Self-Assembly of Hydrogen-Bonded Cage Tetramers of Phosphonic Acid
Abstract
1. Introduction
2. Experimental
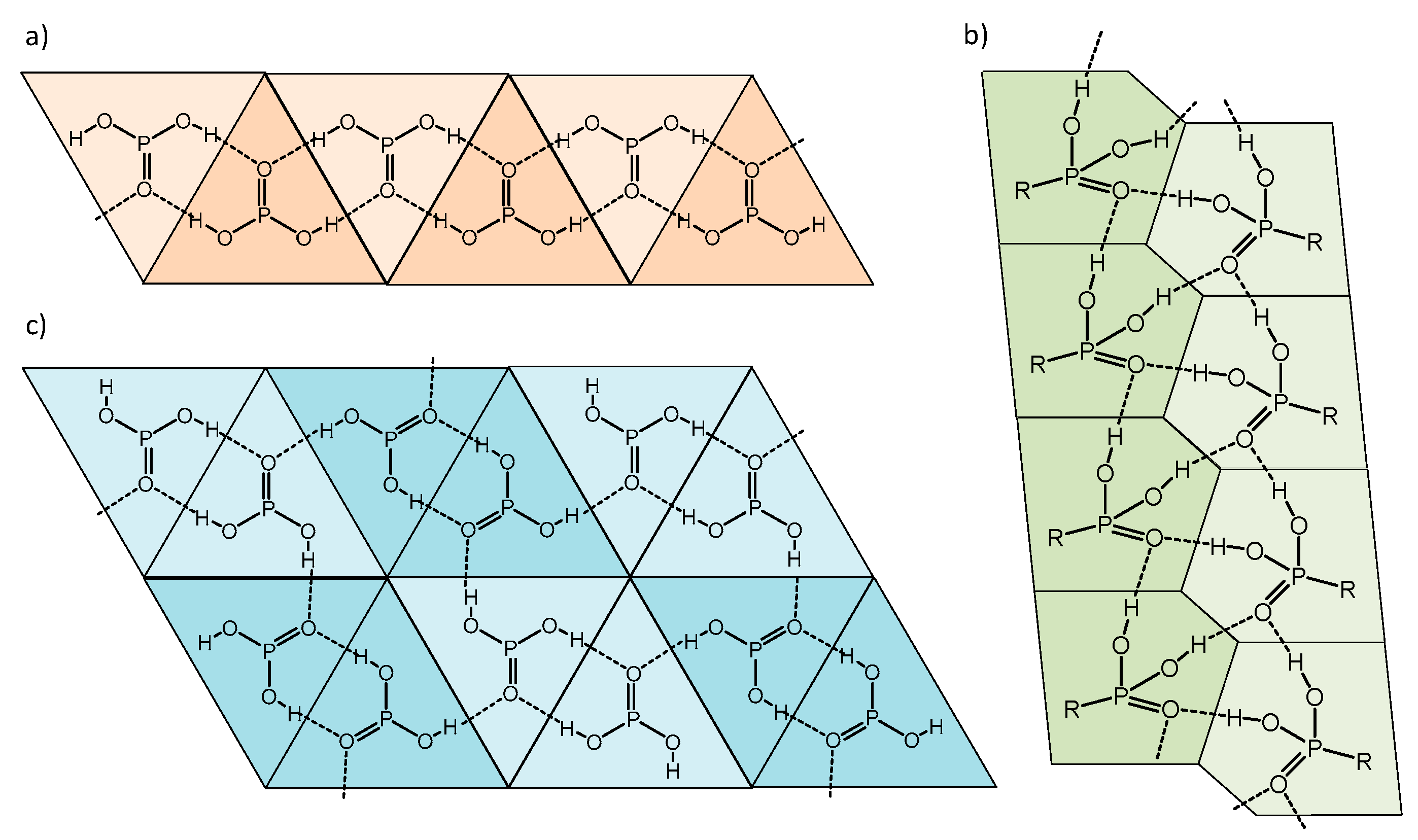
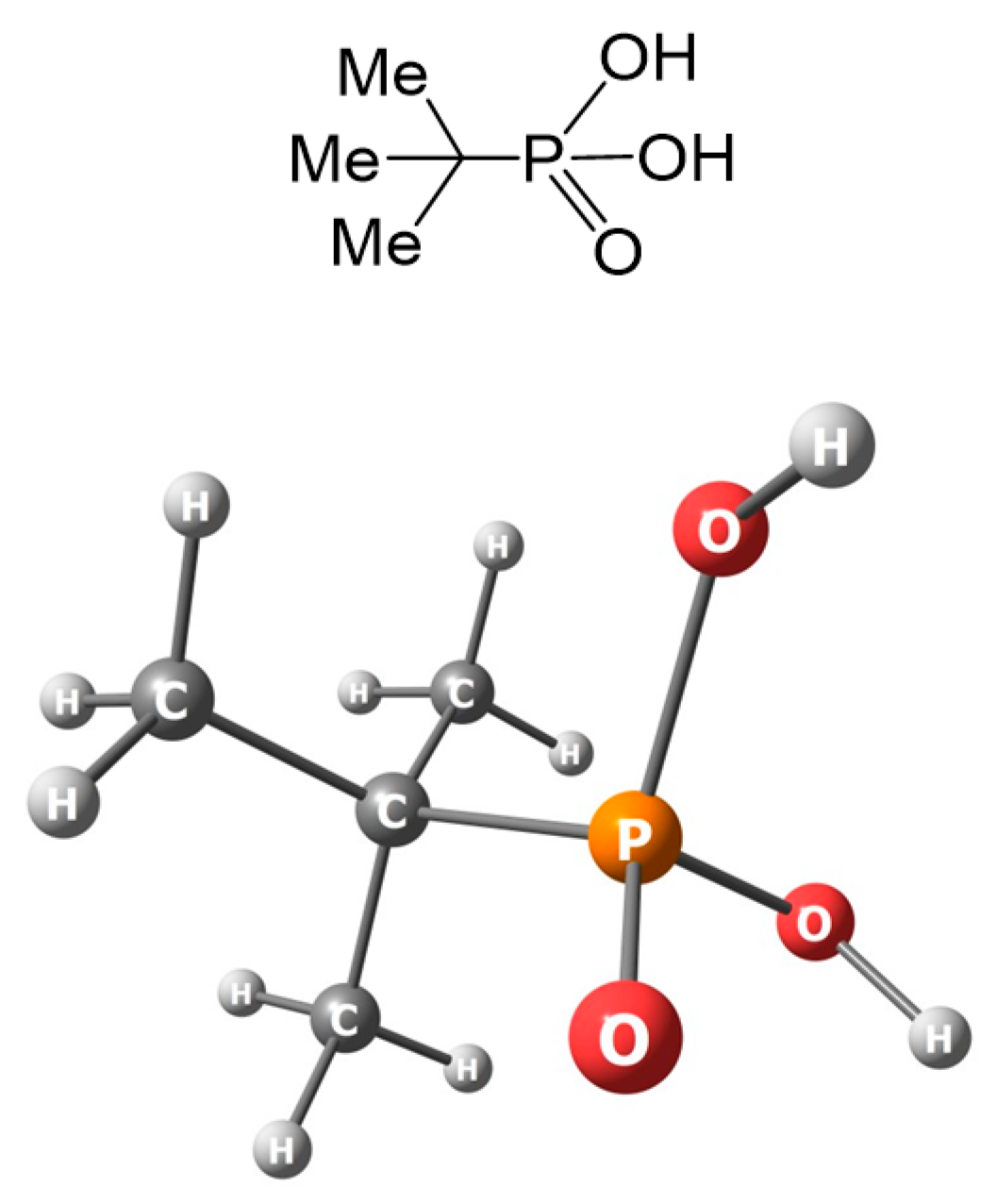
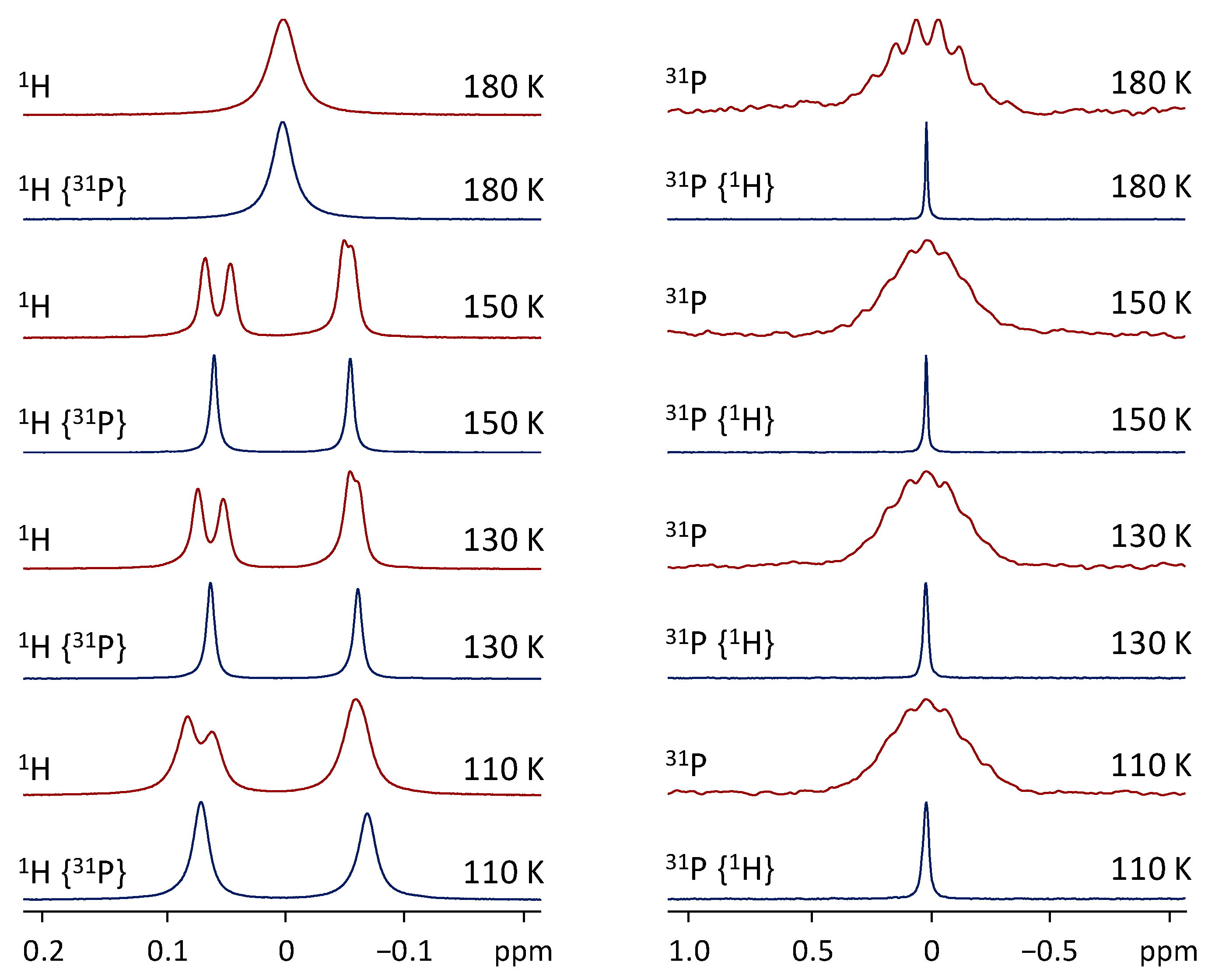
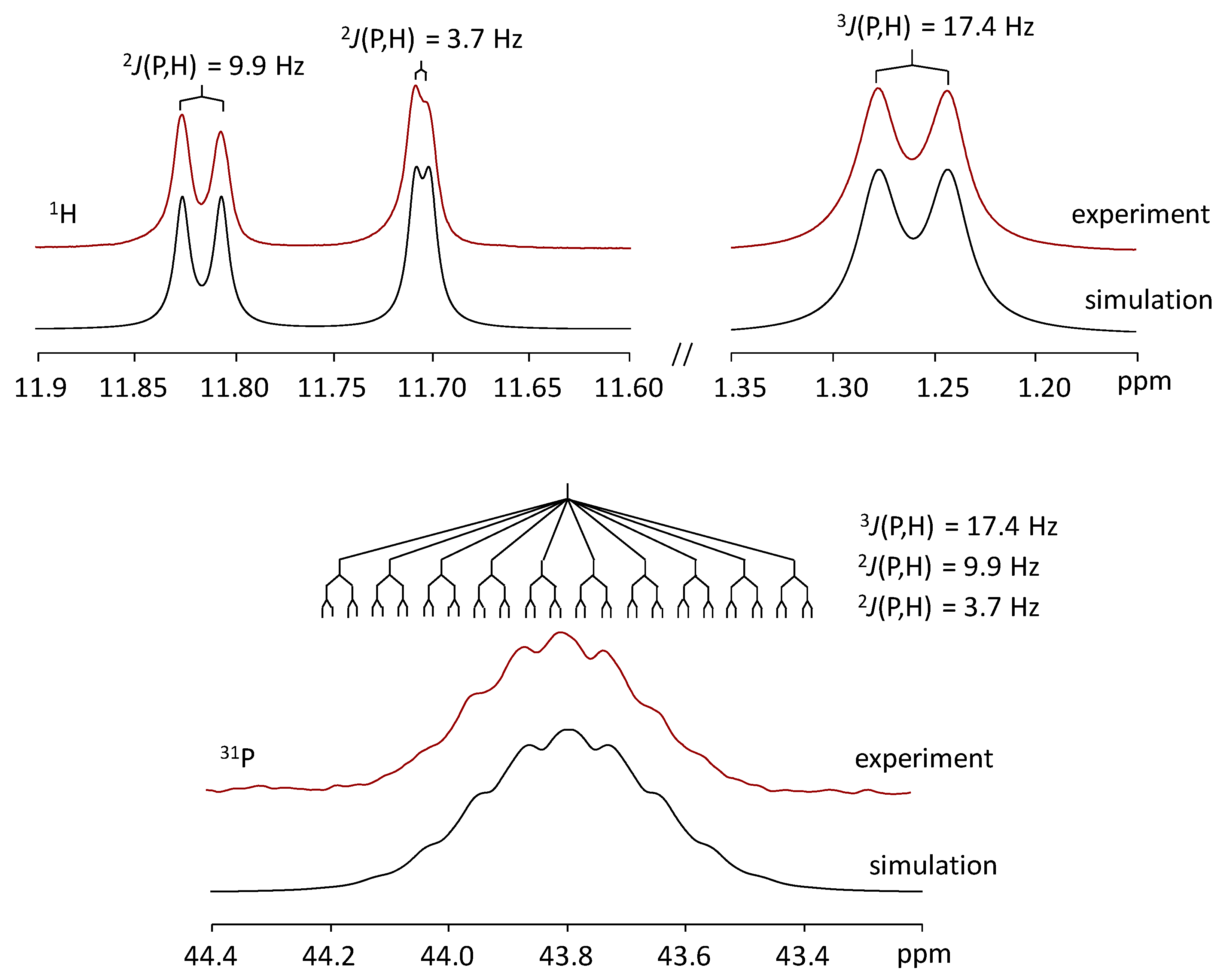
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, O.; Aguilar–Parrilla, F.; Lopez, J.; Jagerovic, N.; Elguero, J.; Limbach, H.-H. Dynamic NMR Study of the Mechanisms of Double, Triple, and Quadruple Proton and Deuteron Transfer in Cyclic Hydrogen Bonded Solids of Pyrazole Derivatives. J. Am. Chem. Soc. 2004, 126, 11718–11732. [Google Scholar] [CrossRef]
- Castaneda, J.P.; Denisov, G.S.; Kucherov, S.Y.; Schreiber, V.M.; Shurukhina, A.V. Infrared and Ab Initio Studies of Hydrogen Bonding and Proton Transfer in the Complexes Formed by Pyrazoles. J. Mol. Struct. 2003, 660, 25–40. [Google Scholar] [CrossRef]
- Limbach, H.-H.; Seiffert, W. Dynamic Processes in Systems with Hydrogen Bonds. I. 1H–NMR Spectroscopic Study of the Cis-Trans Equilibrium and the Hydrogen Bond Association of N,Nˊ–bis(pentadeuterophenyl)–1–amino–3–iminopropene in Carbon Disulfide. Ber. Bunsen Ges. Phys. Chem. 1974, 78, 532–537. [Google Scholar]
- Limbach, H.-H.; Seiffert, W. Dynamic Processes in Systems with Hydrogen Bonds. II. 1H–NMR Spectroscopic Study of the Direct and Indirect Intermolecular Proton Exchange of N,Nˊ–bis(pentadeuterophenyl)–1–amino–3–iminopropene in Carbon Disulfide. Ber. Bunsen Ges. Phys. Chem. 1974, 78, 641–647. [Google Scholar]
- Lopez, J.; Maennle, F.; Wawer, I.; Buntkowsky, G.; Limbach, H.-H. NMR Studies of Double Proton Transfer in Hydrogen Bonded Cyclic N,Nˊ-Bis-arylformamidine Dimers: Conformational Control, Kinetic HH/HD/DD Isotope Effects and Tunneling. Phys. Chem. Chem. Phys. 2007, 9, 4498–4513. [Google Scholar] [CrossRef]
- Torres, V.; Lopez, J.-M.; Langer, U.; Buntkowsky, G.; Vieth, H.-M.; Elguero, J.; Limbach, H.-H. Kinetics of Coupled Double Proton and Deuteron Transfer in Hydrogen-Bonded Ribbons of Crystalline Pyrazole-4-carboxylic Acid. Z. Phys. Chem. 2012, 226, 1125–1147. [Google Scholar] [CrossRef]
- Xue, Q.; Horsewill, A.; Johnson, M.; Trommsdorff, H. Tunneling Dynamics of Double Proton Transfer in Formic Acid and Benzoic Acid Dimers. J. Chem. Phys. 2004, 120, 11107–11119. [Google Scholar] [CrossRef]
- Tolstoy, P.M.; Schah-Mohammedi, P.; Smirnov, S.N.; Golubev, N.S.; Denisov, G.S.; Limbach, H.-H. Characterization of Fluxional Hydrogen-Bonded Complexes of Acetic Acid and Acetate by NMR: Geometries and Isotope and Solvent Effects. J. Am. Chem. Soc. 2004, 126, 5621–5634. [Google Scholar] [CrossRef]
- Tolstoy, P.M.; Smirnov, S.N.; Shenderovich, I.G.; Golubev, N.S.; Denisov, G.S.; Limbach, H.-H. NMR Studies of Solid State—Solvent and H/D Isotope Effects on Hydrogen Bond Geometries of 1:1 Complexes of Collidine with Carboxylic Acids. J. Mol. Struct. 2004, 700, 19–27. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond. Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009; p. 251. [Google Scholar]
- Asfin, R.E.; Denisov, G.S.; Mielke, Z.; Tokhadze, K.G. Particular Features of the ν(OH) Absorption Band of Strongly Hydrogen-Bonded Complexes in the Gas Phase, Low-Temperature Matrices, and Crystalline Films at 12–600 K. Opt. Spectrosc. 2005, 99, 56–67. [Google Scholar] [CrossRef]
- Detering, C.; Tolstoy, P.M.; Golubev, N.S.; Denisov, G.S.; Limbach, H.-H. Vicinal H/D Isotope Effects in NMR Spectra of Complexes with Coupled Hydrogen Bonds. Phosphoric Acids. Doklady Phys. Chem. 2001, 379, 1–4. [Google Scholar] [CrossRef]
- Giba, I.S.; Mulloyarova, V.V.; Denisov, G.S.; Tolstoy, P.M. Influence of Hydrogen Bonds in 1:1 Complexes of Phosphinic Acids with Substituted Pyridines on 1H and 31P NMR Chemical Shifts. J. Phys. Chem. A 2019, 123, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Mulloyarova, V.V.; Giba, I.S.; Kostin, M.A.; Denisov, G.S.; Shenderovich, I.G.; Tolstoy, P.M. Cyclic Trimers of Phosphinic Acids in Polar Aprotic Solvent: Symmetry, Chirality and H/D Isotope Effects on NMR Chemical Shifts. Phys. Chem. Chem. Phys. 2018, 20, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Giba, I.S.; Mulloyarova, V.V.; Denisov, G.S.; Tolstoy, P.M. Sensitivity of 31P NMR Chemical Shifts to Hydrogen Bond Geometry and Molecular Conformation for Complexes of Phosphinic Acids with Pyridines. Magn. Reson. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mulloyarova, V.V.; Giba, I.S.; Denisov, G.S.; Tolstoy, P.M. Conformational Mobility and Proton Transfer in Hydrogen-bonded Dimers and Trimers of Phosphinic and Phosphoric acids. J. Phys. Chem A 2019, 123, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Asfin, R.E.; Denisov, G.S.; Tokhadze, K.G. The Infrared Spectra and Enthalpies of Strongly Bound Dimers of Phosphinic Acids in the Gas Phase. (CH2Cl)2POOH and (C6H5)2POOH. J. Mol. Struct. 2002, 608, 161–168. [Google Scholar] [CrossRef]
- Asfin, R.E.; Denisov, G.S.; Poplevchenkov, D.N.; Tokhadze, K.G.; Velikanova, T.V. IR ν(OH) Band and Dimerization of Phosphorous Acids in the Gas Phase and Solid State. Pol. J. Chem. 2002, 76, 1223–1231. [Google Scholar]
- Mó, O.; Yáñez, M.; Gonzalez, L.; Elguero, J. Spontaneous Self-ionization in the Gas Phase: A Theoretical Prediction. Chem. Phys. Chem. 2001, 2, 465–467. [Google Scholar] [CrossRef]
- Ahmadi, I.; Rahemi, H.; Tayyari, S.F. Structural, Potential Surface and Vibrational Spectroscopy Studies of Hypophosphorous Acid in the Gas Phase and Chain Conformation. A Theoretical Study. J. Korean Chem. Soc. 2005, 49, 129–137. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, L.; Mó, O.; Yáñez, M.; Elguero, J. Very Strong Hydrogen Bonds in Neutral Molecules: The phosphinic acid dimers. J. Chem. Phys. 1998, 109, 2685–2693. [Google Scholar] [CrossRef]
- Picazo, O.; Alkorta, I.; Elguero, J.; Mó, O.; Yáñez, M. Chiral Recognition in Phosphinic Acid Dimers. J. Phys. Org. Chem. 2005, 18, 491–497. [Google Scholar] [CrossRef]
- Rekik, N.; Ghalla, H.; Hanna, G. Explaining the Structure of the OH Stretching Band in the IR Spectra of Strongly Hydrogen-bonded Dimers of Phosphinic Acid and Their Deuterated Analogs in the Gas Phase: A Computational Study. J. Phys. Chem. A 2012, 116, 4495–4509. [Google Scholar] [CrossRef]
- Yue, B.; Yan, L.; Han, S.; Xie, L. Proton Transport Pathways in an Acid–Base Complex Consisting of a Phosphonic Acid Group and a 1,2,3-Triazolyl Group. J. Phys. Chem. B 2013, 117, 7941–7949. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.V.; Krishtal, S.P.; Kiselev, M.G.; Safonova, L.P. Structure of Orthophosphoric Acid-N,N-dimethylformamide Complexes. Russian J. Phys. Chem. 2006, 80, 7–13. [Google Scholar] [CrossRef]
- Yoo, T.; Nguyen, H.D.; Nilsson, M. Molecular Dynamics Investigations of Di-Butyl-Phosphoric Acid—Parameterization and Dimerization. J. Phys. Chem. B 2018, 122, 12040–12048. [Google Scholar] [CrossRef] [PubMed]
- Solka, J.L.; Reis, A.H., Jr.; Mason, G.W.; Lewey, S.M.; Peppard, D.F. Sterically Hindered Solvent Extractants-III: The Molecular and Crystal Structure and Heavy-Element Extraction Properties of the Di-t-pentylphosphinic Acid Dimer. J. Inorg. Nucl. Chem. 1978, 40, 663–668. [Google Scholar] [CrossRef]
- Fenske, D.; Mattes, R.; Lons, J.; Tebbe, K.F. Die Kristallstruktur von Diphenylphosphinsaure. Chem. Ber. 1973, 106, 1139–1144. (In German) [Google Scholar] [CrossRef]
- Reis, A.H., Jr.; Peterson, S.W.; Druyan, M.E.; Gebert, E.; Mason, G.W.; Peppard, D.F. Sterically Hindered Solvent Extractants. 2. A Neutron-Diffraction Study of the Di-tertbutylphosphinic Acid Dimer Showing Strong Asymmetric Hydrogen Bonding. Inorg. Chem. 1976, 15, 2748–2752. [Google Scholar] [CrossRef]
- Druyan, M.E.; Reis, A.H., Jr.; Gebert, E.; Peterson, S.W.; Mason, G.W.; Peppard, D.F. Dimeric Structure of di-tert-butylphosphinic Acid. J. Amer. Chem. Soc. 1976, 98, 4801–4805. [Google Scholar] [CrossRef]
- Bruckmann, J.; Kruger, C.; Lehmann, C.W.; Leitner, W.; Rust, J.; Six, C. Ethylenebis(phosphonic acid). Acta Cryst. C 1999, 55, 695–696. [Google Scholar] [CrossRef]
- Fatila, E.M.; Pink, M.; Twum, E.B.; Karty, J.A.; Flood, A.H. Phosphate–Phosphate Oligomerization Drives Higher Order Co-assemblies with Stacks of Cyanostar Macrocycles. Chem Sci. 2018, 9, 2863–2872. [Google Scholar] [CrossRef]
- Mulloyarova, V.V.; Ustimchuk, D.O.; Filarowski, A.; Tolstoy, P.M. H/D Isotope Effects on 1H NMR Chemical Shifts in Cyclic Heterodimers and Heterotrimers of Phosphinic and Phosphoric Acids. Molecules 2020, 25, 1907. [Google Scholar] [CrossRef]
- Merz, K.; Knüfer, A. Cyclohexylphosphonic Acid. Acta Cryst. 2002, C58, o187–o188. [Google Scholar] [CrossRef] [PubMed]
- Belabassi, Y.; Gushwa, A.F.; Richards, A.F.; Montchamp, J.-L. Structural Analogues of Bioactive Phosphonic Acids: First Crystal Structure Characterization of Phosphonothioic and Boranophosphonic Acids. Elem. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2214–2228. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Blake, A.J.; Lippolis, V.; Schroder, M.; Wilson, C. 4-Methoxyphenylphosphonic Acid: Reactivity of Lawesson’s Reagent. Acta Cryst. 2002, C58, o260–o262. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudkhania, A.H.; Langer, V. Phenylphosphonic Acid as a Building Block for Two-Dimensional Hydrogen-Bonded Supramolecular Arrays. J. Mol. Struct. 2002, 609, 97–108. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Etter, M. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Aiello, D.; Folliet, N.; Laurent, G.; Testa, F.; Gervais, C.; Babonneau, F.; Azaïs, T. Solid state NMR Characterization of Phenylphosphonic Acid Encapsulated in SBA-15 and Aminopropyl-modified SBA-15. Micropor. Mesopor. Mat. 2013, 166, 109–116. [Google Scholar] [CrossRef]
- Kreuer, K.; Paddison, S.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Ye, G.; Janzen, N.; Goward, G. Solid-State NMR Study of Two Classic Proton Conducting Polymers: Nafion and Sulfonated Poly(ether ether ketone)s. Macromolecules 2006, 39, 3283–3290. [Google Scholar] [CrossRef]
- Thompson, E.; Capehart, T.; Fuller, T.; Jorne, J. Investigation of Low-Temperature Proton Transport in Nafion Using Direct Current Conductivity and Differential Scanning Calorimetry. Electrochem. Soc. 2006, 153, A2351–A2362. [Google Scholar] [CrossRef]
- Zhao, R.; Rupper, P.; Gaan, S. Recent Development in Phosphonic Acid-Based Organic Coatings on Aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef]
- Gawalt, E.S.; Avaltroni, M.J.; Koch, N.; Schwart, J. Self-Assembly and Bonding of Alkanephosphonic Acids on the Native Oxide Surface of Titanium. Langmuir 2001, 17, 5736–5738. [Google Scholar] [CrossRef]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic Acid Monolayers for Binding of Bioactive Molecules to Titanium Surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lieberman, I.; Asyuda, A.; Resch, S.; Seim, H.; Kirsch, P.; Zharnikov, M. Thermal Stability of Phosphonic Acid Self-Assembled Monolayers on Alumina Substrates. J. Phys. Chem. C 2020, 124, 2531–2542. [Google Scholar] [CrossRef]
- Cao, L.; Peng, Y.; Li, Z. Phosphonic Acid Self-assembled Monolayer Improved Properties of n-type Organic Field-effect Transistors in Air Ambient. RSC Adv. 2016, 6, 89794–89798. [Google Scholar] [CrossRef]
- Bamba, T.; Ohtake, T.; Ohata, Y.; Nie, H.-Y.; Ban, T.; Yamamoto, S.-I. Octadecylphosphonic Acid Self-assembled Monolayers Obtained Using Rapid Dipping Treatments. Trans. Mat. Res. Soc. Jpn. 2018, 43, 305–309. [Google Scholar] [CrossRef][Green Version]
- Siegel, J.S.; Anet, F.A.I. Dichlorofluoromethane-d: A Versatile Solvent for VT-NMR Experiments. J. Org. Chem. 1988, 53, 2629–2630. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc.: Wallingford, CT, USA. 2016. Available online: http://gaussian.com/citation/ (accessed on 20 January 2021).
- Arey, J.S.; Aeberhard, P.C.; Lin, I.-C.; Rothlisberger, U. Hydrogen Bonding Described Using Dispersion-Corrected Density Functional Theory. J. Phys. Chem. B 2009, 113, 4726–4732. [Google Scholar] [CrossRef] [PubMed]
- Latypov, S.; Polyancev, F.; Yakhvarov, D.; Sinyashina, O. Quantum Chemical Calculations of 31P NMR Chemical Shifts: Scopes and Limitations. Phys. Chem. Chem. Phys. 2015, 17, 6976–6987. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Chemcraft, Version 1.8. Available online: www.chemcraftprog.com (accessed on 20 January 2021).
- Budzelaar, P.H.M. gNMR; Adept Scientific: Letchworth, UK, 2002. [Google Scholar]
- Biegler-König, F.; Schönbohm, J.; Bayles, D. AIM2000—A Program to Analyze and Visualize Atoms in Molecules. J. Comp. Chem. 2001, 22, 545–559. [Google Scholar]
- Biegler-König, F.; Schönbohm, J. An Update to the AIM2000—Program for Atoms in Molecules. J. Comp. Chem. 2002, 23, 1489–1494. [Google Scholar] [CrossRef]
- Shenderovich, I.G.; Burtsev, A.P.; Denisov, G.S.; Golubev, N.S.; Limbach, H.-H. Influence of the Temperature-dependent Dielectric Constant on the H/D Isotope Effects on the NMR Chemical Shifts and the Hydrogen Bond Geometry of the Collidine-HF Complex in CDF3/CDClF2 Solution. Magn. Reson. Chem. 2001, 39, 91–99. [Google Scholar] [CrossRef]
- Dohnal, V.; Tkadlecova, M. A Simple Relation between 1H NMR Data and Mixing Enthalpy for Systems with Complex Formation by Hydrogen Bonding. J. Phys. Chem. B 2002, 106, 12307–12310. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kojima, C.; Ono, A.; Kainosho, M. Developing Model Systems for the NMR Study of Substituent Effects on the N—H···N Hydrogen Bond in Duplex DNA. Magn. Reson. Chem. 2001, 39, 159–165. [Google Scholar] [CrossRef]
- Limbach, H.-H.; Tolstoy, P.M.; Perez-Hernandez, N.; Guo, J.; Shenderovich, I.G.; Denisov, G.S. OHO Hydrogen Bond Geometries and NMR Chemical Shifts: From Equilibrium Structures to Geometric H/D Isotope Effects with Applications for Water, Protonated Water and Compressed Ice. Israel J. Chem. 2009, 49, 199–216. [Google Scholar] [CrossRef]
- Sorgenfrei, N.; Hioe, J.; Greindl, J.; Rothermel, K.; Morana, F.; Lokesh, N.; Gschwind, R.M. NMR Spectroscopic Characterization of cCharge Assisted Strong Hydrogen Bonds in Brønsted Acid Catalysis. J. Am. Chem. Soc. 2016, 138, 16345–16354. [Google Scholar] [CrossRef]
- Silski-Devlin, A.M.; Petersen, J.P.; Liu, J.; Turner, G.A.; Poutsma, J.C.; Kandel, S.A. Hydrogen-bonded Tetramers of Carbamazepine. J. Phys. Chem. C 2020, 124, 5213–5219. [Google Scholar] [CrossRef]
- Dilovic, I.; Matkovic-Calogovic, D.; Kos, M.I. N-Benzyloxy-1H-benzotriazole-1-Carboxamide: A Hydrogen-bonded Tetramer Based upon a Rare R(4)4(20) Structural motif. Acta Cryst. C 2008, 64, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, M.; Marshall, L.J.; de Mendoza, J. Hydrogen-bonded Cyclic Tetramers Based on Ureidopyrimidinones Attached to a 3,6-carbazolyl Spacer. Org. Lett. 2011, 13, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Sagan, B.L.; Walmsley, J.A. Evidence for the Existence of Hydrogen-bonded Cyclic Tetramers in Aqueous Solutions of Tetramethylammonium Guanosine 5′-monophosphate. Biochem. Biophys. Res. Comm. 1985, 128, 980–986. [Google Scholar] [CrossRef]
- Larsen, R.W.; Suhm, M.A. The Benefits of Alternation and Alkylation: Large Amplitude Hydrogen Bond Librational Modes of Alcohol Trimers and Tetramers. Phys. Chem. Chem. Phys. 2010, 12, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Mella, M.; Harris, K.D.M. Pathways for Hydrogen Bond Switching in a Tetrameric Methanol Cluster. Phys. Chem. Chem. Phys. 2009, 11, 11340–11346. [Google Scholar] [CrossRef]
- Denisov, G.S.; Tokhadze, K.G. Ultrastrong Hydrogen Bond in Gas Phase. Dimer of Dimethylphosphinic Acid. Dokl. Phys. Chem. 1994, 337, 117–119. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between Interaction Energy, Intermolecular Distance and Electron Density Properties in Hydrogen Bonded Complexes under External Electric Fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Kumar, G.A.; McAllister, M.A. Theoretical Investigation of the Relationship between Proton NMR Chemical Shift and Hydrogen Bond Strength. J. Org. Chem. 1998, 63, 6968–6972. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Sigalov, M.; Shenderovich, I.G.; Mulloyarova, V.V.; Denisov, G.S.; Tolstoy, P.M. Correlations of NHN hydrogen bond energy with geometry and 1H NMR chemical shift difference of NH protons for aniline complexes. J. Chem. Phys. 2019, 150, 114305. [Google Scholar] [CrossRef] [PubMed]
- Denisov, G.S.; Bureiko, S.F.; Kucherov, S.Y.; Tolstoy, P.M. Correlation Relationships between the Energy and Spectroscopic Parameters of Complexes with F···HF Hydrogen Bond. Doklady Phys. Chem. 2017, 475, 115–118. [Google Scholar] [CrossRef]
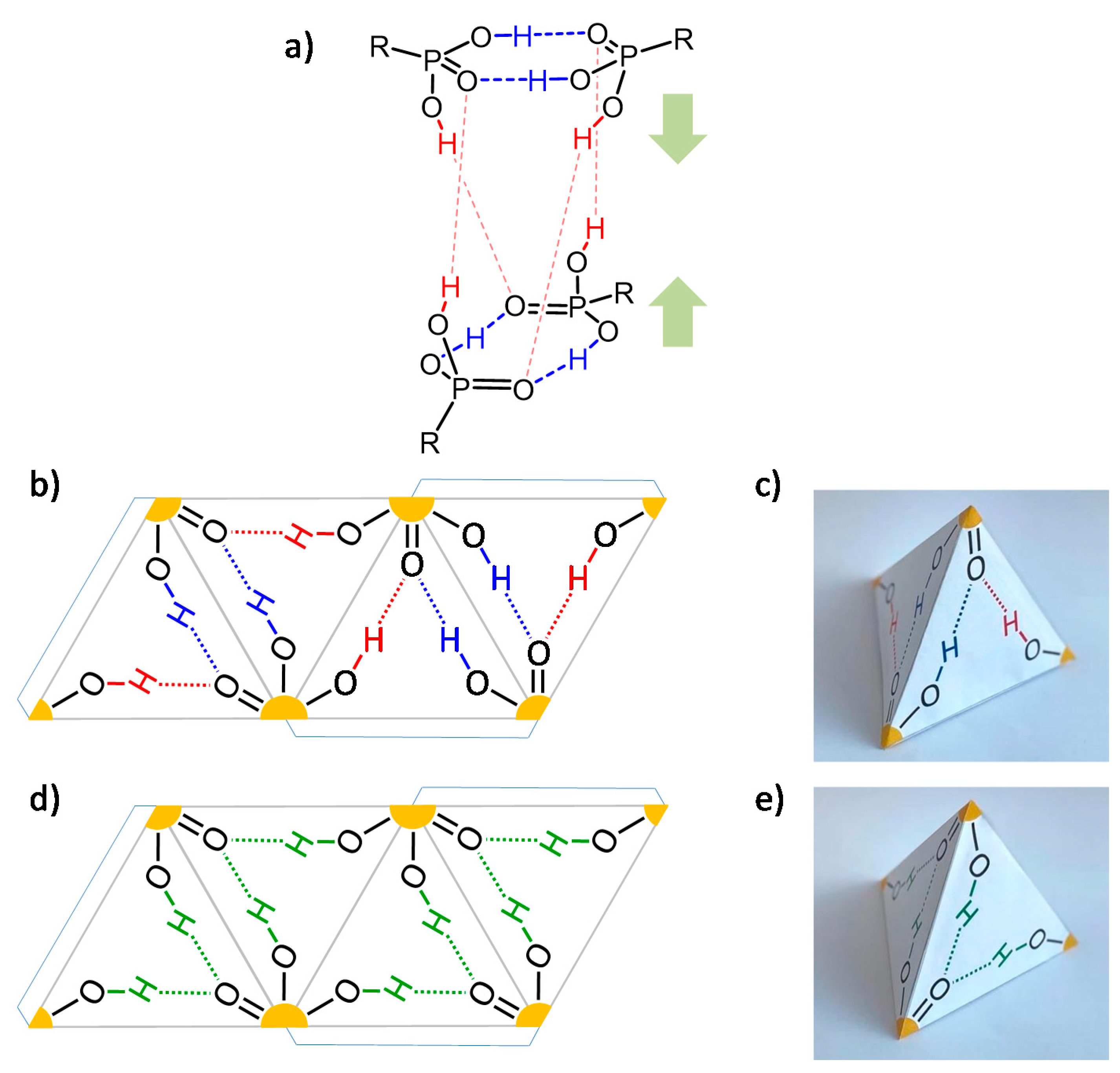
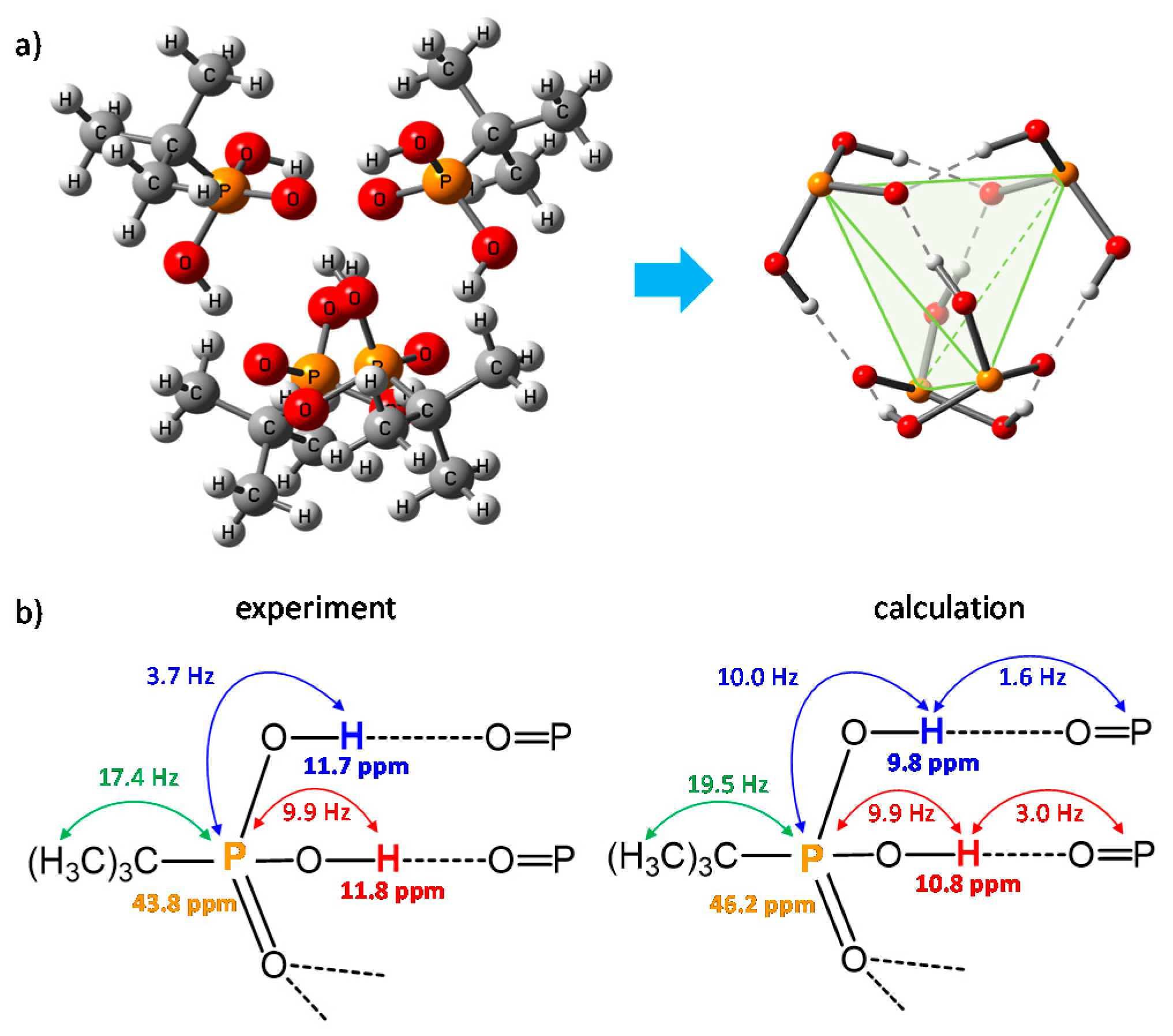
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giba, I.S.; Tolstoy, P.M. Self-Assembly of Hydrogen-Bonded Cage Tetramers of Phosphonic Acid. Symmetry 2021, 13, 258. https://doi.org/10.3390/sym13020258
Giba IS, Tolstoy PM. Self-Assembly of Hydrogen-Bonded Cage Tetramers of Phosphonic Acid. Symmetry. 2021; 13(2):258. https://doi.org/10.3390/sym13020258
Chicago/Turabian StyleGiba, Ivan S., and Peter M. Tolstoy. 2021. "Self-Assembly of Hydrogen-Bonded Cage Tetramers of Phosphonic Acid" Symmetry 13, no. 2: 258. https://doi.org/10.3390/sym13020258
APA StyleGiba, I. S., & Tolstoy, P. M. (2021). Self-Assembly of Hydrogen-Bonded Cage Tetramers of Phosphonic Acid. Symmetry, 13(2), 258. https://doi.org/10.3390/sym13020258






