Abstract
The new homoleptic [Ag(5-nitroquinoline)2]ClO4 centrosymmetric complex was synthesized and its structure aspects were investigated. It crystallized in the monoclinic space group C2/c with a = 10.0279(2) Å, b = 13.2295(3) Å, c = 14.7552(3) Å and β = 102.1050(10)° while V = 1913.96(7) Å3 and half molecule as asymmetric formula. The Ag(I) is coordinated with two symmetrically related 5-nitroquinoline ligand units via the heterocyclic nitrogen atom with Ag-N distance of 2.146(6) Å and N1-Ag-N1 angle of 173.0(3)°. The two coordinated 5-nitroquinoline have anti configuration to one another and the perchlorate anion is set freely uncoordinated. The only Ag…O interactions are Ag1…O2 (3.110 Å) and Ag1…O1 (3.189 Å) which occur between the Ag(I) in one complex unit and the O-atoms from the NO2 groups in the neighbouring complex units. Hence, Ag(I) has coordination number 2 and its coordination geometry is slightly bent. Hirshfeld analysis indicated that the O…H (51.1%), C…H (11.8%), H…H (10.8%) and C…C (8.9%) contacts are the most common. Exclusively, the O…H, C…O, N…O, O…O and Ag…O contacts are the only shorter contacts than the vdWs radii sum of the interacting atoms. The studied Ag(I) complex showed good antimicrobial activity. It has comparable antibacterial activity against P. vulgaris (MIC = 9.7 μg/mL) and S. aureus (39.1 μg/mL) to Gentamycin (4.8 and 9.7 μg/mL, respectively) while better antifungal activity against A. fumigatus (MIC = 39.1 μg/mL) than Ketoconazole (156.2 μg/mL).
1. Introduction
Transition metals and their compounds have many applications in different areas of chemistry [1,2,3]. Silver is one of the coinage metals which is acknowledged to have interesting applications in biology [4,5,6,7,8,9,10,11]. Before the discovery of antibiotics, silver nitrate solution was used as eye drops for new born children to avoid conjunctivitis [12,13]. Also, silver compounds were used as an antibiotic coat for medical devices [14,15,16,17]. Ag and its compounds have promising applications in wound dressing and as cream to treat external infections [14,15,16]. For example silver sulfadiazine (SSD) and nanosilver compounds have great interest in wound dressing to treat external infections [11,17,18]. Preliminary studies indicated that catheters containing silver decrease the possibility of the urinary tract infections [19,20,21]. In the light of this interesting features of silver in its different forms, scientist recommended Ag(I) complexes as a solution for the problems associated with multidrug-resistant bacterial strains (MDRS) [22,23]. Many Ag(I) complexes with pyridine and quinoline ligands have more interesting antibacterial activities against MDRS bacteria than SSD [24,25,26].
On the other hand, quinoline based compounds are important class of nitrogen-containing heterocylces as they are possessing a broad spectrum of biological activities, such as anti-cancer, anti-inflammatory, fungicidal, bactericidal, anti-asthmatic, and anti-malarial activity [27,28,29,30,31,32,33,34,35]. In the light of this interesting antibacterial and antifungal activities of silver(I) complexes and quinolines as well, this work shed the light on the molecular and supramolecular structures of a newly synthesized Ag(I) complex with 5-nitroquinoline (Scheme 1). In addition, antimicrobial activity of this Ag(I) complex is presented.
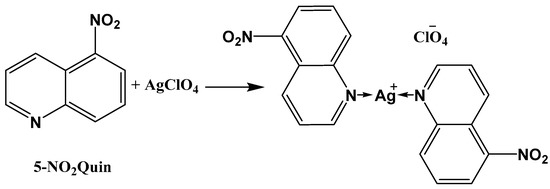
Scheme 1.
Synthesis of [Ag(5-NO2Quin)2]ClO4.
2. Materials and Methods
2.1. Materials and Instrumentation
Details regarding the materials, instruments and crystallographic measurements [36,37] are described in Supplementary Materials. The topology analyses were performed using Crystal Explorer 17.5 program [38].
2.2. Synthesis of [Ag(5-nitroquinoline)2]ClO4
Silver(I) perchlorate (0.2 mmole, 41.5 mg) in 5 mL distilled water was added dropwisely to a 10 mL ethanolic solution of 5-nitroquinoline (0.4 mmole, 67.7 mg) followed by adding 5 mL acetonitrile to dissolve the resulting pale yellow precipitate. The resulting solution was allowed to evaporate slowly at room temperature, pale yellow plate crystals of [Ag(5-nitroquinoline)2]ClO4 were obtained after five days.
[Ag(5-nitroquinoline)2]ClO4; (1): Yield: 91%; Anal. Calcd. C18H12AgClN4O8: C, 38.91; H, 2.18; N, 10.08; Ag, 19.41%. Found: C, 38.80; H, 2.12; N, 9.97; Ag, 19.29%. FTIR (νmax, cm−1): 3109, 3070, 1628, 1594, 1525, 1340, 1146, 1109, 1085. Ligand (5-NO2Quin): 3103, 3072, 2985, 1626, 1592, 1520, 1320, 1242, 1208, 1134, 1073, 1047 (Figure S1, Supplementary Materials).
2.3. Antimicrobial Studies
The antimicrobial activities were tested against selected Gram-positive and Gram-negative bacteria and two fungus as well. Further experimental details are given in the Supplementary Materials (Method S1) [39].
3. Results and Discussion
3.1. Crystal Structure Description
The crystal structure of [Ag(5-NO2Quin)2]ClO4 complex is shown in Figure 1 while the crystallographic data are depicted in Table 1. The structure of the monomeric [Ag(5-NO2Quin)2]ClO4 complex crystallized in the monoclinic centrosymmetric space group C2/c. The unit cell parameters are a = 10.0279(2) Å, b = 13.2295(3) Å, c = 14.7552(3) Å and β = 102.1050(10)° while the unit cell volume is 1913.96(7) Å3. The asymmetric unit consists of half molecule of the formula above.
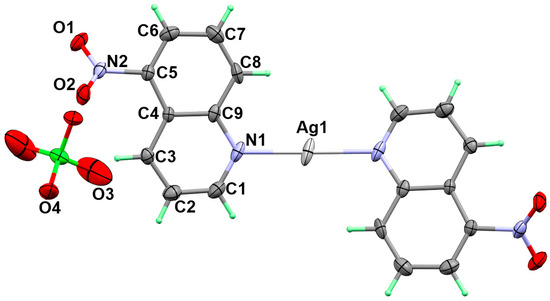
Figure 1.
X-ray structure of [Ag(5-NO2Quin)2]ClO4 complex. Atom numbering of only one half of the complex formula is shown.

Table 1.
Crystallographic data for [Ag(5-NO2Quin)2]ClO4 complex.
In this complex, the Ag(I) is coordinated with two 5-NO2Quin ligand units via the heterocyclic N-atom where the two ligand units are in the anti configuration to one another. Due to symmetry consideration the two Ag-N bonds are equidistant (2.146(6) Å and N1-Ag-N1# bond angle is 173.0(3)° (Symm code: #; Symm code: 1-x,1-y,2-z). These results agree with the structurally related [Ag(5-NO2Quin)2]NO3 complex [23]. The shortest silver to oxygen distance are 3.110 Å (Ag1…O2#; Symm code: x,1-y,1/2+z) and 3.189 Å (Ag1…O1#; Symm code: 1-x,1-y,2-z) with the oxygen atoms from the nitro groups of two neighbouring ligand units. These distances are too long and could not be considered as bonds. Also, the perchlorate anion is not participated in the coordination with silver ion indicating ionic perchlorate. On the other hand, the perchlorate anion represents the outer sphere of the complex and significantly participating in the hydrogen bonding interactions with the neighboring units via its O3 and O4 atoms (Figure 2). Additionally, the O1 and O2 of the nitro group are also participating in the hydrogen bonding interactions.
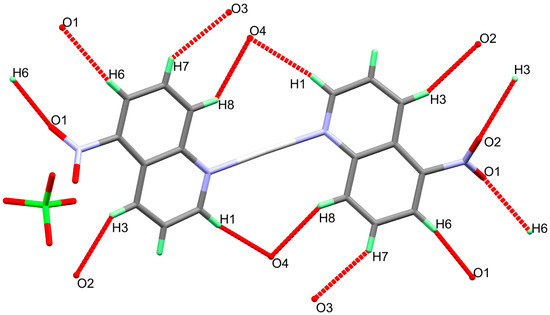
Figure 2.
The hydrogen bond contacts in [Ag(5-NO2Quin)2]ClO4 complex.
As can be seen from Table 2, all the intermolecular interactions belong to the non classical C-H…O interactions. The donor-acceptor distances ranges from 3.317(16) Å (C7-H7…O3) to 3.430(10) Å (C1-H1…O4). The packing scheme of the complex units is shown in Figure 3.

Table 2.
Hydrogen bonds in [Ag(5-NO2Quin)2]ClO4 complex [Å and °].
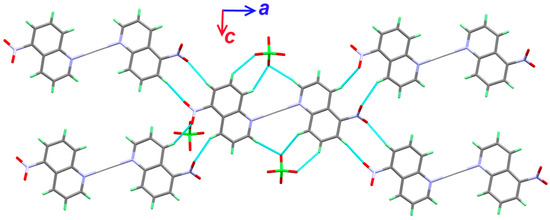
Figure 3.
The packing scheme of the [Ag(5-NO2Quin)2]ClO4 complex units via non classical C-H…O interactions along ac plane.
3.2. Hirshfeld Analysis
Analysis of molecular packing with the aid of Hirshfeld calculations gave quantitative summary of each intermolecular contact occur in the crystal. All Hirshfeld surfaces (dnorm, shape index and curvedness) of the perchlorate complex are presented in Figure 4.
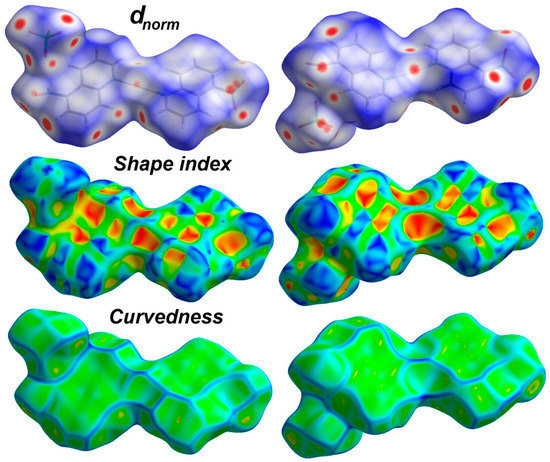
Figure 4.
Hirshfeld surfaces of [Ag(5-NO2Quin)2]ClO4.
The dnorm map comprised three distinct red, white and blue coloured regions which refer to intermolecular contacts shorter, equal and longer than the vdWs radii sum of the interacting atoms, respectively. Hence, the dnorm map revealed many red spots refer to the O…H, C…O, N…O, O…O and Ag…O contacts. The decomposed dnorm map for each interaction is shown in Figure 5 (lower part), while the corresponding fingerprint plots are shown in the upper part of the same figure. The majority of these interactions appeared as sharp spikes in the fingerprint plots indicating short interaction distances (Table 3). The presence of red spots around Ag(1) and the corresponding O(1) and O(2) atoms from the nitro group revealed the presence of short Ag…O interactions among the neighbouring complex units. Interestingly, the Hirshfeld analysis revealed the presence of short O…O and N…O contacts. Additionally, the packing is dominated by large number of O…H interactions which represent about one half the interactions (51.1%) occurred in the crystal.
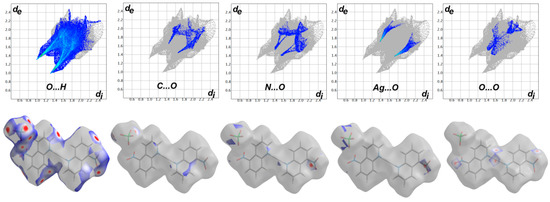
Figure 5.
Decomposed dnorm and fingerprint plots for the detected interactions in [Ag(5-NO2Quin)2]ClO4.

Table 3.
All short contacts in the studied Ag(I) systems.
For the nitrate complex, the O…H, N…O, O…O and Ag…O contacts appeared as red spots in the dnorm map while the C…O contacts have no significance in the molecular packing of this complex (Figure 6). Summary of all short contacts in both complexes are depicted in Table 3. The perchlorate anion is richer in oxygen atoms and also is more bulky than the nitrate anion. As a consequence of these facts, the [Ag(5-NO2Quin)2]ClO4 possessed larger number of O…H contacts which are generally have longer distances than those found in the [Ag(5-NO2Quin)2]NO3 analogue. Also, the Ag…O, N…O and O…O contacts have longer distances in the former than the latter.
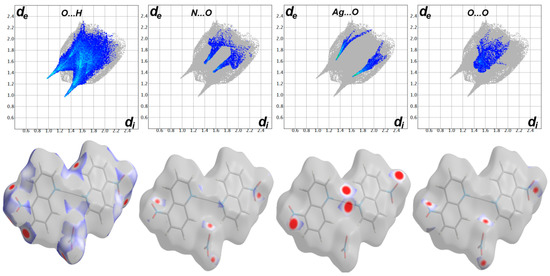
Figure 6.
Decomposed dnorm and fingerprint plots for [Ag(5-NO2Quin)2]NO3. Full Hirshfeld surfaces are shown in Figure S3 (Supplementary Materials).
Another importance from the decomposition of the fingerprint is to obtain a quantitative summary for each contact. The analysis of the fingerprint gave the percentages of all contacts included in the molecular packing of these Ag(I) complexes (Figure 7). The most dominant interactions in the both complexes are the O…H, C…H, H…H and C…C contacts. Their percentage contributions in the molecular packing of the perchlorate complex are 51.1, 11.8, 10.8 and 8.9%, respectively. The corresponding values for the nitrate complex are 39.5, 14.4, 15.4 and 10.1%, respectively. The rest of contacts and their percentages are shown in Figure 7.
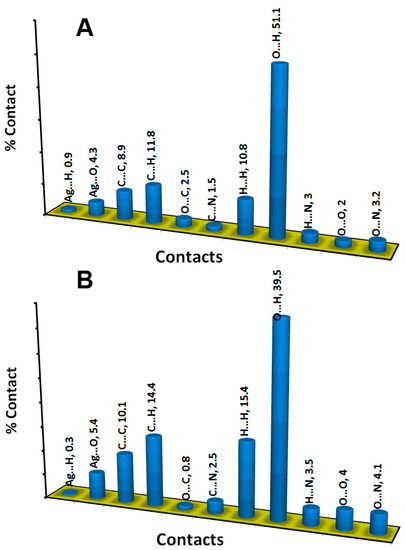
Figure 7.
Possible intermolecular interactions in [Ag(5-NO2Quin)2]ClO4 (A) and its nitrate analogue (B).
On the other hand, the shape index map showed red/blue triangles and the curvedness map has flat green area, both features are clear evidences on the presence of π-π stacking interactions. Careful inspection of the dnorm indicated the absence of red spots corresponding to C…C or C…N contacts among the stacked aromatic π-systems. The C7…C9 (3.424 Å) and C6…C8 (3.458 Å) are the shortest distances between the stacked aromatic π-systems in the perchlorate and nitrate complexes, respectively. These values are larger than the twice of the vdWs radii of carbon. Hence, the π-π stacking interactions are generally weak in both complexes.
3.3. FTIR Spectra
The FTIR spectra of [Ag(5-NO2Quin)2]ClO4 complex showed the characteristic peaks of the free ligand with some variations were detected which confirm the coordination of Ag(I) with 5-NO2Quin ligand. A clear difference in the FTIR spectra of both compounds is the shift of the N–O asymmetric and symmetric stretches [40]. In the FTIR spectra of [Ag(5-NO2Quin)2]ClO4 complex, these bands appeared at 1525 and 1340 cm−1, respectively. The corresponding values in the free 5-NO2Quin are 1520 and 1320 cm−1, respectively. The appearance of three intense bands at 1146, 1109 and 1085 cm−1 corresponding to the perchlorate anion vibrations is another clear difference is shown in the FTIR spectra of [Ag(5-NO2Quin)2]ClO4 complex but not in the free 5-NO2Quin ligand.
3.4. Antimicrobial Activity
The in vitro examination of the antimicrobial activities of [Ag(5-NO2Quin)2]ClO4 complex and 5-NO2Quin ligand is shown in Table 4. Both compounds showed good activity against the selected Gram positive (S. aureus and B. subtilis), Gram negatvie (E. coli and P. vulgaris) bacteria and two fungi (A. fumigatus and C. albicans). The inhibition zones in case of [Ag(5-NO2Quin)2]ClO4 are generally higher than those for the free ligand 5-NO2Quin against all microbes. For example, the inhibition zone diameters are 36 and 20 mm for the Ag(I) complex against A. fumigatus and C. albicans, respectively. The corresponding values for the free ligand are 27 an 18 mm, respectively. Also, the [Ag(5-NO2Quin)2]ClO4 showed inhibition zone diameters of 21, 18, 18 and 27 mm for S. aureus, B. subtilis, E. coli and P. vulgaris, respectively while the corresponding values for the free 5-NO2Quin are 20, 16, 16 and 20 mm, respectively. These results indicated that both compounds have broad spectrum antimicrobial action where the Ag(I) complex has enhanced antimicrobial activity compared to the free ligand. In comparison with Ketoconazole (17 mm), the Ag(I) complex has larger inhibition zone against the fungus A. fumigatus (36 mm) and very close result against P. vulgaris (27 mm) compared to the standard Gentamycin (25 mm).

Table 4.
Inhibition zone diameters of the Ag(I) complex a.
Also, the MIC data revealed the better antimicrobial potency of the Ag(I) complex than the free ligand. The [Ag(5-NO2Quin)2]ClO4 complex has higher potency against the fungus A. fumigatus (MIC = 39.1 μg/mL) than 5-NO2Quin (MIC = 78.1 μg/mL). The antifungal activity of both compounds are considered better than the standard Ketoconazole (156.2 μg/mL). Also, the MIC values is the lowest for the Ag(I) complex as antibacterial agent against P. vulgaris (MIC = 9.7 μg/mL) and S. aureus (39.1 μg/mL). These results are comparable to the antibacterial standard Gentamycin (4.8 and 9.7 μg/mL, respectively). For the free ligand, the MIC values are higher (78.1 μg/mL). Hence, the studied Ag(I) complex has high potency as an antimicrobial agent against these microbes.
4. Conclusions
The [Ag(5-NO2Quin)2]ClO4 complex was synthesized and characterized. This complex does not only have a centrosymmetric space group but also possesses an inversion center at the Ag site. Hence, the asymmetric unit is half of the [Ag(5-NO2Quin)2]ClO4 formula. Its supramolecular structural aspects were investigated based on the X-ray single crystal structure and Hirshfeld analyses. The O…H, C…H, H…H and C…C contacts are the most dominant while the O…H, C…O, N…O, O…O and Ag…O interactions are the only contacts which have shorter distances than the vdWs radii sum of the interacting atoms. Analysis of the molecular packing in the crystal structure of the [Ag(5-NO2Quin)2]NO3 analogue revealed the importance of O…H, N…O, O…O and Ag…O contacts, while the C…O contacts have less importance in this complex. The [Ag(5-NO2Quin)2]ClO4 has broad antimicrobial action against bacteria and fungi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym14030547/s1, Figure S1 FTIR spectra of 5-nitroquinoline (upper) and [Ag(5-nitroquinoline)2]ClO4 complex (lower). Figure S2 Atom numbering of [Ag(5-NO2Quin)2]NO3. Figure S3 Hirshfeld surfaces of [Ag(5-NO2Quin)2]NO3. Physicochemical characterizations; X-ray measurements; Hirshfeld analysis; Method S1 Antimicrobial studies.
Author Contributions
Conceptualization, S.M.S. and A.B.; methodology, M.S.A. and A.A.A.; software, S.M.S.; validation, M.S.A., N.H.A.-S. and A.A.A.; formal analysis, M.S.A., N.H.A.-S. and A.A.A.; investigation, M.S.A.; resources, M.S.A. and A.B.; data curation, A.B. and S.M.S.; writing—original draft preparation, A.B. and S.M.S.; writing—review and editing, A.B. and S.M.S.; visualization, A.B., M.S.A. and N.H.A.-S.; supervision, A.B. and M.S.A.; project administration, A.A.A.; funding acquisition, M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0040).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benedini, S.; Zheng, Y.; Nitti, A.; Mazza, M.M.A.; Dondi, D.; Raymob, F.M.; Pasini, D. Large polarization of push–pull “Cruciforms” via coordination with lanthanide ions. New J. Chem. 2022, 46, 221–227. [Google Scholar] [CrossRef]
- Agnes, M.; Arabi, A.; Caricato, M.; Nitti, A.; Dondi, D.; Yannakopoulou, K.; Patrini, M.; Pasini, D. Helical Nanofibers Formed by Palladium-Mediated Assembly of Organic Homochiral Macrocycles Containing Binaphthyl and Pyridyl Units. ChemPlusChem 2021, 86, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Delforge, A.; Bonifazi, D.; Dondi, D.; Mazzanti, A.; Pasini, D. Chiral nanostructuring of multivalent macrocycles in solution and on surfaces. Org. Biomol. Chem. 2015, 13, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Rowan, R.; Tallon, T.; Sheahan, A.M.; Curran, R.; McCann, M.; Kavanagh, K.; Devereux, M.; McKee, V. Silver bullets in antimicrobial chemotherapy: Synthesis, characterisation and biological screening of some new Ag(I)-containing imidazole complexes. Polyhedron 2006, 25, 1771–1778. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Glisic, B.D.; Senerovic, L.; Comba, P.; Wadepohl, H.; Veselinovic, A.; Milivojevic, D.R.; Djuran, M.I.; Nikodinovic-Runic, J. Silver(I) complexes with phthalazine and quinazoline as effective agents against pathogenic Pseudomonas aeruginosa strains. J. Inorg. Biochem. 2016, 155, 115–128. [Google Scholar] [CrossRef]
- Ahmad, S.; Isab, A.A.; Ali, S.; Al-Arfaj, A.R. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron 2006, 25, 1633–1645. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Fox, C.L.; Modak, S.M. Mechanism of Silver Sulfadiazine Action on Burn Wound Infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; MacDonald, N.E. Preventing ophthalmia neonatorum. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Mallika, P.S.; Asok, T.; Faisal, H.A.; Aziz, S.; Tan, A.K.; Intan, G. Neonatal Conjunctivitis—A Review. Malays. Fam. Physician. 2008, 3, 77–81. [Google Scholar] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327–328, 349–359. [Google Scholar] [CrossRef]
- Bouadma, L.; Wolff, M.; Lucet, J.C. Ventilator-associated pneumonia and its prevention. Curr. Opin. Infect. Dis. 2012, 25, 395–404. [Google Scholar] [CrossRef]
- Fox, C.L., Jr. Silver sulfadiazine for control of burn wound infections. Int. Surg. 1975, 60, 275–277. [Google Scholar]
- Lederer, J.W.; Jarvis, W.R.; Thomas, L.; Ritter, J. Multicenter cohort study to assess the impact of a silver-alloy and hydrogel-coated urinary catheter on symptomatic catheter-associated urinary tract infections. J. Wound Ostomy Cont. Nurs. 2014, 41, 473–480. [Google Scholar] [CrossRef]
- Beattie, M. Can silver alloy catheters reduce infection rates? Nurs. Times 2011, 107, 19–20, 22. [Google Scholar]
- Schumm, K.; Lam, T.B. Types of urethral catheters for management of short-term voiding problems in hospitalized adults: A short version Cochrane review. Neurourol Urodyn 2008, 27, 738–746. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. The Bacterial Challenge: Time to React. EMEA/576176/2009; ECDC/EMEA Joint Technical Report; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2009; ISBN 978-92-9193-193-4.
- Massoud, A.A.; Langer, V.; Gohar, Y.M.; Abu-Youssef, M.A.M.; Janis, J.; Lindberg, G.; Hansson, K.; Ohrstrom, L. Effects of Different Substituents on the Crystal Structures and Antimicrobial Activities of Six Ag(I) Quinoline Compounds. Inorg. Chem. 2013, 52, 4046–4060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Dey, R.; Gohar, Y.; Massoud, A.A.; Öhrström, L.; Langer, V. Synthesis and structure of silver complexes with nicotinate-type ligands having antibacterial activities against clinically isolated antibiotic resistant pathogens. Inorg. Chem. 2007, 46, 5893. [Google Scholar] [CrossRef]
- Clayden, J.P.; Wothers, P.D.; Greeves, N.; Warren, S. Organic Chemistry, 1st ed.; Oxford University: Oxford, UK, 2001; pp. 19–25. [Google Scholar]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pal, M. Quinolines: A new hope against inflammation. Drug Discov. Today 2013, 18, 389–398. [Google Scholar] [CrossRef]
- Chong, C.R.; Sullivan, D.J., Jr. Inhibition of heme crystal growth by antimalarials and other compounds: Implications for drug discovery. Biochem. Pharmacol. 2003, 66, 2201–2212. [Google Scholar] [CrossRef]
- Solomon, R.V.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1508–1510. [Google Scholar] [CrossRef]
- Baba, A.; Kawamura, N.; Makino, H.; Ohta, Y.; Taketomi, S.; Sohda, T. Studies on disease-modifying antirheumatic drugs: Synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J. Med. Chem. 1996, 39, 5176–5182. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 9, 1654–1660. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Franck, X.; Fournet, A.; Prina, E.; Mahieux, R.; Hocquemiller, R.; Figadère, B. Biological evaluation of substituted quinolines. Bioorg. Med. Chem. Lett. 2004, 14, 3635–3638. [Google Scholar] [CrossRef] [PubMed]
- Dorababu, A. Recent update on antibacterial and antifungal activity of quinoline scaffolds. Archiv Pharmazie 2021, 354, 2000232. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; University of Goettingen: Goettingen, Germany, 1996. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western: Perth, Australia, 2017; Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 20 December 2021).
- CLSI. Clinical and Laboratory Standards Institute. Twentieth Informational Supplement. M100-S22. Wayne: PA. 2012. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 20 May 2021).
- Beal, R.W.; Brill, T.B. Vibrational Behavior of the—NO2 Group in Energetic Compounds. Appl. Spectrosc. 2005, 59, 1194–1202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).