Abstract
A single chronic stress is often considered a potential reinforcer in psychiatric disorders. Lithium and ketamine both seem to ameliorate the consequences of stress. Here, male mice were either injected with lithium carbonate (LiCl), ketamine hydrochloride (KET), or sodium chloride (NaCl; controls) over nine consecutive days. Treatment was followed by 2 h of restraint stress over the first seven days. On the 9th day, 2 h after injection, all animals were tested in the open field, and novel object tests and behavior were analyzed using the toolbox ‘DeepLabCut’. To exclude an effect of generally altered locomotion activity on turning behavior, further parameters were assessed. Treatment before chronic stress exposure did not influence the total number of turns, nor the direction of turning behavior in the open field and the novel object test. Additionally, general locomotion did not differ. However, mice treated with LiCl showed a stronger turning bias (i.e., larger absolute lateralization quotients) in the novel object test when compared to mice treated with KET. This study underlines the potential of investigating turning behavior as a sensitive and reliable marker of stress reaction. Additionally, analyzing behavioral asymmetries in the context of psychopharmacological treatment can render new insights.
1. Introduction
While several treatment options for depression are available, between 10–30% of patients do not respond to treatment [1,2,3]. A promising new antidepressant drug that is mostly administered at sub-anesthetic doses by infusion is ketamine [4,5,6]. However, the therapeutic effects of sub-anesthetic ketamine doses have been shown to appear rapidly yet transient upon infusion; thus, repeated treatment is needed to sustain the antidepressant effects [7]. To this end, a systematic review on the maintenance of ketamine treatment in sustaining antidepressant effects in treatment-resistant depression revealed that intravenous, intranasal, oral, and possibly intramuscular and subcutaneous treatment seems to be effective [5]. However, as there are limited studies on this topic, the therapeutic potential is to be further researched [5].
In animals, study results are mixed, so far. In a study with female rats, researchers found that repeated (two-time) intraperitoneal injections of ketamine (10 mg/kg) did not alter anxiety in the elevated plus maze test but increased locomotor behavior in the open field test [8]. In a study exposing male and female mice to chronic mild stress together with intraperitoneal ketamine injection, females proved to be more reactive to the rapid and early antidepressant effects of ketamine treatment and generally responded to lower doses of ketamine but the antidepressant effect of ketamine treatment was long-lasting (days after treatment) in males [9]. Ng et al. showed that an intraperitoneal injection of ketamine (10 mg/kg) before each restraint stress session over 7 days had a protective effect on neuronal plasticity and against the stress-induced loss of dendrites [10]. Similarly, Okine et al. treated male and female mice with a single intraperitoneal injection of ketamine (10 mg/kg) 7 days before chronic stress exposure. Interestingly, ketamine prevented chronic stress-induced neurobiological and behavioral changes in males but not in females [11]. Fitzgerald and colleagues examined male mice that received a single intraperitoneal injection of 10 mg/kg or 30 mg/kg ketamine after two weeks of unpredictable chronic stress and compared it to vehicle-injected mice and non-stressed conditions. When the mice were tested in the forced swim test 24h after injection, mice that were exposed to unpredictable chronic stress showed decreased immobility but increased swimming behavior (representing antidepressant-like effects) [12]. However, a systematic review of studies that investigate the antidepressant effects of ketamine focusing on behavioral despair as a proxy for treatment success concluded that behavioral despair tests such as the forced swim test or tail suspension test, have poor predictive validity for the clinical efficacy of ketamine [13]. Thus, more detailed studies on the effects on several dimensions of behavior and central nervous processes are required.
Another drug that may target the same neuronal circuits as ketamine and that is used primarily to stabilize mood in bipolar disorder is lithium [14,15,16,17]. Bipolar disorder has the highest suicide rate of any mental illness [18] with 35–50% of patients attempting suicide, of which 20% end in death [19]. Lithium salts are often used to treat bipolar disorder, as they prevent the recurrence of a manic episode [20] and act as a suicide preventive [21,22]. However, side effects must be expected when administering lithium [23]. Impaired kidney function, hypothyroidism, hypercalcemia, and severe weight gain can occur [24]. In addition, lithium should not be administered during pregnancy due to a potential teratogenic effect [25].
Besides the apparent beneficial effects of lithium administration on mood stabilization in patients, positive effects have also been reported in animal models [17,26,27]. To investigate whether lithium administration could prevent stress-induced behavioral and neuronal consequences, rats were intraperitoneally injected daily over 2 weeks with lithium chloride (2.5 mEq/kg) and subjected to a chronic mild stress protocol [17]. The results show that lithium treatment during stress exposure prevented the consequences of stress on behavior, corticosterone secretion, and neuronal cell turnover [17]. A study led by Dygalo et al. examined chronic (7 days) intraperitoneal lithium chloride (84 mg/kg) injection together with acute stress for 2 h (on days 6 and 7) in rats. Here, an effect of lithium administration on neuronal plasticity was shown [26]. In a study on the neurochemical effects after intraperitoneal injection of lithium carbonate (25 mg/kg) over 7 consecutive days in glutamine synthetase reporter mice, male animals showed a preventive effect of lithium administration, whereas no effect was found in females [27].
Since stress is considered a frequent cause and intensifier of psychiatric diseases such as depression and bipolar disorder [28,29,30], investigating whether the administration of the selected psychotropic drugs can protect the nervous system from the negative effects of stress exposure is a relevant objective. On the behavioral side, investigating turning behavior as a proxy for hemispheric asymmetries may render new insights into asymmetrical, neuronal alterations associated with stress and psychiatric disorders that are often neglected in psychiatric research.
In general, hemispheric asymmetries exist in all major vertebrate groups [31]. Moreover, these asymmetries are also found in the nervous system of insects and other invertebrates [32]. Developing such asymmetries holds several advantages, especially in highly complex and energy-hungry systems such as the brain [33,34]. Hemispheric asymmetries allow, for example, a more energy-efficient design by avoiding the unnecessary redundancy of processing units and improved action control by avoiding bilateral interference [33]. Given the mentioned benefits of hemispheric asymmetries, it is of interest that most neurodevelopmental and psychiatric disorders have been associated with reduced or atypical asymmetries [35,36,37]. Recent studies point to an important role of atypical asymmetries in detecting more subtle effects of stress exposure [38,39,40,41] and subclinical depressive symptoms [42]. Given that most psychiatric disorders are associated with atypical asymmetries, investigating lateralization in preclinical studies poses a novel and important tool.
There are different ways to determine behavioral asymmetries in rodents: paw preference [43], head-turning [44], or general body-turning behavior [39,45]. One benefit of assessing general body turning is that no pretreatment or training is necessary, and the animals move freely. Moreover, favoring of the right side has been reported for rodents when analyzed in mazes such as the elevated plus or the T-maze [45].
An association between stress exposure and atypical asymmetries has also been reported in preclinical animal models [39,40]. For example, investigating turning behavior in the elevated plus-maze after exposure to early life stress throughout childhood led to atypical leftward turning behavior in rats [39]. Similarly, inducing a maternal immune activation in a rat model for schizophrenia led to atypical turning behavior in the open field test [40]. Interestingly, a study that assessed head-turning asymmetry in rats found that rats with a left-sided head-turning bias showed more behavioral despair in the forced swim test than rats with a right-sided bias [44].
When assessing lateral behavior, laterality research has made great progress over the last decades [46] but still faces methodological problems in terms of objective assessment, especially for lateralized behavior [47]. Advances in deep learning and the development of toolboxes such as ‘DeepLabCut’ can improve “computational ethology” [48] and enable the precise and fast tracking of complex behavior traits [49,50,51].
Generally, behavioral data that are collected through human observation are dependent on the subjective interpretation of the observer [52]. Computational methods that output quantitative, positional data and thus spare the interpretation of behavior at the data-collection stage may enhance the discussion of the meaning of results. Furthermore, behaviors might be missed or assessed incorrectly by the observer during live observation [52]. Conducting the analysis based on video recording, as with ‘DeepLabCut’, enables the objective recording and analyzing of several behaviors. ‘DeepLabCut’ so far facilitates the analysis of, e.g., time spent in a region of interest, behavior clustering, and behavior classification, such as recording when an animal is moving/still, the direction that they are facing, and the average speed of the animals [49,52]. Additionally, ‘DeepLabCut’ enables the tracking of body parts that are hidden only in some frames by calculating likelihood values [49]. The toolbox ‘DeeplabCut’ already was successfully used to analyze, e.g., turning behavior in rats [39,40] or more laborious assessments like the eye use of birds [53]. Using these deep learning toolboxes such as ‘DeeplabCut’ will promote more comparable and replicable results, thus improving the validity of findings.
To further disentangle potential protective effects of ketamine or lithium administration against chronic stress, as a new approach, turning behavior as a proxy for hemispheric asymmetries was investigated in this study. In line with improving “computational ethology”, the deep learning toolbox ‘DeeplabCut’ was applied to track behavioral differences in the open field and novel object tests.
2. Materials and Methods
2.1. Animals
Based on the results of our previous study on the protective effects of lithium injection [27], mice from a Glutamine Synthetase reporter mouse model, B6C3H-Glultm(T2A-LacZ-loxP-T2A-Tk−1-FRT- loxP-T2A-Fluc-FRT)Arte, obtained from the Institute of Experimental and Clinical Pharmacology and Toxicology at the University of Tübingen, Germany (originally prepared by Taconic Artemis, Cologne, Germany) were included in this study. The chosen reporter mouse model enables the detection of rapid changes in promoter activity but does not differ behaviorally from mice strains such as C57Bl6 or C3H. In the previous study ([27]), protective neuronal effects of repeated lithium injection following stress were only found in males but not in females [27]. Thus, in this follow-up study, only male mice were examined. Animals were housed under standard conditions (22 ± 2 °C room temperature, 55 ± 25% humidity) and standard lighting (12 h/12 h) with free access to water and food. All procedures were conducted under the principles of Germany’s Animal Welfare Act after approval by the LANUV (Landesamt für Natur, Umwelt, und Verbraucherschutz Northrhine-Westfalia).
2.2. Treatment and Chronic Stress Paradigm
A total of 36 adult mice (between 50–82 days old at the study start) were included in this study with sample sizes estimated based on the previous study [27]. The mice were exposed to 2 h of restraint stress over seven consecutive days. Before restraining, the mice were randomly assigned to one of three groups and either intraperitoneally injected with lithium carbonate (62470-100G-F from Sigma-Aldrich) (25 mg/kg in 0.1 mL of 0,9% sterile sodium chloride (NaCl; A1671,0250, PanReac AppliChem ITW Reagents), according to our previous study [27]), ketamine hydrochloride (100 mg/mL, 1202, CP-Pharma) (10 mg/kg weight in NaCl, according to similar studies in mice (e.g., [9,11])) or 0.1 mL sterile NaCl. The mice were weighed daily to ensure that the concentration given was according to their body weight. There were no significant changes in body weight throughout the experiments. Therewith, the three groups constitute as follows: (i) NaCl, (ii) ketamine hydrochloride, and (iii) lithium carbonate, with 12 animals per group. For the sake of brevity, we will refer to ‘ketamine hydrochloride’ as ‘ketamine’ and to ‘lithium carbonate’ as ‘lithium’ throughout this manuscript. The experimenter treating the animals was blinded to the substance injected as well as to the groups during behavioral testing. The mice were injected right before the start of their active phase; thus, the restraint stress was conducted under red light (=in the dark).
2.3. Behavioral Analysis
All behavioral testing was conducted under red light (=active phase). On the second day after the restraint stress protocol, all animals were tested 2h after injection in the open field test directly followed by the novel object test. Therefore, the animals were placed in the center of a box of 45 × 45 cm with a 45 cm-high border, facing the same side of the box (Figure 1b), and were allowed to run freely for 10 min while being filmed using an HD Webcam (C920 Pro, Logitech) connected to a laptop. The camera was placed in a plastic frame above the box, with commercially available LED strips in red light attached to the frame on both sides of the camera (Figure 1a). After 5 min, a novel object (wooden cylinder) was placed in the center of the box (Figure 1c), irrespective of the animal’s placement at that moment. The cylinder was always placed from the same side of the box. Both tests thus have the same conditions in terms of light and camera placement. During testing, asymmetry in placing the animal or the object was tried to minimize. However, the experimenter was right-handed, and thus this could impact asymmetry behavior.
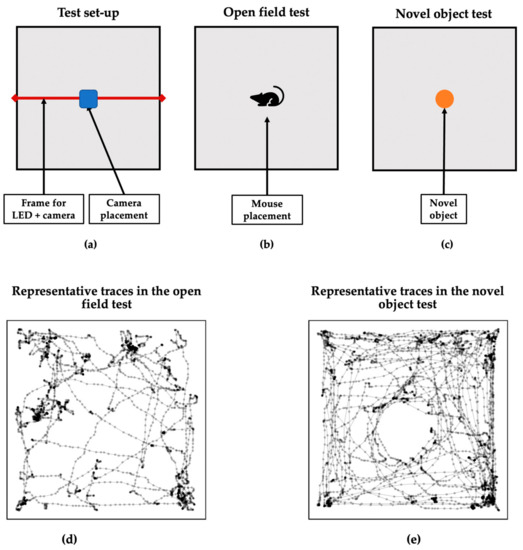
Figure 1.
Layout of the behavioral analyses. Graphical representation of (a) the general test setup, (b) the open field, and (c) novel object tests. Additionally, representative movement traces of one animal in (d) the open field and (e) novel object tests are presented.
Behavior was analyzed offline using video-based tracking via the python software ‘DeepLabCut’ [49,50,51]. A representative movement trace of one animal in the open field (d) and novel object (e) are shown in Figure 1. Extracted x–y coordinates were smoothed, and the noise was removed using the smooth spline function. The extracted coordinates were then used to define several behavior measures. The angular movements were defined by the rotation of body–head orientations using the same definition from Mundorf et al. [40]. Turning behavior was defined as cumulative angular movements of more than 45°. Both left and right turning behaviors were separately counted and used for the subsequent analysis of behavioral asymmetry. Additionally, the body angle was defined as the angular difference between the head–body and body–object orientation. The distance to the object was defined as the Euclidian distance between the novel object and the head position. Based on the distance to the object, the proportion of spending time close to the novel object was measured as the total time that a mouse was closer to the object below <50 pixels, which was approximately two times longer than the mouse’s body.
2.4. Laterality Measures
Based on the extracted number of turns, for each test and mouse, the lateralization quotient (LQ) was calculated according to the formula LQ = ((R − L)/(R + L)) × 100, with R indicating the number of right turns and L indicating the number of left turns. Given that the LQ spans from −100 to 100, positive LQ values reflect a right-sided turning bias, and negative LQ values a left-sided turning bias. To analyze the strength of asymmetry independent of direction, we also determined the absolute, unsigned LQ for each animal.
2.5. Statistical Analyses
All analyses were performed using JASP 0.16.2 [54]. The normality of the data was tested using the Shapiro–Wilk test. The variables number of left and right turns in the novel object test, LQ in the open field and novel object test, absolute LQ in the open field test, and distance traveled to the object were normally distributed (p > 0.05) and thus were compared between the three treatment groups using ANOVAs. The variables number of left and right turns in the open field test, absolute LQ in the novel object test, the proportion of staying, and angle facing the object were not normally distributed (p < 0.05) and thus were further analyzed using the Kruskal–Wallis-Test. Post hoc tests were corrected using the Tukey method, the default in JASP.
3. Results
3.1. Behavioral Asymmetries
3.1.1. Number of Turns
Differences in the number of turns were analyzed for both tests separately, as the open field data were not distributed normally. The mean number of left and right turns per treatment group is given for the open field test in Table 1 and the novel object test in Table 2. First, differences between the treatments in the number of left and right turns in the open field (Figure 2a) were analyzed by a Kruskal–Wallis test. There was no effect of the treatment on the number of left turns (H(2) = 0.478, p = 0. 787) nor on the number of right turns (H(2) = 1.246, p = 0.536).

Table 1.
Average number of left and right turns in the open field test per treatment and test. Additionally, Cohen’s d is given for the difference between left and right turns per treatment group.

Table 2.
Average number of left and right turns in the novel object test per treatment and test. Additionally, Cohen’s d is given for the difference between left and right turns per treatment group.
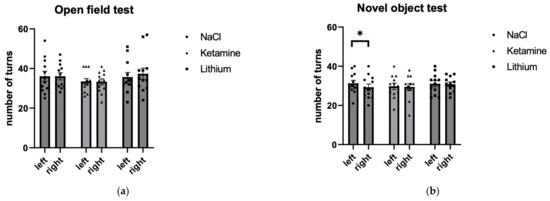
Figure 2.
Number of left and right turns in (a) the open field test and (b) the novel object test. Mice treated with NaCl show more left than right turns in the novel object test. Individual and average (bar plot) data are given with SE for N = 12 mice per group. * p < 0.05.
Differences in the novel object task were analyzed using a 2 × 3 repeated measures ANOVA with the within-subjects factor side (left, right), and the between-subjects factor treatment group (NaCl, ketamine, lithium). The main effect of the test failed to reach significance but indicated a trend (F(1,33) = 13.35; p = 0.094, η2p = 0.082). A follow-up Student’s t-test revealed that in the NaCl group, the number of left and right turns significantly differed (p = 0.016) (Figure 2b and Table 2). Furthermore, to estimate the effect sizes for the difference between the number of left and right turns, Cohen’s d was calculated using a paired-samples Student’s t-test (results are shown in Table 1 and Table 2). The effect sizes were characterized as small (d = ±0.2 − ±0.5), medium (d = ±0.5 − ±0.8), and large (d = ±0.8 or larger).
3.1.2. Lateralization Quotient
In the open field test, the average LQ was 0.61 (SE: ±2.03) in mice treated with NaCl, −0.32 (SE: ±1.41) for mice treated with ketamine, and 1.93 (SE: ±1.35) for mice treated with lithium. In the novel object test, the average LQ was −3.51 (SE: ±1.18) for NaCl, −0.84 (SE: ±1.08) for ketamine, and −0.113 (SE: ±2.12) for lithium. To analyze the difference between the three groups regarding the LQ in the open field test, we performed an ANOVA with the between-subjects factor treatment. An identical ANOVA was also performed for the novel object test. In both ANOVAs, the main effect of the group failed to reach significance (novel object test: p = 0.27, open field test: p = 0.62). The correlation between the turning LQ in the open field test and the novel object test also failed to reach significance (r = −0.19; p = 0.27).
3.1.3. Absolute Lateralization Quotient
To analyze the effect of treatment on the absolute size of asymmetry independent of its direction, the unsigned LQ was analyzed in a second step. In the novel object test, the average LQ was 4.01 (SE: ±1.02) for NaCl, 2.63 (SE: ±0.78) for ketamine treatment, and 6.05 (SE: ±1.08) for the lithium group (Figure 3a). In the open field test, the average LQ was 5.80 (SE: ±1.05) for NaCl, 3.98 (SE: ±0.75) for ketamine, and 4.10 (SE: ±0.80) for lithium (Figure 3b).
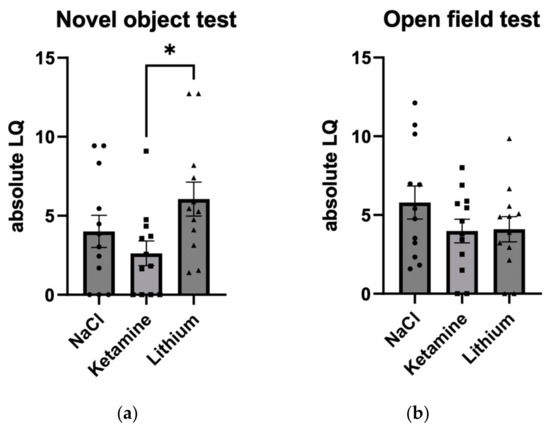
Figure 3.
Absolute lateralization quotient (LQ) in (a) the novel object test and (b) the open field test. Mice treated with lithium show a larger absolute LQ than mice treated with ketamine in the novel object test. Individual and average (bar plot) data are given with SE for N = 12 mice per group. * p < 0.05.
To analyze the difference between the three groups regarding the absolute unsigned LQ in the novel object test, we performed a Kruskal–Wallis test with the between-subjects factor treatment (NaCl, ketamine, lithium) as the data were not normally distributed. A non-significant trend was observed (H(2) = 5.711; p = 0.058). Post hoc tests using Tukey correction revealed a significant difference between the two experimental groups (p = 0.045), with mice treated with lithium showing a larger absolute LQ (average absolute LQ: 6.05; SE: ±0.97) indicating a stronger turning bias than mice treated with ketamine (average absolute LQ: 2.63; SE: ±0.97). Both of the post hoc comparisons between the NaCl and the ketamine groups and between the NaCl and the lithium groups failed to reach significance (all p’s > 0.3). In the ANOVA for absolute LQ in the open field test, the main effect of treatment failed to reach significance (p = 0.27). The correlation between the turning LQ in the open field test and the novel object test failed to reach significance (r = −0.008; p = 0.96).
3.1.4. Direction of Asymmetry
To investigate the effect of treatment on the direction of asymmetry independent of its strength, the sign of the LQ (indicating the direction of asymmetry) was also investigated in a third step. Table 3 shows the distribution of left (negative LQ), right (positive LQ), and ambilateral (LQ = 0) animals in the open field test. Table 4 shows the same distribution for the novel object test.

Table 3.
Direction of asymmetry in the open field test. N = 12 per group.

Table 4.
Direction of asymmetry in the novel object test. N = 12 per group.
3.2. Behavioral Variability in the Novel Object Test
To further disentangle the found differences in the absolute LQ in the novel object test, the average distance to the object, the proportion of time spent close to the novel object, as well as the average angle the mouse body was facing the novel object were analyzed (Table 5).

Table 5.
Average and standard-error (SE) for locomotor behavior in the novel object test. Values are given in pixels in the traveled distance. N = 12 per group.
Therewith, potential confounding effects due to group differences in these locomotor parameters affecting the absolute LQ can be excluded. To analyze differences in the novel object test, Kruskal–Wallis tests with the respective dependent variables proportion of staying, angle facing the object, and the fixed factors treatment (NaCl, ketamine, lithium) were performed. The analyses failed to reach significance for the parameter angle facing the object (H(2) = 0.074, p = 0.964) and the proportion of staying (H(2) = 3.045, p = 0.218). The values for the distance traveled to the object were normally distributed, and thus an ANOVA with the dependent variable distance traveled to the object and the fixed factors treatment (NaCl, ketamine, lithium) was performed but failed to reach significance (F(2,33) = 1.98; p = 0.154). Thus, the groups did not differ in general locomotor activity in the novel object test.
4. Discussion
The study aimed to investigate the potential protective effects of lithium or ketamine treatment prior to chronic stress on turning behavior. Being a sensitive marker for stress-induced changes, turning bias was assessed using a toolbox for high-quality, markerless pose estimation. To exclude an effect of generally altered locomotion activity on turning behavior, further parameters (i.e., distance traveled to the object, proportion of staying, and angle facing the object) were assessed.
Mice treated with lithium show a larger absolute LQ (indicating stronger turning bias) compared to mice treated with ketamine in the novel object test. There was no effect of treatment or stress exposure (NaCl group) on side preference. Interestingly, mice treated with NaCl showed more left than right turns in the novel object task, whereas mice treated with ketamine or lithium showed no difference in the number of left to right turns. In addition, the animals did not differ in any other general locomotor parameter investigated.
Generally, in untreated and unstressed rodents, the favoring of the right side has been reported in mazes [45] as well as in the open field test [40]. It is furthermore known that stress exposure leads to greater right hemispheric activation resulting in atypical leftward-turning behavior in humans and rodents [38,39,54]. Some studies report reduced asymmetric turning behavior after stress exposure in the form of no turning bias instead of a shift towards a leftward turning bias [40]. Interestingly, these attenuated behavioral and neuronal asymmetries have been repeatedly found in patients diagnosed with psychiatric disorders [35,36,37,55]. Consequently, one would have hypothesized that chronic stress exposure or repeated administration of psychopharmaceutic drugs would have either resulted in increased left-sided turning behavior as a consequence of stress exposure on increased right-sided turning behavior as seen in unstressed rodents.
The fact that only the absolute LQ (indicating the absolute strength of asymmetry, independent of its direction) was significantly different between the groups may indicate that stress exposure and psychopharmacological treatment have a strong effect on individual asymmetry but not on the population asymmetry. That is in line with a meta-analysis revealing that rodents show strong individual lateralization of paw preference but no side bias at the population level [43]. However, it must be noted that the found differences in absolute lateralization quotient are small, given the small effect sizes for the difference in the total number of left to right turns for ketamine- or lithium-treated mice in the novel object test.
Another interesting finding is that mice treated with NaCl showed more left than right turns in the novel object task, whereas mice treated with ketamine or lithium showed no difference in the number of left to right turns. Thus, one could hypothesize that ketamine and lithium treatment did affect this difference in left/right turns seen in the untreated (NaCl) group.
Several studies point toward the hypothesis that the right hemisphere is associated with negative emotions and the left hemisphere with positive emotions [56]. For example, a study investigating tail-wagging side preferences in domestic dogs found that dogs show more tail wagging to the right side when seeing their owner but more left-sided tail wagging when seeing a dominant unfamiliar dog [57]. Thus, in this study, left-brain activation (reflected in right-sided tail wagging) was associated with positive emotions and right-brain activation (reflected in left-sided tail wagging) with negative emotions or stress. Interestingly, this effect of laterality and emotions was not observed when investigating dogs’ paw preference and emotionality [58]. However, when investigating eye preference in emotional situations in horses, the effect was found as well. Farmer and colleagues (2010) examined whether domestic riding horses have a preferred eye when observing an unknown person in a neutral area. Indeed, 72% of the horses had a left-eye preference when looking at and approaching an unknown person [59]. The authors hypothesize that horses may prefer the left eye for the assessment and evaluation of a situation [59]. This would be in line with the results showing that mice treated with NaCl before chronic stress exposure had more left than right turns in the novel object test. Here, the novel object is unknown and may induce a stressful situation for the mouse. However, when integrating several aspects of structural and functional lateralization, e.g., for the amygdala, it becomes more likely that functional lateralization is determined by temporal characteristics, emotional valence, and perceptual properties rather than solely emotional valence alone [37].
Another aspect to consider is that, in this study, a repeated administration protocol was tested. In patients, the main advantage of sub-anesthetic ketamine treatment relates to its rapid action, and it is frequently administered via infusion [7]. Repeated treatment in patients usually consists of a weekly infusion and not, as in animals, a daily administration for several days. In contrast, lithium is usually a long-term treatment (over months or years) and is taken orally daily [60]. Consequently, the potential protective effects of ketamine or lithium administration in patients on stress-induced hemispheric asymmetries may have been missed given the study design. Given the different courses of action of both drugs, with ketamine having a rather mood-lifting, psychedelic effect and lithium being used as a mood stabilizer, differential effects on asymmetry are likely.
Older studies investigated whether lithium treatment attenuated asymmetric responsiveness to tyramine on pupil size in human participants suffering from cluster headaches [61]. The authors found that long-term lithium treatment (6 months) improved cluster headache potentially by correcting the abnormal bilateral asymmetries in central neuronal systems represented by symmetric responsiveness to tyramine [61].
Furthermore, the effect of lithium or imipramine (an antidepressant) treatment on paw preference was investigated in cats with the food-reaching task [62]. Interestingly, treating the cats with an intraperitoneal injection of lithium chloride (1 meq/kg) over 40 days resulted in decreased asymmetric paw preference (relative ambilateral), while imipramine (2 mg/kg) treatment increased asymmetric paw use [62]. Thus, studies analyzing laterality after lithium treatment rather point toward a decrease in asymmetric behavior or functioning.
In terms of ketamine treatment and asymmetries, researchers found that the intracerebral injection of ketamine induced a pronounced rotational asymmetry in rats [63]. However, with a dose of 50 mg/kg, the researchers used a substantially higher dose of ketamine [63]. In a study with patients suffering from treatment-resistant depression, neuronal effects of ketamine treatment on electroencephalography (EEG) alpha power were analyzed [64]. They found that patients responding well to ketamine treatment showed higher EEG alpha power but lower EEG alpha asymmetry and theta cordance post-treatment when compared to baseline values [64]. Given this reduced alpha asymmetry, one would have expected a reduced turning bias or smaller absolute LQs after ketamine treatment in mice.
In sum, both lithium and ketamine might have an impact on hemispheric lateralization. To our knowledge, this is the first study to investigate the effects of lithium and ketamine treatment and chronic stress exposure on lateralized behavior in rodents. Our study shows first evidence of the potential effects on turning asymmetry of both psychotropic drugs, even though they show weak and differential effects. Indeed, more studies are needed to further disentangle the direction of effect.
Note, this study holds some limitations. First, the statistical significance is weak and thus should be carefully interpreted, and independent replication in larger samples should be conducted. Then, we did not include a group of mice that was not exposed to stress as a non-stressed control group. Moreover, only male mice were included in this study, but differential effects are known in females [11,65]. Thus, both sexes should be included in future studies. However, females may respond differently to lower doses of, e.g., ketamine [9,65]. Therefore, a more complex study design may be needed. Additionally, in future studies, it would be of great interest to investigate the effects of clozapine treatment as a third compound frequently used in psychiatry treatment. Additionally, clozapine, lithium, and ketamine all show common features (e.g., anti-suicidal, drug resistance) and have a great impact on the course of illness.
5. Conclusions
Repeated lithium treatment prior to chronic stress exposure increases absolute turning bias in male mice compared to ketamine treatment. However, the direction of turning behavior is not affected. The results need further investigation to disentangle potential differential effects and resulting consequences for the animal. Applying computational tools to track behavioral differences allows for objective and reliable results. Moreover, several locomotor parameters, such as turning behavior, should be included in more preclinical studies as they provide a valuable measure for subtle stress-induced changes.
Author Contributions
Conceptualization, A.M. and N.F.; Investigation, A.M.; Software, H.M.; Formal Analysis, A.M., H.M. and S.O.; Validation, A.M., H.M., S.O. and N.F.; Supervision, N.F.; Writing—Original Draft Preparation, A.M.; Writing—Review and Editing, A.M., H.M., S.O. and N.F.; Visualization, A.M. and N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
We thank the whole experimental and molecular psychiatry lab for their support and fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Harbi, K.S. Treatment-Resistant Depression: Therapeutic Trends, Challenges, and Future Directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The Humanistic and Economic Burden of Treatment-Resistant Depression in Europe: A Cross-Sectional Study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhdanava, M.; Pilon, D.; Ghelerter, I.; Chow, W.; Joshi, K.; Lefebvre, P.; Sheehan, J.J. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J. Clin. Psychiatry 2021, 82, 20m13699. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an Antidepressant: Overview of Its Mechanisms of Action and Potential Predictive Biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef]
- Smith-Apeldoorn, S.Y.; Veraart, J.K.; Spijker, J.; Kamphuis, J.; Schoevers, R.A. Maintenance Ketamine Treatment for Depression: A Systematic Review of Efficacy, Safety, and Tolerability. Lancet Psychiatry 2022, 9, 907–921. [Google Scholar] [CrossRef]
- Molero, P.; Ramos-Quiroga, J.A.; Martin-Santos, R.; Calvo-Sánchez, E.; Gutiérrez-Rojas, L.; Meana, J.J. Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review. CNS Drugs 2018, 32, 411–420. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 2019, 176, 401–409. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Gonzalez, C.M.F.; Lucero, D.; Womble, P.D.; Abdel-Rahim, H.; DeVore, J.; Kunkel, M.N.; Quadlander, E.; Stinnett, M.; Boyette-Davis, J. The Effects of Ketamine on Sexual Behavior, Anxiety, and Locomotion in Female Rats. Pharmacol. Biochem. Behav. 2018, 165, 36–44. [Google Scholar] [CrossRef]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex Differences in the Rapid and the Sustained Antidepressant-like Effects of Ketamine in Stress-Naïve and “Depressed” Mice Exposed to Chronic Mild Stress. Neuroscience 2015, 290, 49–60. [Google Scholar] [CrossRef]
- Ng, L.H.L.; Huang, Y.; Han, L.; Chang, R.C.-C.; Chan, Y.S.; Lai, C.S.W. Ketamine and Selective Activation of Parvalbumin Interneurons Inhibit Stress-Induced Dendritic Spine Elimination. Transl. Psychiatry 2018, 8, 272. [Google Scholar] [CrossRef]
- Okine, T.; Shepard, R.; Lemanski, E.; Coutellier, L. Sex Differences in the Sustained Effects of Ketamine on Resilience to Chronic Stress. Front. Behav. Neurosci. 2020, 14, 581360. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Yen, J.Y.; Watson, B.O. Stress-Sensitive Antidepressant-like Effects of Ketamine in the Mouse Forced Swim Test. PLoS ONE 2019, 14, e0215554. [Google Scholar] [CrossRef] [PubMed]
- Viktorov, M.; Wilkinson, M.P.; Elston, V.C.E.; Stone, M.; Robinson, E.S.J. A Systematic Review of Studies Investigating the Acute Effects of N-Methyl-D-Aspartate Receptor Antagonists on Behavioural Despair in Normal Animals Suggests Poor Predictive Validity. Brain Neurosci. Adv. 2022, 6, 23982128221081644. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A Molecular Mechanism for the Effect of Lithium on Development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Marcus, S.R.; Nadiger, H.A.; Chandrakala, M.V.; Rao, T.I.; Sadasivudu, B. Acute and Short-Term Effects of Lithium on Glutamate Metabolism in Rat Brain. Biochem. Pharmacol. 1986, 35, 365–369. [Google Scholar] [CrossRef]
- Petzold, J.; Bauer, M.; Severus, E. Update-Lithium in the Long-Term Treatment of Bipolar Disorders. Fortschr. Neurol. Psychiatr. 2018, 86, 745–753. [Google Scholar] [CrossRef]
- Silva, R.; Mesquita, A.R.; Bessa, J.; Sousa, J.C.; Sotiropoulos, I.; Leão, P.; Almeida, O.F.X.; Sousa, N. Lithium Blocks Stress-Induced Changes in Depressive-like Behavior and Hippocampal Cell Fate: The Role of Glycogen-Synthase-Kinase-3beta. Neuroscience 2008, 152, 656–669. [Google Scholar] [CrossRef]
- Martinowich, K.; Schloesser, R.J.; Manji, H.K. Bipolar Disorder: From Genes to Behavior Pathways. J. Clin. Investig. 2009, 119, 726–736. [Google Scholar] [CrossRef]
- DelBello, M.P.; Adler, C.M.; Cerullo, M.A.; Fleck, D.E.; Strakowski, M.S. Bipolar Disorder. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Geddes, J.R.; Burgess, S.; Hawton, K.; Jamison, K.; Goodwin, G.M. Long-Term Lithium Therapy for Bipolar Disorder: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Psychiatry 2004, 161, 217–222. [Google Scholar] [CrossRef]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the Prevention of Suicide in Mood Disorders: Updated Systematic Review and Meta-Analysis. BMJ 2013, 346, f3646. [Google Scholar] [CrossRef]
- Volkmann, C.; Bschor, T.; Köhler, S. Lithium Treatment Over the Lifespan in Bipolar Disorders. Front. Psychiatry 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Bschor, P.D.T.; Bauer, M. Nebenwirkungs- und Risikoprofil von Lithium. Nervenarzt 2013, 84, 860–863. [Google Scholar] [CrossRef] [PubMed]
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium Toxicity Profile: A Systematic Review and Meta-Analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Giles, J.J.; Bannigan, J.G. Teratogenic and Developmental Effects of Lithium. Curr. Pharm. Des. 2006, 12, 1531–1541. [Google Scholar] [CrossRef]
- Dygalo, N.N.; Bannova, A.V.; Sukhareva, E.V.; Shishkina, G.T.; Ayriyants, K.A.; Kalinina, T.S. Effects of Short-Term Exposure to Lithium on Antiapoptotic Bcl-XL Protein Expression in Cortex and Hippocampus of Rats after Acute Stress. Biochem. Biokhimiia 2017, 82, 345–350. [Google Scholar] [CrossRef]
- Mundorf, A.; Knorr, A.; Mezö, C.; Klein, C.; Beyer, D.K.; Fallgatter, A.J.; Schwarz, M.; Freund, N. Lithium and Glutamine Synthetase: Protective Effects Following Stress. Psychiatry Res. 2019, 281, 112544. [Google Scholar] [CrossRef]
- Andersen, S.L.; Teicher, M.H. Stress, Sensitive Periods and Maturational Events in Adolescent Depression. Trends Neurosci. 2008, 31, 183–191. [Google Scholar] [CrossRef]
- Beyer, D.K.E.; Freund, N. Animal Models for Bipolar Disorder: From Bedside to the Cage. Int. J. Bipolar Disord. 2017, 5, 35. [Google Scholar] [CrossRef]
- Abraham, M.; Mundorf, A.; Brodmann, K.; Freund, N. Unraveling the Mystery of White Matter in Depression: A Translational Perspective on Recent Advances. Brain Behav. 2022, 12, e2629. [Google Scholar] [CrossRef]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Niven, J.E.; Frasnelli, E. Insights into the Evolution of Lateralization from the Insects. Prog. Brain Res. 2018, 238, 3–31. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. A Function for the Bicameral Mind. Cortex J. Devoted Study Nerv. Syst. Behav. 2020, 124, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Mundorf, A. Symmetry and Asymmetry in Biological Structures. Proc. Natl. Acad. Sci. USA 2022, 119, e2204881119. [Google Scholar] [CrossRef] [PubMed]
- Mundorf, A.; Peterburs, J.; Ocklenburg, S. Asymmetry in the Central Nervous System: A Clinical Neuroscience Perspective. Front. Syst. Neurosci. 2021, 15, 733898. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-Z.; Postema, M.C.; Guadalupe, T.; de Kovel, C.; Boedhoe, P.S.W.; Hoogman, M.; Mathias, S.R.; van Rooij, D.; Schijven, D.; Glahn, D.C.; et al. Mapping Brain Asymmetry in Health and Disease through the ENIGMA Consortium. Hum. Brain Mapp. 2022, 43, 167–181. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Peterburs, J.; Mundorf, A. Hemispheric Asymmetries in the Amygdala: A Comparative Primer. Prog. Neurobiol. 2022, 214, 102283. [Google Scholar] [CrossRef]
- Berretz, G.; Wolf, O.T.; Güntürkün, O.; Ocklenburg, S. Atypical Lateralization in Neurodevelopmental and Psychiatric Disorders: What Is the Role of Stress? Cortex J. Devoted Study Nerv. Syst. Behav. 2020, 125, 215–232. [Google Scholar] [CrossRef]
- Mundorf, A.; Matsui, H.; Ocklenburg, S.; Freund, N. Asymmetry of Turning Behavior in Rats Is Modulated by Early Life Stress. Behav. Brain Res. 2020, 393, 112807. [Google Scholar] [CrossRef]
- Mundorf, A.; Kubitza, N.; Hünten, K.; Matsui, H.; Juckel, G.; Ocklenburg, S.; Freund, N. Maternal Immune Activation Leads to Atypical Turning Asymmetry and Reduced DRD2 MRNA Expression in a Rat Model of Schizophrenia. Behav. Brain Res. 2021, 414, 113504. [Google Scholar] [CrossRef]
- Pfeifer, L.S.; Heyers, K.; Ocklenburg, S.; Wolf, O.T. Stress Research during the COVID-19 Pandemic and Beyond. Neurosci. Biobehav. Rev. 2021, 131, 581–596. [Google Scholar] [CrossRef]
- Mundorf, A.; Schmitz, J.; Hünten, K.; Fraenz, C.; Schlüter, C.; Genç, E.; Ocklenburg, S.; Freund, N. MORC1 Methylation and BDI Are Associated with Microstructural Features of the Hippocampus and Medial Prefrontal Cortex. J. Affect. Disord. 2021, 282, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Basbasse, Y.E.; Freund, N.; Ocklenburg, S. Paw Preferences in Mice and Rats: Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 127, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Soyman, E.; Yilmaz, G.D.; Canbeyli, R. Head-Turning Asymmetry: A Novel Lateralization in Rats Predicts Susceptibility to Behavioral Despair. Behav. Brain Res. 2018, 338, 47–50. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Borta, A. Analysis of Behavioral Asymmetries in the Elevated Plus-Maze and in the T-Maze. J. Neurosci. Methods 2005, 141, 251–260. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Berretz, G.; Packheiser, J.; Friedrich, P. Laterality 2020: Entering the next Decade. Laterality 2021, 26, 265–297. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. Laterality for the next Decade: Computational Ethology and the Search for Minimal Condition for Cognitive Asymmetry. Laterality 2021, 26, 303–306. [Google Scholar] [CrossRef]
- Anderson, D.J.; Perona, P. Toward a Science of Computational Ethology. Neuron 2014, 84, 18–31. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless Pose Estimation of User-Defined Body Parts with Deep Learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef]
- Mathis, M.W.; Mathis, A. Deep Learning Tools for the Measurement of Animal Behavior in Neuroscience. Curr. Opin. Neurobiol. 2020, 60, 1–11. [Google Scholar] [CrossRef]
- Nath, T.; Mathis, A.; Chen, A.C.; Patel, A.; Bethge, M.; Mathis, M.W. Using DeepLabCut for 3D Markerless Pose Estimation across Species and Behaviors. Nat. Protoc. 2019, 14, 2152–2176. [Google Scholar] [CrossRef]
- Hardin, A.; Schlupp, I. Using Machine Learning and DeepLabCut in Animal Behavior. Acta Ethologica 2022, 25, 125–133. [Google Scholar] [CrossRef]
- Josserand, M.; Rosa-Salva, O.; Versace, E.; Lemaire, B.S. Visual Field Analysis: A Reliable Method to Score Left and Right Eye Use Using Automated Tracking. Behav. Res. Methods 2022, 54, 1715–1724. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Güntürkün, O. Stress and Laterality-The Comparative Perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mundorf, A.; Ocklenburg, S. The Clinical Neuroscience of Lateralization; Routledge: London, UK, 2021; ISBN 978-1-00-308250-7. [Google Scholar]
- Silberman, E.K.; Weingartner, H. Hemispheric Lateralization of Functions Related to Emotion. Brain Cogn. 1986, 5, 322–353. [Google Scholar] [CrossRef]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymmetric Tail-Wagging Responses by Dogs to Different Emotive Stimuli. Curr. Biol. 2007, 17, R199–R201. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Frasnelli, E.; Guo, K.; Barber, A.; Wilkinson, A.; Mills, D.S. Is There an Association between Paw Preference and Emotionality in Pet Dogs? Animals 2022, 12, 1153. [Google Scholar] [CrossRef]
- Farmer, K.; Krueger, K.; Byrne, R.W. Visual Laterality in the Domestic Horse (Equus caballus) Interacting with Humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef]
- Tondo, L.; Alda, M.; Bauer, M.; Bergink, V.; Grof, P.; Hajek, T.; Lewitka, U.; Licht, R.W.; Manchia, M.; Müller-Oerlinghausen, B.; et al. Clinical Use of Lithium Salts: Guide for Users and Prescribers. Int. J. Bipolar Disord. 2019, 7, 16. [Google Scholar] [CrossRef]
- Fanciullacci, M.; Pietrini, U.; Boccuni, M.; Gatto, G.; Cangi, F. Does Lithium Balance the Neuronal Bilateral Asymmetries in Cluster Headache? Cephalalgia Int. J. Headache 1983, 3 (Suppl. S1), 85–87. [Google Scholar] [CrossRef]
- Tan, U.; Kara, I.; Tan, S. Lithium and Imipramin Effects on Paw Preference in Cats. Int. J. Neurosci. 1990, 52, 25–28. [Google Scholar] [CrossRef]
- Lebedev, S.V.; Petrov, S.V.; Blinov, D.V.; Lazarenko, I.P.; Chekhonin, V.P. Ketamine-Induced Rotational Asymmetry in Evaluation of Motor Disturbances in Rats with Middle Cerebral Artery Occlusion. Bull. Exp. Biol. Med. 2003, 135, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Lin, C.-T.; Ding, W.; Chen, M.-H.; Li, C.-T.; Su, T.-P. Identifying Ketamine Responses in Treatment-Resistant Depression Using a Wearable Forehead EEG. IEEE Trans. Biomed. Eng. 2019, 66, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Cortés, M.; Grace, A.A. Antidepressant Effects of Ketamine on Depression-Related Phenotypes and Dopamine Dysfunction in Rodent Models of Stress. Behav. Brain Res. 2020, 379, 112367. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).