MCD and Induced CD of a Tetraphenoxyperylene-Based Dye in Chiral Solvents: An Experimental and Computational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Calculations
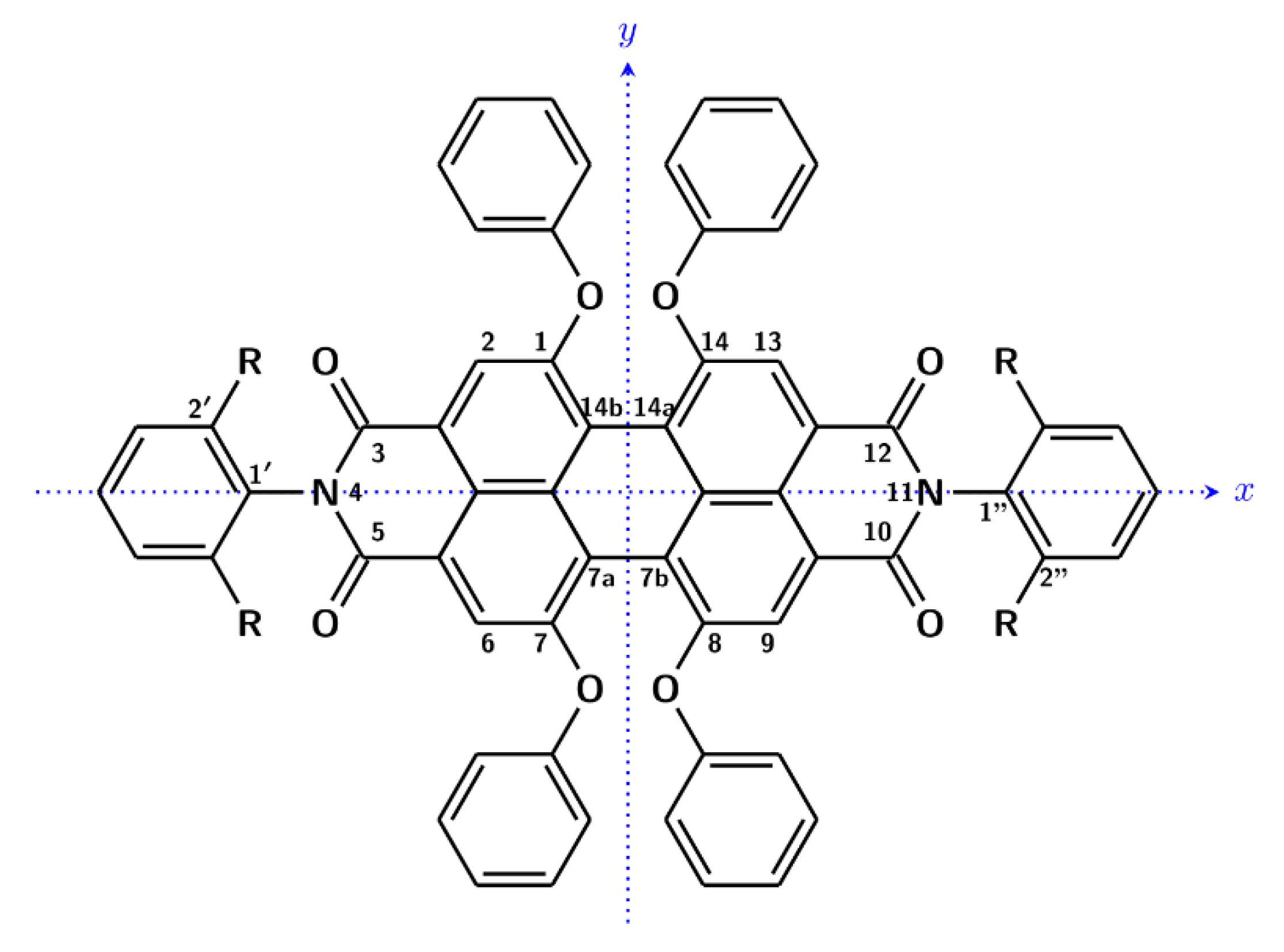
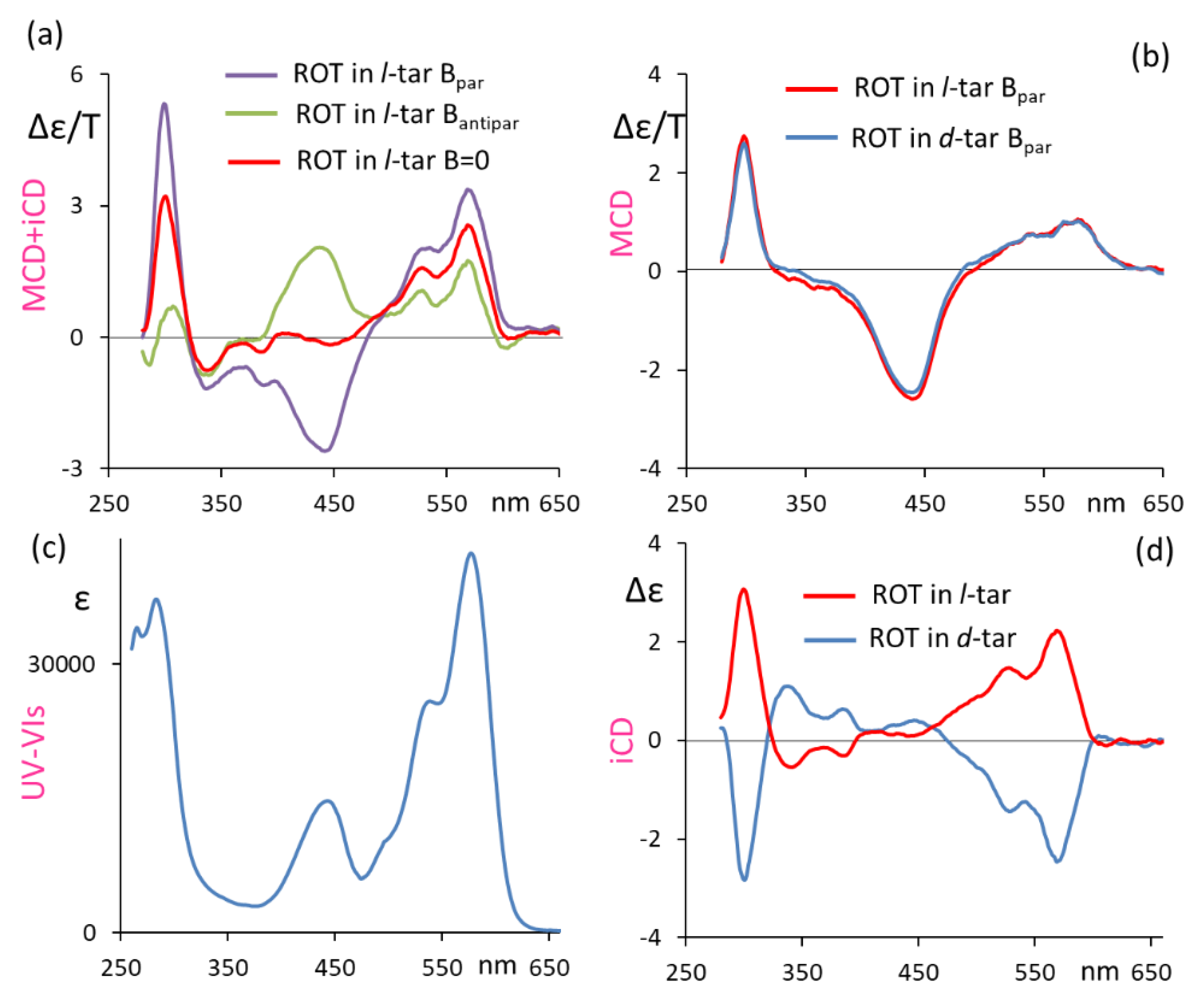
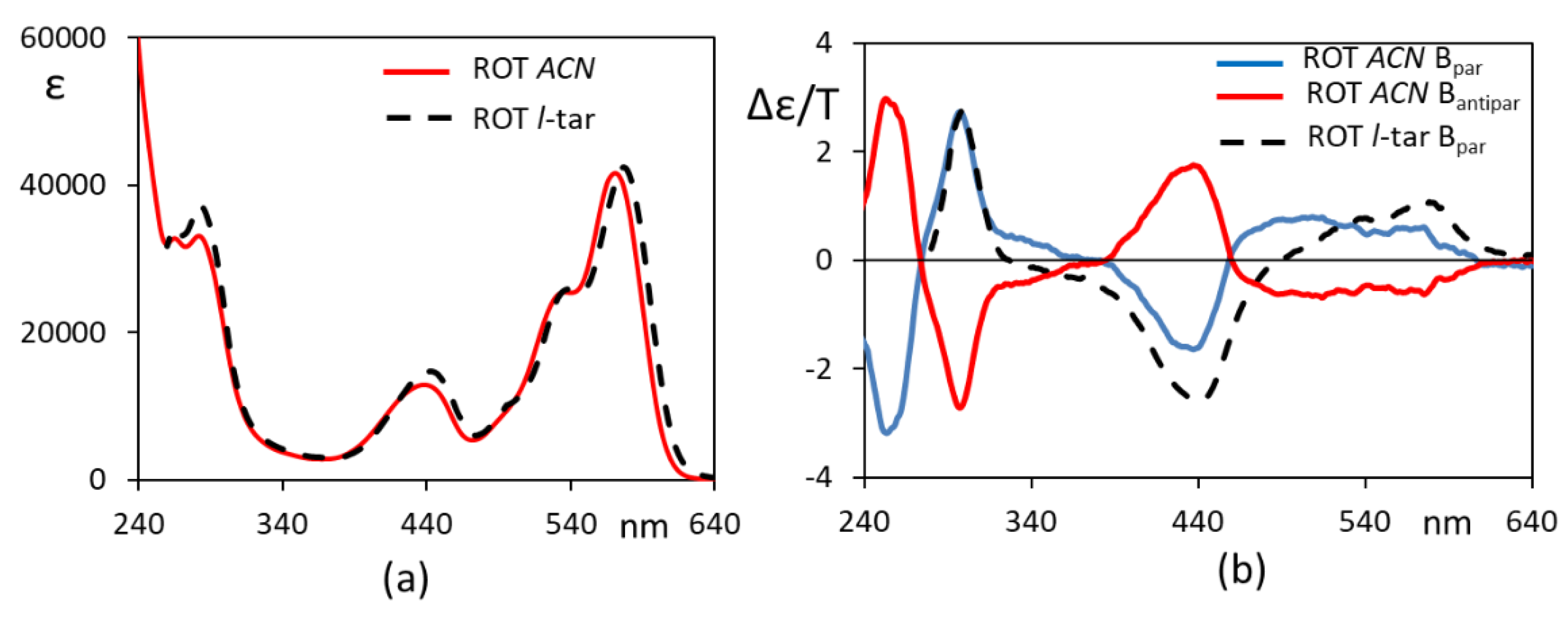
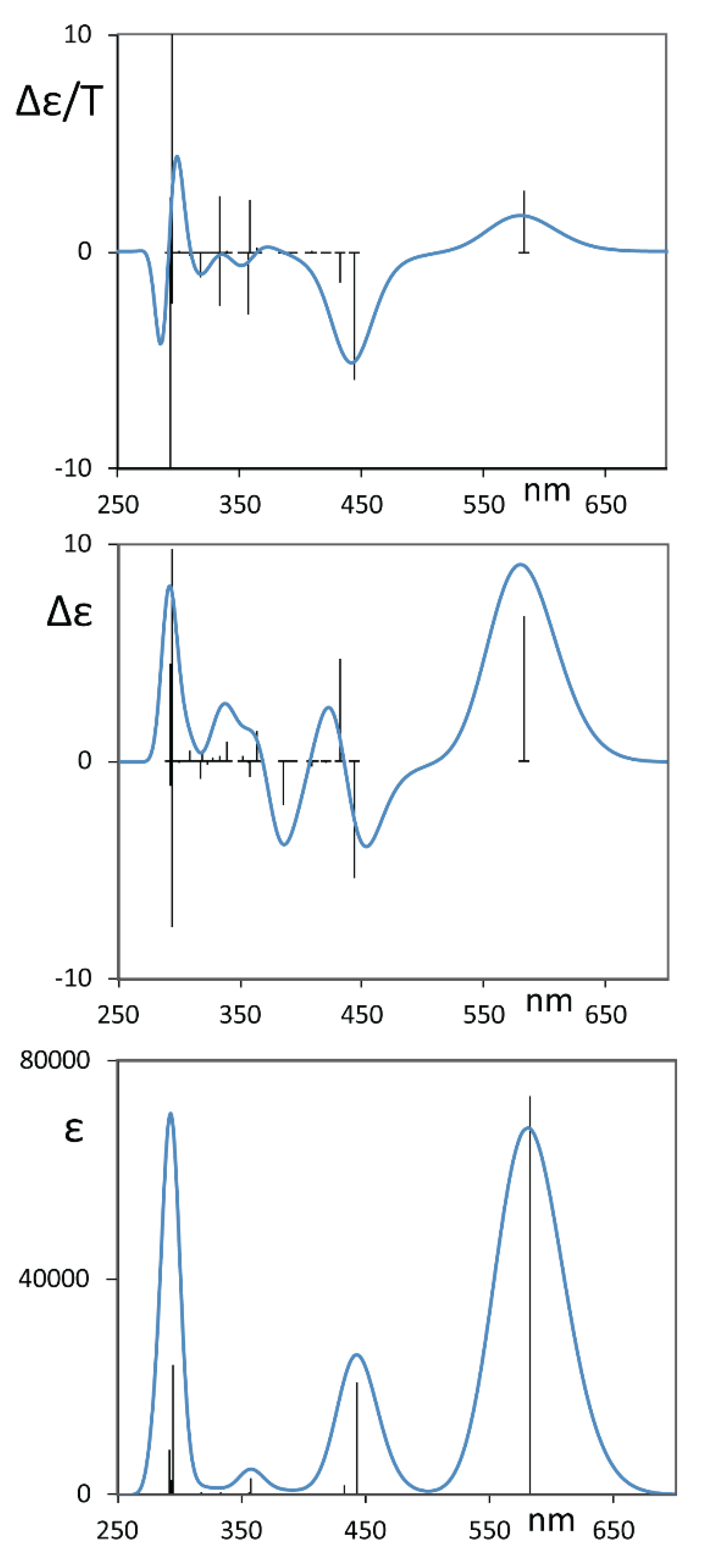
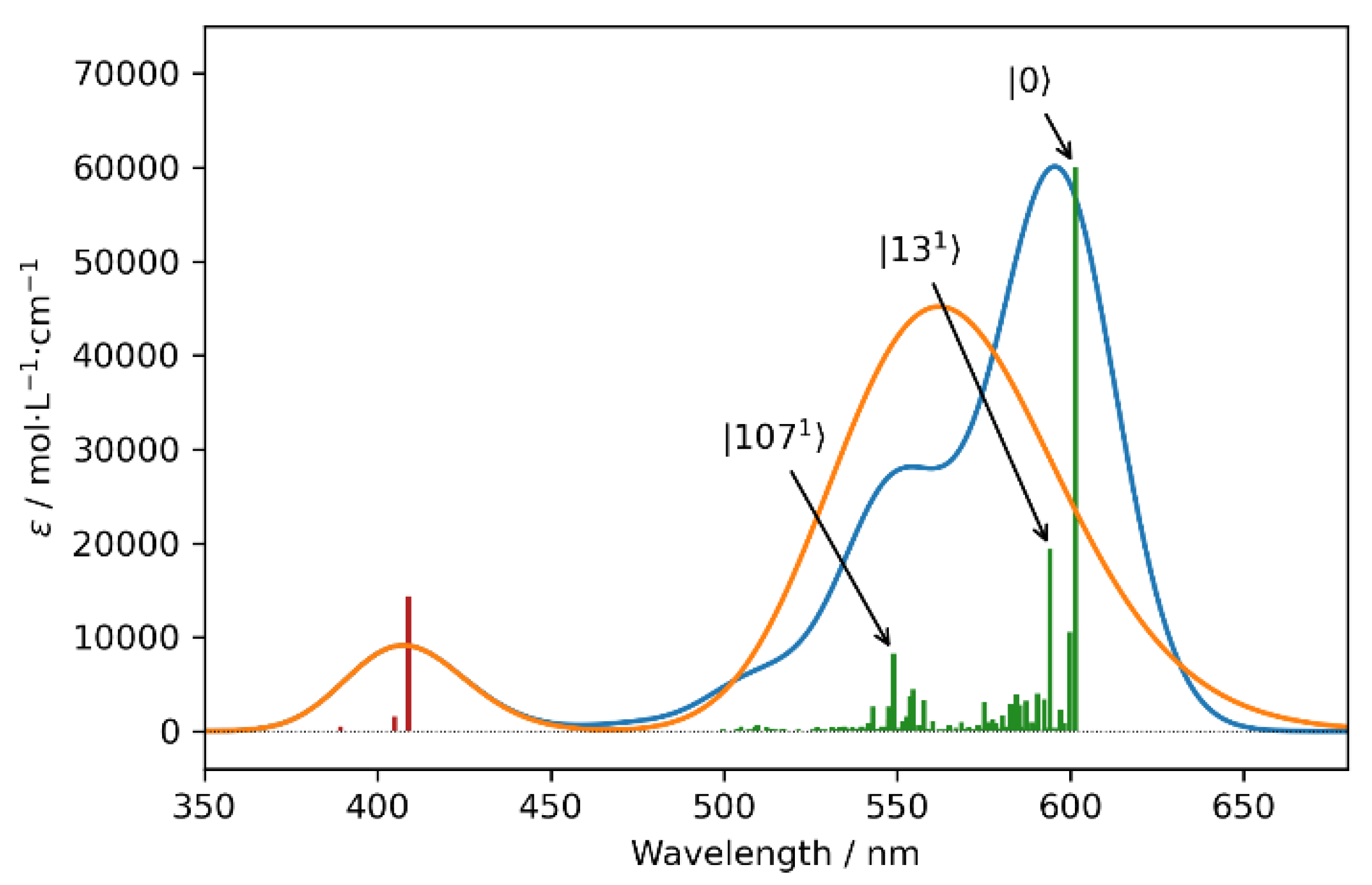
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Mori, T. Chiroptical Properties of Symmetric Double, Triple, and Multiple Helicenes. Chem. Rev. 2021, 121, 2373–2412. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Longhi, G.; Abbate, S.; Lucotti, A.; Tommasini, M.; Villani, C.; Catalano, V.J.; Lykhin, A.O.; Varganov, S.A.; Chalifoux, W.A. Chiral Peropyrene: Synthesis, Structure, and Properties. J. Am. Chem. Soc. 2017, 139, 13102–13109. [Google Scholar] [CrossRef]
- Medel, M.A.; Tapia, R.; Blanco, V.; Miguel, D.; Morcillo, S.P.; Campaña, A.G. Octagon-Embedded Carbohelicene as a Chiral Motif for Circularly Polarized Luminescence Emission of Saddle-Helix Nanographenes. Angew. Chem. 2021, 133, 6159–6165. [Google Scholar] [CrossRef]
- Stephens, P.J.; Schatz, P.N.; Ritchie, A.B.; McCaffery, A.J. Magnetic Circular Dichroism of Benzene, Triphenylene, and Coronene. J. Chem. Phys. 1968, 48, 132–138. [Google Scholar] [CrossRef]
- Michl, J. Magnetic Circular Dichroism of Aromatic Molecules. Tetrahedron 1984, 40, 3845–3934. [Google Scholar] [CrossRef]
- Stagira, S.; Nisoli, M.; Cerullo, G.; Zavelani–Rossi, M.; De Silvestri, S.; Lanzani, G.; Graupner, W.; Leising, G. The Role of Amplified Spontaneous Emission in the Ultrafast Relaxation Dynamics of Polymer Films. Chem. Phys. Lett. 1998, 289, 205–210. [Google Scholar] [CrossRef]
- Gvishi, R.; Reisfeld, R.; Burshtein, Z. Spectroscopy and Laser Action of the “Red Perylimide Dye” in Various Solvents. Chem. Phys. Lett. 1993, 213, 338–344. [Google Scholar] [CrossRef]
- Castiglione, F.; Lanzani, G.; Mele, A.; Monguzzi, A.; Passarello, M.; Ruggirello, A.; Scotognella, F.; Liveri, V.T. Spectroscopic Characterization of Red Perylimide/Surfactant Nanocomposites. J. Mater. Sci. 2011, 46, 6402–6407. [Google Scholar] [CrossRef]
- Abbate, S.; Lebon, F.; Longhi, G.; Passarello, M.; Liveri, V.T. Triggering Dissymmetry in Achiral Dye Molecules by Chiral Solvents: Circular Dichroism Experiments and DFT Calculations. Chirality 2011, 23, 910–915. [Google Scholar] [CrossRef]
- Person, R.V.; Peterson, B.R.; Lightner, D.A. Bilirubin Conformational Analysis and Circular Dichroism. J. Am. Chem. Soc. 1994, 116, 42–59. [Google Scholar] [CrossRef]
- Ghidinelli, S.; Abbate, S.; Mazzeo, G.; Boiadjiev, S.E.; Lightner, D.A.; Longhi, G. Biliverdin Chiral Derivatives as Chiroptical Switches for PH and Metal Cation Sensing. Phys. Chem. Chem. Phys. 2021, 23, 20138–20151. [Google Scholar] [CrossRef] [PubMed]
- Novotná, P.; Králík, F.; Urbanová, M. Chiral Recognition of Bilirubin and Biliverdin in Liposomes and Micelles. Biophys. Chem. 2015, 205, 41–50. [Google Scholar] [CrossRef]
- Lightner, D.A.; Gawronski, J.K.; Gawronska, K. Conformational Enantiomerism in Bilirubin. Selection by Cyclodextrins. J. Am. Chem. Soc. 1985, 107, 2456–2461. [Google Scholar] [CrossRef]
- Rybicka, A.; Longhi, G.; Castiglioni, E.; Abbate, S.; Dzwolak, W.; Babenko, V.; Pecul, M. Thioflavin T: Electronic Circular Dichroism and Circularly Polarized Luminescence Induced by Amyloid Fibrils. Chem. Phys. Chem. 2016, 17, 2931–2937. [Google Scholar] [CrossRef]
- Brittain, H.G.; Richardson, F.S. Solvent Induced Circularly Polarized Emission from Fluorescein. J. Phys. Chem. 1976, 80, 2590–2592. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Lebon, F.; Longhi, G.; Gangemi, F.; Abbate, S.; Priess, J.; Juza, M.; Bazzini, C.; Caronna, T.; Mele, A. Chiroptical Properties of Some Monoazapentahelicenes. J. Phys. Chem. 2004, 108, 11752–11761. [Google Scholar] [CrossRef]
- Bam, R.; Yang, W.; Longhi, G.; Abbate, S.; Lucotti, A.; Tommasini, M.; Franzini, R.; Villani, C.; Catalano, V.J.; Olmstead, M.M.; et al. Four-Fold Alkyne Benzannulation: Synthesis, Properties, and Structure of Pyreno[a]Pyrene-Based Helicene Hybrids. Org. Lett. 2019, 21, 8652–8656. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Cauteruccio, S.; Grecchi, S.; Benincori, T.; Marcaccio, M.; Biroli, A.O.; Longhi, G.; Licandro, E.; Mussini, P.R. Thiahelicene-Based Inherently Chiral Films for Enantioselective Electroanalysis. Chem. Sci. 2019, 10, 1539–1548. [Google Scholar] [CrossRef]
- Schooley, D.A.; Bunnenberg, E.; Djerassi, C. Magnetic Circular Dichroism Studies: A Preliminary Report *. Proc. Natl. Acad. Sci. USA 1965, 53, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Seamans, L.; Moscowitz, A.; Barth, G.; Bunnenberg, E.; Djerassi, C. Magnetic Circular Dichroism Studies. XVIII. Structure and the Magnetic Circular Dichroism of Saturated Ketones. J. Am. Chem. Soc. 1972, 94, 6464–6475. [Google Scholar] [CrossRef]
- Stephens, P.J. Theory of Magnetic Circular Dichroism. J. Chem. Phys. 1970, 52, 3489–3516. [Google Scholar] [CrossRef]
- Stephens, P.J. Magnetic Circular Dichroism. Adv. Chem. Phys. 1976, 35, 197–264. [Google Scholar]
- Ghidinelli, S.; Abbate, S.; Mazzeo, G.; Paoloni, L.; Viola, E.; Ercolani, C.; Donzello, M.P.; Longhi, G. Characterization of Tetrakis(Thiadiazole)Porphyrazine Metal Complexes by Magnetic Circular Dichroism and Magnetic Circularly Polarized Luminescence. Chirality 2020, 32, 808–816. [Google Scholar] [CrossRef]
- Grimme, S. Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Aidas, K.; Angeli, C.; Bak, K.L.; Bakken, V.; Bast, R.; Boman, L.; Christiansen, O.; Cimiraglia, R.; Coriani, S.; Dahle, P.; et al. The Dalton Quantum Chemistry Program System. WIREs Comput. Mol. Sci. 2014, 4, 269–284. [Google Scholar] [CrossRef]
- Dalton, a Molecular Electronic Structure Program. Release V2020.1. 2022. Available online: http://daltonprogram.org (accessed on 1 May 2022).
- Ferrer, F.J.A.; Santoro, F. Comparison of Vertical and Adiabatic Harmonic Approaches for the Calculation of the Vibrational Structure of Electronic Spectra. Phys. Chem. Chem. Phys. 2012, 14, 13549–13563. [Google Scholar] [CrossRef]
- Bloino, J.; Baiardi, A.; Biczysko, M. Aiming at an Accurate Prediction of Vibrational and Electronic Spectra for Medium-to-Large Molecules: An Overview. Int. J. Quantum Chem. 2016, 116, 1543–1574. [Google Scholar] [CrossRef]
- Michl, J. Electronic Structure of Aromatic π-Electron Systems as Reflected in their MCD Spectra. Pure & Appl. Chem. 1980, 52, 1549–1563. [Google Scholar] [CrossRef][Green Version]
- Imai, Y. Circularly Polarized Luminescence (CPL) Induced by an External Magnetic Field: Magnetic CPL (MCPL). Chem. PhotoChem. 2021, 5, 969–973. [Google Scholar] [CrossRef]
- Ghidinelli, S.; Abbate, S.; Mazzeo, G.; Paolesse, R.; Pomarico, G.; Longhi, G. MCD and MCPL Characterization of Luminescent Si(IV) and P(V) Tritolylcorroles: The Role of Coordination Number. ACS Omega 2021, 6, 26659–26671. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, P.; Bouř, P. Computation of Magnetic Circular Dichroism by Sum-over-States Summations. J. Comput. Chem. 2013, 34, 1531–1539. [Google Scholar] [CrossRef]
- Kaminský, J.; Andrushchenko, V.; Bouř, P. Natural and Magnetic Circular Dichroism Spectra of Nucleosides: Effect of the Dynamics and Environment. RSC Adv. 2021, 11, 8411–8419. [Google Scholar] [CrossRef] [PubMed]
- Coriani, S.; Jørgensen, P.; Rizzo, A.; Ruud, K.; Olsen, J. Ab Initio Determinations of Magnetic Circular Dichroism. Chem. Phys. Lett. 1999, 300, 61–68. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. The Classical Theory of Fields, 3rd ed.; Pergamon: Oxford, UK, 1971; pp. 6–18. [Google Scholar]
- Piepho, S.B.; Schatz, P.N. Group Theory in Spectroscopy: With Applications to Magnetic Circular Dichroism; Jon Wiley & Sons: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Rosenfeld, L. Quantenmechanische Theorie der natürlichen optischen Aktivität von Flüssigkeiten und Gasen. Z. Physik 1929, 52, 161–174. [Google Scholar] [CrossRef]
- Khani, S.K.; Faber, R.; Santoro, F.; Hättig, C.; Coriani, S. UV Absorption and Magnetic Circular Dichroism Spectra of Purine, Adenine, and Guanine: A Coupled Cluster Study in Vacuo and in Aqueous Solution. J. Chem. Theory Comput. 2019, 15, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Train, C.; Hillard, E.A.; Avarvari, N.; Rikken, G.L.J.A. Magneto-Chiral Anisotropy: From Fundamentals to Perspectives. Chirality 2021, 33, 844–857. [Google Scholar] [CrossRef] [PubMed]

| Perylene Unit | N-Phenyl * | O-Phenyl | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conf. | Pop | 7-7a-7b-8 | 1-14b-14a-14 | 6-2-13-9 | 3-4-1’-2’ | 7b-8-O-Ph | 7a-7-O-Ph | 14b-1-O-Ph | 14a-14-O-Ph |
| A | 90% | 31.5 | 31.5 | 33.3 | 90.1 | −164.4 | −164.4 | −164.4 | −164.4 |
| B | 7% | 31.3 | 32.2 | 33.2 | 89.8 | −163.7 | −164.2 | −144.4 | 63.3 |
| C | 3% | 31.8 | 30.4 | 32.9 | 89.9 | −164.7 | −167.7 | 63.1 | 63.1 |
| eV | nm | μ 10−18 esu×cm | m 10−21 esu×cm | D | R | B | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.13 | 582 | H->L | a->b3 | μx (b3) | −8.5671 | mx (b3) | −1.4135 | 742,368 | 135 | 42.7 |
| 2 | 2.79 | 444 | H-1->L | b1->b3 | μy (b2) | 4.5427 | my (b2) | −2.9289 | 196,764 | −107 | −89.2 |
| 3 | 2.87 | 433 | H-2->L | b2->b3 | μz (b1) | 1.2996 | mz (b1) | 5.3083 | 17,111 | 96 | −21.3 |
| 30 | 4.21 | 295 | H->L+4 | a->b2 | μy (b2) | 4.9283 | my (b2) | −3.7374 | 238,788 | −152.0 | 316.4 |
| 31 | 4.22 | 294 | H->L + 3 (H-2->L) | a->b1 | μz (b1) | 1.6097 | mz (b1) | 13.4881 | 25,881 | 196.9 | −18.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidinelli, S.; Fusè, M.; Mazzeo, G.; Abbate, S.; Longhi, G. MCD and Induced CD of a Tetraphenoxyperylene-Based Dye in Chiral Solvents: An Experimental and Computational Study. Symmetry 2022, 14, 1108. https://doi.org/10.3390/sym14061108
Ghidinelli S, Fusè M, Mazzeo G, Abbate S, Longhi G. MCD and Induced CD of a Tetraphenoxyperylene-Based Dye in Chiral Solvents: An Experimental and Computational Study. Symmetry. 2022; 14(6):1108. https://doi.org/10.3390/sym14061108
Chicago/Turabian StyleGhidinelli, Simone, Marco Fusè, Giuseppe Mazzeo, Sergio Abbate, and Giovanna Longhi. 2022. "MCD and Induced CD of a Tetraphenoxyperylene-Based Dye in Chiral Solvents: An Experimental and Computational Study" Symmetry 14, no. 6: 1108. https://doi.org/10.3390/sym14061108
APA StyleGhidinelli, S., Fusè, M., Mazzeo, G., Abbate, S., & Longhi, G. (2022). MCD and Induced CD of a Tetraphenoxyperylene-Based Dye in Chiral Solvents: An Experimental and Computational Study. Symmetry, 14(6), 1108. https://doi.org/10.3390/sym14061108







