Abstract
Most biological organisms exhibit different kinds of symmetry; an Animal (Metazoa), which is our Darwinist ancestor, has bilateral symmetry, and many plants exhibit rotational symmetry. It raises some questions: I. How can the evolution from an undifferentiated cell without bilateral symmetry to a complex biological organism with symmetry, which is based on asymmetric DNA and enzymes, lead to the bilateral symmetry? II. Is this evolution to an organism with bilateral symmetry obtained by other factors than DNA and enzymatic reactions? The existing literature about the evolution of the bilateral symmetry has been reviewed, and a new hypothesis has been formulated based on these reviews. The hypothesis is that the morphogenesis of biosystems is connected with the metabolism and that the oscillating kinetics in the Glycolysis have played a role in the polarity of the biological cells and in the establishment of the bilateral symmetry in Animals.
1. Introduction
A biological organism consists of stereo specific molecules.The proteins and enzymes are polymers of L-amino acids and the carbohydrates are polymers of D-carbohydrate units. The structures in a biological organism are obtained from an undifferentiated cell by biochemical reactions with asymmetric enzymes. The development of the organism depends on this quality, whereby the DNA in an undifferentiated cell ensures the complex and unique biological order in the organism.
Nevertheless, most biological organisms exhibit different kinds of symmetry; the first Animals (Metazoa, which our Darwinist ancestor) have bilateral symmetry (Figure 1), and many plants exhibit rotational symmetry, and it raises some questions:

Figure 1.
An example of bilateral symmetry in Homo sapiens: La cueva de las manos (Argentina) 7300 BC. Copyright Wiki Commons.
I. How can the evolution from an undifferentiated cell without bilateral symmetry to a complex biological organism with symmetry, which is based on asymmetric DNA and enzymes, lead to the bilateral symmetry?
II. Is this evolution to an organism with bilateral symmetry obtained by other factors than DNA and enzymatic reactions?
I shall try to answer these two questions based on the existing scientific literature, after first describing the evolution of the bilateral symmetry in Animals.
2. Symmetries in Animals and Plants
Our first ancestor was a bacterium at least 3.5 billion years old [1,2], but the first and simple multicellular organism with bilateral symmetry was a Bilateria or Animal that appeared much later (almost all Animals are Bilaterians with a bilateral symmetry). The earliest fossils of Animals in the Precambrian evolution appeared in the Ediacaran period 635–541 million years ago (Mya) [3,4,5], and the Bilateria was part of the Cambrian explosion at ≈ 541 Mya [6]. The bilateral symmetry in Animals is probably developed from a sea anemone (Cnidaria) with radially symmetry (Nematostella vectensis) [7,8,9,10,11]. In addition, many plants exhibit different kinds of symmetry, e.g., rotational symmetry, but also bilateral symmetry. Today, most plants are flowering plants which are evolved from the Angiospermae. They appeared, however, much later than the Animals [12,13], at ≈ 140–250 Mya ago.
There has been an evolution of the symmetry in Animals after the emergence of the bilateral symmetry. On one hand, there are many examples of a tendency to develop some asymmetry in Animals, and, on the other hand, there is a corresponding evolutionary tendency for ensuring the bilateral symmetry.
The stability of the bilateral symmetry in Animals is maintained by Darwinian natural selections. It is in many contexts an advantage to have the Animal’s organs (ears, eyes, limbs, etc.) placed symmetrically with respect to the symmetry plane. But beyond that, the bilateral symmetry is also maintained by sexual preference. The bilateral symmetry is preserved during the evolution of sexual dimorphism, which, in general, results in a sexual differences, usually with dominating males. The success in male-male competition reflects high quality, and it is normally assumed that the female preference for dominant males should be widespread. But, e.g., investigations in Reference [14] show that females of the rock lizard Lacerta monticola prefer more symmetrical males as potential mates, by which the bilateral symmetry is preserved. The sexual preference among Humans shows, correspondingly, that a high degree of symmetry in the human faces is attractive, and it is found to be masculine for men and to be feminine for females [15]; bilateral symmetry is, in general, aesthetically attractive [16].
But there is also many examples of asymmetry in Animals. Almost all Animals are Bilaterians with bilateral embryos, but many Animals exhibit asymmetric elements. Reviews of asymmetry in Animals are given in References [17,18,19], with the key question [17]: do common rules govern how direction of asymmetry is determined during ontogeny to yield an asymmetrical individual? The answer is that both genes, environment, and chance influence the asymmetry. There is countless examples of local asymmetry in Animals, including asymmetry in Humans [19]. An asymmetry in Animals is, e.g., developed in trilobites relatively early after the emergence of the bilateral symmetry [20]. The asymmetry in a lobster with a cutter and a crusher is established during a lobsters evolution and appears with both the crusher to the left and to the right. While the critical period for crusher determination is genetically determined, the actual trigger is influenced by experience [21]. A similar asymmetry is obtained for crabs [22]. Flat fish have a remarkable asymmetry with both eyes placed on one side of the head. The asymmetry in a flat fish is genetically determined and emerges in its late larval condition, but with the occurrence of both asymmetries in samples of flat fish [23] (Figure 2). A similar example of asymmetry is the Human right-/left-handed asymmetry, which is initialed at an early state of the human embryo [24].

Figure 2.
Two flounder caught in the Baltic Sea. They are asymmetrical Animals but exhibit together a bilateral symmetry. A flounder has both eyes on the same side of its bilateral Animal plane.
The conclusion concerning the bilateral symmetry and asymmetry in Animals is that the bilateral symmetry first appears in the Animal at the late Precambrian evolution and the Cambrian explosion. The asymmetries in Animals appear later and can be caused by genetic, as well as environmental factors, and by chance [17], but with the basic bilateral symmetry maintained in the Animals.
3. The Bilateral Symmetry in Animals
There is an extensive literature about the emergence of the bilateral symmetry. The Darwinist ancestor is as mentioned a sea anemone Cnidaria, which has rotational symmetry. Since oligomeric enzymes in fact can have rotational symmetry [25,26] despite their asymmetric protein units, the morphogenesis of Cnidaria with rotational symmetry could alone be caused by the rotational symmetry of oligomeric enzymes, but this subject is not to debate. Here, I will focus on the evolution from a biological system with rotational symmetry to a system with bilateral symmetry.
The biological evolution is not revolutionary but characterized by gradual changes most often over enormous times, and, despite the general accepted name: the Cambrian explosion, this biological evolution had appeared over millions of years. An example of religious symbols with rotational and bilateral symmetry is shown in Figure 3. The old (and misused) religious Swastika icon has rotational symmetry but contains a cross which also has bilateral symmetries, and the change to the Crucifix with only bilateral symmetry can be obtained by small and continuous changes in the Swastika figure. By analogy with this property, the change from Cnidaria to Animal can be obtained by small and continuous evolutionary changes. As in the case of the emergence of asymmetries in Animals, one shall expect that both genes and environment and chance influence the change of symmetry from rotational to bilateral symmetry.
Figure 3.
Religious symbols with symmetry. The old Indian Swastika icon with rotational symmetry contains a cross with bilateral symmetries. The Crucifix has only a bilateral symmetry. Copyright Wiki Commons.
3.1. The Cellular Polarity and the Cell Division
There exist an extensive review literature about the developmental order and structure in a biological system [27,28,29,30,31,32,33,34,35], and with the focus on the changes in cell polarity. Cell polarity refers to spatial differences in shape, structure, and function within a cell. According to Reference [27], “the origin of large-scale symmetry in biology often lies in asymmetry at a smaller scale”; and, on a cellular level, there is already cell polarity with asymmetries in a simple Bacteria.
A Procariote exhibits a complex cell polarity with asymmetries [36], and, in Reference [28], the authors conclude that such a small scale asymmetry in the polarity of cytoskeletal filaments in two bacteria (Caulobacter crescentus and Bacillus subtilis) plays a central role in the symmetry break at the cellular level through a protrusive force generation and directional transport of molecular assemblies and organelles. In Reference [29], the cellular polarity of RNA in bacterial cells is reviewed. Their cell division is caused by three polarity protein determinants, MinC, MinD, and MinE, that oscillate between the cell poles during cell division. The mechanism for the self-organization of proteins is reviewed in Reference [30], with focus on a possible recruiting of specific proteins to the cell poles by a self-assembling. Another review of articles concerning polarity and growth mechanism in bacteria is given in Reference [31]. The bacterial cell growth consists of two distinct phases: cell elongation and septum formation prior to cell division. They conclude that temporal regulation of the peptidoglycan synthesis is a common theme. The kinetics in cell membranes with emergence of cell polarization is reviewed in Reference [32], where the authors conclude that the membrane kinetics is responsible for the cell polarity. The self-organization of structures in cell with cell polarity and “mechanical forces” is reviewed in Reference [33], with the hypothesis that it is a “ feedback interaction between polarity, mechanics and fate” through self-organizing interactions in this tripartite relationship.
The conclusion from the reviews of polarity in a Bacteria is that it is not at all “chemistry in a bag”, but that already a Bacterium is a highly ordered system and with a complex polarity at the cell division. The polarity includes oscillating peptide locations and, presumably, also regulation by metabolic reactions in the plasma membrane (peptidoglycan synthesis).
The symmetry evolution from Cnidaria to Animal are reviewed in References [7,8,9,10,11], and the evolution in polarity is reviewed in References [34,35]. In Reference [35], the author’s hypothesis is that the emergence of the bilateral symmetry in Animals is the optimal response to mechanical forces, including gravity, on the multicellular biological organism.
The common observation in all the reviews is that there is a polarity in the cells, the cell-membrane, and the location of the cell proteins and genes. The explanation for the emergence of the bilateral symmetry is that it is this structural order in the cell and the genes which, together with an “environmental impact” on the cells, causes the bilateral order at the evolution to an Animal. According to the reviews, the precursor could be structural asymmetries with protrusive forces [28], self-assembling [30,33], gravity and mechanical forces from (growth) constraints [35], mechanical forces and embryogenic cell fate [33], and kinetics in the cell [29] and cell membrane [31,32]. But common for all the reviews is that the genetics alone is not sufficient to ensure the evolution of symmetry, and, if so, it answers the second question raised here.
3.2. Precursors for the Emergence of the Bilateral Symmetry in Animals
In searching for a precursor for the bilateral symmetry in Animals, there are only few guide lines. There is, however, of the order 3 billion years between the emergence of the first simple Bacteria and the Cnidaria in the Ediacaran and Cambrian period. And the evolution of polarity in a simple Bacteria to a (Eukariote-) multicellular Cnidaria with its cell polarity has probably evolved rather continuously in the biological systems in the oceans over this enormous time span. It is, therefore, natural to search for an influence on the cell, its structure, and its genes that has been present throughout this long period of time. Gravity is not good candidate for the simple reason that a bacterium and the polarity of the cells in the biological organisms did not have a constant orientation to the gravity force. The surface tension in a cell and protrusive forces [28] is a possibility because the cell structure with a membrane with non-uniformity in molecular structure will give reason not only to a surface tension but also to local tensions in the membrane [37], e.g., the ion-channels and the membrane proteins are non-uniformly distributed. Self-assembling by self-stabilizing change is another possibility [38]. But the most obvious candidate for the cause of the polarity in the cell and the changes in polarity at the embryonic development is the kinetics in the cells. However, the kinetics in the cells are also determined by the genetics of the biosystem.
4. The Kinetics in a Cell
The kinetics in a cell are very complex, but the central part in the metabolism, the Glycolysis, is common for all living organisms. The biochemical reactions with carbohydrates in the Glycolysis is catalyzed by enzymes, and the consecutive biochemical reactions with stereo specific enzymes ensure the homochirality in the carbohydrates. The emergence of homochirality in carbohydrates is not known, but it could either by obtained endogenously (spontaneous Gibbs free energy favorable) [39,40] or obtained by stereo specific enzymes [41].
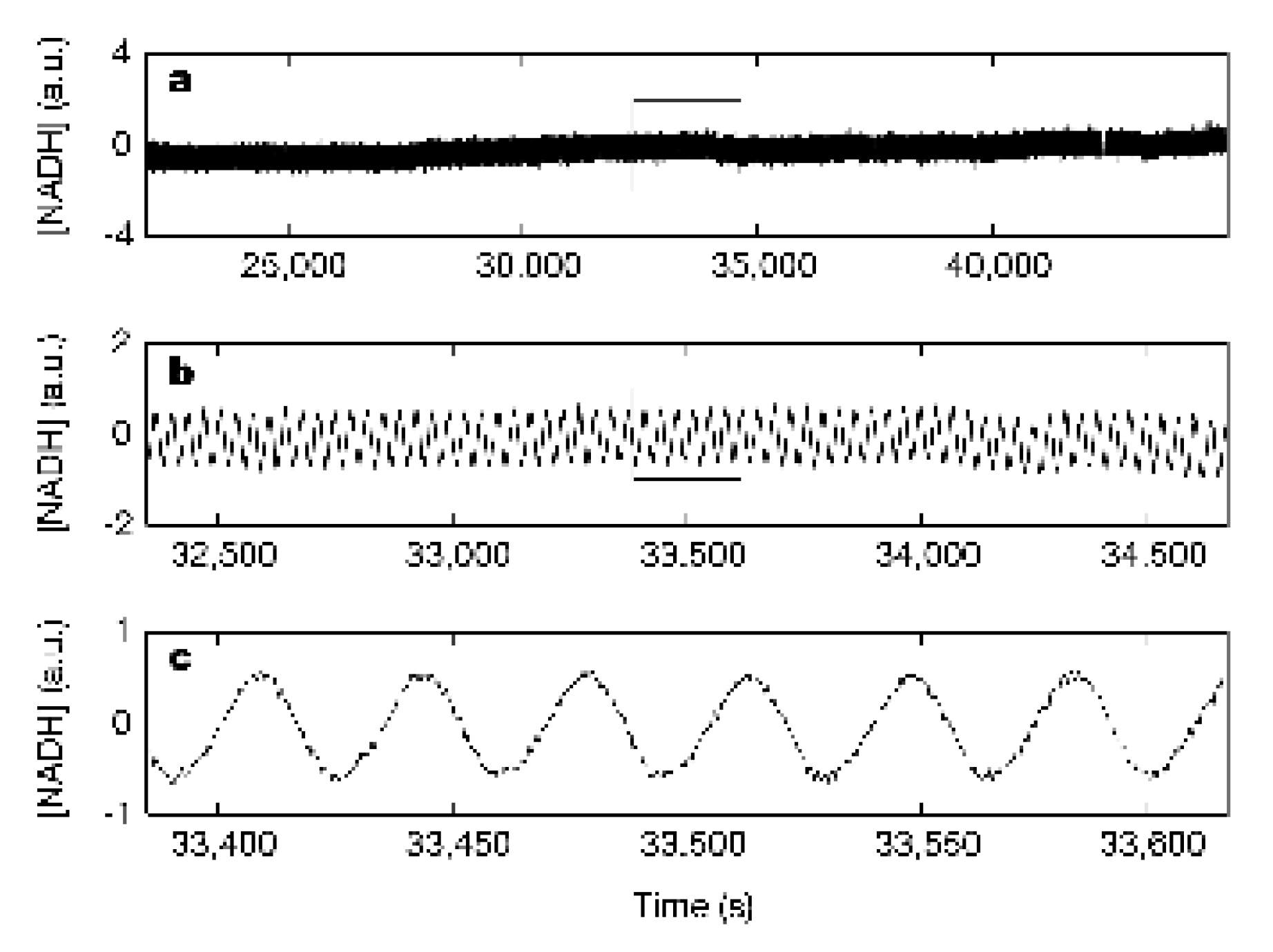
The Glycolysis has a remarkable property: the concentrations of the components in the Glycolysis oscillate. Oscillations in the concentration of NADH in a cell has been known for a long time [42]. Figure 4 shows the oscillation in the in vivo concentration of NADH in a yeast bacteria Saccharomyces cerevisiae [43]. In agreement with this behavior, a comprehensive model for the consecutive metabolic reactions in the glycolysis of the yeast bacteria exhibits oscillations of the concentrations [44]. But the experimentally determined time-oscillating concentration in NADH in the yeast bacteria and the concentrations in the kinetic model for the Glycolysis are mean concentrations in the cells, and the question is whether there could be a connection between the oscillations in these concentrations of the chemical components in the metabolism and the polarity in the cell at the cell division.
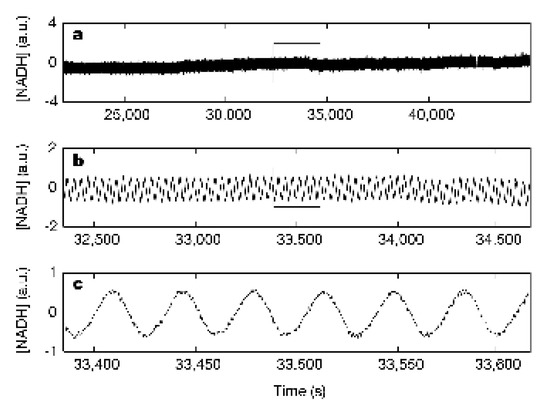
Figure 4.
In vivo, oscillations of NADH concentration in yeast bacteria [43]. The concentration of NADH in arbitrary unit (a.u.) in the yeast bacteria as a function of time after the start of the measurement. The period of the oscillations is 34.8 ± 0.2 s.
The polarity and the cell growth with elongation at cell division are described in Reference [31], with emphasis on polar growth in bacteria with cell elongation and septum formation, and Reference [30] reviews the literature about the “self-organizing” of the genes to the cell pole and summarizes: “In particular, the cell poles-the ends of rod-shaped cells-constitute important platforms for cellular regulation that underlie processes as essential as cell cycle progression, cellular differentiation, ...”. The polarity covers already for Bacteria over a highly ordered gene structure with oscillations (change of local positions) of genes during the cell division [29], and the question is: what kind of evolutionary kinetics could have established this spatial organization and dynamics?
4.1. Early Embryonic Pattern Formation in Drosophila
One of the Animals which appeared at the Cambrian explosion is the small fruit fly Drosophila melanogaster [9], and its metamorphosis has been heavily investigated. One remarkably feature in the evolution of Drosophila is the early embryonic pattern formation with seven stripes with genes for regulation of the development in multicellular organisms with cell differentiation and morphogenesis (Figure 5). The stripes contain the pair-rule genes in the embryo preceding gastrulation (i.e., where the blastula in the embryo goes from a single layer to a multilayer blastula) [45,46]. The stripes in the multicellular embryo also contain genes for the early endosome organization for sorting of the cells in the embryo [47] and homobox genes for the bilateral evolution [48,49]. But the figure of the embryo and the stripes has an apparent rotational symmetry, although the genes for the bilateral evolution may have asymmetrical positions in the stripes. The formation of the three-dimensional bilateral structures from patterned epithelial cell sheets in Drosophila is modeled in Reference [50].

Figure 5.
Early embryonic stripe patterns (violet) of the concentration of RNA in a embryo [51].
4.2. Models for Polarity at the Emergence of the Bilateral Evolution
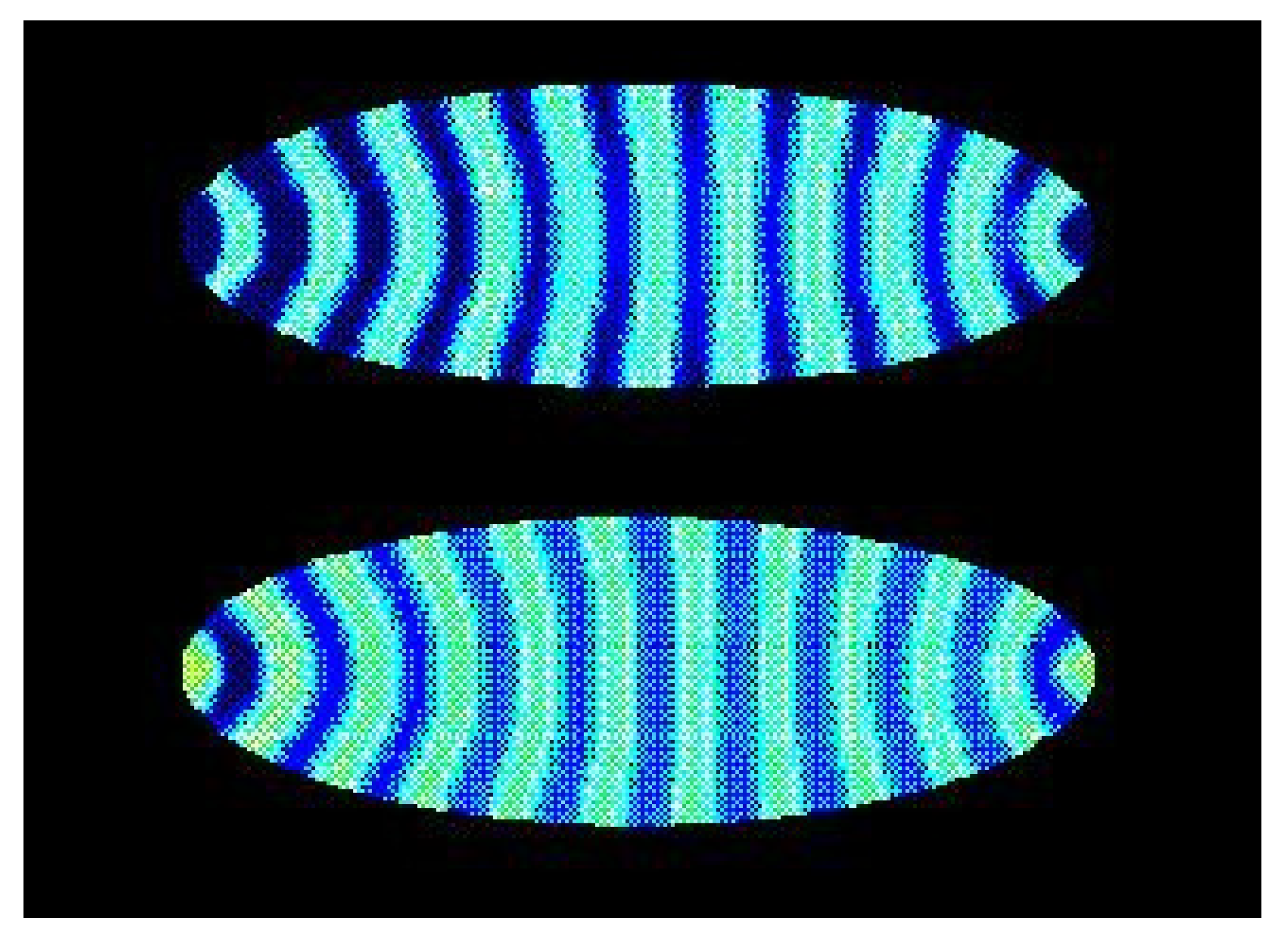
The stripe patterns in Drosophila melanogaster can be obtained by a reaction diffusion model with cross inhibition or activation of the kinetics for a morphogen in a cell [52] (Figure 6), and the embryonic development during gastrulation is correspondingly obtained by an another reaction diffusion model [53]. The patterns obtained by the reaction-diffusion models for the embryogenesis are examples of Turing structures, and these Turing patterns depend on the confinement [54]. In the case of oscillations of a morphogen, the spacial stripes depends on the cross inhibition or cross-activation and on the ratio between the diffusion coefficient for the morphogen and the size R for the biological system [55]. For a bacteria, R is the size of the cell, and, for Drosophila, it is the size of the embryo and, as the embryo grows, the diffusion may be regulated enzymatically in order to maintain the ratio and the pattern in the embryo [55].
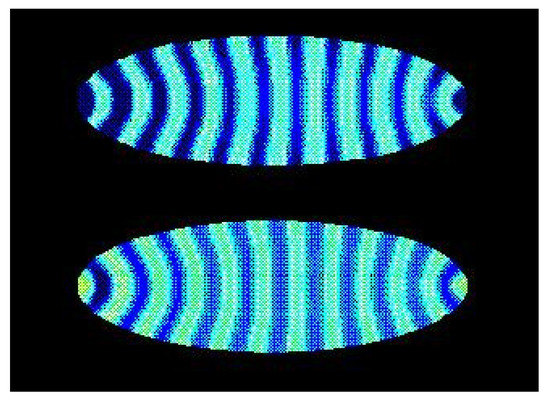
Figure 6.
Turing concentration patterns of a morphogen in an elliptically-shaped cell with reaction-diffusion dynamics [52]. The blue color is for a relative high stationary concentration of the morphogen, and yellow is for a relative small concentration.
Figure 5 and Figure 6 show a striking resemblance, but there is no direct evidence of such a connection between the oscillation in the Glycolysis and the formation of the stripe patterns in the embryo. The polarity and gene patterns in Drosophila obtained in Reference [52] are for a reaction-diffusion model of a morphogen with autocatalysis, but it is in fact also a simple model for the oscillation in the Glycolysis [56] (see Appendix in Reference [52]). And a time-oscillating system can induce stationary patterns in another system. The stationary wavy sand patterns in a beach bottom is an example of such induced patterns caused by the surface waves. So, the oscillations in the Glycolysis can have induced the stripe patterns in the Drosophila.
4.3. The Hypothesis about the Emergence of Bilateral Symmetry in Animals
Two questions were formulated in the Introduction:
I. How can the evolution from an undifferentiated cell without bilateral symmetry to a complex biological organism with symmetry, which is based on asymmetric DNA and enzymes, lead to the bilateral symmetry?
II. Is this evolution to an organism with bilateral symmetry obtained by other factors than DNA and enzymatic reactions?
According to the present analysis, the answer to these two connected questions may be that the evolution of the bilateral symmetry in Animals already started in a simple Bacterium with a polarity of genes and continued in the succeeding three billion years with biological evolution to the multicellular Cnidaria with rotational symmetry. At the end of the Precambrian evolution and subsequent Cambrian explosion, the polarity in the multicellular organism was polarized further and resulted in the emergence of the Bilaterians. The polarity in the multicellular embryo of an Animal exhibits stripe patterns of the genes in the embryo, but whether there already is a similar polarity of the gene structure in the Cnidaria is not reported.
The morphogenesis must be initiated by biochemical reactions, which are part of the chemical network including the metabolism. According to the hypothesis, the evolution of the polarities and the emergence of Bilaterians was induced by the oscillations in the Glycolysis, which is regulated by asymmetrical enzymes. But the evolution in the polarity is not solely controlled by the genetics. The induced Turing patterns also depend on the cell and embryo size.
5. Summary
The biological organisms evolved from Bacteria for more than 3 billion years before the first Bilateria appeared, and a simple Bacterium is already a very complex system with cell polarity. The biological mechanisms that caused this development are most likely also complex. All living organisms have some characteristic features, but a given feature is normal distributed with small deviations from the mean value. Traditionally, the evolution is described as Darwinian with enhancements of a deviation by change and/or a ”survival of the fittest” evolution. An example of the Darwinism survival of the fittest evolution is illustrated in Figure 2, with two flounders that have a bilateral ancestor without the asymmetry of the position of the eyes.
There are non-genetic factors, e.g., change and self-stabilizing change [38], and cell forces (e.g., surface tension) which also might have influenced the evolution toward the bilateral Animals. Most likely, the evolution from a Bacterium toward a multicellular Cnidarian and, finally, an Animal with bilateral symmetry has been continuous over this long time of the evolution before the Cambrian explosion, and with small enhancements of local symmetries, as illustrated by the religious icons in Figure 3. The success of this evolution has been conserved in the DNA of an Animal, and, on a superficial level, it must now seem as if the development from a undifferentiated Animal cell to its embryo, and finally, to an Animal is only genetically determined. But, according to the above hypothesis, this does not have to be the case either. If the above hypothesis is correct, then it is also crucial that this development takes place first in a cell, and then in an embryo with a compact shape, in order to obtain the Turing patterns.
In articles about the origin of life, one finds the view that life is more than chemistry in a bag. But this is an unfortunate formulation because all life takes place in cells. The confined and protected chemical environment in a cell is crucial for the stability of the biochemical reactions [57]. The compact form of a cell is obtained by the surface tension and the osmotic pressure, and they are examples of non-genetic factors in the evolution. According to the hypothesis above, the compact form of a undifferentiated cell, and later the embryo, is also crucial for the stability of the Turing patterns for the evolution of bilateral symmetry in an Animal. So, the answer to the second question formulated here is the fact that all life is obtained inside cells or collections of cells, which are also crucial for the emergence of life and the emergence of Bilaterians.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Axel Hunding is gratefully acknowledged. This work was supported by the VILLUM Foundation’s Matter project, grant No. 16515.
Conflicts of Interest
The author declares no conflict of interest.
References
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Earlist signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kitajima, K.; Spicuzza, M.J.; Kudryavtsev, A.B.; Valley, J.W. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope composition. Proc. Natl. Acad. Sci. USA 2018, 115, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Dunn, F.S.; Liu, A.G.; Donoghue, P.C.J. Ediacaran developmental biology. Biol. Rev. 2018, 93, 914–932. [Google Scholar] [CrossRef] [PubMed]
- Fedonkin, M.A.; Waggoner, B.M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature 1997, 388, 868–871. [Google Scholar] [CrossRef]
- MacGabhann, B.A.; Schiffbauer, J.D.; Hagadorn, J.W.; Van Roy, P.; Lynch, E.P.; Morrison, L.; Murray, J. Resolution of the earliest metazoan record: Differential taphonomy of Ediacaran and Paleozoic fossil molds and casts. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 513, 146–165. [Google Scholar] [CrossRef]
- Bottjer, D.J.; Hagadorn, J.W.; Dornbos, S.Q. The Cambrian Substrate Revolution. GSA Today 2000, 10, 1–7. [Google Scholar]
- Martindale, M.Q.; Henry, J.Q. The Development of Radial and Biradial Symmetry: The Evolution of Bilaterality. Am. Zool. 1998, 38, 672–6840. [Google Scholar] [CrossRef]
- Henry, J.O.; Martindale, M.Q. Evolution of Cleavage Programs in Relationship to Axial Specification and Body Plan Evolution. Biol. Bull. 1998, 195, 363–366. [Google Scholar] [CrossRef]
- Leclère, L.; Horin, C.; Chevalier, S.; Lapébie, P.; Dru, P.; Péron, S.; Jager, M.; Condamine, T.; Pottin, K.; Romano, S.; et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of cnidarian life-clycle. Nat. Ecol. Evol. 2019, 3, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, J.R.; Pang, K.; Burton, P.; Paulson, D.; Martindale, M.Q. Origins of Bilateral Symmetry: Hox and Dpp Expression in a Sea Anemone. Science 2004, 304, 1335–1337. [Google Scholar] [CrossRef]
- Putnam, N.H.; Srivastava, M.; Hellsten, U.; Dirks, B.; Chapman, J.; Salamov, A.; Terry, A.; Shapiro, H.; Lindquist, E.; Kapitonov, V.V.; et al. See Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organisation. Science 2007, 317, 86–94. [Google Scholar] [CrossRef]
- Foster, C.S.P.; Sauquet, H.; Van der Merwe, M.; McPherson, H.; Rossetto, M.; Ho, S.Y.W. Evaluating the Impact of Genomic Data and Priors on Baysian Estimates of the Angiosperm Evolutionary Timescale. Syst. Biol. 2017, 66, 338–351. [Google Scholar] [PubMed]
- Sauquet, H.; von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; de Morais, E.B.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Muñoz, A.; Martín, J. Symmetry, male dominance and female mate preferences in the Iberian rock lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 2002, 52, 342–347. [Google Scholar]
- Little, A.C.; Jones, B.C.; Waitt, C.; Tiddeman, B.P.; Feinberg, D.R.; Perrett, D.I.; Apicella, C.L.; Marlowe, F.W. Symmetry Is Related to Sexual Dimorphism in Faces: Data Across Culture and Scecies. PLoS ONE 2008, 3, e2106. [Google Scholar] [CrossRef]
- McManus, I.C. Symmetry and asymmetry in aesthetics and the arts. Eur. Rev. 2005, 13, 150–180. [Google Scholar] [CrossRef]
- Palmer, A.R. What determines direction of asymmtry: Genes, environment or change? Phil. Trans. R Soc. B 2016, 371, 20150417. [Google Scholar] [CrossRef]
- Grimes, D.T.; Burdine, R.D. Left-Right Patterning: Breaking Symmetry to Asymmetric Morphogenesis. Trends Genet. 2017, 33, 619–628. [Google Scholar] [CrossRef]
- Blum, M.; Ott, T. Animal left-right asymmetry. Curr. Biol. 2018, 28, R293–R305. [Google Scholar] [CrossRef]
- Zong, R.; Gong, Y. Behavioural asymmetry in Devonian trilobites. Palaeogeogr. Paleoclimatol. Plaeocol. 2017, 476, 158–162. [Google Scholar] [CrossRef]
- Govind, C.K. Claw Asymmetry in Lobsters: Case Study in Developmental Neuroethology. J. Neurobiol. 1092, 23, 1423–1445. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.M.; Heatwole, S.J.; Morrell, L.J.; Backwell, P.R.Y. Handedness in fiddler crab fights. Anim. Behav. 2015, 110, 99–104. [Google Scholar] [CrossRef]
- Friedman, M. The evolutionary origin of flatfish asymmetry. Nature 2008, 454, 209–212. [Google Scholar] [CrossRef]
- De Kovel, C.G.; Lisgo, S.; Karlebach, G.; Ju, J.; Cheng, G.; Fisher, S.E.; Francks, C. Left-Right Asymmetry of Maturation Rates in Human Embryonic Neural Development. Biol. Pshychiatry 2017, 82, 204–212. [Google Scholar] [CrossRef]
- Matthews, B.W.; Bernhard, S.A. Oligomeric Enzymes. Annu. Rev. Biophys. Bioeng. 1973, 2, 257–317. [Google Scholar] [CrossRef]
- Kojić-Prodić, B.; Štefanić, Z. Symmetry vercus Asymmetry in the Molecules of Life: Homomeric Protein Assemblies. Symmetry 2010, 2, 885–906. [Google Scholar]
- Li, R.; Bowerman, B. Symmetry Breaking in Biology. Cold Spring Harb. Perspect. Biol. 2009, 1, a0013221. [Google Scholar] [CrossRef]
- Dworkin, J. Cellular Polarity in Prokaryotic Organisms. Cold Spring Harb. Perspect. Biol. 2009, 1, a003368. [Google Scholar] [CrossRef]
- Buskila, A.A.; Kannaiah, S.; Amster-Choder, O. RNA localization in bacteria. RNA Biol. 2014, 11, 1051–1060. [Google Scholar] [CrossRef]
- Laloux, G.; Jacobs-Wagner, C. How do bacteria localize proteins to the cell pole. J. Cell Sci. 2014, 127, 11–19. [Google Scholar] [CrossRef]
- Brown, P.J.B.; Kysela, D.T.; Brun, Y.V. Polarity and the diversity of growth mechanisms in bacteria. Semin. Cell Dev. Biol. 2011, 22, 791–798. [Google Scholar] [CrossRef]
- Orlando, K.; Guo, W. Membrane Organisation and Dynamics in Cell Polarity. Cold Spring Harb. Perspect. Biol. 2010, 2, a003475. [Google Scholar]
- Kim, E.J.Y.; Korotkevich, E.; Hiraga, T. Coordination of Cell Polarity, Mechanics and Fate in Tissue Self-organization. Trends Cell Biol. 2018, 28, 541–550. [Google Scholar] [CrossRef]
- Manuel, M. Early evolution of symmetry and polarity in metazoan body plans. C. R. Biol. 2009, 332, 184–209. [Google Scholar] [CrossRef]
- Holló, G. Demystification of Animal symmetry: Symmetry is a responce to mechanical forces. Biol. Direct 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Spitzer, J. From Water and Ions to Crowded Biomolecules: In Vivo Structuring of a Prokaryotic Cell. Microbiol. Mol. Biol. Rev. 2011, 75, 491–506. [Google Scholar] [CrossRef]
- Toxvaerd, S. Perturbation Theory for Nonuniform Fluids: Surface Tension. J. Chem. Phys. 1971, 55, 3116–3120. [Google Scholar] [CrossRef]
- Toxvaerd, S. Origin of Homochirality in Biosystems. Int. J. Mol. Sci. 2009, 10, 1290–1299. [Google Scholar] [CrossRef]
- Toxvaerd, S. The Role of Carbohydrates at the Origin of Homochirality in Biosystems. Orig. Life Evol. Biosph. 2013, 43, 391–409. [Google Scholar] [CrossRef]
- Mauksch, M.; Wei, S.; Freund, M.; Zamfir, A.; Tsogoeva, S.B. Spontaneous Mirror Symmetry Breaking in the Aldol Reaction and its Potential Relevance in Prebiotic Chemistry. Orig. Life Evol. Biosph. 2010, 40, 79–91. [Google Scholar] [CrossRef]
- Toxvaerd, S. The start of the Abiogenesis: Preservation of homochirality in proteins as a necessary and sufficient condition for the establishment of the metabolism. J. Teor. Biol. 2018, 451, 117–121. [Google Scholar] [CrossRef]
- Ghosh, A.; Change, B. Oscillations of Glycolytic intermediates in yeast cells. Biochem. Biophys. Res. Commun. 1964, 16, 174–181. [Google Scholar] [CrossRef]
- Danø, S.; Sørensen, P.G.; Hynne, F. Sustained oscillations in living cells. Nature 1999, 402, 320–322. [Google Scholar]
- Hynne, F.; Danø, S.; Sørensen, P.G. Full-scale model of glycolysis in Saccharomyces cerevisiae. Biophys. Chem. 2001, 94, 121–163. [Google Scholar] [CrossRef]
- McGinnis, W.; Gerber, R.L.; Wirz, J.; Kuroiwa, A.; Gehring, W.J. A homologous Protein-Coding Sequence in Drosophila Homeotic Genes and Its Conservation in Other Metazoans. Cell 1984, 37, 403–408. [Google Scholar] [CrossRef]
- Carrasco, A.E.; McGinnis, W.; Gehring, W.J.; De Robertis, E.-M. Cloning of an X. laevis Gene Expressed during Early Embryogenesis Coding for a Peptide Region Homologous to Drosophila Homeotic Genes. Cell 1984, 37, 409–414. [Google Scholar] [CrossRef]
- Sakuma, C.; Kawauchi, T.; Haraguchi, S.; Shikanai, M.; Yamaguchi, Y.; Gelfand, V.I.; Luo, L.; Miura, M.; Chihara, T. Drosophila Strip serves as a platform for early endosome organization during axon elongation. Nat. Commun. 2014, 5, 5180. [Google Scholar] [CrossRef]
- Pearson, J.C.; Lemons, D.; McGinnis, W. Modulating hox gene functions during animal body pattering. Nat. Rev. Genet. 2005, 6, 893–905. [Google Scholar] [CrossRef]
- Solnica-Krezel, L.; Sepich, D.S. Gastrulation: Making and Shaping Germ Layers. Annu. Rev. Cell Del. Biol. 2012, 28, 687–717. [Google Scholar] [CrossRef]
- Misra, M.; Audoly, B.; Shvartsman, S.Y. Complex structures from patterned cell sheets. Phil. Trans. R. Soc. B 2017, 372, 20150515. [Google Scholar] [CrossRef]
- Fujioka, M.; Sun, G.; Jaynes, J.B. The Drosophila eve Insolator Homie Promotes eve Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading. PLoS Genet. 2013, 9, e1003883. [Google Scholar] [CrossRef]
- Hunding, A.; Kauffman, S.A.; Goodwin, B.C. Drosophila Segmentation: Supercomputer Simulation of Prepattern Hierarchy. J. Theor. Biol. 1990, 145, 369–384. [Google Scholar] [CrossRef]
- Bozorgui, B.; Komeisky, A.B.; Teimouri, H. Physical-chemical mechanisms of pattern formation during gastrulation. J. Chem. Phys. 2018, 148, 123302. [Google Scholar] [CrossRef]
- Turing, A.M. The chemical basis of Morphogenesis. Phil. Trans. R. Soc. B 1952, 237, 37–72. [Google Scholar]
- Hunding, A.; Sørensen, P.G. Size adaptation to Turing prepatterns. J. Math. Biol. 1988, 26, 27–39. [Google Scholar] [CrossRef]
- Seĺkov, E.E. Self-Oscillations in Glycolysis. Eur. J. Biochem. 1968, 4, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Toxvaerd, S. A Prerequisite for Life. J. Theor. Biol. 2019, 474, 48–51. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).