Cobalt Oxide on a Nanoporous TUD-1 Catalyst for Methylene Blue Dye Interaction DFT Studies and Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Characterization
2.4. Computational Methods
2.5. Fenton Oxidation Reaction
3. Results and Discussion
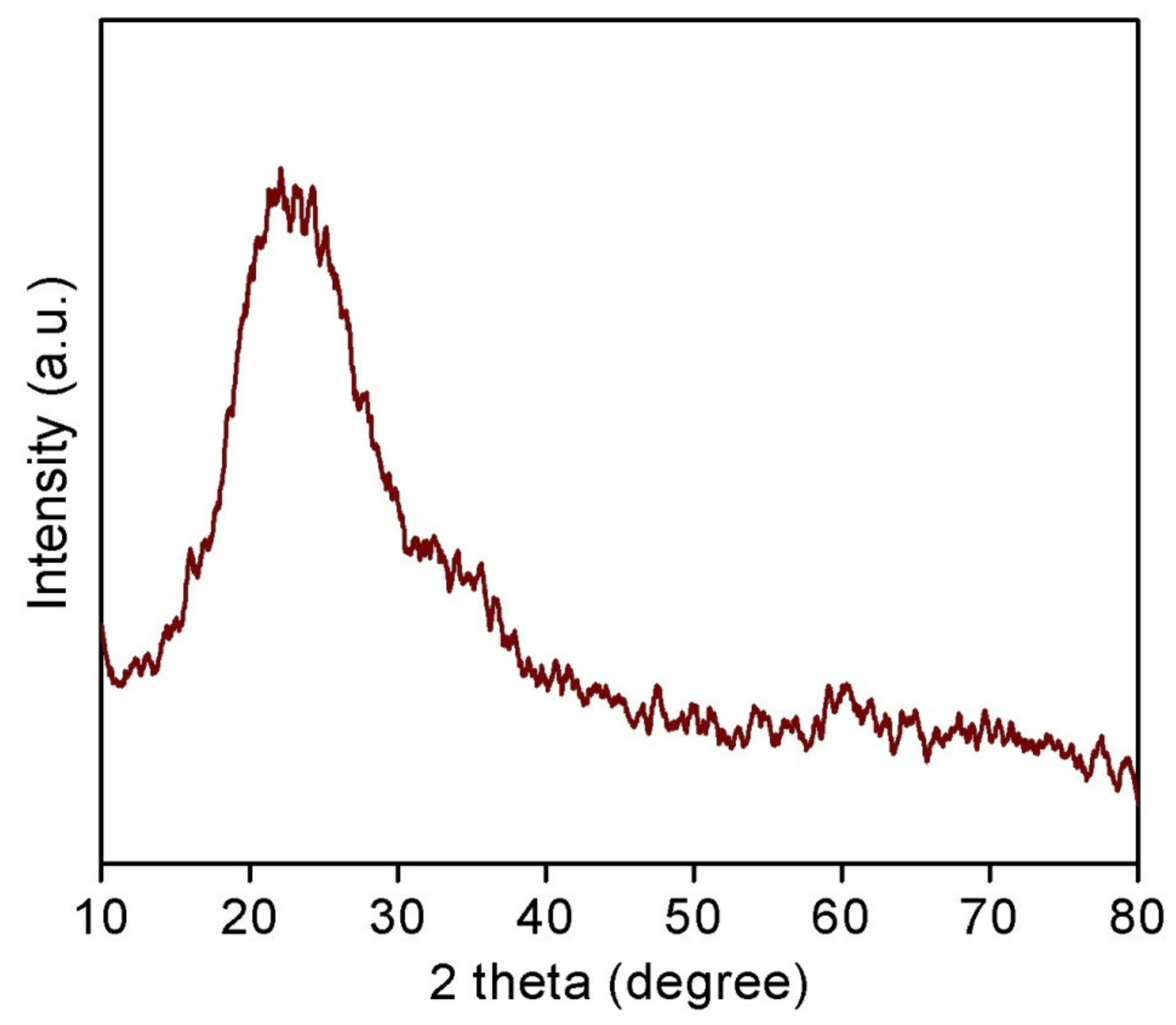
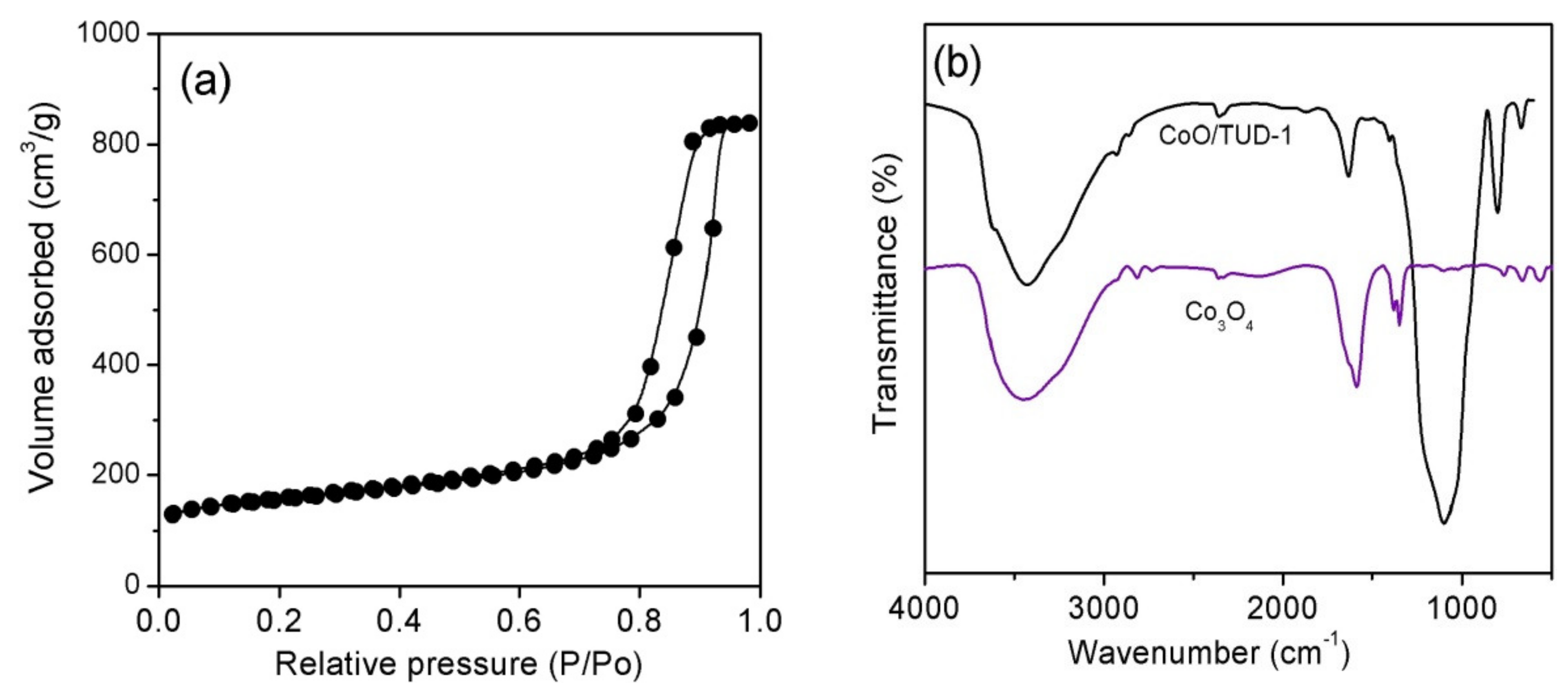
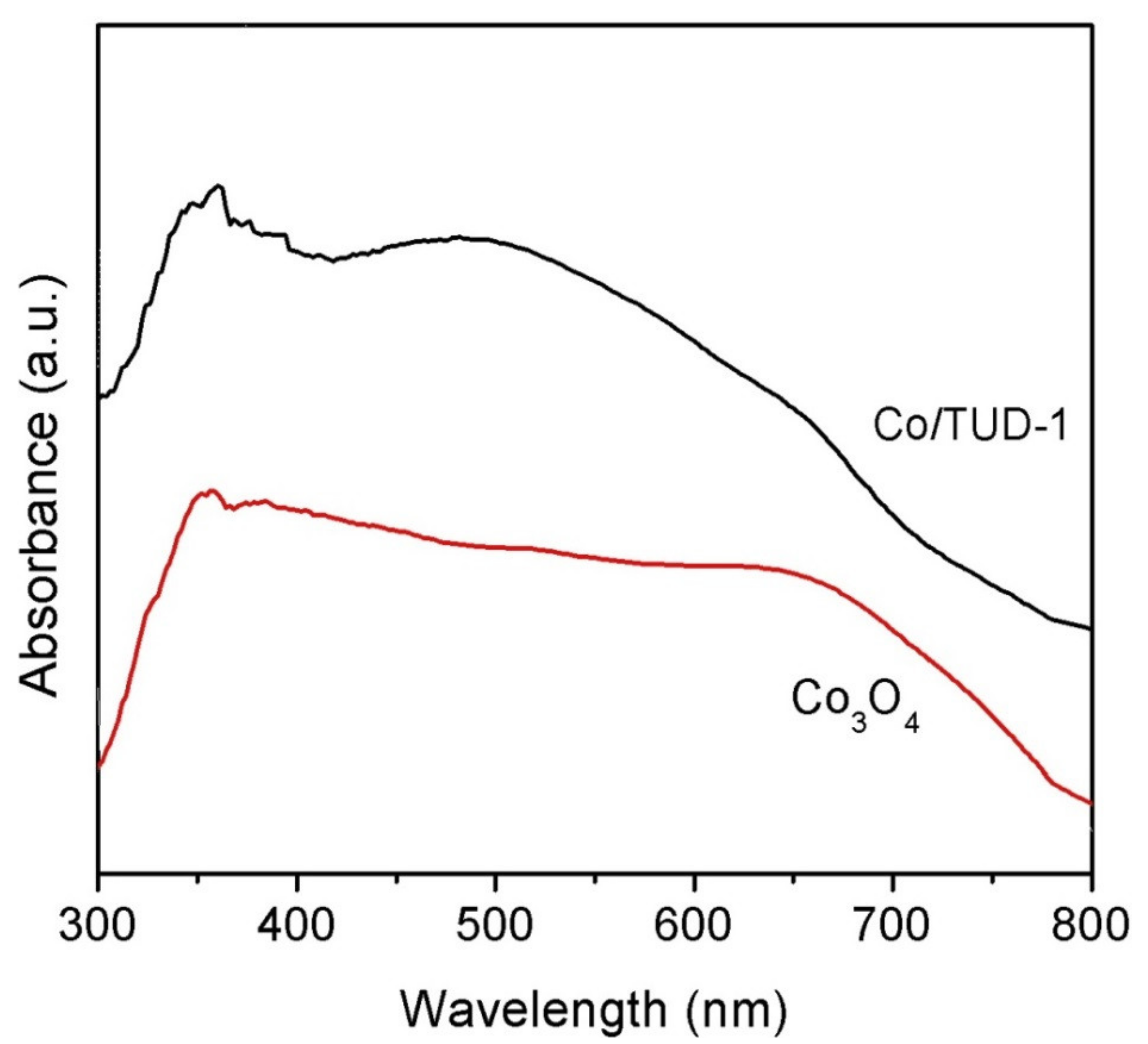
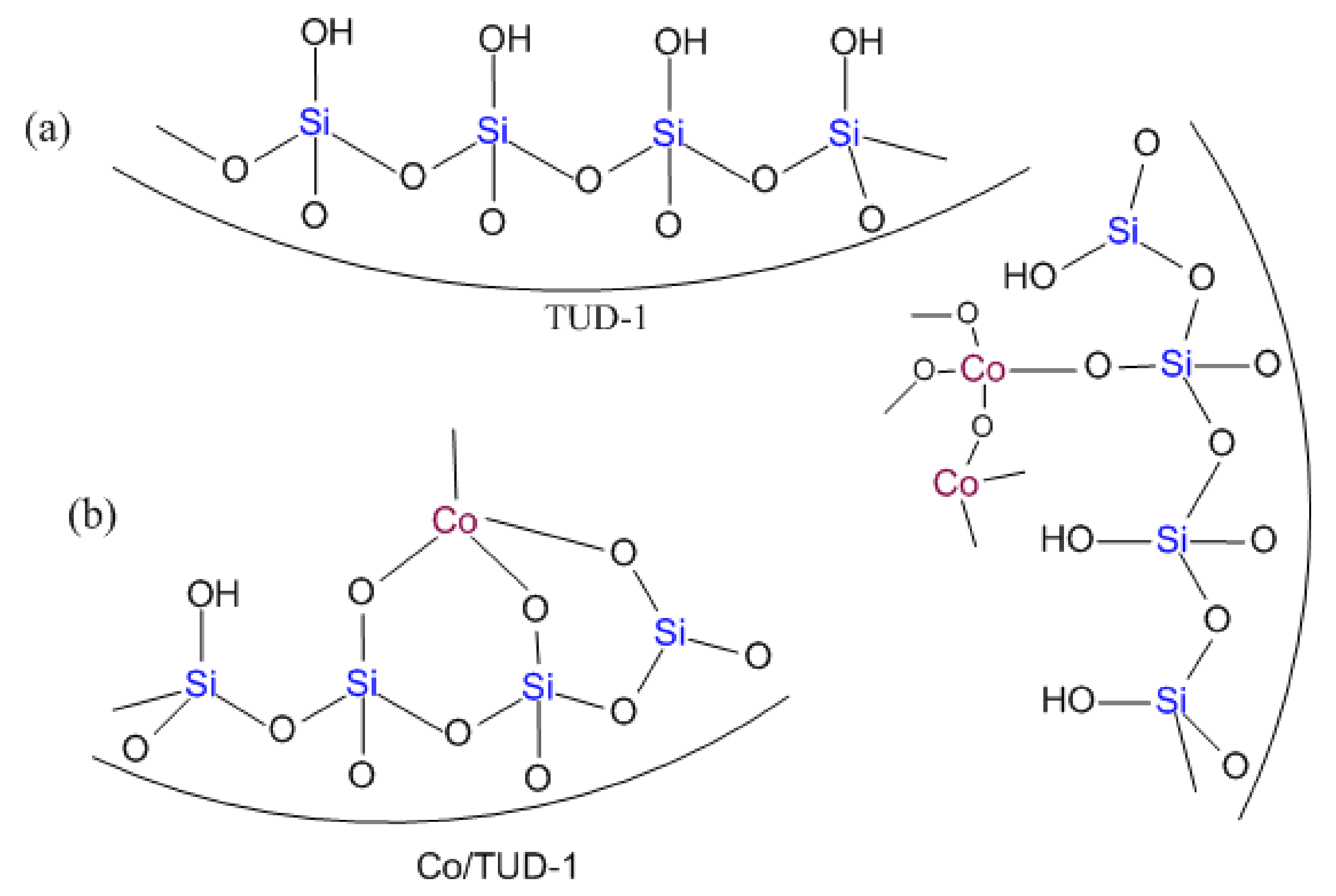
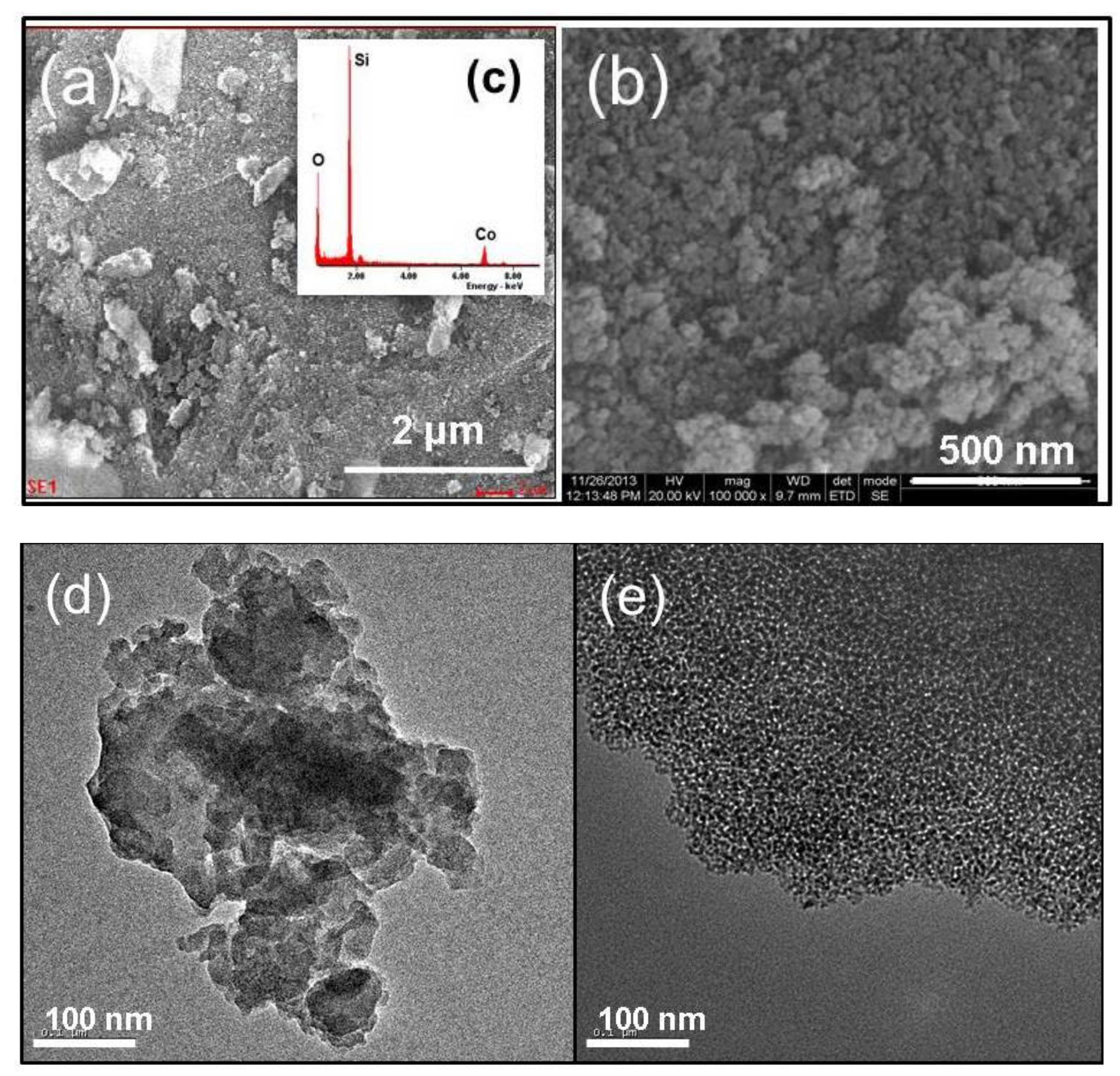
3.1. Characterization of CoO/TUD-1 Material
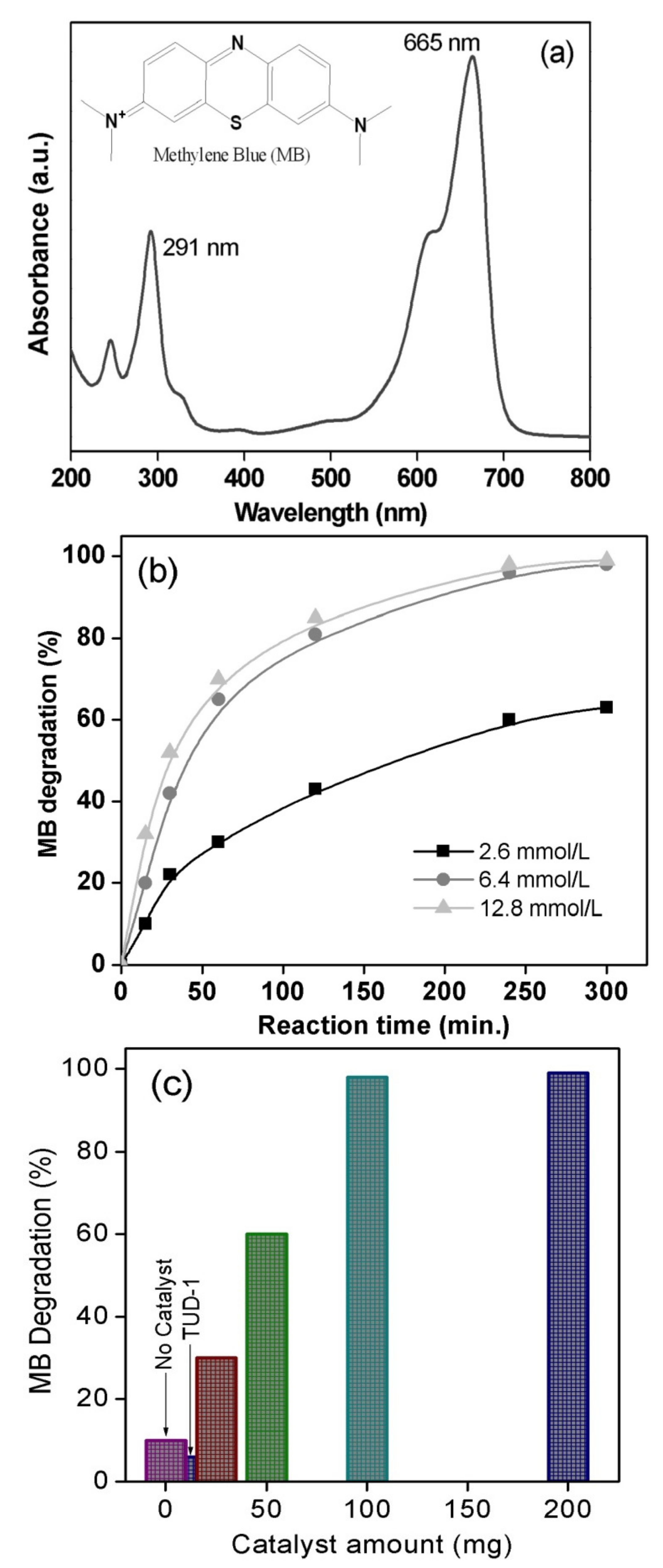
3.2. Methyl Blue Dye Degradation over CoO/TUD-1
3.2.1. About the Model
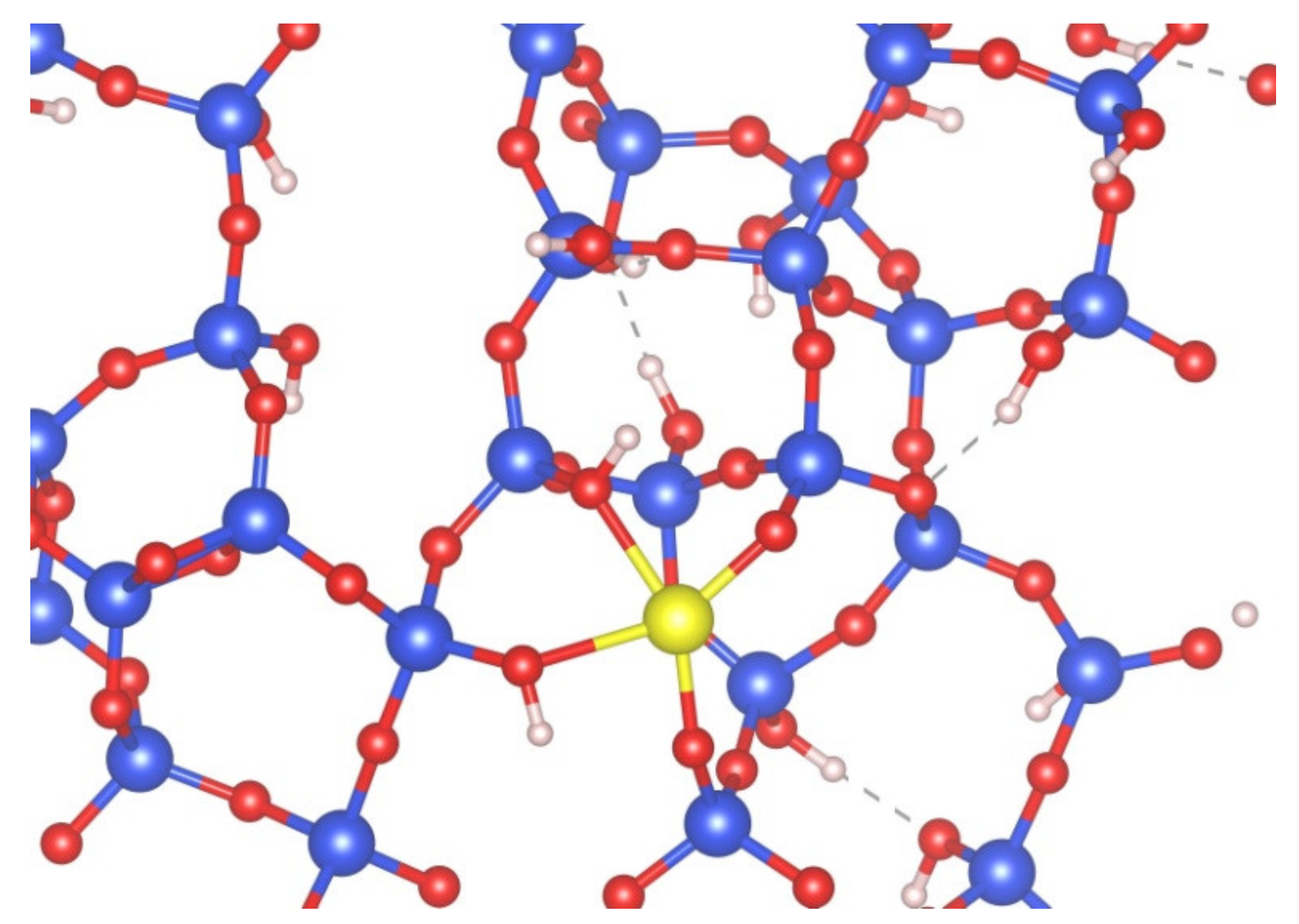
3.2.2. Adsorption of Single Co Atom
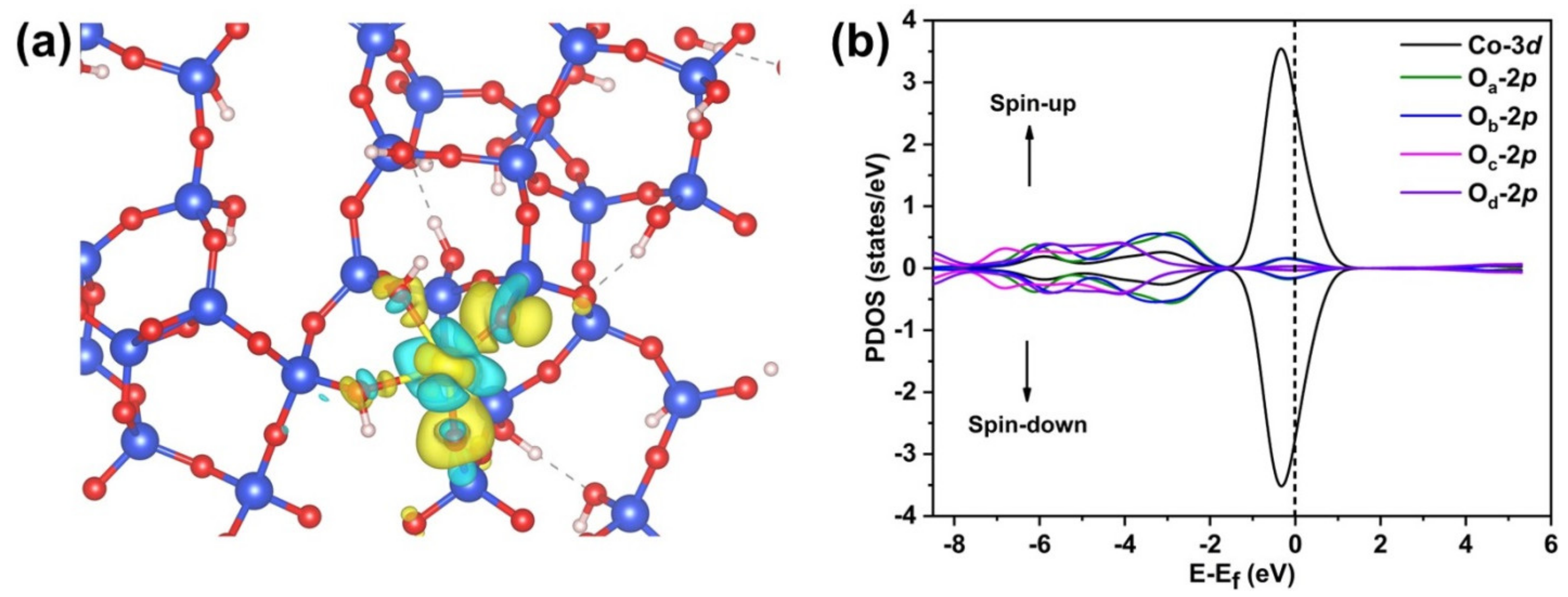
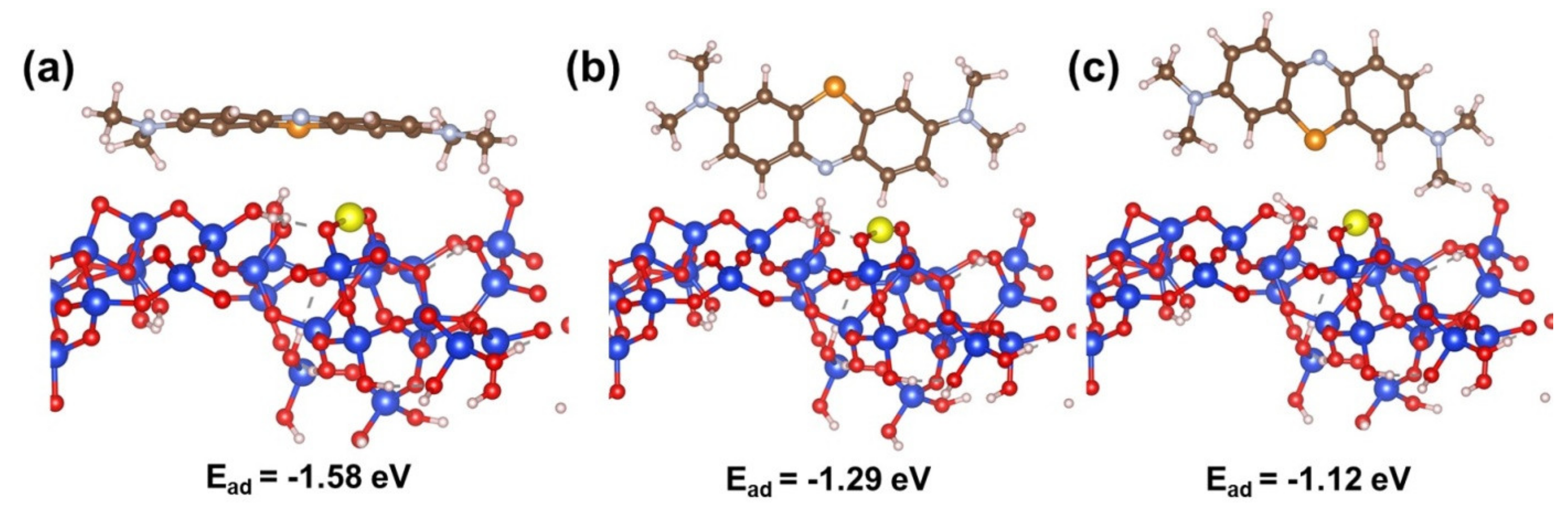
3.2.3. Adsorption of Methylene Blue on CoO/TUD-1
3.2.4. Degradation of Methylene Blue on CoO/TUD-1 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronym and Abbreviations
| TUD-1 | Technische Universiteit Delft |
| DFT | Density Functional Theory |
| MB | methylene blue |
| TEOS | Tetraethyl orthosilicate |
| TEA | Triethanolamine |
| TEAOH | Tetraethyl ammonium hydroxide |
| VASP | Vienna ab initio simulation package |
| PAW | Projector augmented wave |
| PBE | Perdew−Burke−Ernzerhof |
| EDD | Electron density differences |
| EF | Fermi level |
| Ead | adsorption energy |
References
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment, Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sangpour, P.; Hashemi, F.; Moshfegh, A.Z. Photoenhanced degradation of methylene blue on cosputtered M:TiO2 (M = Au, Ag, Cu) nanocomposite systems: A comparative study. J. Phys. Chem. C 2010, 114, 13955–13961. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Zhou, L.; Bao, C.; Zhu, H.; Zhang, Y. Synthesis and application of rGO/CoFe2O4 composite for catalytic degradation of methylene blue on heterogeneous Fenton-like oxidation. J. Taiwan Inst. Chem. Eng. 2016, 67, 484–494. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Ong, S.A.; Toorisaka, E.; Hirata, M.; Hano, T. Biodegradation of redox dye Methylene Blue by up-flow anaerobic sludge blanket reactor. J. Hazard. Mater. 2005, 124, 88–94. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced catalytic degradation of methylene blue by A-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B Environ. 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Cheng, S.; Oatley, D.L.; Williams, P.M.; Wright, C.J. Characterisation and application of a novel positively charged nanofiltration membrane for the treatment of textile industry wastewaters. Water Res. 2012, 46, 33–42. [Google Scholar] [CrossRef]
- Kim, S.; Yu, M.; Yoon, Y. Fouling and Retention Mechanisms of Selected Cationic and Anionic Dyes in a Ti3C2Tx MXene-Ultrafiltration Hybrid System. ACS Appl. Mater. Interfaces 2020, 12, 16557–16565. [Google Scholar] [CrossRef]
- Mudliar, R.; Umare, S.S.; Ramteke, D.S.; Wate, S.R. Energy efficient—Advanced oxidation process for treatment of cyanide containing automobile industry wastewater. J. Hazard. Mater. 2009, 164, 1474–1479. [Google Scholar] [CrossRef]
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Ferrocene-catalyzed heterogeneous fenton-like degradation of methylene blue: Influence of initial solution pH. Ind. Eng. Chem. Res. 2014, 53, 6334–6340. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B Environ. 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Lin, P.; Cui, Z.; Chu, C.; Shen, B. Investigation of FePC amorphous alloys with self-renewing behaviour for highly efficient decolorization of methylene blue. J. Mater. Chem. A 2018, 6, 10686–10699. [Google Scholar] [CrossRef]
- Liang, S.X.; Wang, X.; Zhang, W.; Liu, Y.; Wang, W.; Zhang, L.C. Selective laser melting manufactured porous Fe-based metallic glass matrix composite with remarkable catalytic activity and reusability. Appl. Mater. Today 2020, 19, 100543. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.; Zhang, W.; Wang, W.; Lu, J.; Zhang, L.C. Metallic Glasses: Compelling Rejuvenated Catalytic Performance in Metallic Glasses. Adv. Mater. 2018, 30, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kirchon, A.; Zhang, P.; Li, J.; Joseph, E.A.; Chen, W.; Zhou, H.C. Effect of Isomorphic Metal Substitution on the Fenton and Photo-Fenton Degradation of Methylene Blue Using Fe-Based Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Cheng, H.; Ma, J.; Qin, Y.; Kong, Y.; Komarneni, S. Catalytic degradation of methylene blue through activation of bisulfite with CoO nanoparticles. Sep. Purif. Technol. 2020, 239, 116561. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043. [Google Scholar] [CrossRef]
- Hu, P.; Long, M.; Environ, A.C.B. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Shukla, P.R.; Wang, S.; Sun, H.; Ang, H.M.; Tadé, M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B Environ. 2010, 100, 529–534. [Google Scholar] [CrossRef]
- Stoyanova, M.; Slavova, I.; Christoskova, S.; Ivanova, V. Catalytic performance of supported nanosized cobalt and iron–cobalt mixed oxides on MgO in oxidative degradation of Acid Orange 7 azo dye with peroxymonosulfate. Appl. Catal. A Gen. 2014, 476, 121–132. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Ramanathan, A.; Maschmeyer, T. Co-TUD-1: A Ketone-Selective Catalyst for Cyclohexane Oxidation. Chem. Eur. J. 2006, 1782–1789. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Mul, G.; Wei, W.; Anand, R.; Hanefeld, U. Fe, Co and Cu-incorporated TUD-1: Synthesis, characterization and catalytic performance in N2O decomposition and cyclohexane oxidation. Catal. Today 2005, 110, 264–271. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Karthikeyan, S.; Maheswari, R.; Lee, A.F.; Ramanathan, A. Fenton-like degradation of Bisphenol A catalyzed by mesoporous Cu/TUD-1. Appl. Surf. Sci. 2017, 393, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Pachamuthu, M.P.; Rajalakshmi, R. RSC Advances Direct glycol assisted synthesis of an amorphous mesoporous silicate with framework incorporated Co2+: Characterization and catalytic application in ethylbenzene oxidation. RSC Adv. 2014, 4, 29909–29916. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter. 2009, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Li, X.F.; Wang, W.M.; Lin, H.C.; Zhang, L.C. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B Environ. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Miao, F.; Wang, Q.; Di, S.; Yun, L.; Zhou, J.; Shen, B. Enhanced dye degradation capability and reusability of Fe-based amorphous ribbons by surface activation. J Material Sci. Tech. 2020, 53, 163–173. [Google Scholar] [CrossRef]
- Anand, R.; Hamdy, M.S.; Hanefeld, U.; Maschmeyer, T. Liquid-phase oxidation of cyclohexane over Co-TUD-1. Catal. Lett. 2006, 95, 113–117. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Shanthi, K.; Luque, R.; Ramanathan, A. SnTUD-1: A Solid Acid Catalyst for Three Component coupling reactions at room temperature. Green Chem. 2013, 15, 2158–2166. [Google Scholar] [CrossRef]
- Islam, M.M.; Costa, D.; Calatayud, M.; Tielens, F. Characterization of Supported Vanadium Oxide Species on Silica: A Periodic DFT Investigation. J. Phys. Chem. C. 2009, 113, 10740–10746. [Google Scholar] [CrossRef]
- Wojtaszek, A.; Sobczak, I.; Ziolek, M.; Tielens, F. Gold Grafted to Mesoporous Silica Surfaces, a Molecular Picture. J. Phys. Chem. C 2009, 113, 13855–13859. [Google Scholar] [CrossRef]
- Tranca, D.C.; Wojtaszek-Gurdak, A.; Ziolek, M.; Tielens, F. Supported and inserted monomeric niobium oxide species on/in silica: A molecular picture. Phys. Chem. Chem. Phys. 2015, 17, 22402–22411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siodla, T.; Sobczak, I.; Ziolek, M.; Tielens, F. Theoretical and experimental insight into zinc loading on mesoporous silica. Microporous Mesoporous Mater. 2018, 256, 199–205. [Google Scholar] [CrossRef]
- Handzlik, J.; Grybos, R.; Tielens, F. Structure of Monomeric Chromium(VI) Oxide Species Supported on Silica: Periodic and Cluster DFT Studies. J. Phys. Chem. C 2013, 117, 8138–8149. [Google Scholar] [CrossRef]
- Vankimmenade, E.; Kuiper, A.; Tamminga, Y.; Thune, P.; Niemantsverdriet, J. A surface science model for the Phillips ethylene polymerization catalyst: Thermal activation and polymerization activity. J. Catal. 2004, 223, 134–141. [Google Scholar] [CrossRef]
- Hu, B.; Schweitzer, N.M.; Das, U.; Kim, H.; Niklas, J.; Poluektov, O.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; Hock, A.S.; et al. Selective propane dehydrogenation with single-site Coll on SiO2 by a non-redox mechanism. J. Catal. 2015, 322, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Vandarkuzhali, S.A.A.; Karthikeyan, S.; Viswanathan, B.; Pachamuthu, M.P. Arachis hypogaea derived activated carbon/Pt catalyst: Reduction of organic dyes. Surf. Interfaces 2018, 13, 101–111. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachamuthu, M.P.; Baskaran, S.; Manivannan, C.; Kishore, S.C.; Bellucci, S.; Boopathy, R. Cobalt Oxide on a Nanoporous TUD-1 Catalyst for Methylene Blue Dye Interaction DFT Studies and Degradation. Symmetry 2021, 13, 1754. https://doi.org/10.3390/sym13091754
Pachamuthu MP, Baskaran S, Manivannan C, Kishore SC, Bellucci S, Boopathy R. Cobalt Oxide on a Nanoporous TUD-1 Catalyst for Methylene Blue Dye Interaction DFT Studies and Degradation. Symmetry. 2021; 13(9):1754. https://doi.org/10.3390/sym13091754
Chicago/Turabian StylePachamuthu, Muthusamy Poomalai, Sambath Baskaran, Chandrakumar Manivannan, Somasundaram Chandra Kishore, Stefano Bellucci, and Ramasamy Boopathy. 2021. "Cobalt Oxide on a Nanoporous TUD-1 Catalyst for Methylene Blue Dye Interaction DFT Studies and Degradation" Symmetry 13, no. 9: 1754. https://doi.org/10.3390/sym13091754
APA StylePachamuthu, M. P., Baskaran, S., Manivannan, C., Kishore, S. C., Bellucci, S., & Boopathy, R. (2021). Cobalt Oxide on a Nanoporous TUD-1 Catalyst for Methylene Blue Dye Interaction DFT Studies and Degradation. Symmetry, 13(9), 1754. https://doi.org/10.3390/sym13091754







