1. Introduction
Let
B be a continuous body undergoing a thermomechanical transformation, whose evolution in the spacetime is ruled by the system of balance laws
with
as the components of the velocity field on
B entering the total time derivative,
as the components of the flux of
, and
as the production of
(for the sake of simplicity, we assume that the supplies are zero). Moreover, the symbols
and
mean the partial derivatives of function
f with respect to time and to the spatial coordinate
, respectively.
For instance, in classical rational thermodynamics, Equation (
1) is the balance of mass, linear momentum, angular momentum, and energy [
1,
2],
where
denotes the mass density,
the specific internal energy,
r the energy supply,
,
, and
the components of velocity, heat flux, and body force, respectively, and
are the components of the Cauchy stress [
1,
2], while in the extended non-equilibrium thermodynamic theories, taking the fluxes as independent variables, the set of field equations includes the balance laws for the independent fluxes [
3,
4,
5,
6,
7].
We suppose that the fields
, the fluxes
, and the productions
depend on
unknown fields
and on their spatial derivatives
. Then, suitable constitutive equations must be assigned for them. Such equations must account for material symmetry, namely, the invariance of certain physical properties with respect to a group of coordinate transformations (symmetry group). Thus, suitable representation theorems for symmetric and/or isotropic functions must be applied [
8]. Through the constitutive equations, material symmetry reflects itself in the Equation (
1) field, which inherit from them particular mathematical properties. Meaningful examples of such reciprocal influences are illustrated in [
9], where some important equations of continuum physics are studied by the Lie symmetry analysis of differential equations. On the other hand, the mathematical representation of material symmetry cannot neglect the constraint dictated by the second law of thermodynamics, which imposes the local entropy production
where
s is the specific entropy,
are the components of the entropy flux, and
the absolute temperature, are nonnegative, namely,
whatever the thermodynamic process is [
1,
2]. Indeed, the unilateral differential constraint (
7) can be interpreted either as a restriction on the solutions of Equation (
1), or as a restriction on the constitutive equations that characterize the fields
,
,
,
s, and
. In the first case, it leads to the following assumption:
Assumption 1. Among the mathematical solutions of Equation (1), we must find those that are physically realizable by substituting them into the Inequality (7) and checking the sign. In the second case, instead, it leads to a different assumption, namely:
Assumption 2. The constitutive equations for , , , s, and must be assigned in such a way that the constraint (7) is satisfied along arbitrary thermodynamic processes. Modern constitutive theories of continuum thermodynamics are based on the second statement, which was formulated for the first time by Coleman and Noll [
1] in 1963, and is universally known as the entropy (or dissipation) principle [
10]. On the other hand, since the determination of solutions of Equation (
1) is, in general, very difficult, the Coleman–Noll approach is the most convenient one used for determining the consequences of the second law. Two rigorous mathematical procedures for analyzing the restrictions dictated by the second law on the constitutive equations are based on such an assumption, namely, the Coleman–Noll and Liu procedures [
1,
11,
12].
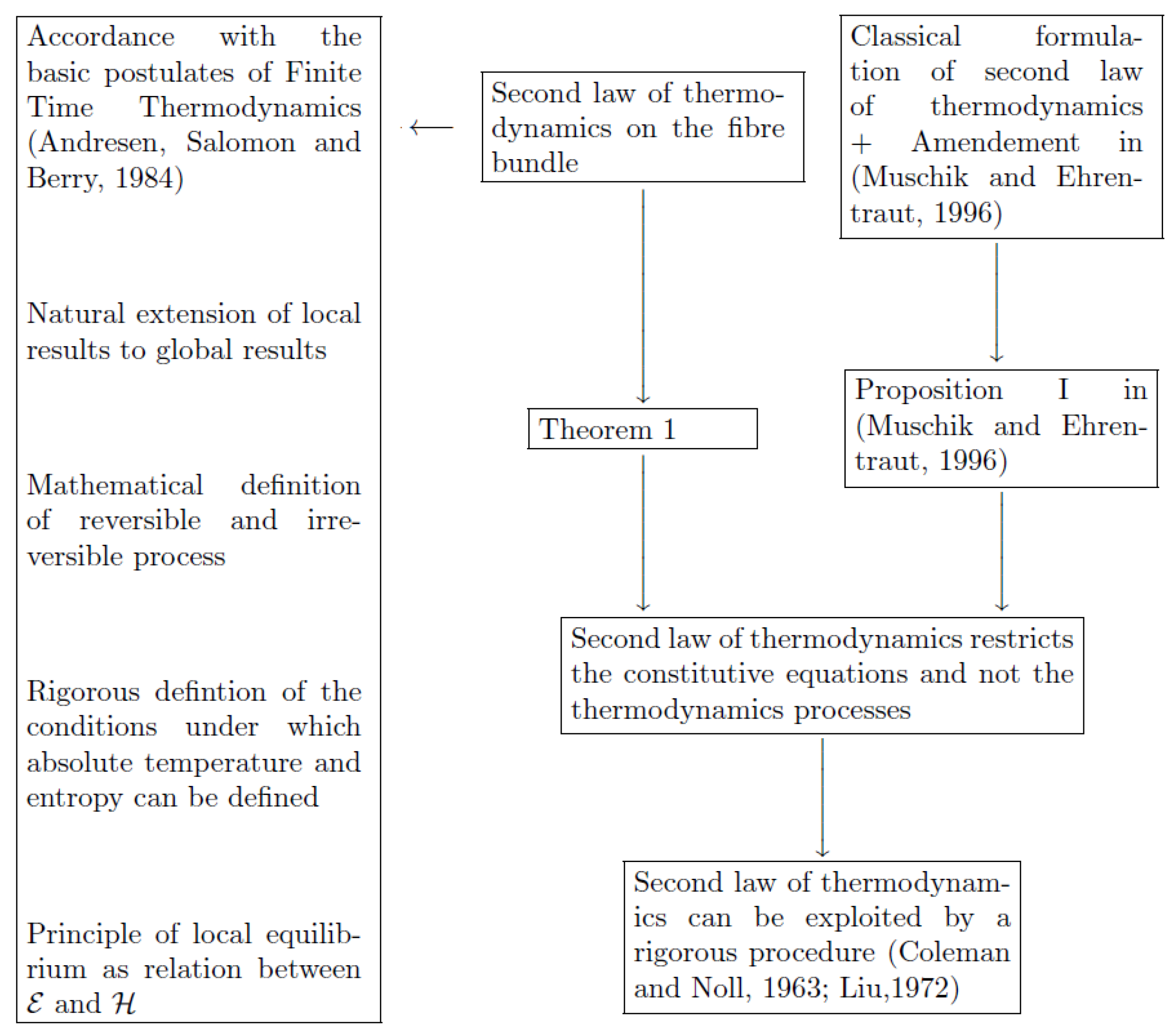
In order to investigate if the entropy principle, as formulated by Coleman and Noll, is just an operative assumption or a consequence of a general physical law, Muschik and Ehrentraut in 1996 proved their celebrated theorem in [
13], which we revisit here within a geometric perspective. Since the theorem has a complex mathematical formulation, herein we limit ourselves to provide a sketch of the result, referring the reader to reference [
13] for details. Their starting point is the following concept of equilibrium for a thermodynamic system.
A thermodynamic system is said to be in equilibrium (stable or metastable), if isolation of the system from its environment does not affect the observables of the system (or in other words, if the state of the isolated system is the same as the state of the system prior to insulation).
Then, they define the process direction vectors as those vectors that are tangent to the curve representative of a process in the state space. Moreover, they show that in a fixed point of such a curve, the entropy production is linear in the process direction vectors . The vectors are said irreversible, if , reversible, if , non-real, if . In particular, a vector such that , is called the reversible process direction.
At this point, Muschik and Ehrentraut prove their fundamental.
Proposition 1. If in a point of non-equilibrium of the curve representative of the process in the state space there exist both irreversible and non-real process directions, then, in that point, it is possible to obtain a reversible process direction as a linear combination of the latter.
Such a result is unphysical, since in a non-equilibrium point the entropy production can be zero. In order to overcome such a discrepancy, Muschik and Ehrentraut add to the classical formulation of the second law, represented by the Inequality (
7), the following amendment.
Except in equilibria, reversible process directions in state space do not exist.
The consequences of the amendment are severe, because it implies that, in a given point, the process directions are either all irreversible (or reversible, in the limit of quasi-static transformation), or all non-real. On the other hand, since non-real process directions do not exist in nature, we must choose the first option. As a consequence, non-real process directions that must be excluded by the second law do not exist. Moreover, since the point on the curve of the process is arbitrary, we are led to conclude that there are no non-real thermodynamic processes to be forbidden by the second law and, hence, the last necessarily restricts the constitutive equations and not the thermodynamic processes. In this way, the classical Coleman–Noll approach follows by a rigorous proof.
It is worth observing that Proposition 1 and its consequences are not aimed at giving more deep physical insight on some basic concepts of thermodynamics, such as equilibrium and reversibility, since in [
13] those concepts are the classical ones. Indeed, Proposition 1 aims at answering the following question, which is fundamental from the methodological point of view: is the Coleman–Noll interpretation of the second law, as a constraint on the constitutive equations, a mathematical consequence of the basic laws of thermodynamics or an additional operative assumption that does not follow from those laws? The answer, given by Proposition 1, is that such an interpretation is a consequence of the second law, provided the amendment is postulated. In the absence of the amendment, the Coleman–Noll approach would be an arbitrary assumption and, hence, it could be questioned. The present paper is motivated by the observation that the important result illustrated above can be put in a more general and accessible form within a geometric framework. To achieve that task, we use the results in references [
14,
15], where a geometric perspective on non-equilibrium thermodynamics has been given. The chosen state space is different with respect to that considered reference [
13], because we do not include in it the time derivatives. In this way, the constitutive equations we deal with are suitable to satisfy the principle of material indifference [
2]. After defining the space of the higher derivatives, we introduce the definitions of the real, ideal, and over-ideal vector of the higher derivatives. For thermodynamic processes, we give the definitions of irreversible, reversible, and over-reversible process, by analyzing the properties of its representative curve in the fiber bundle of the configuration spaces. Once the geometric framework is complete, we reformulate the second law of thermodynamics, both locally and globally, in space and time, in order to encompass the amendment. In this way, we are able to prove a new formulation of the Muschik and Ehrentraut theorem. In the discussion in
Section 5, we highlight some of the main advantages of the geometric approach presented here. We underline that our generalized formulation of the second law seems to be more adapt for some recent thermodynamic theories that analyze real transformations, which occur in a finite time, and not by quasi-static transformations, which require an infinite time to be realized [
16,
17,
18]. Moreover, we will see that the use of the fiber bundle allows to provide, in a very intuitive manner, the mathematical definition of reversible and irreversible processes, and to generalize, in a natural way, local results to global ones. Finally, our approach leads to a physically sound interpretation of the principle of local equilibrium [
19], which provides the theoretical justification of the definition of entropy and temperature outside the equilibrium.
The paper runs as follows.
In
Section 2, we construct a new thermodynamic framework for non-equilibrium processes. In
Section 3, we present a new formulation, both locally and globally, of the second law of thermodynamics. In
Section 4, we prove the Muschik and Ehrentraut theorem. In
Section 5, we discuss our results and present some open problems that will be considered in future research.
2. The Thermodynamic Framework
In this section, we construct a geometric framework where our main results can be formulated. To this end, we begin by providing some basic definitions.
Definition 1. The space of the configurations at the instant t is represented by a ω-dimensional vector space spanned by the solutions of Equation (1) with a structure of a finite-dimensional manifold. We assume that the total configuration space is given by the disjointed union
with a given natural structure of a fiber bundle over the real line
where time flows [
14,
15].
Definition 2. is called the configuration bundle.
Under the natural assumption that
does not vary in time, namely,
, then
has the topology of the Cartesian product
Definition 3. A vector valued function is said to be a thermodynamic process π of duration τ. Moreover, is the parametric equation of the curve representative of π in .
Definition 4. For , a vector valued function is said to be a restricted thermodynamic process p of initial point and duration , reference [14]. Moreover, is the parametric equation of the curve γ representative of p in . Remark 1. For we have , for , is the process of duration 0, i.e., the null process.
As said in
Section 2, in order to find the fields
, i.e., to solve the system (
1), for the quantities
,
and
constitutive equations must be assigned on a suitable state space.
Definition 5. The -dimensional vector space with the structure of a finite-dimensional manifoldfor any value of the time variable t, it represents a local in the time state space and it is called state space at the instant t. Definition 6. The disjoint unionwith a given natural structure of a fiber bundle over the real line where time flows, it represents the total configuration space and it is said to be the thermodynamic bundle. Again, under the natural assumption that
does not vary in time, namely,
, then
has the topology of the Cartesian product
The balance Equation (
1) on the local in time state space
reads
In Equation (
14), we may individuate the
higher derivatives
, which are the space and time derivatives of the elements of
.
Definition 7. The local in time -dimensional vector spaceand the fiber bundlerepresent the space of the higher derivatives at time t and its fiber bundle, respectively. Definition 8. A point is said to be in a state of equilibrium at the instant t, ifand The Definition 8 leads to
Definition 9. The equilibrium subspace of and its fiber bundle are given byand Analogously, the entropy inequality on the state space reads
Definition 10. The local in time -dimensional vector space at time tand the fiber bundledefine the vector space and the fiber bundle of the higher derivatives, respectively, whose state vectors satisfy the entropy inequality. Moreover, the equilibrium subspace of and its fiber bundle are given byand Remark 2. We defined two different spaces of the higher derivatives, one for the balance equations and another for the entropy inequality, related to the fundamental focus of the present investigation, namely, to determine the conditions, if any, under which all of the solutions of the balance laws are also solutions of the entropy inequality. This will be discussed in detail in the next section.
The relations in Equations (
14) and (
19) can be arranged as follows
Let us now define the
column vector function
the
column vector
and the
matrix
with
and
defined on
. In this way, the balance Equation (
24) can be rearranged as
Analogously, after defining the
column vector function
and the scalar function
we can write the Inequality (
25) as
Remark 3. It is worth observing that the higher derivatives entering the system (29) are elements of , while those entering the Inequality (32) are elements of . From now on, we pursue our analysis under the hypothesis that
B occupies the whole space. Then, for arbitrary
, we consider the restricted process
p of the initial instant
and duration
, and suppose that it corresponds to the solution of the Cauchy problem for the system (
29) with initial conditions
If
and
are regular, and
is invertible, the theorem of Cauchy–Kovalevskaya ensures that the Cauchy problem (
29) and (
33) has a unique solution continuously depending on the initial data (
33), reference [
20]. However, such a solution does not necessarily correspond to a thermodynamic process that is physically realizable, since the physically admissible solutions of (
29) and (
33) are only those solutions, which additionally satisfy the unilateral differential constraint (
32). On the other hand, the problems (
29) and (
33) are very difficult to solve, in general, so that finding a solution, and verifying ex post if it also satisfies (
32), does not seem to be a convenient procedure. For that reason, Coleman and Noll [
1] in 1963 postulated the constitutive principle referred in
Section 2, reference [
10]. It is important to investigate if the Coleman and Noll postulate is a consequence of a general physical law or an arbitrary, although very useful, assumption, as observed by Muschik and Ehrentraut [
13]. Such a study will be carried out in the following sections.
3. Local and Global Formulations of the Second Law of Thermodynamics
Let us consider now a fixed point
whose vector position will be indicated by
, a fixed instant of time
. We note that, whatever is
, it can can ever be considered as the initial time of a restricted process of duration
. Moreover, let
,
, and
the vector spaces
,
, and
. When evaluated in
, the balance Equation (
29) and the entropy Inequality (
32) transform in the algebraic relations:
In this way, we can regard the
matrix
as a linear morphism from
to the
-dimensional Euclidean vector space defined on
. Analogously, the vector
can be regarded as a linear application from
in
, so that
belongs to the dual space
of
. It is worth observing that, since
is supposed to be invertible (otherwise the Cauchy problem (
29) and (
33) would not admit a unique solution), the algebraic relations (
34) allow to determine
of the
components of
. Moreover, by spatial derivation of the initial conditions (
33), we have
which, once evaluated in
, allow to determine
components of
. It is worth observing that, since the initial conditions can be assigned arbitrarily, such
quantities can assume arbitrary values. Moreover, there are further
components of the vector
that remain completely arbitrary, since the system (
34) and the initial relations (
36) allow to determine only
of the
components of
. It is not guaranteed that the Inequality (
35) is satisfied whatever is
. Thus, we define the space
constituted by the vectors of
, which satisfy both Equation (
34) and the Inequality (
35).
Remark 4. It is worth observing that, although it is not guaranteed that the Inequality (
35)
is satisfied, whatever is , at this stage, we do not have elements to exclude such a possibility. In other words, we do not have elements to decide if, actually, is a proper subspace of or if it coincides with . In order to decide if
, or
, we follow the way paved by Muschik and Ehrentraut [
13], who observed that such a decision cannot be ensued solely by the second law of thermodynamics, because such a law does not contain information regarding Equation (
34) or the initial conditions (
36). In order to fill this gap, Muschik and Ehrentraut completed the information contained in the Inequality (
35) by an amendment that clarifies how the reversible transformations can be realized from the operative point of view. Here, we followed their strategy, but we propose a more general approach that includes the amendment into a new formulation of the second law. To achieve that task, we needed some preliminary definitions. To this end, we observed that, in the real world, reversible thermodynamic transformations do not exist, but they are approximated by very slow (quasi-static) transformations in which, in any point
, the system is close to the thermodynamic equilibrium. From an ideal point of view, a quasi-static transformation requires an infinite time to occur, and in any point of the system, the value of the state variable is constant in time.
Remark 5. Alternative formulations of the thermodynamic laws that consider realistic transformations occurring in a finite time are proposed within the framework of finite time thermodynamics [16,17,18]. As far as the thermodynamic framework is concerned, if
B undergoes a quasi-static transformation, along with Muschik and Ehrentraut [
13], we say that, in any point
, the vectors of the higher derivatives are elements of
. Such an observation suggests the following definitions.
Definition 11. A vector is said:
Real, if it satisfies the relation ;
Ideal, if it satisfies the relation ;
Over-ideal, if it satisfies the relation .
We can establish the following, owing to the definitions above:
Postulate 1 (A local formulation of the second law of thermodynamics). Let B be a body, and let the couple represent an arbitrary point of B at an arbitrary instant . Suppose B undergoes an arbitrary thermodynamic process of initial instant and duration . Then, the local space of the higher derivatives does not contain over-ideal vectors. Moreover, a vector is ideal if, and only if, is in thermodynamic equilibrium.
The postulate, the above traduces the experimental evidence, in that, in a thermodynamic process, the entropy production cannot be negative at any point of B at any instant . Moreover, it also expresses the further experimental fact, which is often tacit in the formulations of the second law of thermodynamics—that the entropy production can be zero only in the points of B that are in equilibrium. In particular, if the point at the instant is in a thermodynamic equilibrium, then . Hence, is ideal if, and only if, is in a thermodynamic equilibrium. Finally, a vector is either real or over-ideal if, and only if, is not in a thermodynamic equilibrium.
Remark 6. We should note that the local formulation of the second law of thermodynamics prohibits that over-ideal vectors be in , but it does not prevent that they be in . Whether contains over-ideal vectors (or not) is the focus of the present investigation.
Definition 12. Let B be a body undergoing an arbitrary thermodynamic process p of the initial instant and duration , and let γ be the curve representative of the process in . The process p is said to be:
Irreversible, if there exists at least a point of γ in which the vector of the higher derivatives is real;
Reversible, if in any point of γ the vector of the higher derivatives is ideal;
Over-reversible, if there exists at least a point of γ in which the vector of the higher derivatives is over-ideal.
The definitions above allow us to enunciate the following:
Postulate 2 (The global formulation of the second law of thermodynamics). Over-reversible processes do not occur in nature. Moreover, a thermodynamic process is reversible if, and only if, any point , at any instant t, is in a thermodynamic equilibrium.
The previous formulations (local and global) of the second law of thermodynamics include information not present in the classical ones—that the reversible transformations are necessarily quasi-static and, hence, they need an infinite time to occur. Thus, they represent ideal processes, which, in nature, are approximated by very slow transformations. Here, we take into account such a situation by admitting that this is possible if, and only if, at any point of the body, at any instant t, there is a state of thermodynamic equilibrium. On the other hand, this implies that at any point of , the vector of the higher derivatives lie in the equilibrium bundle , and is ideal.