizing free flap donor-site morbidity. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 447–452. [Google Scholar] [CrossRef]
- Lee, M.; Sim, S.; Jiemin, Y. Y-Balance Test But Not Functional Movement Screen Scores Are Associated With Peak Knee Valgus Moments During Unplanned Sidestepping: Implications For Assessing Anterior Cruciate Ligament Injury Risk. Int. Soc. Biomech. Sport. 2017, 35, 77–80. [Google Scholar]
- Shah, K.C.; Peehal, J.P.; Shah, A.; Crank, S.; Flora, H.S. Star excursion balance test for assessment of dynamic instability of the ankle in patients after harvest of a fibular free flap: A two-centre study. Br. J. Oral Maxillofac. Surg. 2017, 55, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Diefenbach, J.; Schmermund, D.; Böttger, S.; Pons-Kühnemann, J.; Scheibelhut, C.; Heiss, C.; Howaldt, H.P. Donor-site morbidity after fibula transplantation in head and neck tumor patients: A split-leg retrospective study with focus on leg stability and quality of life. Cancers 2020, 12, 2217. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Kohlmeier, C.; Suling, A.; Assaf, A.T.; Catala-Lehnen, P.; Amling, M.; Heiland, M.; Riecke, B. Prospective biomechanical analysis of donor-site morbidity after fibula free flap. J. Cranio-Maxillofac. Surg. 2016, 44, 155–159. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, C.Y.; Myoung, H.; Kim, M.J.; Yun, P.Y. Gait analysis of donor leg after free fibular flap transfer. Int. J. Oral Maxillofac. Surg. 2008, 37, 625–629. [Google Scholar] [CrossRef]
- Macdonald, K.I.; Taylor, S.M.; Trites, J.R.B.; Fung, E.W.; Barnsley, P.G.; Dunbar, M.J.; Leahey, J.L.; Hart, R.D. Effect of fibula free flap harvest on the gait of head and neck cancer patients: Preliminary results. J. Otolaryngol. Head Neck Surg. 2011, 40, S34–S40. [Google Scholar]
- Queen, R.M.; Attarian, D.E.; Bolognesi, M.P.; Butler, R.J. Bilateral symmetry in lower extremity mechanics during stair ascent and descent following a total hip arthroplasty: A one-year longitudinal study. Clin. Biomech. 2015, 30, 53–58. [Google Scholar] [CrossRef]
- Baj, A.; Beltramini, G.A.; Massarelli, O.; Youssef, D.A.; Giannì, A.B. Minimally invasive harvest of free fibula flap. Plast. Reconstr. Surg. 2013, 131, 474e–477e. [Google Scholar] [CrossRef]
- Avrin, R.; Davis, I.; Higginson, J.; Royer, T. The symmetry angle: A novel, robust method of quantifying asymmetry. Gait Posture 2008, 27, 622–627. [Google Scholar]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; McFadyen, B.J.; Malouin, F. Frontal and sagittal plane analyses of the stair climbing task in healthy adults aged over 40 years: What are the challenges compared to level walking? Clin. Biomech. 2003, 18, 950–959. [Google Scholar] [CrossRef]
- Mundi, N.; Ghasemi, F.; Zeng, P.Y.F.; Prokopec, S.D.; Patel, K.; Kim, H.A.J.; Di Gravio, E.; MacNeil, D.; Khan, M.I.; Han, M.W.; et al. Sex disparities in head & neck cancer driver genes: An analysis of the TCGA dataset. Oral Oncol. 2020, 104, 104614. [Google Scholar] [PubMed]
- Maben, D.; Anehosur, V.; Kumar, N. Assessment of Donor Site Morbidity Following Fibula Flap Transfer. J. Maxillofac. Oral Surg. 2021, 20, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hölzle, F.; Kesting, M.R.; Hölzle, G.; Watola, A.; Loeffelbein, D.J.; Ervens, J.; Wolff, K.D. Clinical outcome and patient satisfaction after mandibular reconstruction with free fibula flaps. Int. J. Oral Maxillofac. Surg. 2007, 36, 802–806. [Google Scholar] [CrossRef]
- Schardt, C.; Schmid, A.; Bodem, J.; Krisam, J.; Hoffmann, J.; Mertens, C. Donor site morbidity and quality of life after microvascular head and neck reconstruction with free fibula and deep-circumflex iliac artery flaps. J. Cranio-Maxillofac. Surg. 2017, 45, 304–311. [Google Scholar] [CrossRef]


| Patients | Age (Years) | Height (m) | BMI (kg/m2) | VFFF 1 Variant | Receiving Site Pathology | Notes |
|---|---|---|---|---|---|---|
| Bone | ||||||
| M2 | 23 | 1.78 | 21.9 | Left | R mandibular body keratocyst | |
| M4 | 30 | 1.58 | 33.6 | Left | R mandibular ramus ameloblastoma recurrence | Pain |
| F2 | 38 | 1.76 | 32.6 | Right | R parasymphysis jaw keratocyst | Pain |
| F4 | 45 | 1.60 | 26.0 | Left | L mandibular ramus keratocyst | |
| F5 | 53 | 1.57 | 18.3 | Left | R mandibular ramus adenoid cystic carcinoma | |
| F8 | 28 | 1.72 | 19.6 | Right | R mandibular ramus pseudarthrosis | |
| M6 | 59 | 1.68 | 33.3 | Mini Left | L retromolar trigone mucoepidermoid carcinoma | Flexor hallucis longus deficit—Stiffness |
| Osteocutaneous | ||||||
| F1 | 54 | 1.69 | 17.9 | Left | L parasymphysis jaw pseudarthrosis | - |
| Osteo-myocutaneous | ||||||
| M1 | 64 | 1.78 | 33.1 | Right | L mandibular ramus squamous cell carcinoma | Radiotherapy |
| M3 | 27 | 1.73 | 22.4 | Right | L retromolar trigone squamous cell carcinoma | |
| M5 | 61 | 1.72 | 18.9 | Right | L mandibular body and mouth floor squamous cell carcinoma | Extensor digitorum and hallucis longus deficit—Pain—Paraesthesia—Wound dehiscence |
| M7 | 59 | 1.78 | 25.2 | Right | Jaw symphysis and mouth floor squamous cell carcinoma | Radiotherapy |
| F3 | 70 | 1.60 | 22.7 | Right | L mandibular ramus squamous cell carcinoma | |
| F7 | 61 | 1.47 | 23.6 | Mini Left | R mandibular body mucoepidermoid carcinoma | - |
| Mean | 48 | 1.68 | 24.9 | |||
| SD | 16 | 0.10 | 5 | |||
| Healthy Limb | Donor Limb | Comparison | Effect Size | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | 2-Way ANOVA | Partial η2 | |||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p Time | p Side | p × | η2 Time | η2 Side | η2 × |
| Ascent | ||||||||||||||
| Step cadence (step/s) | 0.79 | 0.10 | 0.77 | 0.09 | 0.78 | 0.12 | 0.78 | 0.13 | 0.907 | 0.426 | 0.390 | 0.000 | 0.003 | 0.001 |
| Stance (%) | 0.61 | 0.02 | 0.62 | 0.03 | 0.62 | 0.03 | 0.61 | 0.04 | 0.899 | 0.757 | 0.098 | 0.000 | 0.001 | 0.009 |
| Swing (%) | 0.39 | 0.02 | 0.38 | 0.03 | 0.38 | 0.03 | 0.39 | 0.04 | 0.899 | 0.757 | 0.098 | 0.000 | 0.001 | 0.009 |
| Double support (%) | 0.14 | 0.02 | 0.13 | 0.03 | 0.14 | 0.03 | 0.12 | 0.03 | 0.964 | 0.079 | 0.261 | 0.000 | 0.069 | 0.006 |
| Step duration (s) | 1.31 | 0.15 | 1.32 | 0.15 | 1.32 | 0.24 | 1.32 | 0.21 | 0.753 | 0.793 | 0.753 | 0.001 | 0.000 | 0.000 |
| Step width (m) | 0.09 | 0.04 | 0.08 | 0.04 | 0.10 | 0.03 | 0.09 | 0.03 | 0.076 | 0.062 | 0.691 | 0.017 | 0.022 | 0.001 |
| Velocity (m/s) | 0.53 | 0.06 | 0.49 | 0.14 | 0.53 | 0.08 | 0.53 | 0.09 | 0.340 | 0.416 | 0.341 | 0.011 | 0.008 | 0.008 |
| Descent | ||||||||||||||
| Step cadence (step/s) | 0.88 | 0.12 | 0.88 | 0.12 | 0.88 | 0.16 | 0.89 | 0.15 | 0.933 | 0.976 | 0.705 | 0.000 | 0.000 | 0.000 |
| Stance (%) | 0.61 | 0.03 | 0.61 | 0.03 | 0.60 | 0.03 | 0.60 | 0.03 | 0.258 | 0.677 | 0.563 | 0.017 | 0.002 | 0.004 |
| Swing (%) | 0.39 | 0.03 | 0.39 | 0.03 | 0.40 | 0.03 | 0.40 | 0.03 | 0.256 | 0.681 | 0.567 | 0.017 | 0.002 | 0.004 |
| Double support (%) | 0.12 | 0.02 | 0.11 | 0.02 | 0.12 | 0.02 | 0.12 | 0.02 | 0.641 | 0.952 | 0.820 | 0.003 | 0.000 | 0.000 |
| Step duration (s) | 1.15 | 0.15 | 1.19 | 0.20 | 1.10 | 0.37 | 1.16 | 0.20 | 0.511 | 0.222 | 0.841 | 0.008 | 0.012 | 0.000 |
| Step width (m) | 0.08 | 0.05 | 0.08 | 0.04 | 0.10 | 0.03 | 0.08 | 0.02 | 0.565 | 0.321 | 0.245 | 0.004 | 0.008 | 0.012 |
| Velocity (m/s) | 0.59 | 0.08 | 0.60 | 0.08 | 0.60 | 0.11 | 0.60 | 0.10 | 0.808 | 0.658 | 0.961 | 0.001 | 0.000 | 0.000 |
| Pre | Post | |||||
|---|---|---|---|---|---|---|
| Variable | p-Value | ES | ||||
| Ascent | ||||||
| Step cadence | 0.7 | 3.0 | 0.2 | 1.8 | 0.431 | 0.234 |
| Stance | −0.4 | 1.4 | 0.2 | 1.6 | 0.097 | 0.391 |
| Swing | 0.7 | 2.1 | −0.3 | 2.4 | 0.104 | 0.410 |
| Double support | 2.8 | 7.5 | 4.7 | 8.9 | 0.353 | 0.233 |
| Step duration | −0.2 | 2.5 | −0.1 | 2.0 | 0.805 | 0.061 |
| Step width | 2.2 | 12.1 | 4.2 | 7.4 | 0.500 | 0.211 |
| Velocity | 2.9 | 11.5 | 0.0 | 1.9 | 0.341 | 0.359 |
| Descent | ||||||
| Step cadence | 0.1 | 2.5 | −0.2 | 2.1 | 0.659 | 0.164 |
| Stance | −0.4 | 1.2 | 0.0 | 2.4 | 0.571 | 0.211 |
| Swing | 0.5 | 1.9 | −0.1 | 3.4 | 0.546 | 0.219 |
| Double support | 0.2 | 4.3 | −0.4 | 7.1 | 0.710 | 0.114 |
| Step duration | −1.0 | 3.2 | 0.2 | 2.1 | 0.236 | 0.448 |
| Step width | −3.2 | 18.3 | 4.2 | 9.6 | 0.209 | 0.498 |
| Velocity | −0.2 | 1.9 | −0.2 | 2.1 | 0.922 | 0.044 |
| Healthy Limb | Donor Limb | Comparison | Effect Size | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | 2-Way ANOVA | Partial η2 | |||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p Time | p Side | p × | η2 Time | η2 Side | η2 × |
| Ascent | ||||||||||||||
| Ankle flexion | 42.8 | 11.6 | 49.9 | 17.9 | 43.0 | 10.0 | 42.2 | 16.6 | 0.060 | 0.623 | 0.010 | 0.017 | 0.012 | 0.019 |
| Ankle inversion | 7.7 | 4.3 | 9.5 | 6.3 | 8.1 | 3.6 | 9.9 | 3.4 | 0.567 | 0.287 | 0.956 | 0.002 | 0.039 | 0.000 |
| Knee flexion | 65.2 | 19.5 | 73.0 | 15.8 | 61.2 | 20.6 | 69.0 | 17.1 | 0.090 | 0.403 | 0.987 | 0.012 | 0.045 | 0.000 |
| Hip abduction | 11.7 | 3.97 | 11.8 | 5.0 | 12.5 | 4.2 | 12.7 | 5.5 | 0.218 | 0.943 | 0.923 | 0.009 | 0.000 | 0.000 |
| Hip flexion | 53.2 | 5.8 | 53.8 | 4.7 | 54.3 | 6.4 | 54.8 | 3.0 | 0.440 | 0.701 | 0.964 | 0.011 | 0.003 | 0.000 |
| Pelvis obliquity | 12.8 | 4.3 | 13.5 | 5.4 | 13.4 | 7.3 | 13.5 | 5.5 | 0.818 | 0.467 | 0.598 | 0.001 | 0.001 | 0.001 |
| Pelvis rotation | 10.1 | 3.9 | 10.0 | 2.7 | 10.6 | 4.7 | 11.4 | 3.6 | 0.143 | 0.631 | 0.525 | 0.018 | 0.002 | 0.003 |
| Pelvis tilt | 7.1 | 2.2 | 6.9 | 2.5 | 6.5 | 2.1 | 7.3 | 2.5 | 0.760 | 0.612 | 0.283 | 0.001 | 0.005 | 0.012 |
| Descent | ||||||||||||||
| Ankle flexion | 64.4 | 7.7 | 62.4 | 3.5 | 58.4 | 7.7 | 60.2 | 8.9 | 0.056 | 0.981 | 0.101 | 0.076 | 0.000 | 0.016 |
| Ankle inversion | 17.2 | 6.4 | 15.6 | 5.9 | 14.2 | 4.7 | 17.7 | 7.5 | 0.748 | 0.615 | 0.028 | 0.001 | 0.007 | 0.042 |
| Knee flexion | 84.3 | 7.3 | 88.8 | 7.1 | 84.9 | 8.3 | 85.1 | 4.4 | 0.148 | 0.401 | 0.044 | 0.012 | 0.028 | 0.024 |
| Hip abduction | 10.4 | 3.5 | 9.2 | 3.5 | 10.4 | 4.5 | 8.5 | 1.9 | 0.403 | 0.287 | 0.640 | 0.003 | 0.052 | 0.002 |
| Hip flexion | 24.6 | 4.1 | 25.6 | 3.6 | 25.2 | 4.1 | 28.6 | 4.7 | 0.129 | 0.035 | 0.173 | 0.045 | 0.064 | 0.020 |
| Pelvis obliquity | 6.3 | 1.1 | 6.8 | 2.4 | 6.4 | 1.6 | 6.8 | 2.2 | 0.934 | 0.146 | 0.938 | 0.000 | 0.015 | 0.000 |
| Pelvis rotation | 9.6 | 3.4 | 9.1 | 3.2 | 9.8 | 3.5 | 8.9 | 2.6 | 0.921 | 0.227 | 0.686 | 0.000 | 0.013 | 0.001 |
| Pelvis tilt | 5.3 | 1.4 | 5.0 | 1.3 | 5.1 | 1.5 | 5.0 | 1.4 | 0.815 | 0.296 | 0.753 | 0.001 | 0.006 | 0.001 |
| Pre | Post | |||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p-Value | ES |
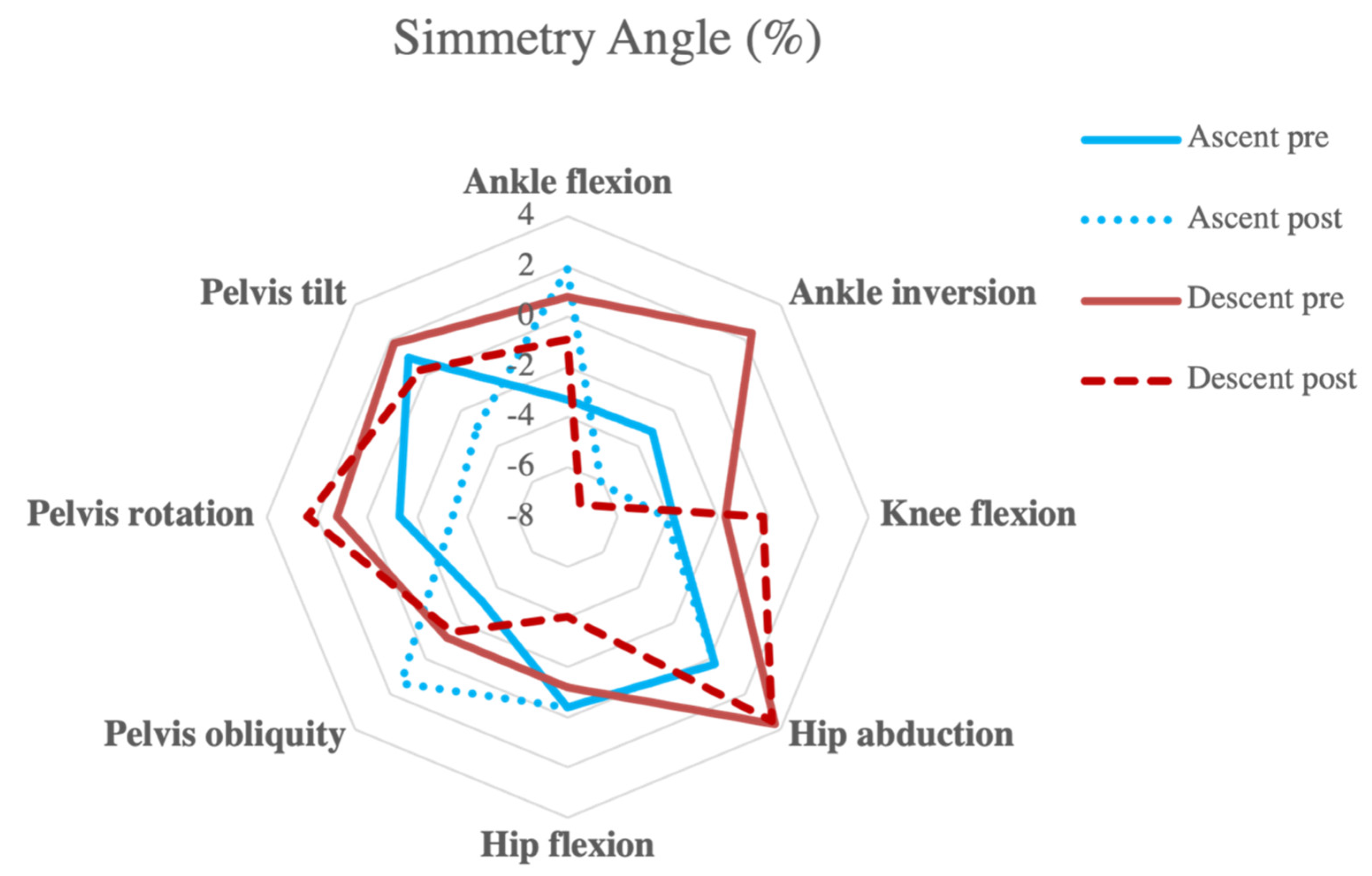
| Ascent | ||||||
| Ankle flexion | −3.3 | 16.6 | 1.9 | 17.0 | 0.014 | 0.315 |
| Ankle inversion | −3.2 | 24.6 | −6.1 | 17.1 | 0.512 | 0.139 |
| Knee flexion | −3.8 | 14.6 | −4.1 | 17.6 | 0.857 | 0.017 |
| Hip abduction | 0.3 | 15.5 | 0.3 | 17.5 | 0.994 | 0.001 |
| Hip flexion | −0.4 | 2.5 | −0.4 | 4.4 | 0.984 | 0.006 |
| Pelvis obliquity | −3.2 | 7.3 | 1.4 | 4.6 | 0.080 | 0.715 |
| Pelvis rotation | −1.3 | 9.5 | −3.4 | 12.7 | 0.517 | 0.182 |
| Pelvis tilt | 1 | 8.7 | −2.9 | 14.3 | 0.313 | 0.327 |
| Descent | ||||||
| Ankle flexion | 0.8 | 3.2 | −0.9 | 5.0 | 0.176 | 0.400 |
| Ankle inversion | 2.4 | 15.1 | −7.3 | 14.2 | 0.027 | 0.643 |
| Knee flexion | −1.7 | 4.1 | −0.2 | 3.9 | 0.049 | 0.373 |
| Hip abduction | 3.7 | 17.3 | 3.5 | 17.6 | 0.958 | 0.011 |
| Hip flexion | −1.2 | 5.9 | −4.0 | 5.8 | 0.213 | 0.462 |
| Pelvis obliquity | −1.2 | 7.6 | −1.5 | 6.8 | 0.927 | 0.042 |
| Pelvis rotation | 1.2 | 8.4 | 2.4 | 9.2 | 0.686 | 0.132 |
| Pelvis tilt | 1.8 | 5.1 | 0.3 | 8.2 | 0.642 | 0.216 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zago, M.; Di Giuli, R.; Beltramini, G.; Bolzoni, A.; Baj, A.; Galli, M.; Giannì, A.B.; Sforza, C. Donor-Site Morbidity after Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Stair Climbing Asymmetry and Functional Outcome. Symmetry 2022, 14, 1888. https://doi.org/10.3390/sym14091888
Zago M, Di Giuli R, Beltramini G, Bolzoni A, Baj A, Galli M, Giannì AB, Sforza C. Donor-Site Morbidity after Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Stair Climbing Asymmetry and Functional Outcome. Symmetry. 2022; 14(9):1888. https://doi.org/10.3390/sym14091888
Chicago/Turabian StyleZago, Matteo, Riccardo Di Giuli, Giada Beltramini, Alessandro Bolzoni, Alessandro Baj, Manuela Galli, Aldo Bruno Giannì, and Chiarella Sforza. 2022. "Donor-Site Morbidity after Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Stair Climbing Asymmetry and Functional Outcome" Symmetry 14, no. 9: 1888. https://doi.org/10.3390/sym14091888
APA StyleZago, M., Di Giuli, R., Beltramini, G., Bolzoni, A., Baj, A., Galli, M., Giannì, A. B., & Sforza, C. (2022). Donor-Site Morbidity after Osteocutaneous Free Fibula Transfer: Longitudinal Analysis of Stair Climbing Asymmetry and Functional Outcome. Symmetry, 14(9), 1888. https://doi.org/10.3390/sym14091888








