Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Apparatus
2.2. Experimental Apparatus and Behavioral Tasks
2.3. Data Analysis
3. Results
3.1. The Assumptions Underlying the Race Model Were All Satisfied
3.2. Reactive and Proactive Inhibitory Control Do Not Differ According to Handedness
3.3. Correlations between the Laterality Quotient Scores and the Behavioral Parameters of the Go-Only and Stop-Signal Tasks
4. Discussion
4.1. Inhibition, Handedness, and Laterality
4.2. RTs, MTs, Handedness, and Laterality
4.3. The Left Hemisphere Role’s in Actions Planning
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabella, G. Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front. Syst. Neurosci. 2014, 8, 206. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, G. Inhibitory control and impulsive responses in neurodevelopmental disorders. Dev. Med. Child Neurol. 2021, 63, 520–526. [Google Scholar] [CrossRef]
- Wostmann, N.M.; Aichert, D.S.; Costa, A.; Rubia, K.; Moller, H.J.; Ettinger, U. Reliability and plasticity of response inhibition and interference control. Brain Cogn. 2013, 81, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Mirabella, G.; Pani, P.; Ferraina, S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. J. Neurophysiol. 2011, 106, 1454–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattia, M.; Pani, P.; Mirabella, G.; Costa, S.; Del Giudice, P.; Ferraina, S. Heterogeneous attractor cell assemblies for motor planning in premotor cortex. J. Neurosci. 2013, 33, 11155–11168. [Google Scholar] [CrossRef] [PubMed]
- Mattia, M.; Spadacenta, S.; Pavone, L.; Quarato, P.; Esposito, V.; Sparano, A.; Sebastiano, F.; Di Gennaro, G.; Morace, R.; Cantore, G.; et al. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements. Front. Neuroeng. 2012, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coxon, J.P.; Stinear, C.M.; Byblow, W.D. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 2006, 95, 3371–3383. [Google Scholar] [CrossRef] [PubMed]
- Swick, D.; Ashley, V.; Turken, A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, G.; Iaconelli, S.; Romanelli, P.; Modugno, N.; Lena, F.; Manfredi, M.; Cantore, G. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients. Cereb. Cortex 2012, 22, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, G.; Iaconelli, S.; Modugno, N.; Giannini, G.; Lena, F.; Cantore, G. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson’s patients. PLoS ONE 2013, 8, e62793. [Google Scholar] [CrossRef] [Green Version]
- Mancini, C.; Modugno, N.; Santilli, M.; Pavone, L.; Grillea, G.; Morace, R.; Mirabella, G. Unilateral Stimulation of Subthalamic Nucleus Does Not Affect Inhibitory Control. Front. Neurol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- van den Wildenberg, W.P.; van Boxtel, G.J.; van der Molen, M.W.; Bosch, D.A.; Speelman, J.D.; Brunia, C.H. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J. Cogn. Neurosci. 2006, 18, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, N.C.; Cai, W.; Conner, C.R.; Pieters, T.A.; Claffey, M.P.; George, J.S.; Aron, A.R.; Tandon, N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage 2012, 59, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- van den Wildenberg, W.P.M.; van Wouwe, N.C.; Ridderinkhof, K.R.; Neimat, J.S.; Elias, W.J.; Bashore, T.R.; Wylie, S.A. Deep-brain stimulation of the subthalamic nucleus improves overriding motor actions in Parkinson’s disease. Behav. Brain Res. 2021, 402, 113124. [Google Scholar] [CrossRef]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-Stage Parkinson’s Patients Show Selective Impairment in Reactive But Not Proactive Inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef]
- Bundy, D.T.; Leuthardt, E.C. The Cortical Physiology of Ipsilateral Limb Movements. Trends Neurosci. 2019, 42, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Cabibel, V.; Hordacre, B.; Perrey, S. Implication of the ipsilateral motor network in unilateral voluntary muscle contraction: The cross-activation phenomenon. J. Neurophysiol. 2020, 123, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Mazzocchio, R.; Dambrosia, J.; Murase, N.; Olivier, E.; Cohen, L.G. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb. Cortex 2005, 15, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp. Brain Res. 1999, 124, 520–524. [Google Scholar] [CrossRef]
- Carson, R.G. Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J. Physiol. 2020, 598, 4781–4802. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.U.; Röricht, S.; Gräfin von Einsiedel, H.; Kruggel, F.; Weindl, A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 1995, 118, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Sunderland, A.; Gowland, P.A. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage 2005, 24, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hutchinson, S.; Schlaug, G.; Pascual-Leone, A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage 2003, 20, 2259–2270. [Google Scholar] [PubMed]
- Bundy, D.T.; Szrama, N.; Pahwa, M.; Leuthardt, E.C. Unilateral, 3D Arm Movement Kinematics Are Encoded in Ipsilateral Human Cortex. J. Neurosci. 2018, 38, 10042–10056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, A.; Georgesco, M. Interhemispheric facilitation and inhibition studied in man with double magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 395–403. [Google Scholar]
- Muellbacher, W.; Facchini, S.; Boroojerdi, B.; Hallett, M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin. Neurophysiol. 2000, 111, 344–349. [Google Scholar] [CrossRef]
- Perez, M.A.; Cohen, L.G. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J. Neurosci. 2008, 28, 5631–5640. [Google Scholar] [CrossRef]
- Waters, S.; Wiestler, T.; Diedrichsen, J. Cooperation Not Competition: Bihemispheric tDCS and fMRI Show Role for Ipsilateral Hemisphere in Motor Learning. J. Neurosci. 2017, 37, 7500–7512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Ashe, J.; Georgopoulos, A.P.; Merkle, H.; Ellermann, J.M.; Menon, R.S.; Ogawa, S.; Ugurbil, K. Functional imaging of human motor cortex at high magnetic field. J. Neurophysiol. 1993, 69, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, A.; Gut, M.; Binder, M.; Forsberg, L.; Rymarczyk, K.; Urbanik, A. Switching handedness: fMRI study of hand motor control in right-handers, left-handers and converted left-handers. Acta Neurobiol. Exp. 2012, 72, 439–451. [Google Scholar]
- Buetefisch, C.M.; Revill, K.P.; Shuster, L.; Hines, B.; Parsons, M. Motor demand-dependent activation of ipsilateral motor cortex. J. Neurophysiol. 2014, 112, 999–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276–291. [Google Scholar] [CrossRef]
- Faurie, C.; Raymond, M. Handedness frequency over more than ten thousand years. Proc. Biol. Sci. 2004, 271, S43–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corballis, M.C. From mouth to hand: Gesture, speech, and the evolution of right-handedness. Behav. Brain Sci. 2003, 26, 199–208; discussion 208–160. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadalupe, T.; Willems, R.M.; Zwiers, M.P.; Arias Vasquez, A.; Hoogman, M.; Hagoort, P.; Fernandez, G.; Buitelaar, J.; Franke, B.; Fisher, S.E.; et al. Differences in cerebral cortical anatomy of left- and right-handers. Front. Psychol. 2014, 5, 261. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, A.; Ng, M.; Al Omran, Y.; Alfaro-Almagro, F.; McCarthy, P.; Marchini, J.; Bennett, D.L.; Smith, S.; Douaud, G.; Furniss, D. Handedness, language areas and neuropsychiatric diseases: Insights from brain imaging and genetics. Brain 2019, 142, 2938–2947. [Google Scholar] [CrossRef] [Green Version]
- Amunts, K.; Schlaug, G.; Schleicher, A.; Steinmetz, H.; Dabringhaus, A.; Roland, P.E.; Zilles, K. Asymmetry in the human motor cortex and handedness. Neuroimage 1996, 4, 216–222. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Klöppel, S.; Rivière, D.; Perrot, M.; Frackowiak, R.; Siebner, H.; Mangin, J.-F. The effect of handedness on the shape of the central sulcus. Neuroimage 2012, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Deus, J.; Losilla, J.M.; Capdevila, A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology 1999, 52, 1038–1043. [Google Scholar] [CrossRef]
- Joliot, M.; Tzourio-Mazoyer, N.; Mazoyer, B. Intra-hemispheric intrinsic connectivity asymmetry and its relationships with handedness and language Lateralization. Neuropsychologia 2016, 93, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, R.; Yamada, K.; Kinomura, S.; Yamaguchi, T.; Matsui, H.; Yoshioka, S.; Fukuda, H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993, 623, 33–40. [Google Scholar] [CrossRef]
- Solodkin, A.; Hlustik, P.; Noll, D.C.; Small, S.L. Lateralization of motor circuits and handedness during finger movements. Eur. J. Neurol. 2001, 8, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Pool, E.M.; Rehme, A.K.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Functional resting-state connectivity of the human motor network: Differences between right- and left-handers. Neuroimage 2015, 109, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civardi, C.; Cavalli, A.; Naldi, P.; Varrasi, C.; Cantello, R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin. Neurophysiol. 2000, 111, 624–629. [Google Scholar] [CrossRef]
- Hiraoka, K.; Igawa, K.; Kashiwagi, M.; Nakahara, C.; Oshima, Y.; Takakura, Y. The laterality of stop and go processes of the motor response in left-handed and right-handed individuals. Laterality 2018, 23, 51–66. [Google Scholar] [CrossRef]
- Serrien, D.J.; Sovijarvi-Spape, M.M. Cognitive control of response inhibition and switching: Hemispheric lateralization and hand preference. Brain Cogn. 2013, 82, 283–290. [Google Scholar] [CrossRef]
- Mirabella, G.; Pani, P.; Ferraina, S. Context influences on the preparation and execution of reaching movements. Cogn. Neuropsychol. 2008, 25, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Edlin, J.M.; Leppanen, M.L.; Fain, R.J.; Hackländer, R.P.; Hanaver-Torrez, S.D.; Lyle, K.B. On the use (and misuse?) of the Edinburgh Handedness Inventory. Brain Cogn. 2015, 94, 44–51. [Google Scholar] [CrossRef]
- Online Resources: Open Science Framework Platform. Available online: https://osf.io/wrkah/ (accessed on 16 August 2021).
- Cortex and Cortex Explorer: Real-Time Software and Data Analysis Tools. Available online: https://www.nimh.nih.gov/research/research-conducted-at-nimh/research-areas/clinics-and-labs/ln/shn/software-projects.shtml (accessed on 16 August 2021).
- Poffenberger, A.T. Reaction Time to Retinal Stimulation, with Special Reference to the Time Lost in Conduction through Nerve Centers; The Science Press: New York, NY, USA, 1912. [Google Scholar]
- Alberto Marzi, C. Asymmetry of interhemispheric communication. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Levitt, S.; Gutin, B. Multiple choice reaction time and movement time during physical exertion. Res. Q. 1971, 42, 405–410. [Google Scholar] [CrossRef]
- Logan, G.D. Attention, automaticity and the ability to stop a speeded choice response. In Attention and Performance IX; Long, J., Baddley, A., Eds.; Erlbaum: Hillsdale, NJ, USA, 1981; pp. 205–222. [Google Scholar]
- Mirabella, G.; Pani, P.; Pare, M.; Ferraina, S. Inhibitory control of reaching movements in humans. Exp. Brain Res. 2006, 174, 240–255. [Google Scholar] [CrossRef]
- Boucher, L.; Stuphorn, V.; Logan, G.D.; Schall, J.D.; Palmeri, T.J. Stopping eye and hand movements: Are the processes independent? Percept. Psychophys. 2007, 69, 785–801. [Google Scholar] [CrossRef]
- Verbruggen, F.; Chambers, C.D.; Logan, G.D. Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychol. Sci. 2013, 24, 352–362. [Google Scholar] [CrossRef] [Green Version]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 16 August 2021).
- Savage, C.R.; Thomas, D.G. Information processing and interhemispheric transfer in left- and right-handed adults. Int. J. Neurosci. 1993, 71, 201–219. [Google Scholar] [CrossRef]
- Klöppel, S.; Vongerichten, A.; van Eimeren, T.; Frackowiak, R.S.; Siebner, H.R. Can left-handedness be switched? Insights from an early switch of handwriting. J. Neurosci. 2007, 27, 7847–7853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Factors influencing the latency of simple reaction time. Front. Hum. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Velay, J.L.; Benoit-Dubrocard, S. Hemispheric asymmetry and interhemispheric transfer in reaching programming. Neuropsychologia 1999, 37, 895–903. [Google Scholar] [CrossRef]
- Goodin, D.S.; Aminoff, M.J.; Ortiz, T.A.; Chequer, R.S. Response times and handedness in simple reaction-time tasks. Exp. Brain Res. 1996, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Marzi, C.A.; Bisiacchi, P.; Nicoletti, R. Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 1991, 29, 1163–1177. [Google Scholar] [CrossRef]
- Rabbitt, P. Hand dominance, attention, and the choice between responses. Q. J. Exp. Psychol. 1978, 30, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, S.; Boulinguez, P. Manual asymmetries in the directional coding of reaching: Further evidence for hemispatial effects and right hemisphere dominance for movement planning. Exp. Brain Res. 2002, 147, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Carson, R.G.; Chua, R.; Goodman, D.; Byblow, W.D.; Elliott, D. The preparation of aiming movements. Brain Cogn. 1995, 28, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Hodges, N.J.; Lyons, J.; Cockell, D.; Reed, A.; Elliott, D. Hand, space and attentional asymmetries in goal-directed manual aiming. Cortex 1997, 33, 251–269. [Google Scholar] [CrossRef]
- Carey, D.P.; Hargreaves, E.L.; Goodale, M.A. Reaching to ipsilateral or contralateral targets: Within-hemisphere visuomotor processing cannot explain hemispatial differences in motor control. Exp. Brain Res. 1996, 112, 496–504. [Google Scholar] [CrossRef]
- Mieschke, P.E.; Elliott, D.; Helsen, W.F.; Carson, R.G.; Coull, J.A. Manual asymmetries in the preparation and control of goal-directed movements. Brain Cogn. 2001, 45, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, K.C.; Churchland, M.M. Motor cortex signals for each arm are mixed across hemispheres and neurons yet partitioned within the population response. Elife 2019, 8, e46159. [Google Scholar] [CrossRef] [PubMed]
- Haaland, K.Y. Left hemisphere dominance for movement. Clin. Neuropsychol. 2006, 20, 609–622. [Google Scholar] [CrossRef]
- De Renzi, E.; Lucchelli, F. Ideational Apraxia. Brain 1988, 111, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Na, D.L.; Kang, E.; Lee, K.M.; Lee, S.W.; Na, D.G. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp. Brain Res. 2001, 139, 311–317. [Google Scholar] [CrossRef]
- Johnson-Frey, S.H.; Newman-Norlund, R.; Grafton, S.T. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex 2005, 15, 681–695. [Google Scholar] [CrossRef] [Green Version]
- Vingerhoets, G.; Acke, F.; Alderweireldt, A.S.; Nys, J.; Vandemaele, P.; Achten, E. Cerebral lateralization of praxis in right- and left-handedness: Same pattern, different strength. Hum. Brain Mapp. 2012, 33, 763–777. [Google Scholar] [CrossRef]
- Merrick, C.M.; Dixon, T.C.; Breska, A.; Lin, J.J.; Edward, F.C.; King-Stephens, D.; Laxer, K.D.; Weber, P.B.; Carmena, J.M.; Knight, R.T.; et al. Left Hemisphere Dominance for Bilateral Kinematic Encoding in the Human Brain. bioRxiv 2021. [Google Scholar] [CrossRef]
- Elliott, D.; Lyons, J.; Chua, R.; Goodman, D.; Carson, R.G. The influence of target perturbation on manual aiming asymmetries in right-handers. Cortex 1995, 31, 685–697. [Google Scholar] [CrossRef]
- Goodale, M.A. Hemispheric differences in motor control. Behav. Brain Res. 1988, 30, 203–214. [Google Scholar] [CrossRef]
- Floegel, M.; Kell, C.A. Functional hemispheric asymmetries during the planning and manual control of virtual avatar movements. PLoS ONE 2017, 12, e0185152. [Google Scholar] [CrossRef] [PubMed]
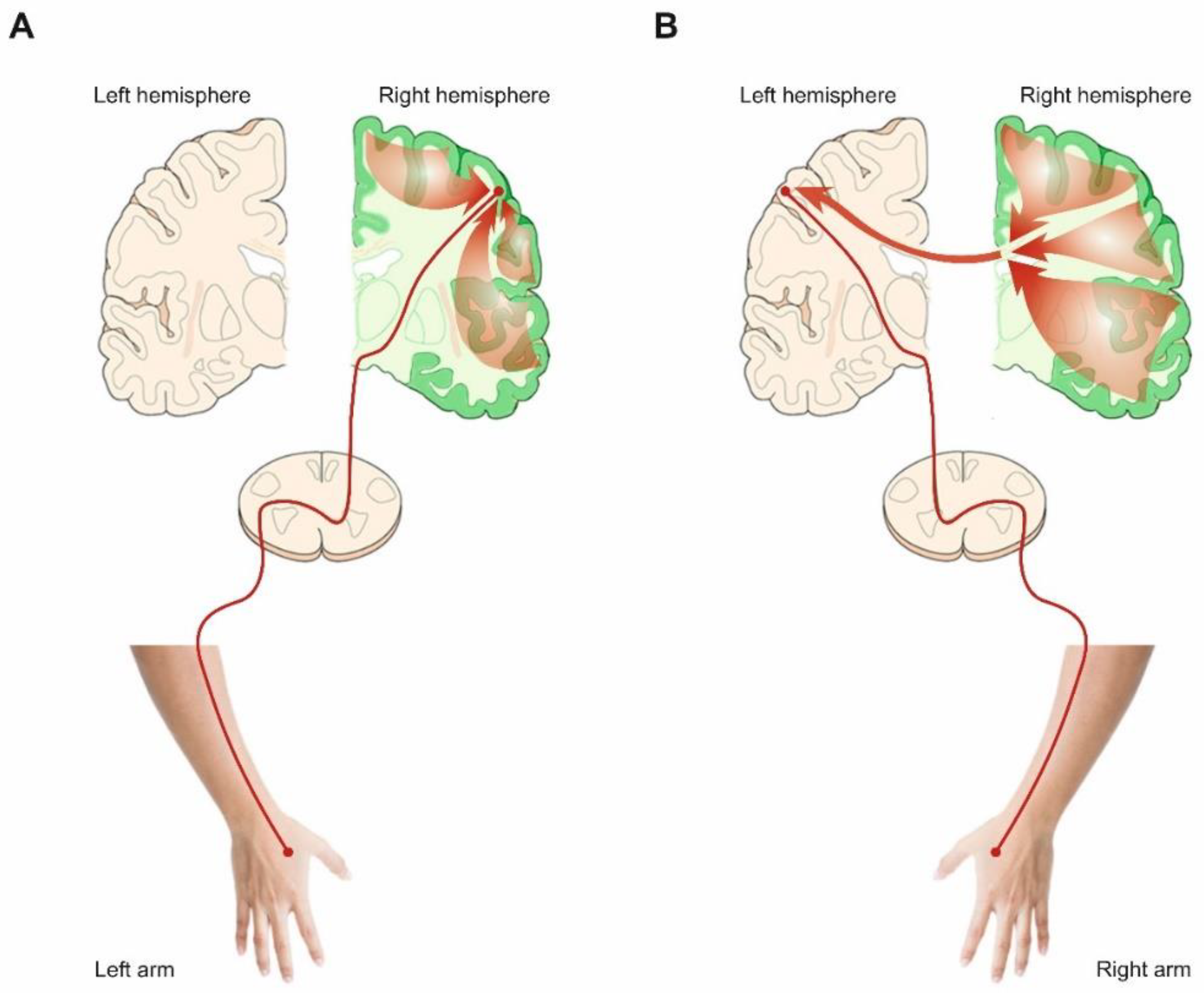
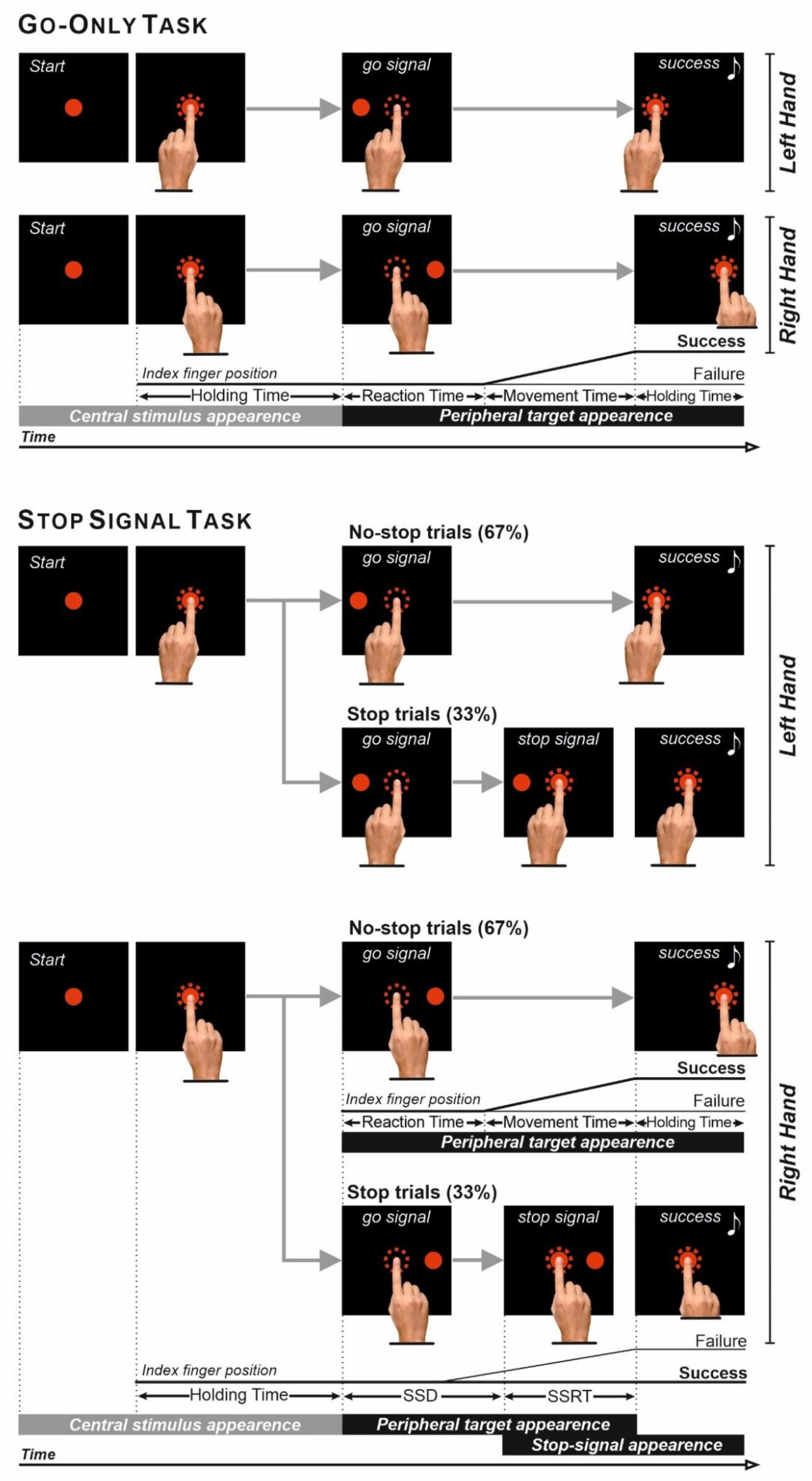
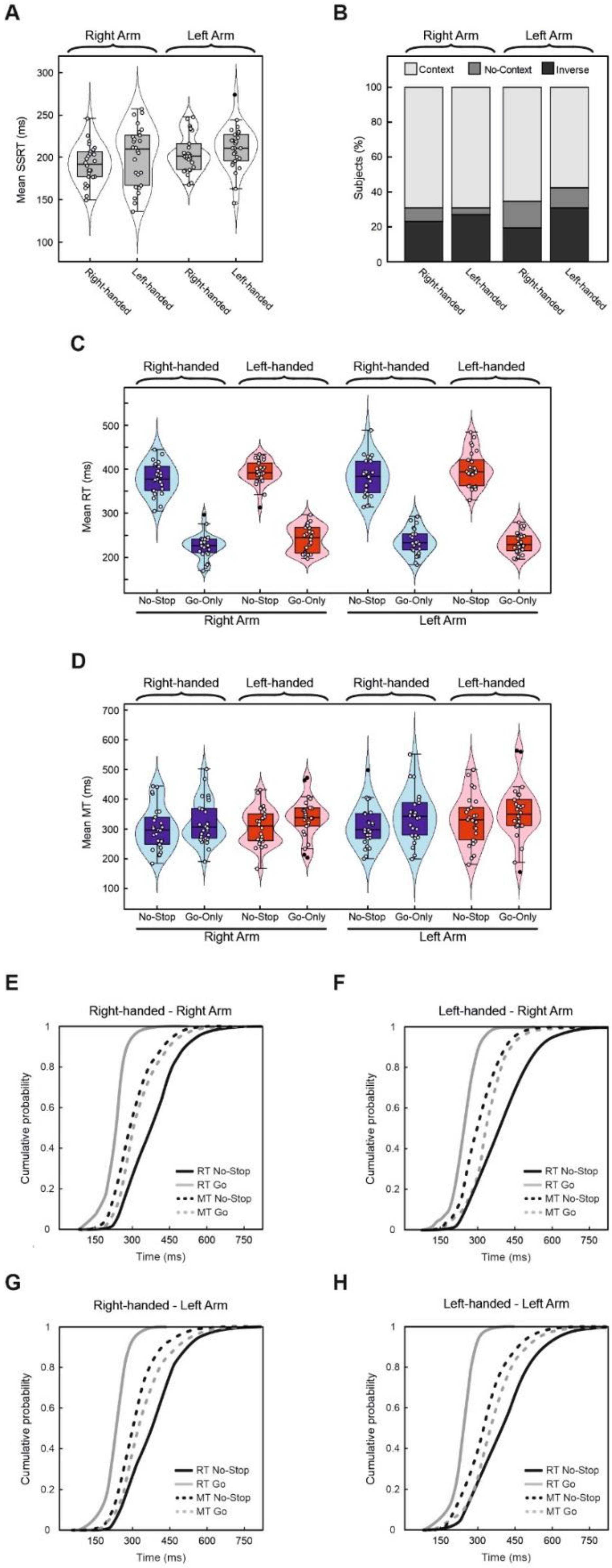
| Right-Handers | Left-Handers | |||
|---|---|---|---|---|
| Right Reaching Arm | Left Reaching Arm | Right Reaching Arm | Left Reaching Arm | |
| Mean SSD | 180.9 ± 47.5 | 176.5 ± 51.9 | 189.8 ± 41.1 | 192.4 ± 47.8 |
| P(failure) | 0.51 ± 0.02 | 0.52 ± 0.03 | 0.52 ± 0.03 | 0.52 ± 0.03 |
| SSRT | 192.1 ± 22.6 | 204.3 ± 22.4 | 201.6 ± 35.0 | 210.5 ± 25.8 |
| RT No-Stop trials | 377.5 ± 35.4 | 382.0 ± 42.5 | 392.8 ± 27.3 | 400.7 ± 39.2 |
| RT Stop-Failure trials | 328.2 ± 34.6 | 330.7 ± 35.3 | 330.4 ± 37.5 | 327.7 ± 38.6 |
| RT Go-Only trials | 225.1 ± 27.7 | 234.4 ± 28.4 | 241.2 ± 30.1 | 233.2 ± 23.5 |
| MT No-Stop trials | 300.4 ± 72.8 | 308.7 ± 68.6 | 306.1 ± 62.9 | 329.3 ± 82.5 |
| MT Go-Only trials | 322.8 ± 74.5 | 339.2 ± 83.7 | 335.5 ± 66.0 | 353.2 ± 91.5 |
| Accuracy Go-Only trials | 0.95 ± 0.06 | 0.95 ± 0.05 | 0.94 ± 0.05 | 0.95 ± 0.04 |
| Accuracy No-Stop trials | 0.97 ± 0.02 | 0.96 ± 0.04 | 0.95 ± 0.04 | 0.96 ± 0.03 |
| Three-Way ANOVA of RTs: Trial Type (No-Stop Trials, Stop-Failure Trials); Handedness (Left-Handers, Right-Handers); Reaching Arm (Left Arm, Right Arm) | ||||||
|---|---|---|---|---|---|---|
| Value of Parameters | p-Values | Mdiff | 95% CI | Effect Size | BF10 | |
| Main effect: Trial Type | F[1, 50] = 350.84 | p < 0.001 | 58.99 | [48.50, 69.48] | ηp2 = 0.88 | >100 |
| Interaction: Trial TypexReaching Arm | F[1, 50] = 5.53 | p = 0.023 | ηp2 = 0.54 | 0.34 | ||
| Post hoc Tests | ||||||
| Right Arm No-Stop vs. Stop-Failure | t(67.3) = 16.33 | p < 0.001 | 55.86 | [43.02, 68.70] | d = 2.26 | >100 |
| Left Arm No-Stop vs. Stop-Failure | t(67.3) = 18.17 | p < 0.001 | 62.12 | [47.55, 76.69] | d = 2.52 | >100 |
| Right Arm No-Stop vs. Left Arm Stop-Failure | t(99.2) = 11.97 | p < 0.001 | 55.95 | [42.52, 69.38] | d = 1.66 | >100 |
| Left Arm No-Stop vs. Right Arm Stop-Failure | t(99.2) = 13.27 | p < 0.001 | 62.03 | [47.11, 76.95] | d = 1.84 | >100 |
| No-Stop Right vs. Left Arm | t(64.5) = −1.66 | p = 0.602 | −6.17 | [−14.40, 2.07] | d = 0.23 | 0.44 |
| Stop-Failure Right vs. Left Arm | t(64.5) = 0.02 | p = 1 | 0.09 | [−6.45, 6.62] | d < 0.01 | 0.15 |
| Interaction: Trial TypexHandedness | F[1, 50] = 7.67 | p = 0.008 | ηp2 = 0.13 | 14.8 | ||
| Post hoc Tests | ||||||
| Right-handers No-Stop vs. Stop-Failure | t(50) = 11.29 | p < 0.001 | 50.27 | [36.10, 64.43] | d = 1.57 | >100 |
| Left-handers No-Stop vs. Stop-Failure | t(50) = 15.20 | p < 0.001 | 67.71 | [53.54, 81.88] | d = 2.11 | >100 |
| Right-handers No-Stop vs. Left-handers Stop-Failure | t(62.4) = 5.38 | p < 0.001 | 50.72 | [35.75, 65.68] | d = 0.75 | >100 |
| Left-handers No-Stop vs. Right-handers Stop-Failure | t(62.4) = 7.13 | p < 0.001 | 67.26 | [54.53, 80.00] | d = 0.99 | >100 |
| No-Stop Left- vs. Right-handers | t(62.4) = 1.80 | p = 0.458 | 16.99 | [3.20, 30.79] | d = 0.25 | 2.53 |
| Stop-Failure Left- vs. Right-handers | t(62.4) = −0.05 | p = 1 | −0.45 | [−14.90, 14.01] | d < 0.01 | 0.21 |
| Two-Way ANOVA of SSRT: Handedness (Left-Handers, Right-Handers); Reaching Arm (Left Arm, Right Arm) | ||||
|---|---|---|---|---|
| Value of Parameters | p-Values | Effect Size | BF10 | |
| Main effect: Reaching Arm | F[1, 50] = 6.33 | p = 0.015 | ηp2 = 0.11 | 3.31 |
| Main effect: Handedness | F[1, 50] = 1.62 | p = 0.209 | ηp2 = 0.03 | 0.54 |
| Interaction: Reaching Arm xHandedness | F[1, 50] = 0.17 | p = 0.685 | ηp2 < 0.01 | 0.35 |
| Three-Way ANOVA of RTs: Handedness (Left-Handers, Right-Handers); Reaching Arm (Left Arm, Right Arm); Trial Type (No-Stop, Go-Only) | ||||
|---|---|---|---|---|
| Value of Parameters | p-Values | Effect Size | BF10 | |
| Main effect: Trial Type | F[1, 50] = 777.45 | p < 0.001 | ηp2 = 0.94 | >100 |
| Main effect: Handedness | F[1, 50] = 4.07 | p = 0.049 | ηp2 = 0.08 | 1.29 |
| Main effect: Reaching Arm | F[1, 50] = 2.10 | p = 0.154 | ηp2 = 0.04 | 0.22 |
| Interaction: Reaching ArmxHandedness | F[1, 50] = 2.15 | p = 0.149 | ηp2 = 0.04 | 0.33 |
| Interaction: Trial TypexHandedness | F[1, 50] = 0.73 | p = 0.396 | ηp2 = 0.01 | 0.41 |
| Interaction: Trial TypexReaching Arm | F[1, 50] = 1.05 | p = 0.311 | ηp2 = 0.02 | 0.28 |
| Interaction: Trial TypexReaching ArmxHandedness | F[1, 50] = 3.65 | p = 0.062 | ηp2 = 0.07 | 0.77 |
| Three-Way ANOVA of MTs: Handedness (Left-Handers, Right-Handers); Reaching Arm (Left Arm, Right Arm); Trial Type (No-Stop, Go-Only) | ||||
|---|---|---|---|---|
| Value of Parameters | p-Values | Effect Size | BF10 | |
| Main effect: Trial Type | F[1, 50] = 16.15 | p < 0.001 | ηp2 = 0.24 | >100 |
| Main effect: Reaching Arm | F[1, 50] = 7.56 | p = 0.008 | ηp2 = 0.13 | 8.67 |
| Main effect: Handedness | F[1, 50] = 0.50 | p = 0.484 | ηp2 = 0.01 | 0.54 |
| Interaction: Reaching ArmxHandedness | F[1, 50] = 0.47 | p = 0.498 | ηp2 = 0.01 | 0.28 |
| Interaction: Trial TypexHandedness | F[1, 50] < 0.01 | p = 0.989 | ηp2 < 0.01 | 0.19 |
| Interaction: Trial TypexReaching Arm | F[1, 50] = 0.03 | p = 0.866 | ηp2 < 0.01 | 0.19 |
| Interaction: Trial TypexReaching ArmxHandedness | F[1, 50] = 0.89 | p = 0.350 | ηp2 = 0.02 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, C.; Mirabella, G. Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control. Symmetry 2021, 13, 1602. https://doi.org/10.3390/sym13091602
Mancini C, Mirabella G. Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control. Symmetry. 2021; 13(9):1602. https://doi.org/10.3390/sym13091602
Chicago/Turabian StyleMancini, Christian, and Giovanni Mirabella. 2021. "Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control" Symmetry 13, no. 9: 1602. https://doi.org/10.3390/sym13091602
APA StyleMancini, C., & Mirabella, G. (2021). Handedness Does Not Impact Inhibitory Control, but Movement Execution and Reactive Inhibition Are More under a Left-Hemisphere Control. Symmetry, 13(9), 1602. https://doi.org/10.3390/sym13091602







