The results of the Akrosorb soda-lime, raw Bentonite, and molded Bentonite clay (with Na2SiO3 binder) adsorbent characterized using XRD, FTIR, BET, and SEM were presented below.
4.1. XRD Result of Akrosorb and Bentonite Clay
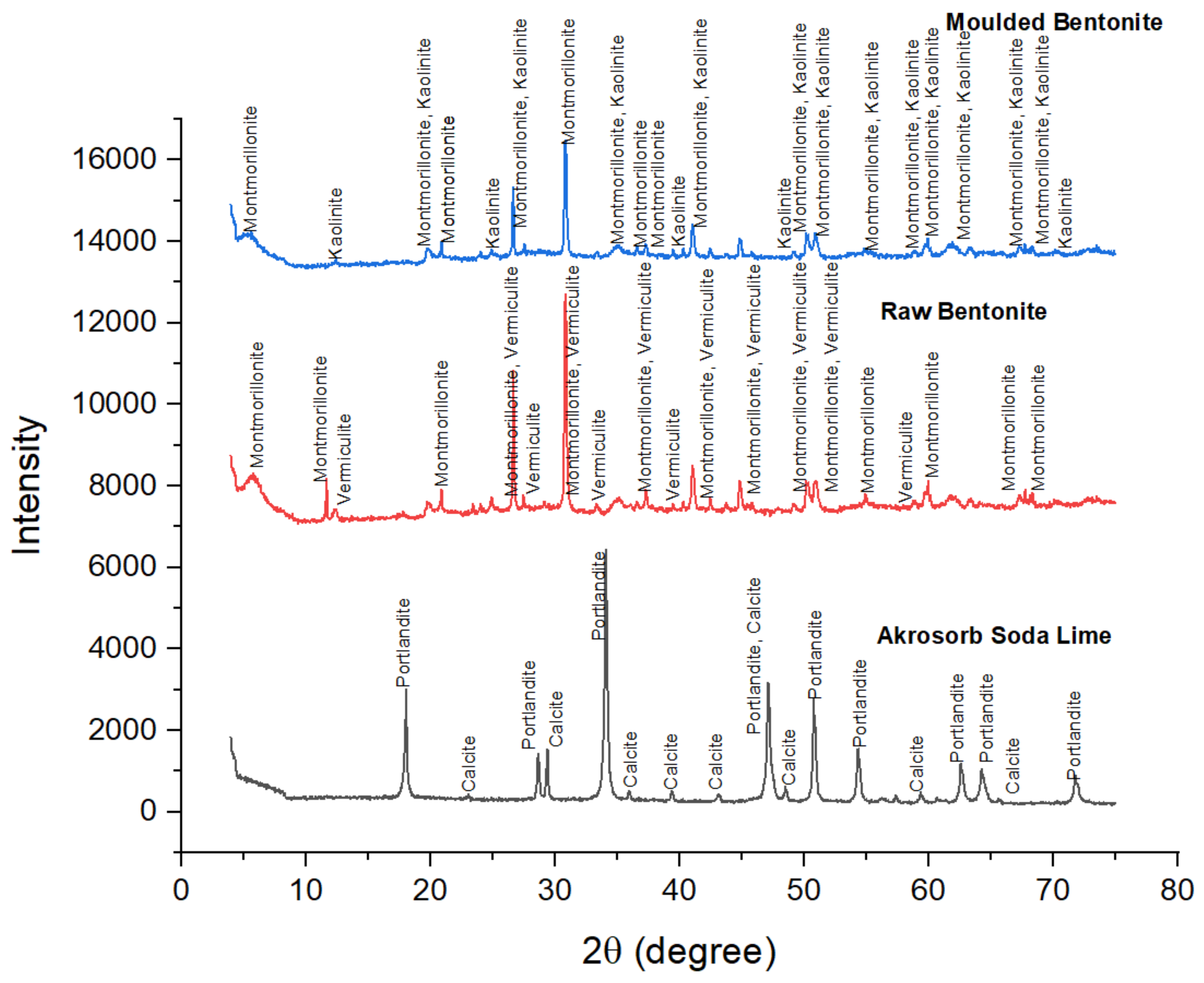
The crystallographic features of Akrosorb soda-lime and Bentonite clay adsorbent used for CO
2 and GHG capture in the adsorption column were determined as shown in
Figure 7. The crystallographic study of Akrosorb soda-lime adsorbents showed that it contains mainly Ca(OH)
2 (87.5%) and a small quantity of CaCO
3 (12.5%) and that the crystal system is trigonal with hexagonal axes for the portlandite and calcite phases present. The degree of crystallinity of the Akrosorb soda-lime adsorbents is 26% with 74% amorphous content, which portends high adsorption capacity for Akrosorb soda-lime due to its high amorphous content, which corresponds to the available composition from the Akrosorb soda-lime adsorbents data sheet (Ca(OH)
2 > 80% w/w) obtained from the manufacturer. It was also found that the crystal structure of Akrosorb soda-lime (contains Ca(OH)
2 and CaCO
3) in portlandite and calcite phases is made up of a trigonal crystal system with hexagonal axes (unit cell parameter of a = 3.59247 Å and c = 4.90820 Å for portlandite and a = 4.99150 Å and c = 17.08800 Å for calcite phase).
The crystallography of the molded Bentonite clay was also examined using XRD analysis as presented in
Figure 7. The
Figure 7 result shows the diffraction peaks of molded Bentonite at 2θ angles of 5.66°, 20.92°, 25.01°, 26.69°, 30.87°, 35.15°, 36.59°, 37.30°, and 44.87° are typical of those of Montmorillonite silicate phase and correspond to the diffraction peak of Montmorillonite in the raw Bentonite clay. The most intense peak is observed at the 2θ angle of 30.87°, which corresponds to those of Montmorillonite, showing that the molding of the Bentonite clay using Na
2SiO
3 binder did not affect the structure of the Montmorillonite silicate phase. However, it was observed that the XRD pattern of the vermiculite phase in the Bentonite clay changes after molding with Na
2SiO
3 binder to the kaolinite silicate phase.
Figure 7 shows that the diffraction peaks of molded Bentonite at 2θ angles of 12.43°, 24.96°, 39.51°, 49.12°, and 70.10 are typical of the kaolinite silicate phase. The characteristic peaks of the kaolinite were for crystalline silica. It can be seen that the other peaks that represent the kaolinite phase were much lower; they generally had an intensity of fewer than 900 counts and even lower, which implies that the kaolinite content is lower than Montmorillonite content in the molded Bentonite. The change in the phases in the molded Bentonite clay indicates that the binder utilized affected the crystal structure of the Bentonite from vermiculite phase to kaolinite phase. It was further observed that the crystal system of the molded Bentonite clay is anorthic. The analysis further shows that the degree of crystallinity of the molded Bentonite clay reduces to about 9.98% with a 90.02% amorphous silica phase. The slight increase in the amorphous silica phase would further enhance the adsorptive properties of the molded Bentonite compared to the raw Bentonite.
Figure 7 also presents the XRD pattern of the raw Bentonite clay and the molded Bentonite clay.
Figure 7 indicates that the diffraction peaks for raw Bentonite at 2θ angles of 5.82°, 11.70°, 17.84°, 20.92°, 26.69°, 30.87°, 35.15°, 44.87°, 50.20°, 50.96°, 54.95°, 58.81°, 59.99°, 61.73°, 63.43°, 67.79°, and 68.37° correspond to the diffraction peak of Montmorillonite. The most intense peak was observed at the 2θ angle of 30.87°, which corresponds to those of Montmorillonite and vermiculite, which shows that the raw Bentonite clay comprises mainly of Montmorillonite and vermiculite silicate phases with monoclinic and anorthic structures, respectively. These peaks correspond to (001), (002), (003), (012), (110), and (111) crystallographic planes assigned to the Montmorillonite phase, respectively, and are consistent with basal spacing reported in the literature for the Montmorillonite silicate phase. In addition, the peaks of the raw Bentonite at 2θ angles of 12.46°, 20.92°, 23.46°, 24.93°, 26.69°, 30.87°, 33.39°, 37.33°, and 41.06° correspond to those of vermiculite. These peaks are consistent with basal spacing of (004), (114), and (008) reported in the literature for the vermiculite silicate phase. The basal spacing for Montmorillonite and the vermiculite phase was observed to be 15.004 Å and 14.334 Å, which correspond to (001) and (002) planes respectively, indicating both Montmorillonite and vermiculite phases to be present in almost equal quantity. The XRD analysis shows that the raw Bentonite comprises mainly of Montmorillonite and vermiculite silicate phases in almost equal quantity, as shown in
Figure 5, and the crystal system comprising mainly of anorthic and monoclinic crystal with three unequal axes, a = 5.18 Å, b = 8.98 Å, and c = 15.0 Å for the Montmorillonite phase and a = 5.349 Å, b = 9.255 Å, and c = 28.7217 Å for the vermiculite phase. The XRD analysis further shows that the degree of crystallinity of the raw Bentonite clay is 11.96% with 88.04% amorphous silica phase, which indicates high adsorption.
The crystal structure of the Bentonite and Akrosorb soda-lime adsorbents was determined by the XRD technique.
Figure 7 presents the XRD pattern of the Akrosorb soda-lime adsorbents. It can be seen that the diffraction peaks at 2θ angles of 18.08°, 28.72°, 34.13°, 47.15°, 50.80°, 54.35°, 56.18°, 59.20°, 64.30°, and 71.81° were typical diffraction peaks of portlandite (Ca(OH)
2), and the most intense peak was observed at the 2θ angle of 34.13°, which corresponds to portlandite (Ca(OH)
2). This shows that Akrosorb soda-lime adsorbents are comprised mostly of Ca(OH)
2 phase with a hexagonal structure. These peaks correspond to (001), (110), (011), (012), (110), and (111) crystallographic planes assigned to the Ca(OH)
2 phase respectively. The XRD result of Ca(OH)
2 is consistent with those reported for the Ca(OH)
2 phase by Guerrero et al. (2017). In addition, the basal spacing of the Akrosorb soda-lime adsorbents at 2θ angles of 18.08° and 28.72° was 4.9060 Å and 2.6272 Å respectively, indicating that the (001) and (110) planes show portlandite to be in excess. In addition, the diffraction peaks at 2θ angles of 23.07°, 29.42°, 35.96°, 39.43°, 43.16°, 47.15°, 48.52°, 57.42°, 60.67°, and 65.64° were typical diffraction peaks of calcite (CaCO
3), and the low intensity of the calcite peaks shows that the Akrosorb soda-lime adsorbents contain a small quantity of calcite. The calcite peaks correspond to (012), (104), (110), (113), (202), (211), and (122) crystallographic planes assigned to the CaCO
3 phase, respectively. This corresponds with peaks at 2θ of 23.1°, 29.4°, 35.9°, 39.4°, 43.3°, and 57.3°, representing (112), (104), (110), (113), (202), (211), and (122) reported in the literature.
4.2. Brunauer–Emmett–Teller Result of Adsorbent
The BET technique was used to find out the total pores volume area, using a Quanta chrome instrument. The DH, DFT, and BJH methods were used to evaluate the mesoporus and micropores of adsorbent materials, while the t-method was also used to examine the surface/external area, and the DR techniques were used to examine the micropores. The porosity is classified into three groups of pore dimensions: these are micropores (width < 2 nm), mesoporous (width: 2–50 nm), and macrospores (width > 50 nm).
Table 2 presents the BET surface area analysis of the Akrosorb soda-lime and Bentonite adsorbent.
Table 2 presents the surface and pore properties of the Akrosorb soda-lime and Bentonite adsorbents. It can be seen that the Akrosorb soda-lime adsorbent is highly micro porous with a 1072 m
2/g micropore area when compared to that of raw Bentonite clay and molded Bentonite adsorbents with 498 m
2/g and 498.2 m
2/g micropore area, respectively. It was observed that there was no change in the micropore area of the Bentonite clay after molding using Na
2SiO
3 as a binder, as the micropore area only increases from 498 to 498.2 m
2/g. Hence, Akrosorb soda-lime adsorbent has a higher micropore area than molded Bentonite, which could be due to the high amorphous content in the Akrosorb soda-lime adsorbent crystal structure established from the XRD analysis. This implies that a higher micropore area signifies the availability of more adsorption sites for CO
2 and other GHG. In addition, it was observed that the cumulative adsorption surface area of Akrosorb soda-lime adsorbent was higher than that of molded Bentonite.
The pore volume of adsorbent is another crucial parameter of adsorbent materials. The pore volume of Akrosorb soda-lime and molded Bentonite adsorbent are presented in
Table 2. Substantiating the micropore area and cumulative adsorption surface area of the adsorbents, it can be seen that the micropore volume of the Akrosorb soda-lime and molded Bentonite adsorbents are 0.02478–0.3810 m
3/g and 0.02364–0.1770 m
3/g respectively, while the cumulative adsorption pore volumes are 0.5444–0.5566 m
3/g and 0.2533–0.2665 m
3/g, respectively. This further confirms that the Akrosorb soda-lime adsorbent possesses a higher adsorption capacity than molded Bentonite adsorbents. This could be attributed to the high development of the pores in Akrosorb soda-lime.
The pore size of the adsorbent is also another significant parameter that gives insight into the adsorption capacity of an adsorbent. According to the classification of IUPAC, pore sizes are classified into three categories: < 2.0 nm are microporous, 2.0–50 nm are mesoporous, and > 50 nm macrospores. From
Table 2, it can be seen that the diameter of the pores of the Akrosorb soda-lime adsorbent, raw Bentonite clay, and molded Bentonite adsorbents are mostly in the range of mesoporous pore size of 2.647–3.479 nm, 2.647–3.479 nm, and 2.647–2.800 nm, respectively. However, the HK method shows the presence of microporous pore sizes of 1.847, 1.847, and 0.3675 nm for Akrosorb soda-lime, raw Bentonite clay, and molded Bentonite adsorbents, respectively. In addition, the cumulative adsorption pore diameter for Akrosorb soda-lime, raw Bentonite clay, and molded Bentonite adsorbents are in the range of 2.105–2.426 nm, 2.100–2.427 nm, and 2.132 nm, respectively. It can be seen that the Akrosorb adsorbent and raw Bentonite clay have equal cumulative adsorption pore diameters, while that of molded Bentonite is slightly smaller. This shows that Akrosorb soda-lime has higher pore size compared to that of molded Bentonite adsorbent and both have microporous and mesoporous pore surface sizes.
Additionally, the micropores pore width formed in the interlayer spacing was found to be 6.503, 6.538, and 5.749 nm for Akrosorb soda-lime, raw Bentonite clay, and molded Bentonite, respectively, which indicates the formation of mesoporous (2.0–50 nm). These observations indicate that Akrosorb soda-lime and molded Bentonite both can adsorb CO2 and GHG. It was also found that adsorption takes place mainly through micropores and mesopores. Hence, from the adsorbents characterization, it can be concluded that Akrosorb soda-lime adsorbent has a higher adsorption capacity for CO2 and other GHG than Bentonite adsorbent. Therefore, both Akrosorb soda-lime and molded Bentonite adsorbents indicate good adsorption potential for CO2 and other GHG, and the combined usage of both adsorbents will enhance the capture of CO2 and other GHG at a low cost compared to the usage of Akrosorb soda-lime alone as adsorbent.
4.3. SEM/EDX Result of Akrosorb Soda-Lime and Bentonite Clay
A scanning electron microscope (SEM) was employed to examine the morphology of the surface and pore development of the adsorbent.
Figure 8 shows the SEM image of the Akrosorb soda-lime adsorbent. It was observed that the SEM image of the Akrosorb adsorbent shows a crystalline and smooth surface morphology with smaller pores. The SEM images showed the formation of a dense and rough surface and the formation of nano crystalline powder particles. The surface roughness observed from
Figure 9 is attributed to the relatively high amorphous phase associated with the Ca(OH)
2 phase, which corroborates the high amorphous phase established from XRD analysis.
The high composition of Ca Akrosorb adsorbent further confirms the XRD result, which predominantly shows Ca(OH)2 and CaCO3 phases and that the Akrosorb adsorbent constitutes primary Ca.
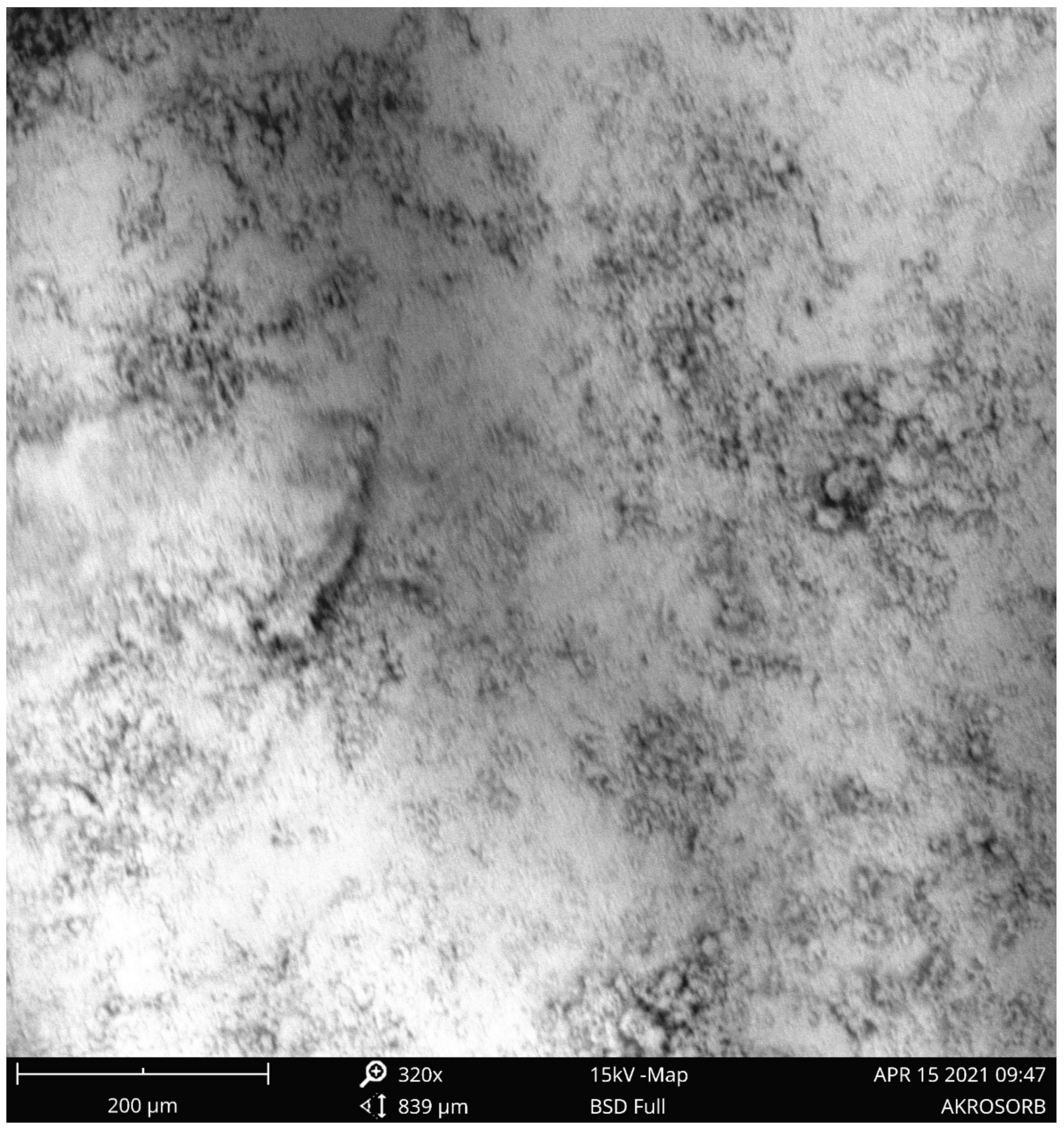
The raw Bentonite clay and molded Bentonite were also characterized using the SEM technique.
Figure 10 indicated raw Bentonite clay SEM image at 320 and 750 magnifications. It was observed that the image shows a scattered and rough surface morphology with the formation of a nano crystalline particle. The surface roughness observed from
Figure 10 is attributed to the relatively high amorphous silicate phase associated with the raw Bentonite clay phase, which validates the high amorphous phase established from XRD analysis.
Figure 11shows that the minimum peak intensity of Akrosorb soda lime was larger than that of Bentonite, which is attributed to the presence of portlandite and calcite phases as well as the crystallographic structure of Akrosorb when compared to those of Bentonite, which contains Montmorillonite, Vermiculite, and Kaolinite.
The molded Bentonite was also characterized using the SEM technique.
Figure 12 shows the SEM image of the molded Bentonite. It was observed that the SEM image of the molded Bentonite shows a crystalline and smooth surface morphology. The SEM images showed the formation of a dense and slightly rough surface with smaller pores. The change in surface morphology could be attributed to the effect of the binder (Na
2SiO
3) used, which was established from the XRD analysis to affect the crystal structure of the Bentonite. The image in
Figure 13 shows some pore development; this could be attributed to the presence of kaolinite identified in the molded Bentonite from the XRD analysis. This is because the kaolinite phase has an empty framework structure that is almost 50% void, which makes the special structure of kaolinite have a lot of micropores and mesopores of less than one nanometer.
It can be seen that there was an increase in the silica content from 31.91% in the raw Bentonite clay to 40.43% in the molded Bentonite. This could be attributed to the use of Na2SiO3 as a binder and also responsible for the increases in the sodium content in the molded Bentonite. The Bentonite clay is a calcium Montmorillonite that contains mainly silicon oxide, ferric oxide, calcium oxide, and aluminum oxide, which corroborates the finding. Calcium Bentonite is a useful adsorbent; this makes Bauchi LGA Bentonite a useful adsorbent.
4.4. Fourier Transform (FTIR) Result of Akrosorb Soda-Lime and Bentonite Clay
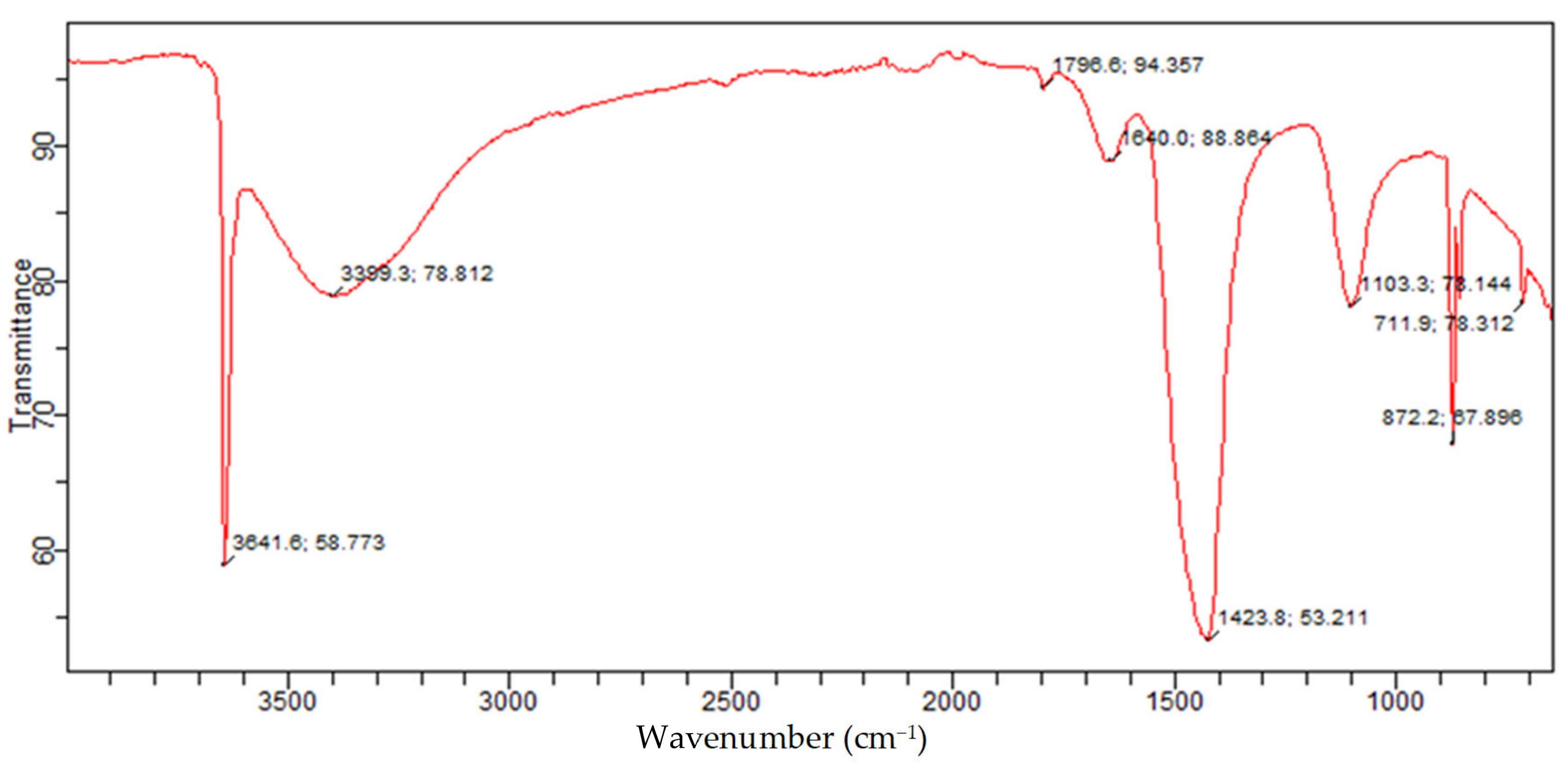
The FTIR is a measure of quantitative and qualitative analysis of the functional group of organic and inorganic samples in a material. The FTIR of the Akrosorb adsorbents indicated in
Figure 14 shows the bands of a constituent functional group identified from the Akrosorb adsorbents FTIR. It was observed that the sharp band at 711.9 cm
−1 indicates a carbonate ion (CO
32−), which is attributed to the presence of a crystalline calcite phase. The bands at 872.2 cm
−1 and 1103.3 cm
−1 indicating CO
32− bending vibration and CO
32− stretching respectively correspond to the presence of amorphous calcium carbonate phase of calcite. However, the band at 1423.8 cm
−1 is attributed to the presence of the CO
3−2 asymmetric stretching mode of the carbonate ion bending vibration band, which corresponds to the carbonate phase of calcite. This is consistent with the XRD analysis, which also indicated the presence of calcite as well as amorphous and crystalline phases. Furthermore, it can be seen from
Figure 14 that the characteristic band at 1640.0/cm is due to the H-O-H bending vibration of water. The bands at 3399.3/cm and 3641.6/cm are because of the presence of O-H broad band vibration as well as Ca-O-H and O-H stretching mode, which is similar to the stretching mode of portlandite. The O-H stretching mode also corresponds to structural water within the amorphous calcite phase. This is also consistent with the findings established from the XRD analysis of the presence of the portlandite phase.
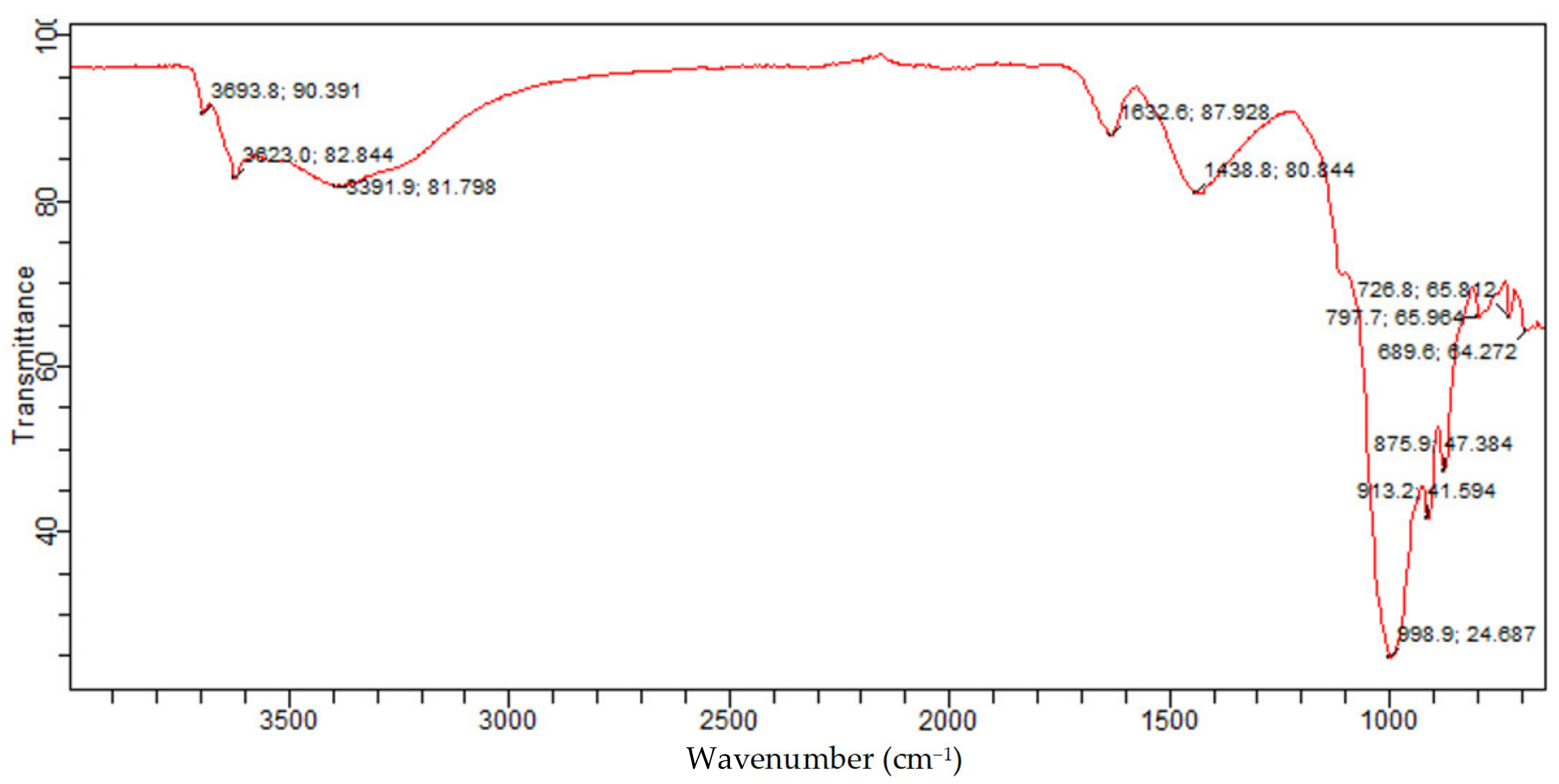
The FTIR of raw Bentonite depicted in
Figure 15 is attributed to the presence of quartz silicate Montmorillonite and vermiculite phases in the Bentonite. In addition, the 875.9 cm
−1 and 913.2 cm
−1 band is related to Al-Al-OH; this band indicates the presence of silicate type of Montmorillonite and vermiculite. The band at 1438.8/cm is because of the Ca-O broadband vibration, which is attributed to the Montmorillonite phase, while the bands at 1632.6/cm, 3391.9/cm, and 3623 cm
−1 are because of the -OH bending mode, -OH vibration, and -OH stretching mode for the interlayer adsorbed water in the Montmorillonite and vermiculite phases. This is consistent with the OH band for water reported. Furthermore, the band at 3693.8 cm
−1 is associated with O-H. This is consistent with the bands reported for the Si-OH and Al-OH groups of tetrahedral sheets of Montmorillonite and vermiculite silicate. This corroborates with the presence of Montmorillonite and vermiculite in the Bentonite clay established from XRD analysis.
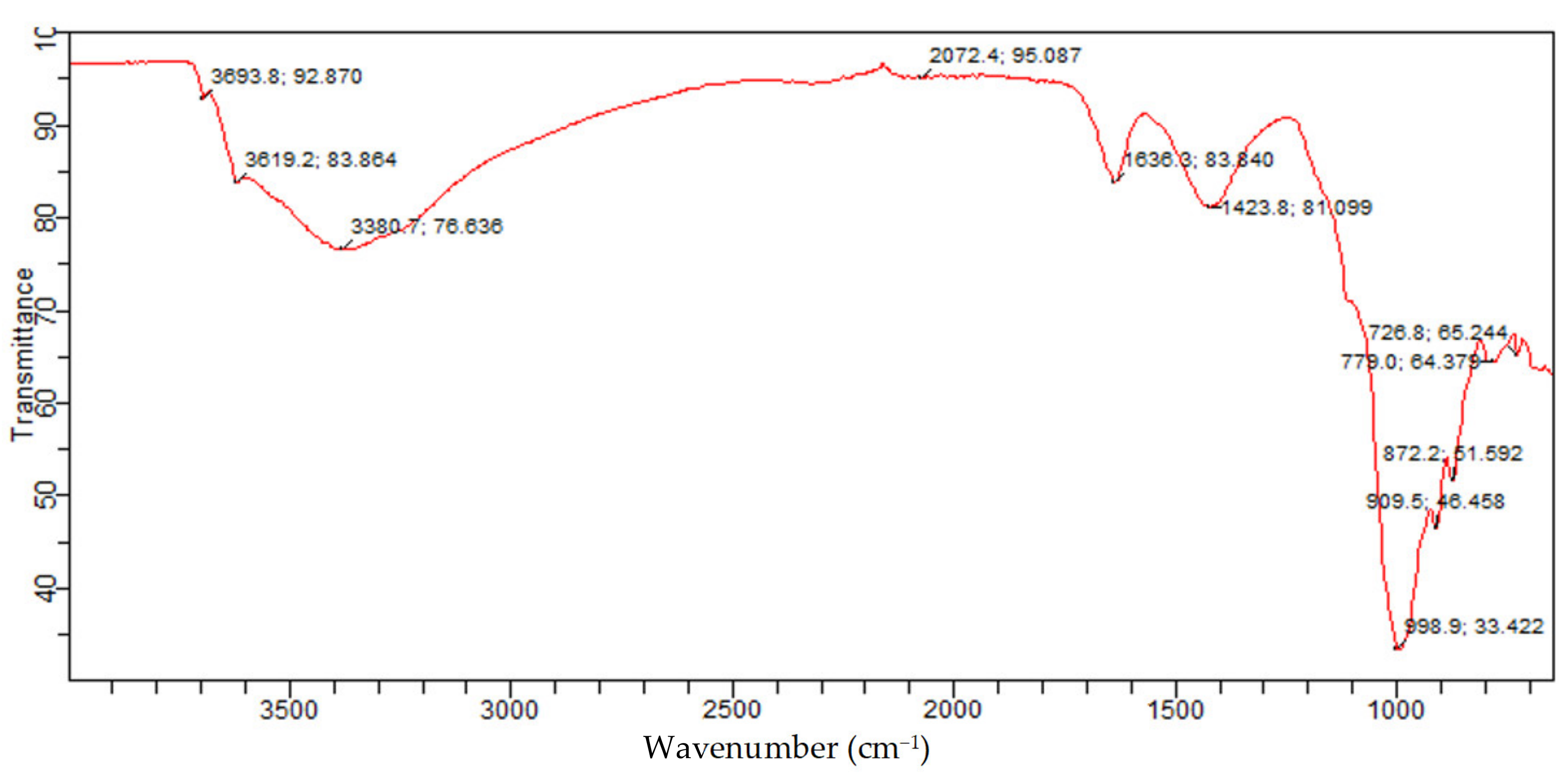
Figure 16 presents the identified functional groups from the FTIR analysis and associated constituents for the molded Bentonite. The bands of the molded Bentonite clay show similar bands with those of the raw Bentonite clay. However, slight changes were observed in the molded Bentonite band. These changes could be attributed to the effect of the binder (Na
2SiO
3) used in molding the Bentonite clay, resulting in the structural change of the crystals.
From
Figure 12, the bands at 726.8 cm
−1 and 779.0 cm
−1 were because of Si-O bending vibration and indicative of the Al-O-Si inner surface vibration of silicate of kaolinite quartz and Montmorillonite, which were similar to those reported in the literature for silicate of kaolinite and Montmorillonite phases. The bands at 872.2 cm
−1, 909.5 cm
−1, and 998.9 cm
−1 are set to the Si-O-Al stretching mode and Al-Al-OH vibrations, indicating the presence of kaolinite and Montmorillonite silicate. These bands are similar to those reported. The bands at 1423.8 cm
−1 and 1636.3/cm
−1 are assigned to Si-O stretching and H-O-H bending, respectively. These bands are consistent with those of Montmorillonite silicate and water. The absorption bands at 3380.7 cm
−1, 3619.2 cm
−1, and 3693.8 cm
−1 are attributed to asymmetric and symmetric stretching of structural hydroxyl groups, which are attributed to adsorbed crystal water in Montmorillonite and kaolinite phases of the Bentonite balls. This further confirms that after molding the Bentonite clay using Na
2SiO
3 as binder, the phase composition changes to Montmorillonite and kaolinite. The established band for the Montmorillonite and kaolinite phases of the Bentonite balls are consistent with those reported in the literature. Hence, the presence of Montmorillonite and kaolinite phases of the Bentonite balls portends the removal capacity for CO
2 due to their high exchange capacity.
4.5. Result of Optimization Process for Enhanced Removal Efficiency
The response surface methodology (RSM) optimization of the process parameter of the flare emission capture plant was carried out using the Central Composite Design approach (CCD), with a three-level, three-factor (temperature, pressure, and time) experimental design. The Design Expert
® 12 software package was utilized for implementing the RSM optimization. The experimental values of the factors in actual terms and the responses (removal efficiency of CO
2, CO, NO
x, and HC) are presented in
Table 3. The novel plant RSM optimization result shows that 93.24% of CO
2 and 62.18% of CO were absorbed, while 86.14% of NO
x and 55.87% of HC were absorbed. The established optimum conditions of CO
2, NO
x, HC, and CO removal efficiency are 22 °C, 2 atm, and 60 min.
Table 3 shows the various experimental runs performed on the flare emission capture pilot plant in assessing the optimum parameter for emission removal efficiency. This indicates that the Akrosorb and Bentonite adsorbent used in the column has a relatively low affinity for hydrocarbon (HC) removal.
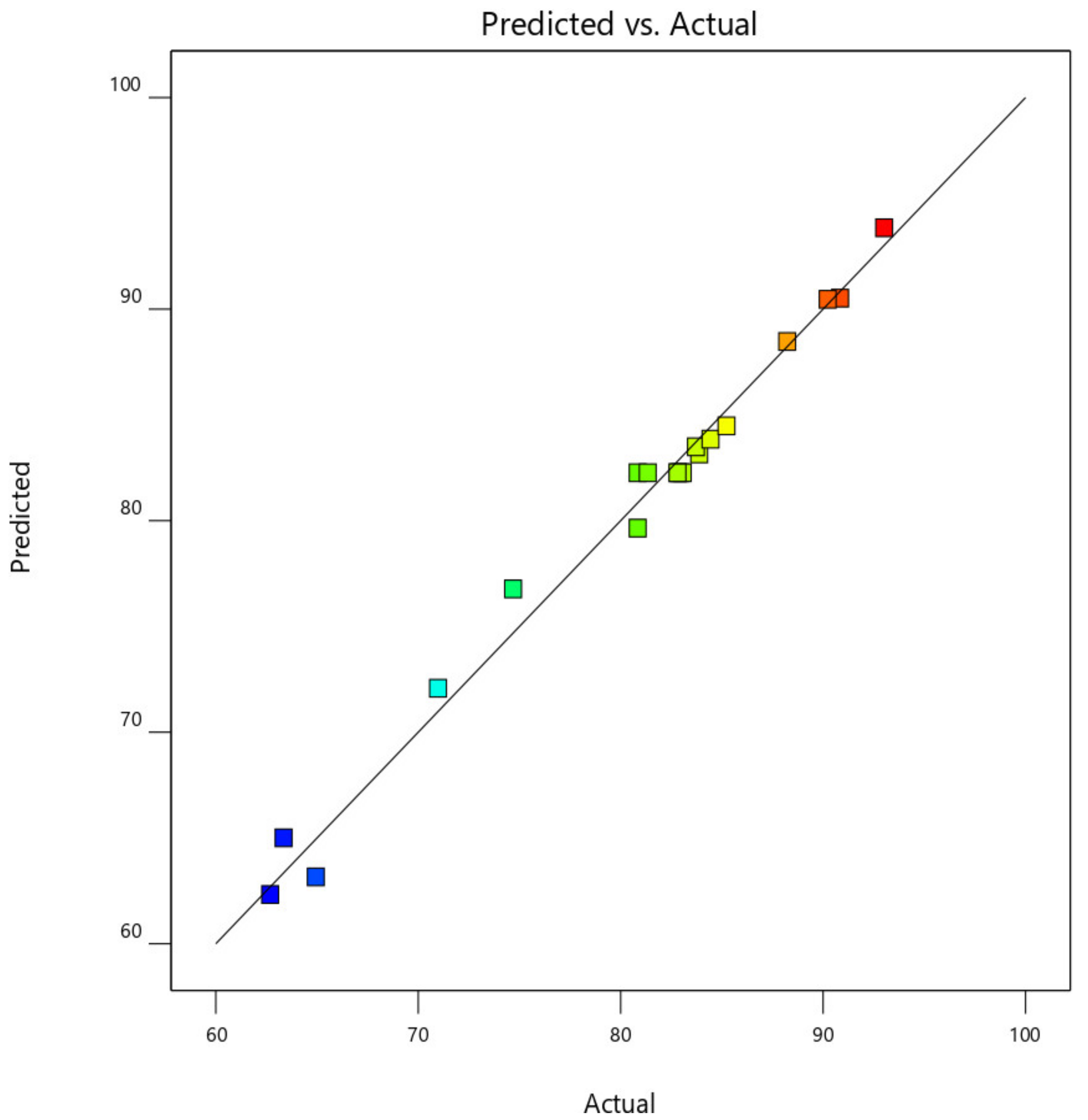
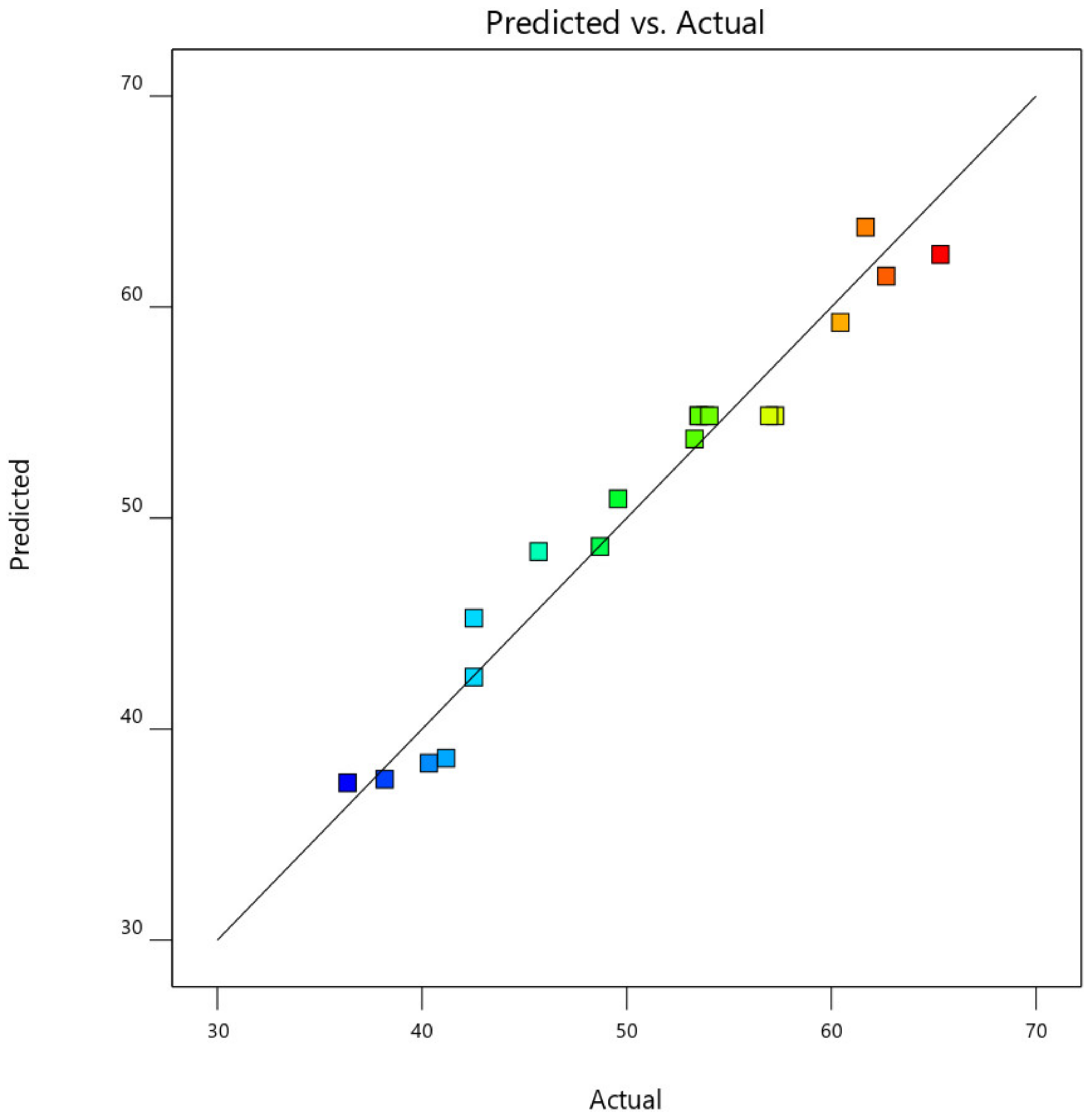
This was further demonstrated by the plot of predicted CO
2, CO, NO
x, and HC removal efficiency against the actual experimental CO
2, CO, NO
x, and HC removal efficiency values, as shown in
Figure 17. From
Figure 18, the data point was found to be very closer to the diagonal lines, which indicates the closeness of the model’s predicted result with that of the actual experimental results, which further demonstrates and validates the goodness of the fitted models.
4.5.1. Result of Optimum Process Parameter of Emission Removal Efficiency
The primary objective of the study is to optimize the process parameters for maximum CO
2, CO, NO
x, and HC removal efficiency by the pilot plant. As such, the process parameters were optimized using response surface CCD experiments. The optimization tool in the Design Expert 12 application package was used to determine the optimum parameter that maximizes CO
2, CO, NO
x, and HC removal efficiency using the optimum desirability function with the setup constraint for process parameters in the range of input examined in this study as show in
Table 4, while the constraint for the responses was maximized for all responses.
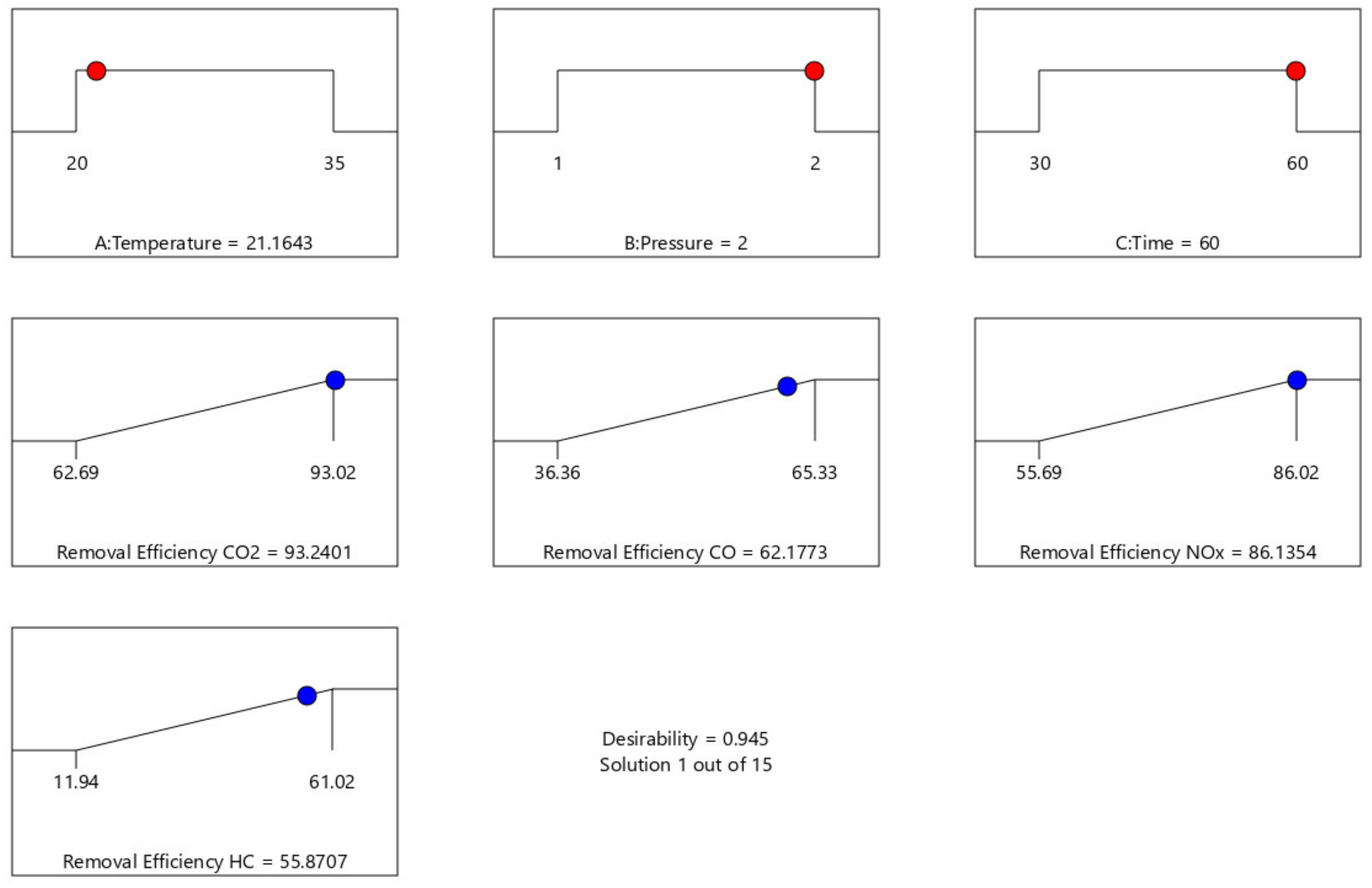
Table 5 and
Figure 19 show the result of the optimization of the process parameter for maximum response.
Table 5 shows the optimization result generated at different experimental design points. The optimum condition of the process parameter that maximizes CO
2, CO, NO
x, and HC removal efficiency are 21.164 °C, 2 atm, and 60 min to give 93.24%, 62.18%, 86.14%, and 55.87% for CO
2, CO, NO
x, and HC removal efficiency, respectively, at a desirability factor of 0.945 (a mathematical method to find the optimum). Additionally, a Ramp plot, which is a graphical view of each optimal solution, was used to depict the established optimum process parameters as well as the responses depict in
Figure 19.
4.5.2. Validation Result of the Optimized Process Parameter
A validation experiment was conducted to test and validate the reliability of the optimum process conditions of 22 °C, 2 atm, and 60 min obtained from the optimization study. The validation experiment was carried out in triplicate. The average experimental CO
2, CO, NO
x, and HC removal efficiencies were determined, as shown in
Figure 19. The CO
2, CO, NO
x, and HC removal efficiencies obtained from the validation test were found to be very close to the predicted optimum values for CO
2, CO, and NO
x with less than 1% margin of error, except for and HC removal efficiency with about 9.4% margin of error. Hence, the results indicated that there is no significant difference between the established optimum and the experimental validation results. This shows that the RSM predictions were in good agreement with experimental values, and the developed model with minimum percent errors <1% for CO
2, CO, and NO
x and 9.4% error for HC removal efficiency. Therefore, this indicated that the optimization achieved in the present study was reliable, and the established optimum process parameters for CO
2, CO, NO
x, and HC removal efficiency are 22 °C, 2 atm, and 60 min.
4.6. Result of Analysis of Variance (ANOVA) for GHGs Removal Efficiency
The ANOVA was used to transform the single values into statistical values and to test the significant difference between the mean values of the inlet and outlet, within and among the compounds. Statistical analysis of the removal efficiency of the pilot plant was performed using ANOVA test to evaluate and check the effect of inlet and outlet gas concentration on the removal efficiency of the pilot plant. The results of the ANOVA test are summarized in
Table 6.
The
p-value, which is an index measuring the discrepancy of the strength of evidence against the null hypothesis (the hypothesis that there is no association between the factors and response variable), was examined. To quantify the strength of evidence against the null hypothesis,
p < 0.05 (5% significance) is used as a standard level for concluding that there is evidence against the hypothesis tested. The significance difference at the inlet and outlet concentration on the removal efficiency was evaluate using
p-values as well as F-value and F-critical value to examine the interaction strength of each parameter. From
Table 6, it can be seen that the sample collection
p-value is 0.9918 (
p > 0.05), which indicates that the sample collection period is not significant and does not have an effect on the removal efficiency of the pilot plant. However, the
p-value of the inlet and outlet concentration of the gases is 0.0074 (
p < 0.05), which indicates that the inlet and outlet concentration of the gases is significant and has a significant effect on the removal efficiency of the pilot plant. Statistically, the variation in flare gas emission could impact the removal efficiency of the plant.
In addition, the interaction between the sample collection period and inlet and outlet concentration of the gases was examined to check the impact on removal efficiency of the plant. From
Table 7, it was found that the
p-value of the interaction between the sample collection and the inlet and outlet concentration of the gases is 0.9999 (
p > 0.05), which indicates that there is no significant interaction between the sample collection period, inlet, and outlet concentration of the gases with the removal efficiency of the pilot plant. Therefore, this implies that the relationship between the sample collection period and inlet concentration has no significant effect on the removal efficiency of the pilot plant and that the removal efficiency is dependent on the inlet concentration of the flare gas emitted. The ANOVA estimated marginal means for the inlet and the outlet emission.
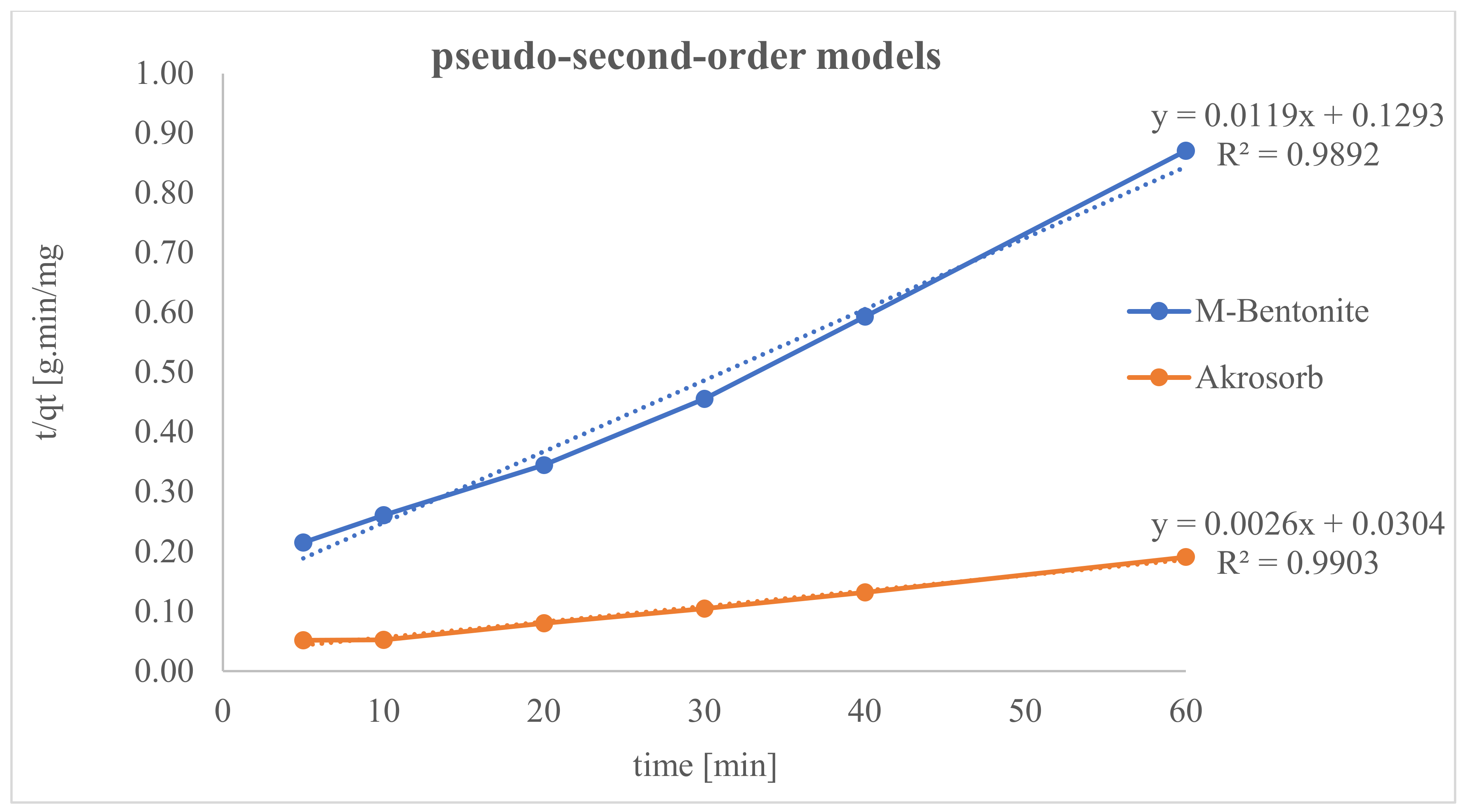
4.7. Results of Adsorption Capacity
Figure 20 depicts the adsorption trend of CO
2 gas in Akrosorb soda-lime and molded Bentonite ball adsorbents for 0–60 min and 35 °C temperature. A sharp rise in adsorption of CO
2 was observed in the first 0–10 min to attain adsorption of 192.0 mg CO
2/g and 38.35 mg CO
2/g for Akrosorb soda-lime and molded Bentonite ball adsorbents, respectively. This could be attributed to the availability of a sufficient adsorption site per unit mass of adsorbent in the first 0–10 min of adsorption. In addition, for both Akrosorb soda-lime and molded Bentonite ball adsorbents, the rate of CO
2 adsorption increases slightly from 192 to 286.40 mg/g and 38.35 to 65.87 mg/g respectively as time increases from 10 to 30 min. However, as the adsorption time increases beyond 30 min to 60 min, the curve for both Akrosorb soda-lime and molded Bentonite ball adsorbents tends to flatten, reaching maximum adsorption of 314.30 mg/g and 68.90 mg/g at 60 min. This is attributed to the fact that a further increase in time beyond 30 min resulted in the adsorption sites getting saturated gradually and the acceptance rate being regulated by the rate at which the CO
2 gas is been moved from the exterior of the adsorbent to the internal part, and as such, the adsorption rate became much slower.
Furthermore, it was noted that the Akrosorb soda-lime adsorbent exhibited a higher adsorption capacity than the molded Bentonite balls adsorbent. This could be attributed to the higher surface and pore area associated with the Akrosorb soda-lime adsorbent with 1072 m2/g surface area when compared to that of the molded Bentonite adsorbent with 498.2 m2/g surface area, respectively. In addition, the pore volume of the Akrosorb soda-lime adsorbent was in the range of 0.02478–0.3810 m3/g, which was much higher than those of molded Bentonite balls adsorbents in the range of 0.02364–0.1770 m3/g, while the cumulative adsorption pore volume was also established from the Branuer–Emmett–Teller analysis to be 0.5444–0.5566 m3/g for the Akrosorb adsorbent, which was more than twice that of the molded Bentonite ball (0.2533–0.2665 m3/g). The low adsorption capacity associated with Bentonite could be attributed to the fact that Bentonite, though proven to be efficient in removing many toxic materials, particularly metallic, organic, and gaseous contaminants, has a low ability to absorb organic and gaseous molecules such as CO2 and the active site is not uniform. As such, a solution is needed to improve its adsorption performance. Hence, the adsorption rate for both Akrosorb soda-lime and molded Bentonite ball adsorbents decreases with increases in adsorption time due to the attainment of equilibrium.