1. Introduction
A shock wave is a paradigm of non-equilibrium process with irreversible character. It drives the system out of equilibrium and, at the same time, causes irreversible changes observed through the increase of entropy [
1,
2,
3]. In idealized models, shock waves are described as singular surfaces on which jumps of field variables occur. However, when dissipation is taken into account, shock waves are smeared out into continuous profiles, thus creating the so-called shock structure. [
4].
Analysis of the shock structure in gaseous media is strongly influenced by the model taken to describe dissipation. For the present study, two approaches are significant—continuum (macroscopic) and kinetic (mesoscopic) one. Continuum approach uses macroscopic field variables and their governing equations to describe the system. Within it, classical continuum models assume non-local constitutive relations for non-convective fluxes like stress tensor, heat flux, or diffusion flux. Typical examples are Navier–Stokes model, Fourier law and Fick law [
5]. Such models usually lead to parabolic partial differential equations, or their generalizations. In the same (continuum) setting, dissipation may also be described by means of relaxation, which assumes that non-equilibrium variables converge (relax) towards their equilibrium values during the process. They yield models which usually consist of the hyperbolic systems of balance laws. It is remarkable that in the classical (thermodynamic) limit, i.e., in the small relaxation time approximation, one may recover the classical parabolic models starting from the hyperbolic ones [
6,
7,
8,
9]. In more physical terms, classical models can be regarded as approximations of the hyperbolic ones when processes occur in the neighborhood of the local equilibrium state [
10].
The kinetic approach is focused on the smaller (meso) scale. It takes statistical description—single particle velocity distribution function—and mutual interaction between the particles which causes its change. It is built around the Boltzmann equation and inherits dissipation by construction, through the collision operator [
11]. A remarkable feature of the kinetic theory is that it can recover classical continuum models in the so-called hydrodynamic limit, when the Knudsen number is small, using the Chapman-Enskog asymptotic expansion. On the other hand, by means of the moment method kinetic theory yields hyperbolic systems of balance laws for macroscopic observables, taken as moments of the velocity distribution function. Under appropriate assumptions, relaxation continuum models of rarefied gases are completely equivalent to moment equations of the kinetic theory of gases [
12,
13].
Either approach described above has its advantages and shortcomings. For example, kinetic theory is obviously more refined than the continuum one, but evolution of the system is eventually monitored through macroscopic observables. In this study we shall try to take the benefits from both approaches and analyze the shock structure in the hyperbolic model of the multi-component mixture of Euler fluids. By an Euler fluid we consider the fluid in which viscous and heat conducting effects are negligible. By a phrase multi-component mixture, although it seems to be a pleonasm, we reveal our intention to analyze the mixture of more than two constituents (as opposed to binary, i.e., two-component mixture). In fact, we shall analyze the shock structure in a three-component mixture as a paradigmatic example of the multi-component one. It is known that a multi-component mixture exhibits peculiar phenomena with respect to the binary one, as for instance the uphill diffusion [
14,
15,
16]. Before we embark on the shock structure analysis, a short review of modelling issues and important results in this particular problem relevant to our study will be given.
Our analysis will be based upon the continuum model of mixture developed within the framework of rational extended thermodynamics (RET) [
13,
17] (for a review of the model one may consult [
18]). This model is rather peculiar from macroscopic point of view since it takes velocities and temperatures of the constituents as field variables. RET formalism, i.e., Galilean invariance of the governing equations and their compatibility with the entropy inequality, makes the model thermodynamically consistent. The model is hyperbolic, and dissipation is taken into account through mutual interaction between the constituents, which turns out to be of the relaxation type. However, this approach is limited in the sense that phenomenological coefficients, which appear in the closure process, cannot be explicitly determined within its framework. To resolve this problem, we turn to the kinetic approach based upon the polyatomic gas mixture model, i.e., the system of Boltzmann equations, with the continuous internal energy variable [
19,
20,
21], which is more tractable than the semi-classical model with discrete energy levels [
22,
23,
24,
25], yet shown to reproduce physical requirements [
26,
27,
28,
29] and to be well posed mathematically in the case of space homogeneity [
30]. Moreover, the multi-temperature (MT) model for mixture of Euler fluids is fully compatible with moment equations derived from this system at an Euler level [
31,
32]. In particular, we find the complete closure of the MT model by using the phenomenological coefficients from the weak form of the continuous collision operator at an Euler level [
33].
In this study, the shock wave will be treated as a continuous traveling wave solution of the governing equations which converges to equilibrium states at infinity. Such an assumption makes feasible the use of dynamical systems approach. This kind of theoretical shock structure analysis in continuum models of single-component fluids has its roots in [
34], where it was applied to Navier–Stokes–Fourier model. Further application of this method to hyperbolic continuum models was studied in [
35] (see also Chapter 12 in [
13] for a comprehensive review). The main feature of this approach is that the shock structure is described by a heteroclinic orbit in the state space which connects stationary points related to upstream and downstream equilibrium. More precisely, this orbit is an intersection of unstable and stable manifolds of these stationary points [
36], and can be related to the bifurcation of the upstream equilibrium point [
37]. This could also be useful for the numerical computation of the shock profiles, albeit in the limited way. Namely, it was shown [
38] that in hyperbolic systems of balance laws, endowed with a convex entropy density, continuous shock structure does not exist if the shock speed is greater than the highest characteristic speed of the system; instead, there appears a continuous solution with the so-called sub-shock.
Apart from single-component models of gases, shock waves were also studied in the context of mixtures. In the early stage, they were analyzed by means of classical models [
39] and DSMC [
40]. Efficient computing devices moved the focus towards development of numerical methods for the solution of Boltzmann equations for mixtures [
41,
42,
43]. On the other hand, shock structure was also analyzed for the moment equations of mixtures, derived from the kinetic theory for various types of interaction [
44,
45]. At the same time, detailed and systematic analysis of the shock structure in hyperbolic MT mixture of Euler fluids was given in [
46,
47], which revealed certain new features of the temperature overshoot. In this context, existence of continuous profiles and appearance of sub-shocks turned out to be more delicate than in the one-component case, as discussed in [
48,
49,
50]. Shock structure in the mixture of noble gases was revisited by DSMC in [
51]. Recently, attention was given to the entropy growth and the entropy production analysis in the mixture of Euler fluids [
52], and the mixture with viscous and thermal dissipation [
53]. It was found out that in former case diffusion makes the major contribution to dissipation, while in the latter one it is the thermal conduction.
It is remarkable that most of the studies mentioned above were concerned with binary mixtures. They certainly reveal the properties which distinguish them from single-component fluids, like temperature overshoot or influence of diffusion on the shock thickness. However, there are phenomena which can be observed only when the multi-component mixture is studied. This gives us strong motivation to analyze the shock structure problem in three-component mixture of Euler fluids. So far, only few references were found which analyze the shock waves in multi-component mixtures within the frameworks indicated above. In [
54], the shock structure was analyzed in a mixture of gases with disparate molecular masses, and viscous and thermal dissipation; ternary mixture is studied as a final example, with a special attention on the presence of two zones within the shock profile. In [
55], the analysis was based upon numerical solution of the Boltzmann equations; although the main focus was on binary mixture, three-species mixture was studied for the computation of parallel temperatures which exhibit an overshoot. Finally, in [
56], three- and four-component mixtures are studied by numerical solution of the Boltzmann equations; profiles of macroscopic variables are found to have good agreement with experiments and computations by other authors.
Our study will be focused on the shock waves in mixtures of Euler fluids, using the MT model developed in [
17], together with phenomenological coefficients from [
33]. It will be assumed that the mixture is homogeneous in the sense that in each infinitesimal volume element all the constituents are present. It will also be assumed that all the constituents are ideal gases, described by usual thermal and caloric equations of state, and that chemical reactions do not occur between them. Once equipped with the closed, thermodynamically consistent system of governing equations, we shall analyze numerically computed shock structures in three-component mixtures. Our aim is to reveal and bring to light possible qualitative novelties which appear when the mixture contains more than two components.
In the rest of the paper, model of the mixture of Euler fluids is presented in
Section 2. It brings together continuum modeling and kinetic theory results to get the closed system of equations. In
Section 3, the shock structure problem for a three-component mixture is formulated. It contains all the information needed for derivation of equations, their transformation into dimensionless form, and building up an appropriate numerical procedure.
Section 4 contains numerical solutions of the shock structure equations and their analysis. Shock profiles are analyzed for different values of parameters of the model (Mach number, equilibrium concentrations and molecular mass ratios), which provides an insight of their importance in the shock structure analysis. Additionally, multi-temperature shock profiles are compared with single-temperature ones, and the norm of their difference is computed. Finally,
Section 5 brings the analysis of relaxation processes captured by the model. It is of utmost importance since they manifest dissipation. Therefore, relative magnitudes of relaxation times for mechanical and thermal relaxations are computed throughout the profile. Paper ends up with proper conclusions and an outlook of future research directions.
4. Analysis of the Shock Structure
Analysis of the shock structure in three-component mixture of Euler fluids has several goals. First, we want to validate our model, especially phenomenological coefficients, by computing typical profiles that are comparable with the ones obtained by other methods. Second, we want to recognize the features typical for polyatomic gases. Third, we want get a glimpse of the influence of Mach number on the shock structure. Fourth, we want set up the data which mimic the binary mixture and find out what are the differences that three-component model brings. Finally, we shall compare the multi-temperature shock structure with the single-temperature one, and try to estimate the effect obtained by the MT assumption.
Computation are performed in the way explained in
Section 3. However, field variables are scaled to change in the
interval, where it is appropriate.
4.1. Shock Structure in the MT Model
Our model contains several parameters, whose values have to be given prior to numerical computation. First, phenomenological coefficients (
21) depend on the type of particle interaction through the parameter
of the cross-section, (
22) and (
23). In the sequel, we shall assume
, which corresponds to the hard-sphere model of particle interaction. Furthermore, in all the computations we shall assume the following values of ratios of specific heats:
,
. In most of the computations mass ratios will be
and
, unless stated otherwise (see Case 4). This assumption tends to cover the most interesting case from the MT point of view—the one with a mixture whose constituents have disparate masses.
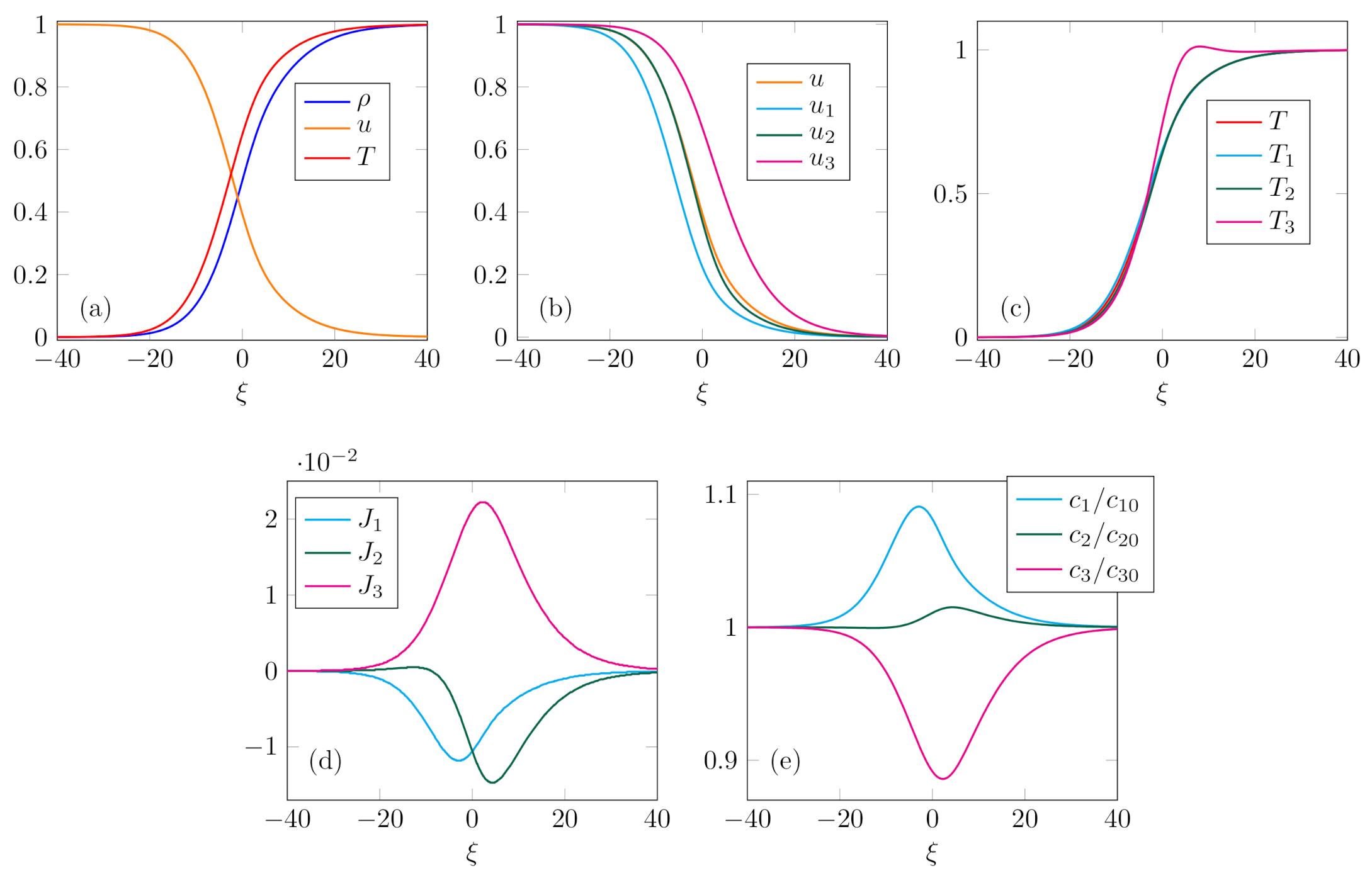
Case 1. In this case it is analyzed the situation with small equilibrium concentration
of the heaviest constituent, and large equilibrium concentration
. Shock structure is computed for
,
and
, and
, and shown in
Figure 1. Average mixture variables
,
u and
T have proper monotonic profiles, as well as velocities of the constituents. However, it may be observed that temperature
of the heaviest constituent has an overshoot—the zone in which the variable has the value greater than the downstream equilibrium value. This result corresponds to similar observations in binary mixtures, where an overshoot appears when heavier constituents have small concentration [
41,
42,
47]. It may also be noted that the difference between the velocities of the constituents is significant. Therefore, this case corresponds to typical profiles which can be found in [
55,
56]. Implicitly, it validates the structure of phenomenological coefficients (
21) obtained by matching the continuum and kinetic approach.
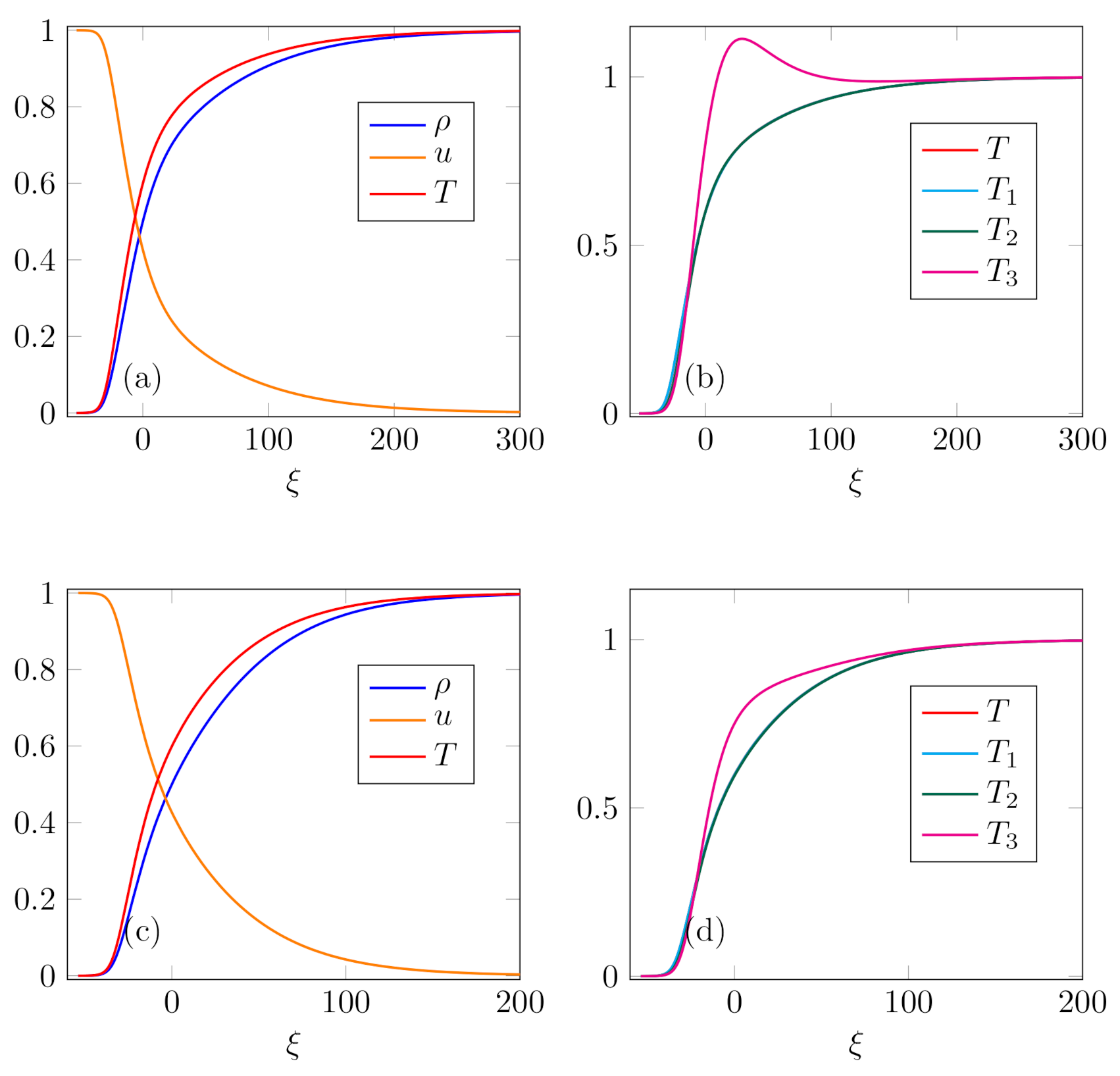
Case 2. In the second case, two profiles were computed for the same value of Mach number,
. The difference appears in equilibrium concentrations: for
,
, and
, profiles are presented in
Figure 2, graphs (a) and (b); for
,
, and
, profiles are presented in
Figure 2, graphs (c) and (d). The profiles consist of two zones: upstream one with steeper gradients of field variables, and downstream one with moderate gradients. This appears to be more prominent in the profiles of mixture variables, especially on graph (a). This kind of profile, named Type-C, was firstly observed in [
59] for the shock structure in ET14 model of a polyatomic gas (see also [
18] for a contextualized account). The result was further supported by numerical solution of the Boltzmann equation for polyatomic gases [
60], as well as two-temperature Navier–Stokes equations for polyatomic gases [
61]. The main cause of such a profile is attributed to the presence of dynamic pressure and its relaxation (bulk viscosity), which is longer than the relaxation of viscous stress and heat flux. Although our model does not take into account these processes, there exists certain resemblance to this phenomenon in our profiles as well. To the best of our knowledge, such profiles do not appear in monatomic gases and their mixtures, and we are convinced that polyatomic molecular structure is the cause for this result. Note that such profiles appear in the case with an overshoot and without it alike.
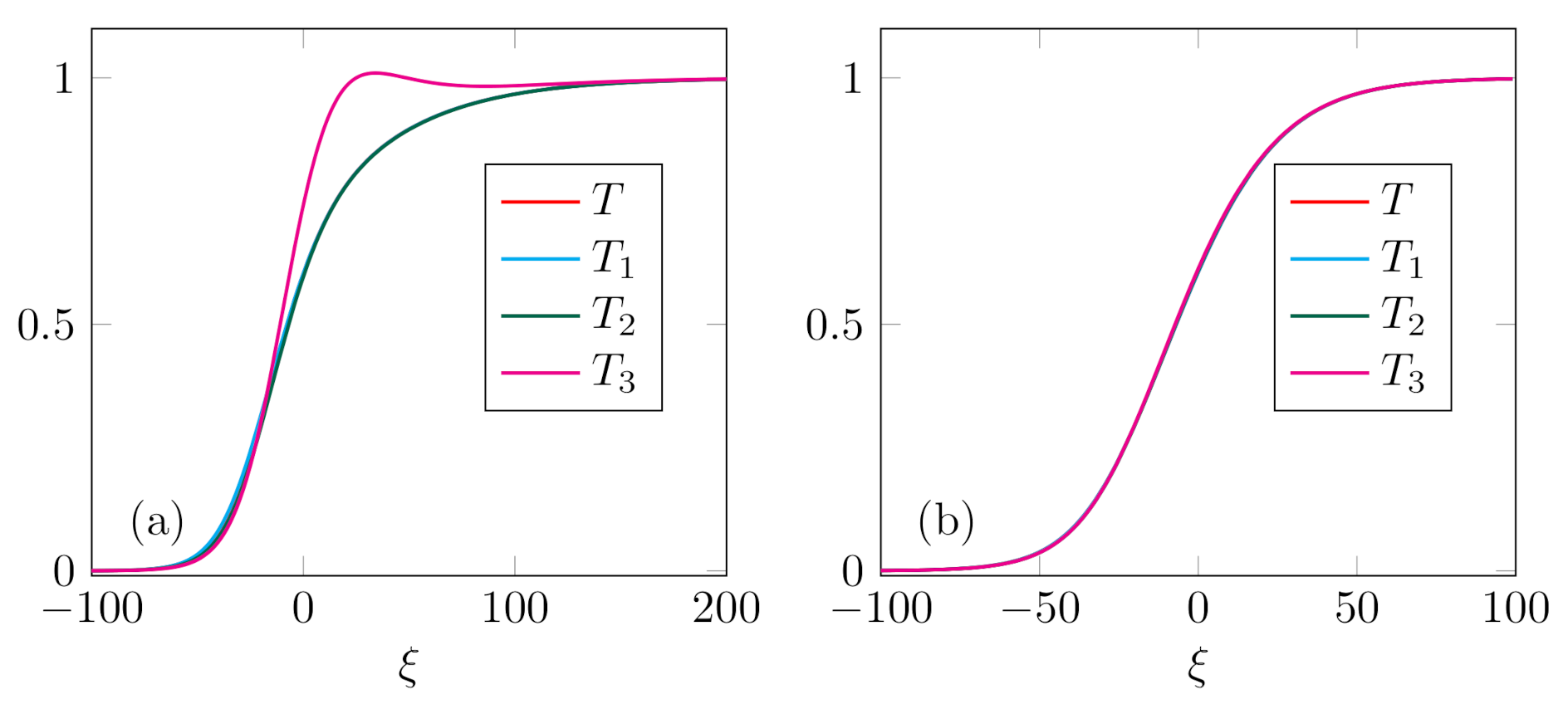
Case 3. In this case we examined the profiles with the highest equilibrium concentration
. In particular, we computed the profiles for
,
and
, and for two different values of Mach number,
and
, presented in
Figure 3a,b, respectively. For these profiles it is remarkable that temperatures
of the constituents have negligibly small difference, which is almost indistinguishable on the scale of graph (and thus not presented here). Therefore, we present the two cases which are in accordance with the results for binary mixture [
47]—shock thickness decreases with the increase of Mach number. This conclusion is valid for small values of
.
Case 4. Here we computed the profiles for
,
and
. However, we analyzed two different cases: (a)
and
, and (b)
and
, presented in
Figure 4. These results give an insight into the influence of mass ratio on the shock structure. In case (a), in the mixture with disparate masses of constituents, one may observe the temperature overshoot in
. However, in case (b) where constituents 2 and 3 have comparable masses, temperatures of the constituents are almost indistinguishable.
4.2. Comparison with a Single-Temperature Model
There always appears a question of the relevance of the MT assumption in the mixtures. Certainly, there are non-equilibrium processes in which this assumption cannot be dropped (e.g., in plasma). On the other hand, it also proved to be relevant in mixtures whose constituents have disparate atomic/molecular masses, like noble gases. Within the framework of RET, even if the multi-temperature assumption is dropped, one ends up with a multi-velocity model which is still hyperbolic and does not produce the paradox of infinite speed of pulse propagation.
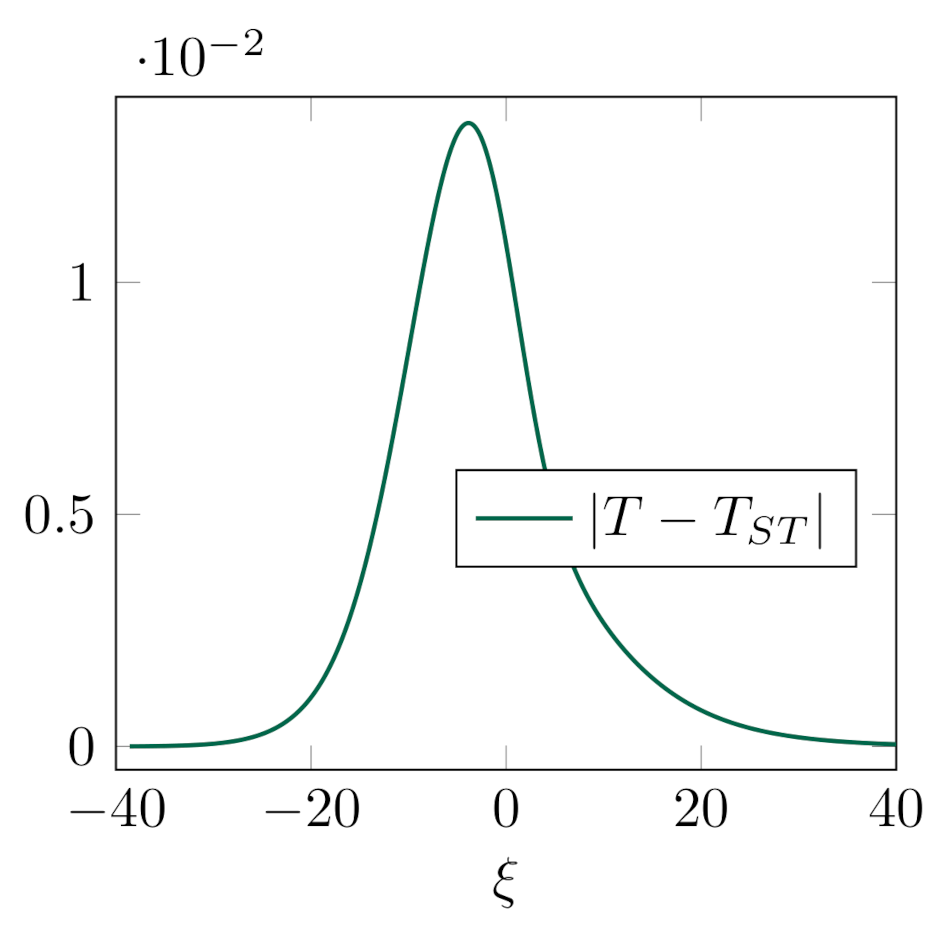
Our intention is to quantify the discrepancy in mixture field variables, primarily in the average temperature of the mixture, which appears within the shock profile computed for MT model, and the one computed for ST model. To that end, we compute the sup-norm of the difference of numerically computed solutions, i.e., , where denotes the temperature field along the shock profile in ST model.
For the shock structure with a temperature overshoot, presented in Case 1. of this study, sup-norm of the difference of normalized temperature profiles (the ones whose equilibrium values are set to
and
) is
(see
Figure 5). This implies that processes of energy exchange between the constituents, which appear due to the temperature difference, do not have a significant influence on the average value of temperature in MT model. However, this does not mean that they may be neglected. It was shown that appearance of temperature overshoot is directly related to insufficient energy exchange/transfer [
43,
47]. Additionally, preliminary computations, not reported here, show that there are cases with extremely large temperature overshoot,
, in which ST is not capable of proper capturing the behavior of the mixture temperature. This will be the subject of our prospective study.
5. Relaxation in the Shock Structure
One of the main features of hyperbolic systems of balance laws is that dissipation is achieved through the relaxation process, i.e., convergence of non-equilibrium variables to a certain (local) equilibrium state. Quantitative measure of the rate of convergence towards equilibrium is relaxation time. In simpler mathematical models, relaxation time may be unique for the whole system. However, if the model describes complex physical processes, there may be a need for more than one relaxation time. This is the case in our model of multi-component mixtures.
Mechanical (diffusion) and thermal relaxation times were introduced in (
25), while their influence on the source terms was described in (
26). It was shown recently [
53] that the rate of convergence may not be the same for different processes. Therefore, our aim is to compare different relaxation times and to get an insight to the rate of convergence towards equilibrium for different processes. We shall compare the relaxation times in a relative sense, i.e., by computing the ratio of relaxation times
and
to the reference relaxation time
along the shock profiles. After straightforward calculation, one may find the explicit dimensionless form of ratios of the diffusion relaxation times to the reference one:
Ratios of the thermal relaxation times to the reference one in dimensionless form read:
Ratios of the phenomenological coefficients needed to complete these expressions are given in the
Appendix A.
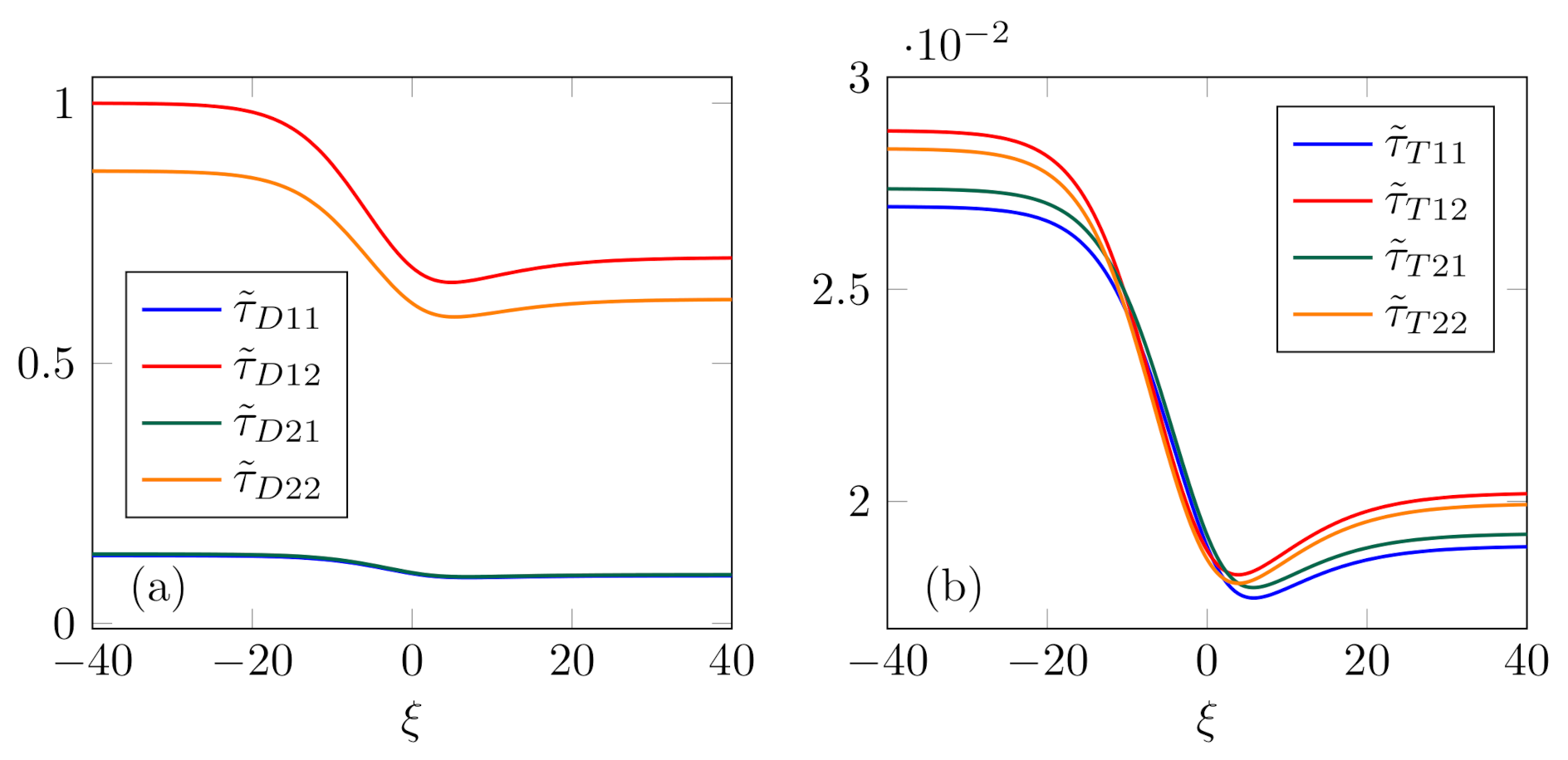
Relaxation times, presented in
Figure 6, are computed for the profiles analyzed in Case 1. They share one common property—a decrease along the shock profile, although it may not be monotonic. However, the most remarkable feature of the relaxation times in the multi-component mixture, at least in this case, is that the thermal relaxation times
are considerably smaller than the diffusion relaxation times
. These results oppose the ones obtained in the case of binary mixture [
40,
47], even when the viscous and thermal dissipation are taken into account [
53]. This certainly calls for deeper analysis of relaxation processes in the mixture. Additionally, it must be kept in mind that relaxation times may be strictly related to the rate of convergence towards equilibrium only in the simplest possible space-homogeneous cases. Therefore, relaxation times (
25) in our model cannot be simply identified as the time-rates of convergence of non-equilibrium variables.
6. Conclusions and Outlook
This paper was devoted to the study of shock structure in multi-component mixture of Euler fluids. Analysis was based upon the hyperbolic MT model for mixtures developed within the framework of RET. Explicit form of phenomenological coefficients, which appear in the source terms, was determined by matching with a linearized weak form of the collision operator in the system of Boltzmann equations for mixtures of polyatomic gases. The analysis was restricted to the normal shock waves, smeared out into a shock structure due to dissipation. Therefore, the shock structure was assumed in the form of a traveling wave. The original governing equations are transformed into a system of ODE’s that determine the shock structure as a heteroclinic orbit which asymptotically connects stationary points. Shock profiles were found as numerical solutions to the system of shock structure equations in dimensionless form, and analyzed for the several different sets of values of the parameters.
Our attention was focused on cases which possess certain distinguishing features, and thus can be taken as representatives of a broader class of qualitatively similar shock profiles. In particular, the following representative cases were recognized:
- (a)
shock profiles with an overshoot in (the temperature of the heaviest constituent); this appears in the mixture with disparate masses of the constituents, , , and when the equilibrium concentration of the heaviest constituent is small;
- (b)
shock profiles with two zones; in this case one distinguishes the front part of the profile, with steep gradients of field variables, and the rear part with moderate gradients of field variables;
- (c)
shock profiles with small magnitude of the diffusion temperatures, and decreasing thickness with the increase of Mach number; this case is typical for large equilibrium concentration of the heaviest constituent;
- (d)
shock profiles which depend on the mass ratio; increase of the mass ratio leads to disappearance of the temperature overshoot, and resembles the behavior of binary mixture.
We also analyzed the ST model and compared its profiles with the MT ones. It was recognized that MT assumption does not produce significant difference between the profiles of the average temperatures in MT and ST case. However, certain profiles exist with larger magnitude of temperature overshoot, and systematic analysis of MT assumption in these cases is a matter of ongoing research.
Finally, to perceive the rate at which dissipation occurs, we computed the relaxation times which appear in the source terms. It was found out that relaxation times decreased along the shock profile (but not monotonically). Analysis also shed new light on their magnitudes by giving an estimate that thermal relaxation times could be smaller than the diffusion ones.
The present study opens a new chapter in analysis of the shock structure in gaseous mixtures. It is important to note that the model took advantage of the continuum approach, which secured thermodynamic consistency and peculiar structure of the source terms, and the kinetic theory, which equipped us with explicit form of the phenomenological coefficients. In such a way we obtain reliable and tractable mathematical model at the same time. Results obtained in this way are in good agreement with the results of other studies, and therefore confirm validity of the model. Further analysis, which is a work in progress, will be concerned with a systematic study of the shock structure in the regions of parameter space in which continuous solutions exist. This will give a deeper insight into the influence of certain parameters on quantitative characteristics of the shock profiles, like the temperature overshoot or the shock thickness. Additionally, in continuation, it is our intention to extend the analysis of the entropy growth and the entropy production rate, given in [
52] for the binary mixture, to three-component mixtures. Our aim is also to take into account viscous and thermal dissipation and evaluate their influence on the shock structure in multi-component mixtures, like it was done in [
53] for binary mixtures. Finally, it is our intent to analyze the cases of specific mixtures. To that end we plan to use novel models of polyatomic gases, derived in the kinetic theory [
21], to get better estimates of phenomenological coefficients and to relate them to measurable physical quantities like diffusivity. This will also lead to possible explicit estimate of the shock thickness in terms of the average mean free path of the molecules.