Abstract
A series of new bidentate N,S-ligands—aziridines containing a para-substituted phenyl sulfide group—was synthesized and evaluated in the Pd-catalyzed Tsuji–Trost reaction and addition of diethylzinc and phenylethynylzinc to benzaldehyde. A high enantiomeric ratio for the addition reactions (up to 94.2:5.8) was obtained using the aziridine ligand bearing a p-nitro phenyl sulfide group. Collected results reveal a specific electronic effect that, by the presence of particular electron-donating or electron-withdrawing groups in the PhS- moiety, influences the σ-donor–metal binding and the enantioselectivity of the catalyzed reactions.
1. Introduction
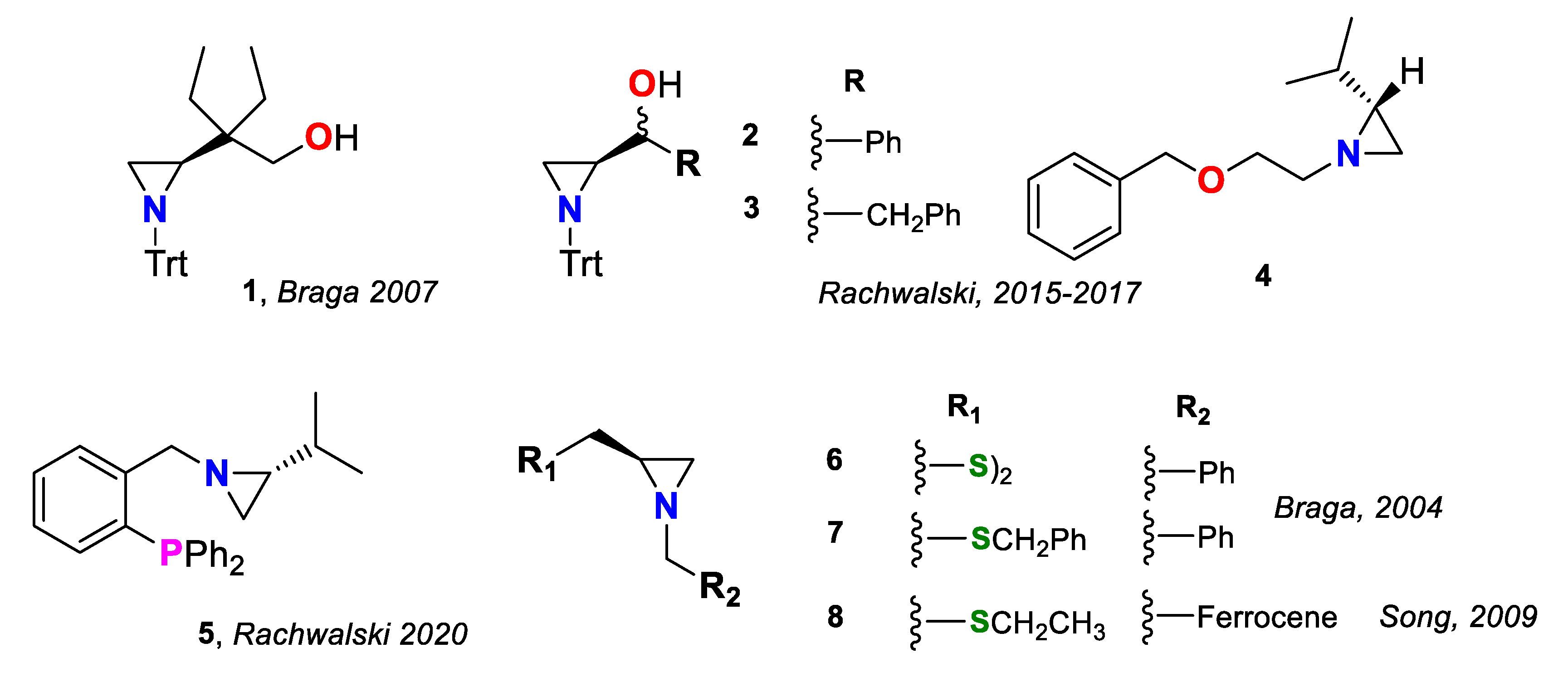
Aziridines have established their position in modern organic synthesis thanks to a fortunate combination of properties—reactivity, stability and multidirectional transformability with high atom economy [1]. These small heterocycles can be variously functionalized on the nitrogen or both carbon atoms and serve as stable intermediates, which, through a facile ring opening with various nucleophiles, can efficiently introduce a specific puzzle to a more complex molecule [2,3]. Their utility is even greater when chirality is considered. In a fused three-membered ring system, the chiral center is always adjacent to the nitrogen donor, constituting an alluring ligand for asymmetric synthesis [4,5]. Till now, enantiopure aziridines were broadly evaluated as reagents and catalysts in asymmetric reactions [6]. As heterobidentate ligands, with incorporated heteroatoms such as oxygen, in the form of alcohols, ethers or phenols; phosphorus, as phosphines; and sulfur, as sulfides and disulfides, they can efficiently improve the stereoselectivity of the transition metal-catalyzed carbon–carbon bond formation in various types of reactions, including alkenylzinc addition to aldehydes (compounds 1 [7], 2 [8], 3 [9,10], 6 [11]) and to enones (derivative 4 [12]), Friedel–Crafts alkylation of indoles (phosphine 5 [13]) and Pd-catalyzed allylic alkylation (N,S-ligands 7 [14] and 8 [15])—examples are presented in Figure 1.
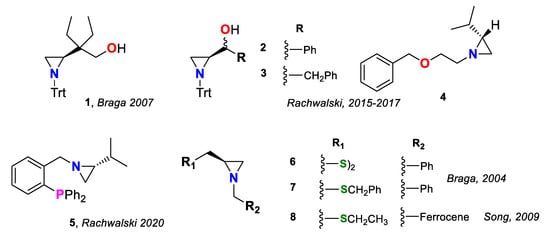
Figure 1.
N,heteroatom-bidentate ligands 1–8 for carbon–carbon bond formation.
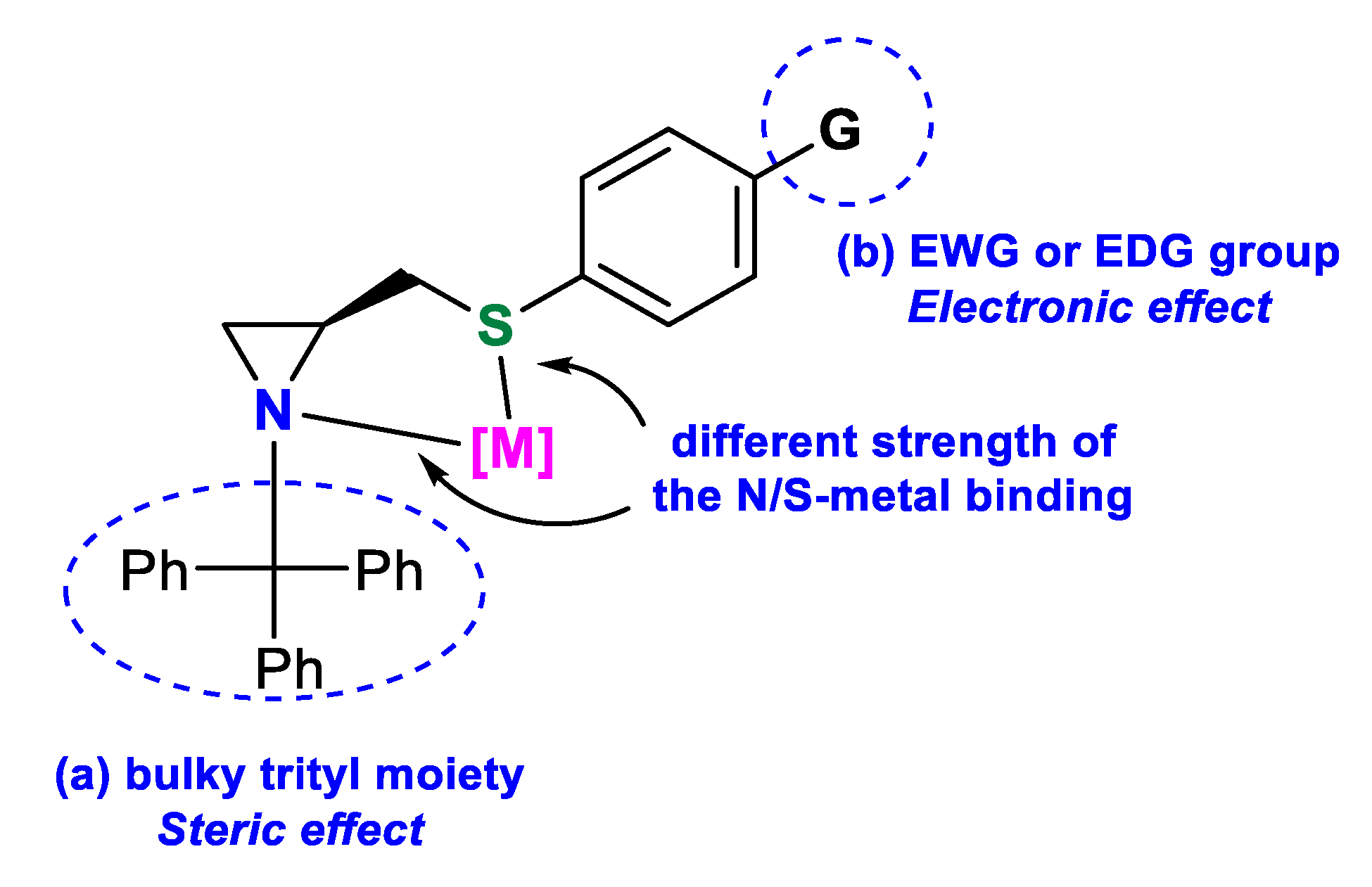
Divergent electronic effects of heteroatoms can improve the selectivity of the reaction due to the different bindings of the two donors to the central metal atom. N(sp3),S-bidentate aziridine-based ligands, presented in Figure 1, are known to enantioselectively promote metal-catalyzed asymmetric reactions; however, the scope of investigations is limited to the work of Braga [11,14] and Song [15]. The aim of this work was to obtain a series of new aziridine sulfide ligands and evaluate which specific feature—steric or electronic effect—influences the enantioselectivity of the carbon–carbon bond formation by two common protocols: synthesis of secondary alcohols through diethylzinc and phenylethynylzinc addition to aldehydes and palladium-catalyzed asymmetric allylic alkylation. The obtained compounds will combine (a) a bulky trityl moiety attached to the aziridine nitrogen, providing a steric hindrance that may direct the side of metal–donor coordination, and (b) an electron withdrawing (EWG) or electron donating (EDG) group, at the phenylsulfanyl substituent, decreasing or increasing the electron density on the sulfur atom, thus modulating the strength of the S–[M] binding (Figure 2).
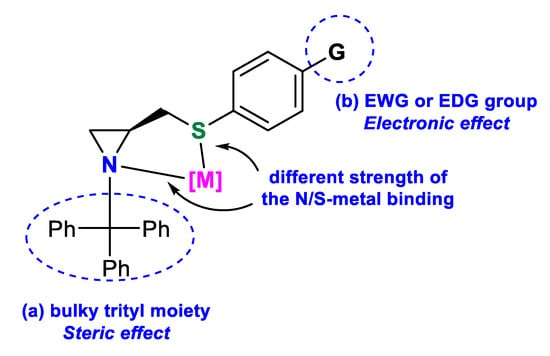
Figure 2.
Construction of the designed ligands.
2. Results and Discussion
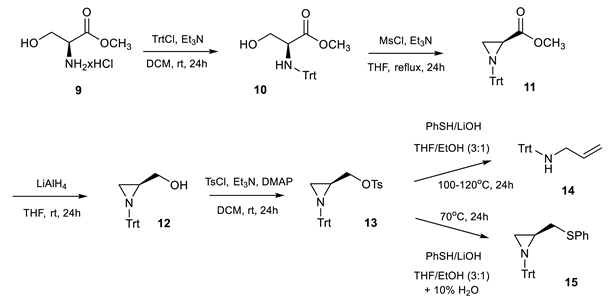
The first step of the research involved the synthesis of N-trityl aziridine tosylate 13 that was performed by a multistep procedure presented by Schneider et al. [16] starting from L-serine methyl ester 9. The substrate 9 was transformed to the corresponding N-trityl derivative 10 which was cyclized to aziridine 11 by treatment with mesyl chloride. Further reduction with lithium aluminum hydride yielded the aziridine alcohol 12, next converted to the tosylate 13. To obtain the final sulfanylaziridines, further nucleophilic substitution of the tosyl group was planned to be performed through the formation of a thiophenolate PhS−M+. However, at elevated temperatures, cleavage of the aziridine ring was observed, furnishing the allylic amine 14. Optimization involved modification of the temperature and solvent and estimation of the influence of base exchange. Obtained data are summarized in Table 1.

Table 1.
Synthesis of sulfanylaziridines—reaction optimization.
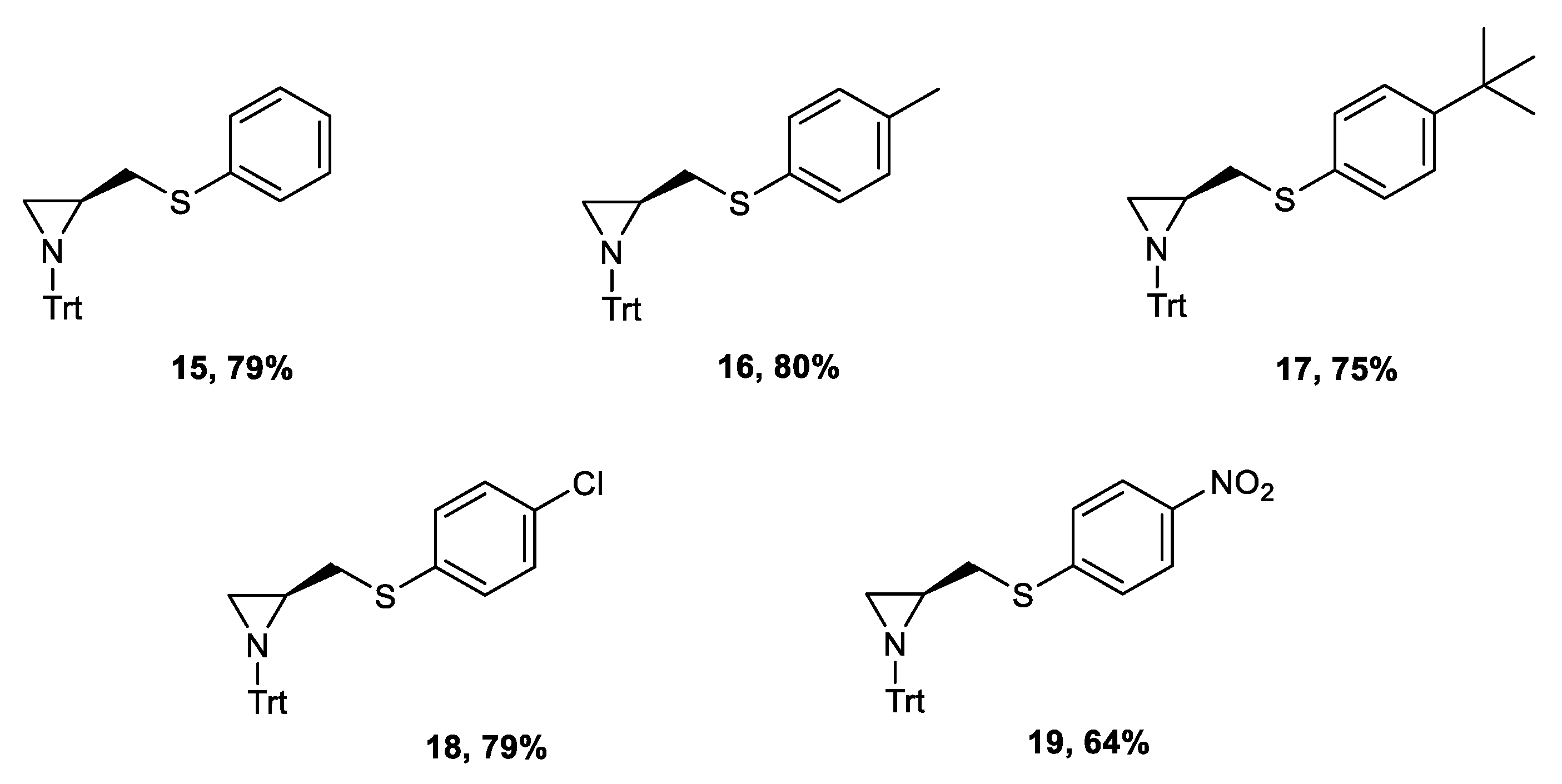
The highest yield of the final aziridine was obtained when 10% of water was added to the solvent. The increased solubility of the used base—lithium hydroxide—elevated the rate of the product formation. The procedure was efficient for all variously p-substituted ligands (yields: 64–80%) (Figure 3).
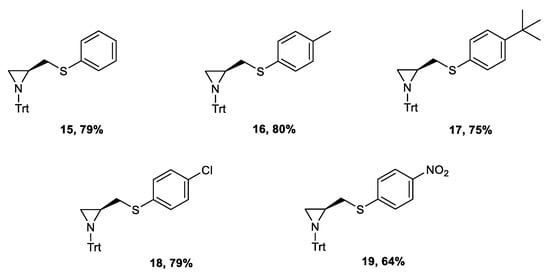
Figure 3.
Structure of the synthesized ligands 15–19.
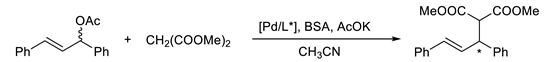
Next, the catalytic properties of synthesized sulfanylaziridines were evaluated. The Pd-catalyzed Tsuji–Trost reactions between racemic 1,3-diphenyl-2-propenyl acetate and dimethyl malonate were carried out on a 0.2 mmol scale with 10 mol% of chiral ligands 15–19. The selected reaction conditions were based on our previous research. We observed that increasing the amount of catalyst does not improve the yield and enantiomeric excess of the process. Alternatively, lowering the quantity of the catalyst decreases the overall enantioselectivity [17,18,19]. Results are presented in Table 2.

Table 2.
Results of palladium-catalyzed Tsuji–Trost reaction with chiral ligands 15–19 a.
The highest enantiomeric excess was observed for the unsubstituted aziridine 15. The overall enantioselectivity of all reactions was low, indicating that the N-trityl group prevents an efficient Het–[M] binding. This demonstrates that when the nitrogen atom is too sterically hindered, weak N–Pd coordination decreases the power of the nitrogen atom as the π-acceptor. In coordination with the weak S–Pd binding, the selectivity of the nucleophilic attack towards the carbon atoms of the formed palladium–allyl complex is only moderate.
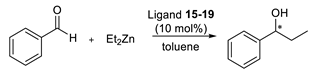
Further activity evaluation involved the synthesis of secondary alcohols by the addition of diethylzinc to benzaldehyde. Basing on known literature reports [20,21,22], we used 10 mol% of catalyst as the most common reaction condition. Results are collected in Table 3.

Table 3.
Results of diethylzinc addition to benzaldehyde promoted by ligands 15–19.
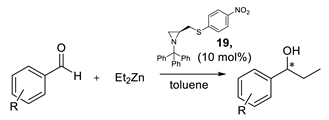
For unsubstituted aziridine 15 and compounds with additional electron-donating groups 16–19, the reaction proceeded with low enantiomeric excess. However, in the case of 4-nitrophenyl derivative 19, the final secondary alcohol was obtained with a high excellent enantiomeric ratio. We can assume that when the binding power of the sulfur atom is diminished, through the electron-withdrawing effect of the p-NO2 group, the increased affinity of the N(sp3) atom to the metal center significantly increases the formation of one enantiomer.
The catalytic properties of compound 19 were further tested on several benzaldehydes. In all cases, the result was repetitively selective, confirming the promising catalytic activity of ligand 19 (Table 4).

Table 4.
Results of diethylzinc addition to substituted benzaldehydes promoted by ligand 19.
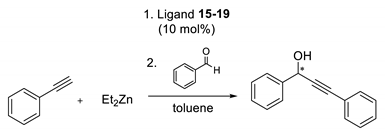
Finally, the catalytic activity of all ligands 15–19 was tested in the addition of phenylethynylzinc to benzaldehyde (Table 5).

Table 5.
Results of phenylethynylzinc addition to benzaldehyde promoted by ligands 15–19.
The resultant enantiomeric ratio was moderate for all derivatives; however, the best result was also observed for the p-nitrosubstituted aziridine 19.
3. Materials and Methods
3.1. General
Melting points were measured with a Büchi Tottoli SPM-20 heating unit (Büchi Labortechnik AG, Flawil, Switzerland) and were uncorrected. NMR spectra were recorded on a Bruker Avance III/400 or Bruker Avance III/700 (Karlsruhe, Germany) for 1H and 176.1 MHz or 100.6 MHz for 13C. Chemical shifts were recorded relative to SiMe4 (δ0.00) or solvent resonance (CDCl3 δ7.26, CD3OD δ3.31). Multiplicities were given as: s (singlet), d (doublet), dd (double doublet), ddd (double double doublet), t (triplet), dt (double triplet) and m (multiplet). NMR spectra were carried out using ACD/NMR Processor Academic Edition. Elemental analyses were performed on a Vario MACRO CHN analyzer. Commercially available solvents DMF, DCM and MeOH (Aldrich, St. Louis, MO, USA) and chemicals were used without further purification. Column chromatography was performed using Merck 40-63D 60Å silica gel (Merck, Darmstadt, Germany) [23]. HPLC analysis was performed on SHIMADZU NEXERA X2 apparatus using CHIRALPAK® IA-3 (4.6 mm × 25 cm) (Chiral Technologies INC. West Chester, PA, USA) without a guard column. Each HPLC analysis was controlled by comparison with the pure sample and the racemate.
3.2. Procedures and Analysis Data
3.2.1. Synthesis of (S)-Methyl 3-Hydroxy-2- (Tritylamino)Propanoate 10
A mixture of (S)-methyl 2-amino-3-hydroxypropanoate hydrochloride 9 (5.0 g, 32 mmol) and Et3N (8.94 mL, 64 mmol) in DCM (90 mL) was stirred under an argon atmosphere at room temperature until it dissolved. Next, the solution was cooled to 0 °C, tritylchloride (8.95 g, 32 mmol) was added portion-wise and the mixture was stirred at ambient temperature. After 24 h, the solution was washed with aqueous citric acid (10%) (3 × 30 mL) and water (2 × 30 mL). The combined organic layers were dried over MgSO4, filtered and concentrated on a rotary evaporator. The crude product was purified by crystallization (solvent: methanol) [16]. Yield: 60%.
3.2.2. Synthesis of (S)-Methyl 1-Tritylaziridine-2-Carboxylate 11
To a solution of trityl serine methyl ester 10 (7.0 g, 19.38 mmol) in THF (80 mL), cooled to 0 °C, Et3N (5.93 mL, 42.64 mmol) was added dropwise under an argon atmosphere. Next, methanesulfonyl chloride (1.80 mL, 23.26 mmol) was added, and the solution was stirred at 0 °C for 30 min and then refluxed for 72 h. The solvent was evaporated under vacuo, and the reaction mixture was dissolved in ethyl acetate and washed with 10% citric acid solution (3 × 50 mL), sodium bicarbonate (2 × 40 mL) and water. The combined organic layers were dried over anhydrous magnesium sulfate and evaporated. The crude product was purified by crystallization (solvent: methanol) [16]. Yield: 65%.
3.2.3. Synthesis of f (S)-(1-Tritylaziridin-2-yl)Methanol 12
The aziridine ester 11 (4.30 g, 12.56 mmol) was dissolved in THF (70 mL) and cooled to 0 °C, and LiAlH4 (1.19 g, 31.33 mmol) was added portion-wise. After 24 h of stirring at room temperature, the mixture was cooled to 0 °C and 2 M aqueous NaOH was added dropwise until the gray residue turned to white solid. The resulting suspension was washed with Et2O and decantated several times. The combined organic layers were dried over anhydrous magnesium sulfate and evaporated. The crude product was used without further purification [16]. Yield: 96%.
3.2.4. Synthesis of (S)-(1-Tritylaziridin-2-yl)Methyl 4- Methylbenzenesulfonate 13
To a solution of (S)-(1-tritylaziridin-2-yl)-methanol 12 (3.80 g, 12.06 mmol) in DCM (60 mL), cooled to 0 °C, triethylamine (1.36 g, 13.54 mmol), tosyl chloride (2.53 g, 13.27 mmol) and DMAP (0.015 g, 1.20 mmol) were added. The mixture was stirred at room temperature for 24 h. Next, the solution was washed with sodium bicarbonate (3 × 30mL) and water. The combined organic layers were dried over anhydrous magnesium sulfate and evaporated. The crude product was used without further purification [16]. Yield: 87%.
3.2.5. Synthesis of 2-Thiophenylaziridines 15–19
To a solution of p-substituted thiophenol (1.00 mmol) in a mixture of THF (3 mL) and ethanol (2 mL), an aqueous solution of lithium hydroxide (2.30 mmol/0.8 mL H2O) was added and stirred for 2 h in reflux. Next, tosylate (1.00 mL), dissolved in THF (3 mL), was added and the reaction mixture was stirred for an additional 24 h at 70 °C. The reaction was cooled to room temperature and the solvents were evaporated on a rotary evaporator. The obtained residue was dissolved in DCM and washed with water. The combined organic layers were dried over magnesium sulfate and evaporated. The obtained crude product was purified by column chromatography on basic aluminum oxide, using hexane/diethyl ether (98:2) as eluent.
(S)-2-(Phenylsulfanylmethyl)-1-Tritylaziridine 15
Yield: 79%; crystalline solid, mp 92–94 °C; = −65.00 (c = 1, CHCl3)
1H NMR (700 MHz, CDCl3) δ = 1.17 (d, J = 5.6 Hz, 1H), 1.53—1.56 (m, 1H), 1.75 (d, J = 3.5 Hz, 1H), 3.08 (dd, J1 = 13.3 Hz, J2 = 7.0 Hz, 1H); 3.45 (dd, J1 = 7.7 Hz, J2 = 4.2 Hz, 1H), 7.17 −7.31 (m, 14H), 7.51 (d, J = 7.7 Hz, 6H) ppm; 13C NMR (100.6 MHz, CDCl3) δ = 27.4 (CH2), 32.3 (CH), 37.4 (CH2), 74.2 (C), 126.1 (CH), 126.7 (3 × CH), 127.5 (6 × CH), 128.9 (2 × CH), 129.5 (6 × CH), 129.7 (2 × CH), 136.4 (C), 144.5 (3 × C) ppm; IR: 3057, 3018, 2920, 2851, 1579, 1487, 1445, 1385, 1242, 1214, 1185, 1154, 1085, 1067, 1021, 1004 cm−1; Elemental Anal. Calcd for C28H25NS (407.58): C, 82.51; H, 6.18; N, 3.44 Found: C, 82.46; H, 6.10; N, 3.43 (see Supplementary Materials S1).
(S)-2-(p-Tolylsulfanylmethyl)-1-Tritylaziridine 16
Yield: 80%; oil, = −17.00 (c = 1, CHCl3)
1H NMR (700 MHz, CDCl3) δ = 1.11 (d, J = 6.3 Hz, 1H), 1.46—1.49 (m, 1H), 1.67 (d, J = 4.2 Hz, 1H), 2.30 (s, 3H), 2.98 (dd, J1 = 12.6 Hz, J2 = 7.7 Hz, 1H); 3.36 (dd, J1 = 13.3 Hz, J2 = 4.2 Hz, 1H), 7.03 (d, J = 7.7 Hz, 2H), 7.19−7.26 (m, 11 H), 7.46 (d, J = 7.7 Hz, 6H) ppm 13C NMR (100.6 MHz, CDCl3) δ = 21.1 (CH3), 27.5 (CH2), 32.5 (CH), 38.2 (CH2), 74.2 (C), 126.8 (3 × CH), 127.5 (6 × CH), 129.6 (6 × CH), 129.7 (2 × CH), 130.7 (2 × CH), 132.7 (C), 136.3 (C), 144.6 (3 × C) ppm; IR: 3054, 3018, 2976, 2917, 1961, 1893, 1808, 1595, 1489, 1446, 1386, 1316, 1245, 1213, 1182, 1151, 1118, 1088, 1032, 1013 cm−1; Elemental Anal. Calcd for C29H27NS (421.60): C, 82.62; H, 6.46; N, 3.32 Found: C, 82.72; H, 6.63; N, 3.24 (see Supplementary Materials S2).
(S)-2-((4-Tert-Butylphenylsulfanyl)Methyl)-1-Tritylaziridine 17
Yield: 75%; oil, = −6,5 (c = 1, CHCl3)
1H NMR (700 MHz, CDCl3) δ = 1.12 (d, J = 5.6 Hz, 1H), 1.29 (s, 6H), 1.46—1.49 (m, 1H), 1.53 (s, 3H), 1.70 (d, J = 3.5 Hz, 1H), 3.00 (dd, J1 = 16.3 Hz, J2 = 7 Hz, 1H); 3.40 (dd, J1 = 16.3 Hz, J2 = 4.2 Hz, 1H), 7.19–7.26 (m, 13 H), 7.46 (d, J = 7.7 Hz, 6H) ppm; 13C NMR (100.6 MHz, CDCl3) δ = 27.0 (CH2), 30.9 (3 × CH3), 31.9 (C), 34.0 (CH), 37.3 (CH2), 73.7 (C), 125.4 (2 × CH), 126.2 (3 × CH), 127.0 (6 × CH), 129.1 (6 × CH), 129.5 (2 × CH), 132.2 (C), 144.0 (3 × C), 149.0 (C) ppm;IR: 3056, 3030, 2956, 2866, 1595, 1488, 1459, 1446, 1393, 1316, 1267, 1246, 1201, 1184, 1151, 1120, 1084, 1052, 1012 cm−1; Elemental Anal. Calcd for C32H33NS (463.68): C, 82.89; H, 7.17; N, 3.02 Found: C, 83.05; H, 7.21; N, 2.92 (see Supplementary Materials S3).
(S)-2-((4-Chlorophenylsulfanyl)Methyl)-1-Tritylaziridine 18
Yield: 79%; crystalline solid, mp: 112-114 °C; = −6,2 (c = 1, CHCl3)
1H NMR (700 MHz, CDCl3) δ = 1.13 (d, J = 6.3 Hz, 1H), 1.44—1.47 (m, 1H), 1.70 (d, J = 2.8 Hz, 1H), 3.01 (dd, J1 = 16.3 Hz, J2 = 7.0 Hz, 1H); 3.60 (dd, J1 = 16.3 Hz, J2 = 4.2 Hz, 1H), 7.15—7.29 (m, 13H), 7.45 (d, J = 7.7 Hz, 6H) ppm; 13C NMR (100.6 MHz, CDCl3) δ = 27.0 (CH2), 31.7 (CH), 37.1 (CH2), 74.0 (C), 126.3 (3 × CH), 127.1 (6 × CH), 128.6 (2 × CH), 129.0 (6 × CH), 130.5 (2 × CH), 134.4 (C), 143.9 (3 × C), 145.83 (C) ppm; IR: 3051, 3033, 2921, 2851, 1594, 1487, 1446, 1376, 1240, 1219, 1175, 1155, 1111, 1092, 1047, 1032, 1006 cm−1; Elemental Anal. Calcd for C28H24ClNS (442.02): C, 76.08; H, 5.47; N, 3.17 Found: C, 76.09; H, 5.48; N, 3.12 (see Supplementary Materials S4).
(S)-2-((4-Nitrophenylsulfanyl)Methyl)-1-Tritylaziridine 19
Yield: 64%; crystalline solid, mp: 125–127 °C; = −16,4 (c = 1, CHCl3)
1H NMR (700 MHz, CDCl3) δ = 1.19 (d, J = 5.6 Hz, 1H), 1.48—1.52 (m, 1H), 1.81 (d, J = 3.5 Hz, 1H), 3.17 (dd, J1 = 16.3 Hz, J2 = 7.0 Hz, 1H); 3.47 (dd, J1 = 15.6 Hz, J2 = 4.2 Hz, 1H), 7.22—7.30 (m, 13H), 7.47 (d, J = 10.7 Hz, 6H), ppm; 13C NMR (100.6 MHz, CDCl3) δ =: 27.0 (CH2), 31.0 (CH), 35.0 (CH2), 73.9 (C), 123.5 (2 × CH), 126.2 (2 × CH), 126.5 (3 × CH), 127.2 (6 × CH), 129.0 (6 × CH), 143.8 (3 × C), 145.6 (C), 147.0 (C) ppm; IR: 3052, 3020, 2913, 2852, 1594, 1485, 1470, 1445, 1380, 1272, 1242, 1181, 1153, 1087, 1056, 1031, 1002 cm−1; Elemental Anal. Calcd for C28H24N2O2S (452.57): C, 74.31; H, 5.35; N, 6.19 Found: C, 76.66; H, 5.95; N, 6.79 (see Supplementary Materials S5).
3.3. General Procedure for AAA (Trost–Tsuji) Reaction
The solution of chiral ligand (0.020 mmol, 10 mol%) and [Pd(η3-C3H5)Cl]2 (1.9 mg, 0.005 mmol) in acetonitrile (0.5 mL) was stirred under argon atmosphere at room temperature for 15 min. To this mixture, the solution of rac-1,3-diphenyl-2-propenyl acetate (0.20 mmol, 0.050 g) in CH3CN (1.0 mL) was added followed by dimethyl malonate (0.069 mL, 0.6 mmol), N,O-bis(trimethylsilyl)acetamide (BSA, 0.148 mL, 0.6 mmol) and anhydrous potassium acetate (0.5 mg, 0.006 mmol). The solution was stirred at room temperature and the reaction was monitored by TLC. After 3–4 days, the solvent was evaporated and the residue was purified by column chromatography (n-hexane/ethyl acetate, 5:1 v/v) and analyzed by 1H NMR, and the enantiomeric excess was determined using chiral HPLC (Chiracel IA-3 column, n-hexane/i-PrOH 95:5, flow rate 1.0 mL/min, λ 251 nm, t(R) 9.4 min, t(S) 11.7 min) [24].
3.4. General Procedure for the Asymmetric Addition of Diethylzinc to Aldehydes
Toluene (0.5 mL) and Et2Zn (1 M in hexane, 0.376 mmol) were added to a 25 mL round-bottom flask under argon atmosphere. The catalyst (15–19) (10% mol, 0.0126 mmol) in toluene (0.5 mL) was then added and the mixture was stirred for 20 min at room temperature. After cooling to 0 °C in an ice bath, the solution of benzaldehyde (1 M in toluene, 0,126 mmol, 126 μL) was added to the reaction flask. After stirring overnight, the reaction was quenched with a saturated solution of NH4Cl (3 mL). The reaction mixture was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed with 1 M HCl (5 mL) and saturated NaCl solution (5 mL) and dried over anhydrous MgSO4. After filtration, the solvent was removed on a rotary evaporator to afford the product alcohol, which was analyzed on GC [25].
3.5. General Procedure for the Asymmetric Addition of Phenylethynylzinc to Benzaldehyde
In a 25 mL flask under argon toluene (0.5 mL), Et2Zn (1 M in hexane, 0.26 mmol, 0.26 mL) and phenylacetylene (0.286 mmol) were placed and stirred for 30 min at room temperature. Then, the catalyst (15–19) (10% mol, 0.013 mmol) in toluene (0.5 mL) was added and the mixture was stirred for 20 min at room temperature. The flask was cooled to 0 °C in an ice bath and the solution of benzaldehyde (1 M in toluene, 0,13 mmol, 130 μL) was added. After stirring overnight, the reaction was quenched with a saturated solution of NH4Cl (3 mL). The reaction mixture was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed with 1 M HCl (5 mL) and saturated NaCl solution (5 mL) and dried over anhydrous MgSO4. After filtration, the solvent was removed on a rotary evaporator under vacuum to give the product alcohol, in agreement with published data [26], which was analyzed on a chiral HPLC column (Chiracel OJ column, n-hexane/i-PrOH 80:20, flow rate 0.7 mL/min, λ 254 nm, t(1) 14.34 min, t(2) 24.32 min) [25].
4. Conclusions
This article presents an efficient methodology for the synthesis of new N(sp3),S-bidentate aziridine-based ligands. The addition of 10% of water to the used solvent significantly increased the yield of the product, indicating that the solubility of the used base is crucial for the nucleophile (PhS−M+) formation. The catalytic activity of all ligands was tested in the Pd-catalyzed Tsuji–Trost reaction and addition of diethylzinc and phenylethynylzinc to benzaldehyde. The obtained results conclude that the N-trityl moiety can presumably prevent the N–Pd coordination, decreasing the enantioselectivity of the reaction. In the case of the diethylzinc addition to benzaldehyde, the highest e.r. (94.2:5.8) was observed for the (S)-2-((4-nitrophenylsulfanyl)methyl)-1-tritylaziridine. This suggests that the EWG, through the reduction in the electron density on the sulfur atom, increases the N–Zn coordination. It can be concluded that in the case of this type of ligand, 2-(phenylsulfanyl)methylaziridines’ steric and electronic effects that enhance the N–[M] coordination correspond to the improvement in the enantioselectivity of the performed reactions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/13/3/502/s1.
Author Contributions
Conceptualization, J.Ś. and A.J.P.-M.; data curation, M.P.K., A.L., A.K. and M.Z.-B.; formal analysis, A.L., A.K. and M.Z.-B.; investigation, M.P.K., A.L., A.K., M.Z.-B. and A.J.P.-M.; writing—original draft, A.J.P.-M. and J.Ś.; writing—review and editing, M.P.K., A.J.P.-M. and J.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S. Advances in the synthesis and chemistry of aziridines. Adv. Heterocycl. Chem. 2019, 129, 245–335. [Google Scholar]
- Botuha, C.; Chemla, F.; Ferreira, F.; Pérez-Luna, A. Aziridines in Natural Product Synthesis. In Heterocycles in Natural Product Synthesis; Majumdar, K.C., Chattopadhyay, S.K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 154–196. [Google Scholar]
- Macha, L.; D’hooghe, M.; Ha, H.-J. Deployment of Aziridines for the Synthesis of Alkaloids and Their Derivatives. Synthesis 2019, 51, 1491–1515. [Google Scholar]
- Tanner, D. Chiral Aziridines—Their Synthesis and Use in Stereoselective Transformations. Angew. Chem. Int. Ed. 1994, 33, 599–619. [Google Scholar] [CrossRef]
- McCoull, W.; Davies, F.A. Recent Synthetic Applications of Chiral Aziridines. Synthesis 2000, 10, 1347–1365. [Google Scholar] [CrossRef]
- Braga, A.L.; Paixao, M.W.; Westermann, B.; Schneider, P.H.; Wessjohann, L.A. Aziridine-Modified Amino Alcohols as Efficient Modular Catalysts for Highly Enantioselective Alkenylzinc Additions to Aldehydes. Synlett 2007, 6, 917–920. [Google Scholar] [CrossRef]
- Jarzyński, S.; Utecht, G.; Leśniak, S.; Rachwalski, M. Highly enantioselective asymmetric reactions involving zinc ions promoted by chiral aziridine alcohols. Tetrahedron Asymmetry 2017, 28, 1774–1779. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Jarzyński, S.; Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Highly enantioselective addition of arylzinc reagents to aldehydes promoted by chiral aziridine alcohols. Tetrahedron Asymmetry 2016, 27, 1238–1244. [Google Scholar] [CrossRef]
- Jarzyński, S.; Leśniak, S.; Pieczonka, A.M.; Rachwalski, M. N-Trityl-aziridinyl alcohols as highly efficient chiral catalysts in asymmetric additions of organozinc species to aldehydes. Tetrahedron Asymmetry 2015, 26, 35–40. [Google Scholar] [CrossRef]
- Braga, A.L.; Milani, P.; Paixao, M.W.; Zeni, G.; Rodrigues, O.E.D.; Alves, E.F. Aziridine sulfides and disulfides as catalysts for the enantioselective addition of diethylzinc to aldehydes. Chem. Comm. 2004, 21, 2488–2489. [Google Scholar] [CrossRef] [PubMed]
- Jarzyński, S.; Rachwalski, M.; Pieczonka, A.M.; Wujkowska, Z.; Leśniak, S. Highly efficient conjugate additions of diethylzinc to enones promoted by chiral aziridine alcohols and aziridine ethers. Tetrahedron Asymmetry 2015, 26, 924–927. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Braga, A.L.; Paixao, M.W.; Milani, P.; Silveira, C.C.; Rodrigues, O.E.D.; Alves, E.F. New Aziridine Sulfide Ligands for Palladium-Catalyzed Asymmetric Allylic Alkylation. Synlett 2004, 7, 1297–1299. [Google Scholar] [CrossRef]
- Niu, J.-L.; Wang, M.-C.; Kong, P.-P.; Chen, Q.-T.; Zhu, Y.; Song, M.-P. Origin of enantioselectivity with heterobidentate sulfide-tertiary amine (sp3) ligands in palladium-catalyzed allylic substitution. Tetrahedron 2009, 65, 8869–8878. [Google Scholar] [CrossRef]
- Borges, R.; Andrade, F.C.D.; Schwab, R.S.; Sousa, F.S.S.; de Souza, M.N.; Savegnago, L.; Schneider, P.H. Straightforward synthesis and antioxidant studies of chalcogenoaziridines. Benzeneperoxyseleninic Acids—Synthesis and Properties. Tetrahedron 2016, 57, 3501–3504. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Rewucki, P.; Walenczak, S. Sulfur-containing derivatives from (1R)-(-)-myrtenal designed as chiral ligands. Tetrahedron 2016, 72, 3851–3857. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Siedlecka, R.; Skarżewski, J. Chiral phenylselenyl derivatives of pyrrolidine and Cinchona alkaloids: Nitrogen-selenium donating ligands in palladium-catalyzed asymmetric allylic alkylation. Tetrahedron Asymmetry 2007, 18, 131–136. [Google Scholar] [CrossRef]
- Wojaczyńska, E.; Zielińska-Błajet, M.; Turowska-Tyrk, I.; Skarżewski, J. Sulfoxides derived from Cinchona alkaloids—Chiral ligands in palladium-catalyzed asymmetric allylic alkylation. Tetrahedron Asymmetry 2010, 21, 853–858. [Google Scholar] [CrossRef]
- Huang, Z.; Lai, H.; Qin, Y. Syntheses of Novel Chiral Sulfinamido Ligands and Their Applications in Diethylzinc Additions to Aldehydes. J. Org. Chem. 2007, 72, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Prause, F.; Wagner, S.; Breuning, M. Enantioselective addition of diethylzinc to aldehydes catalyzed by 5-cis-substituted proline derivatives. Tetrahedron 2019, 75, 94–101. [Google Scholar] [CrossRef]
- Raji, M.; Le, T.M.; Fülöp, F.; Szakonyi, Z. Synthesis and Investigation of Pinane-Based Chiral Tridentate Ligands in the Asymmetric Addition of Diethylzinc to Aldehydes. Catalysts 2020, 10, 474. [Google Scholar] [CrossRef]
- Obieziurska, M.; Pacuła, A.J.; Laskowska, A.; Długosz-Pokorska, A.; Janecka, A.; Ścianowski, J. Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability. Materials 2020, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Van Vrankel, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Wang, M.-C.; Zhang, Q.-J.; Zhao, W.-X.; Wang, X.-D.; Ding, X.; Jing, T.-T.; Song, M.-P. Evaluation of Enantiopure N-(Ferrocenylmethyl)azetidin-2-yl(diphenyl)methanol for Catalytic Asymmetric Addition of Organozinc Reagents to Aldehydes. J. Org. Chem. 2008, 73, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-X.; Wu, J.; Au-Yeung, T.T.-L.; Yip, C.-W.; Haynes, R.K.; Chan, A.S.C. Highly Enantioselective Phenyl Transfer to Aryl Aldehydes Catalyzed by Easily Accessible Chiral Tertiary Aminonaphthol. J. Org. Chem. 2005, 70, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).