Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics
Abstract
1. Introduction
2. Materials and Methods
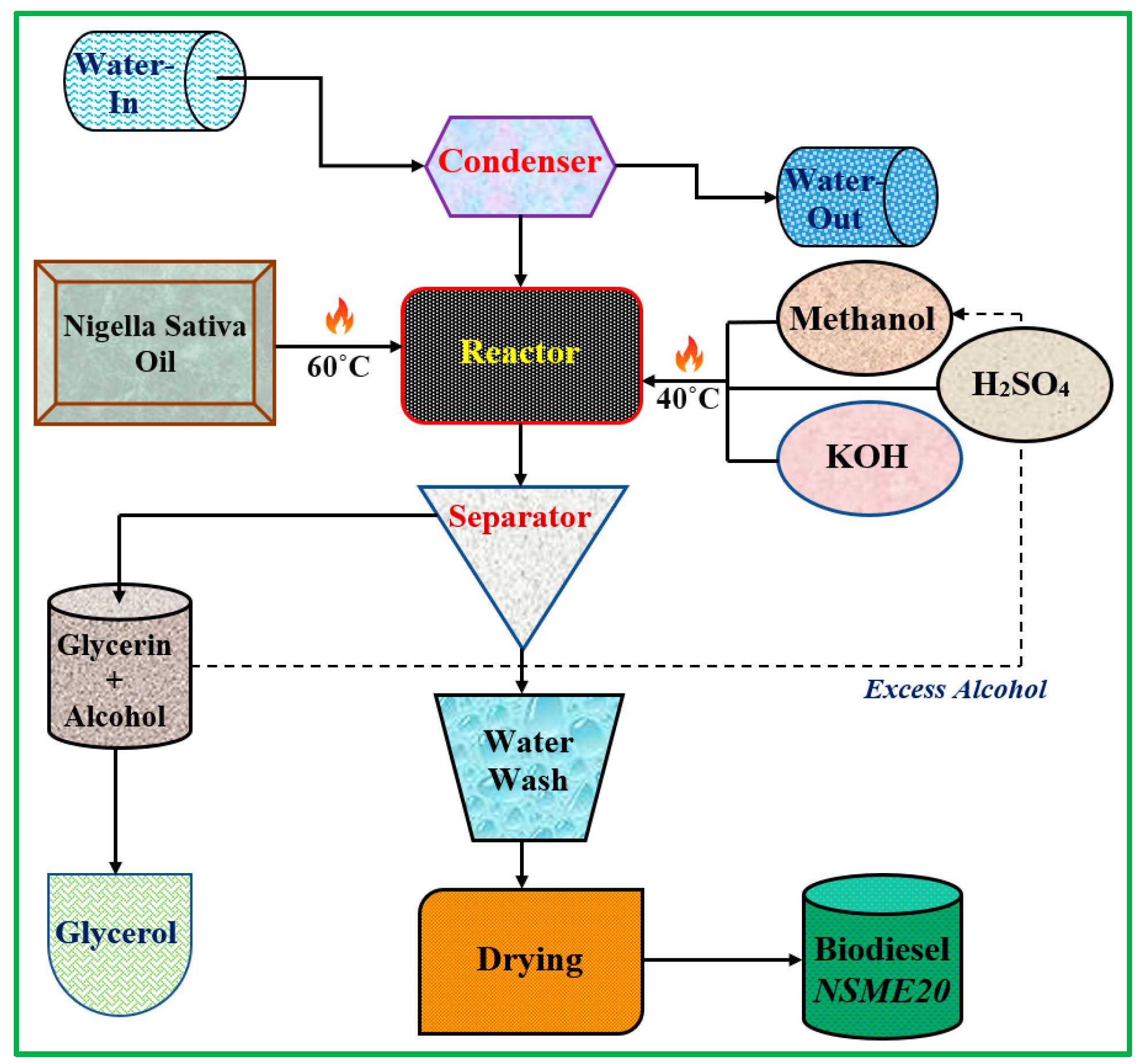
2.1. Preparation of Nigella sativa Methyl Ester
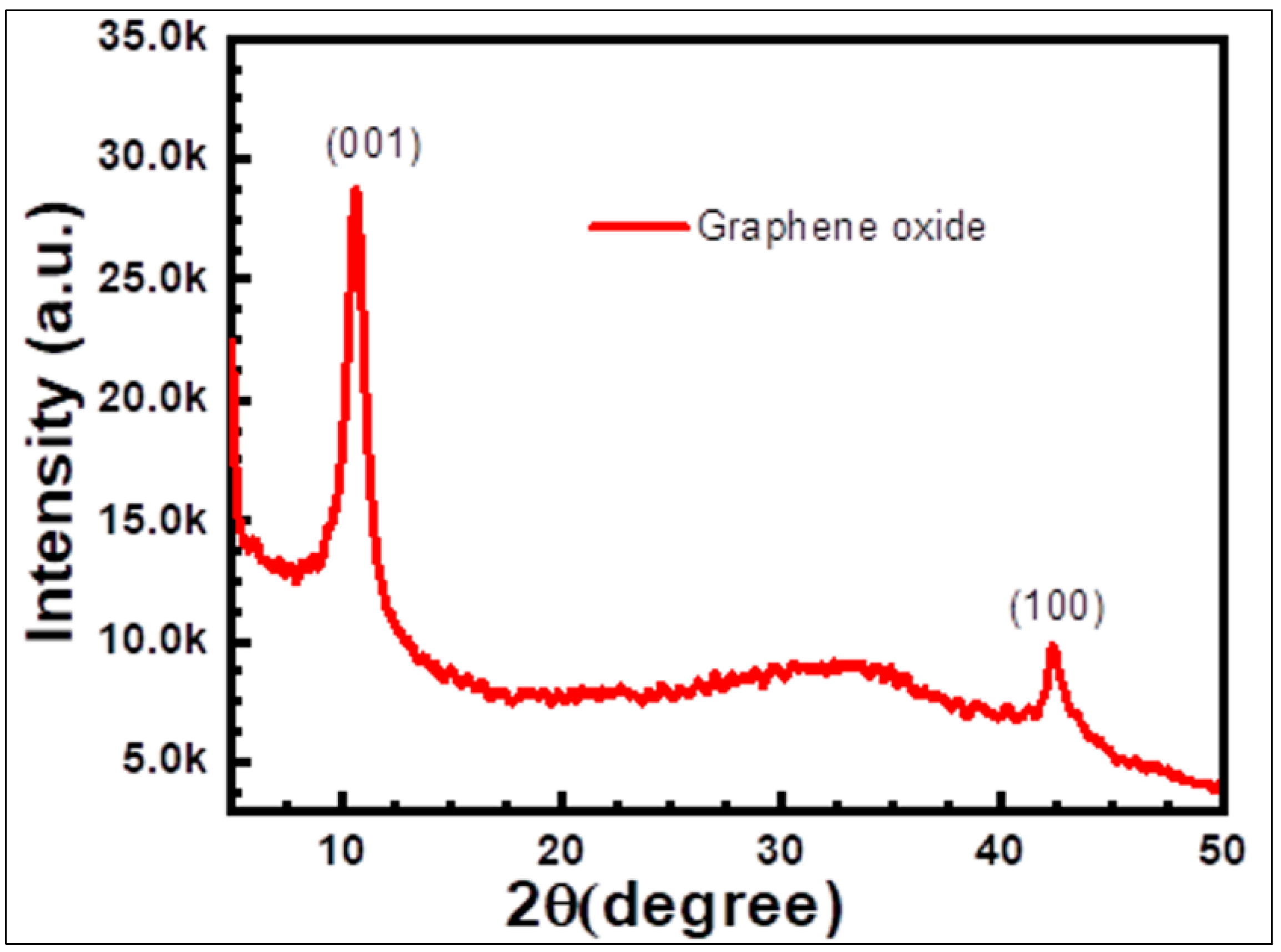
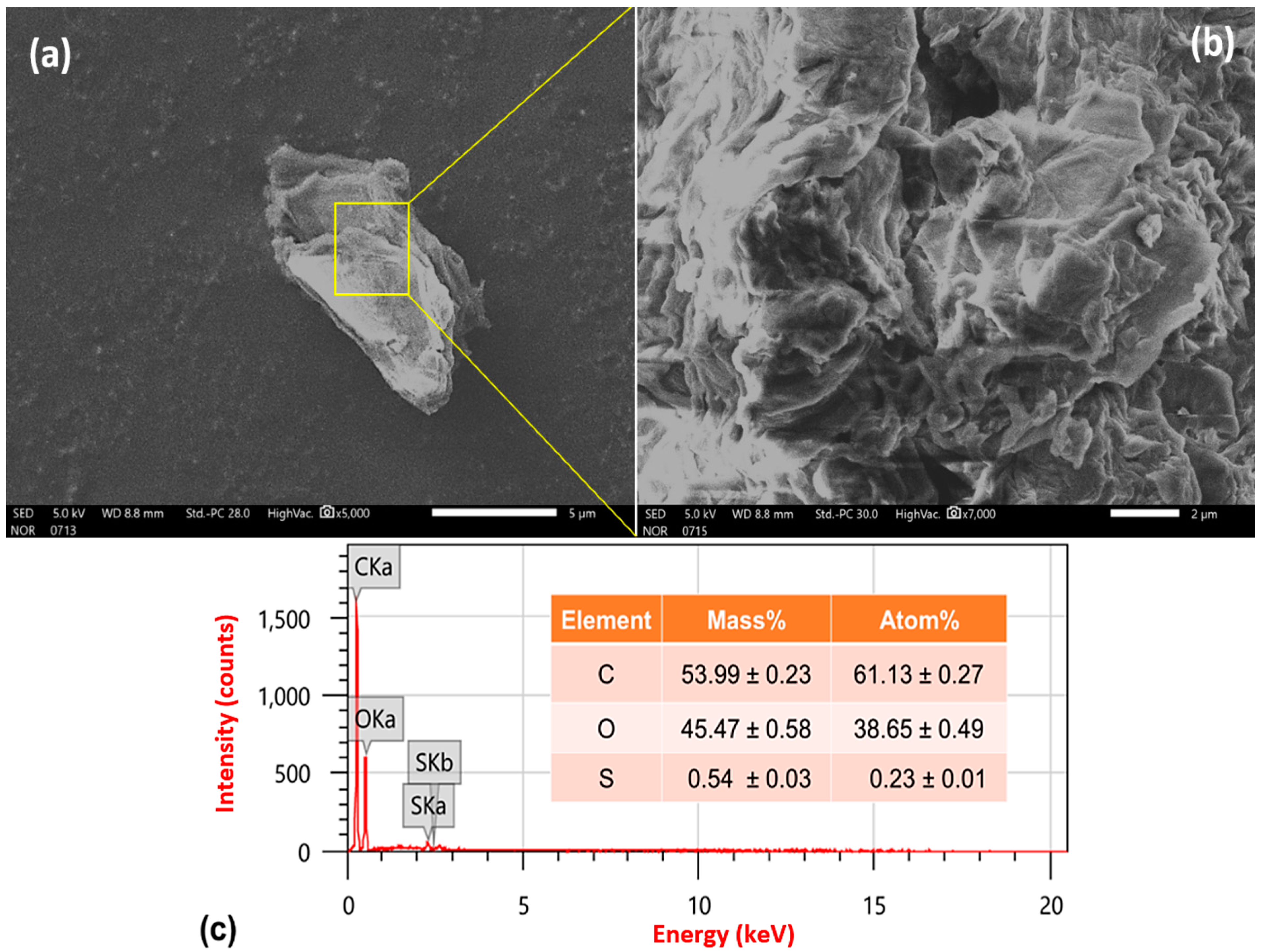
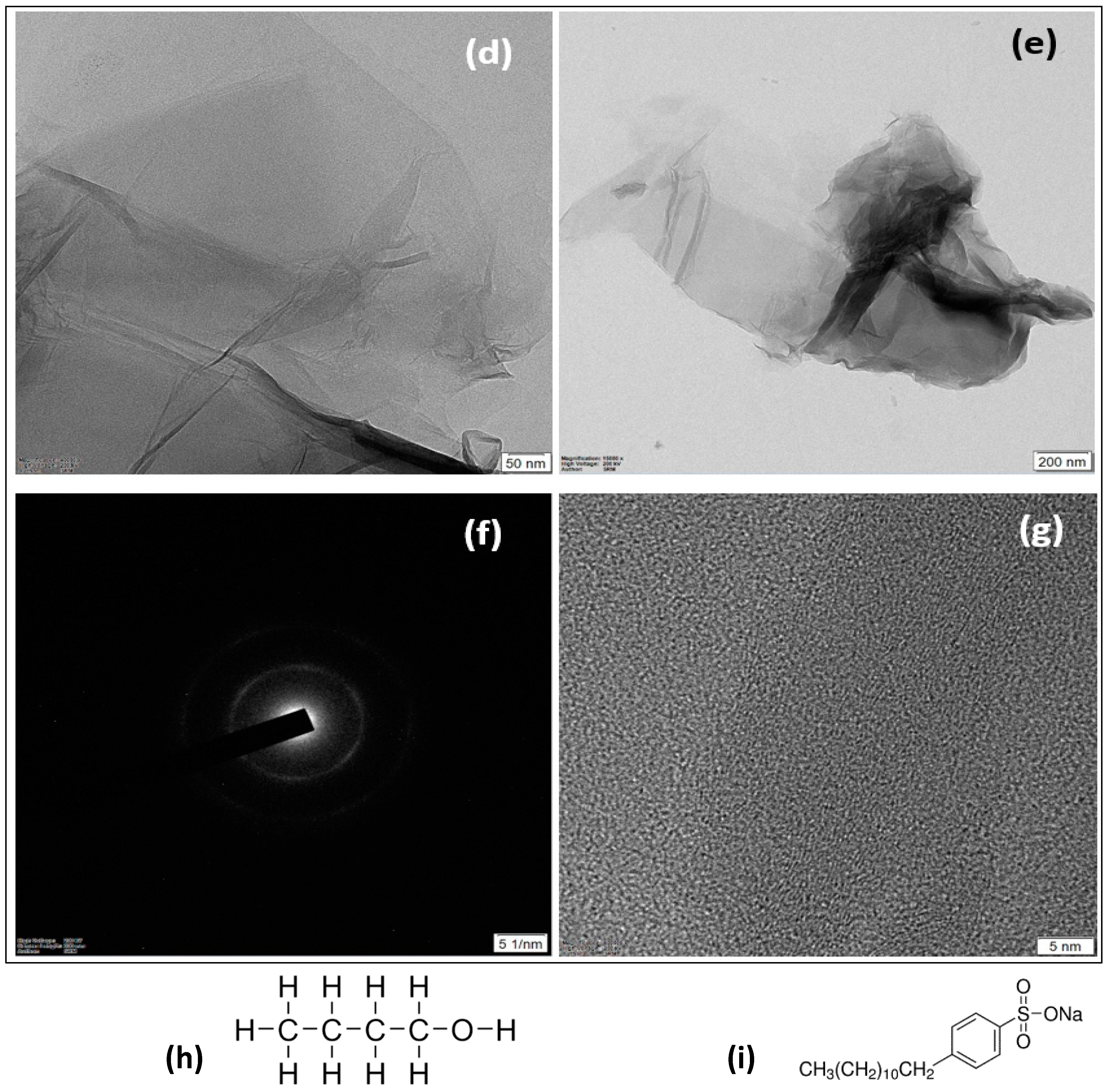
2.2. Properties and Characterization of Graphene Oxide Nanoparticles
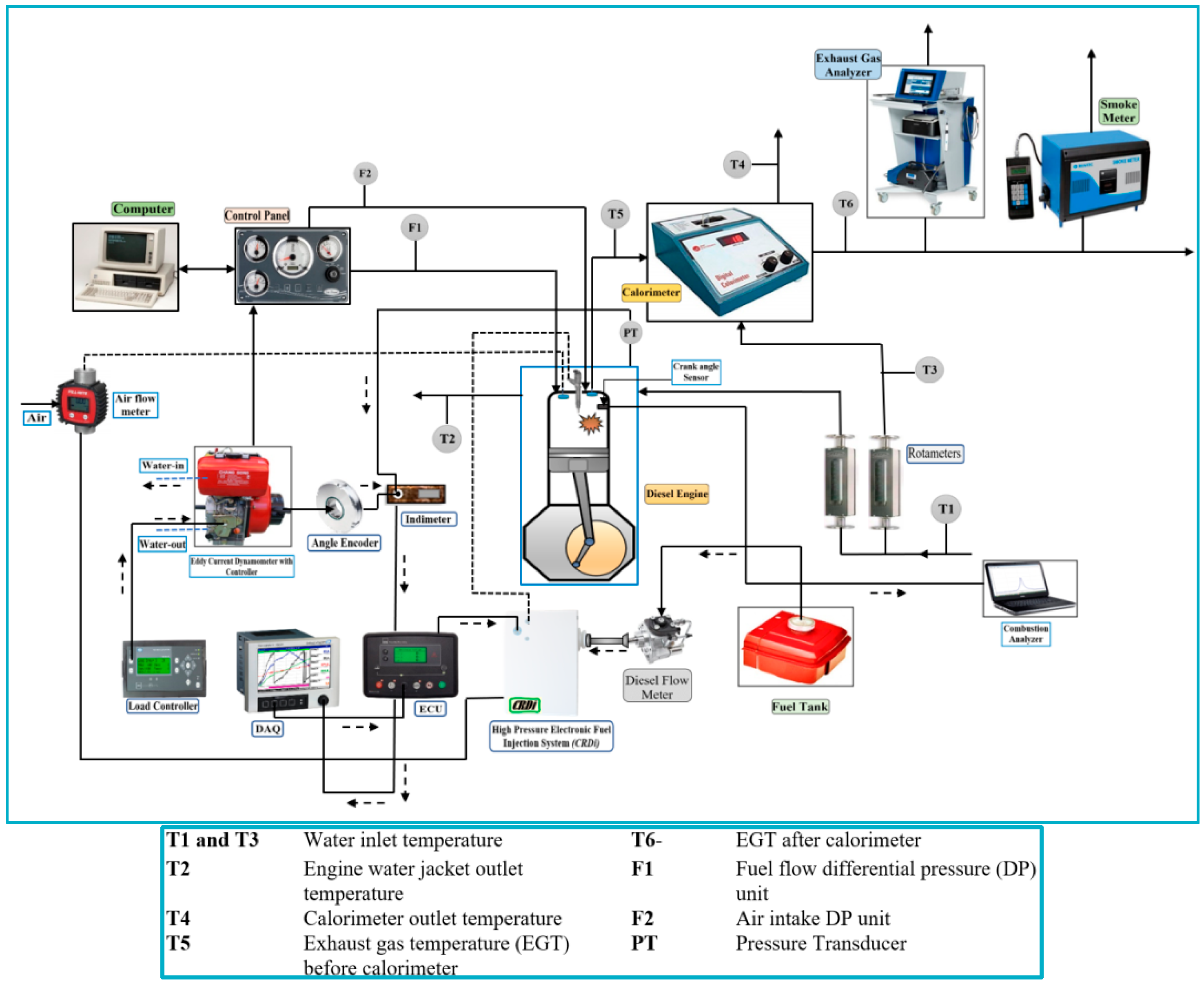
2.3. Test Engine Setup
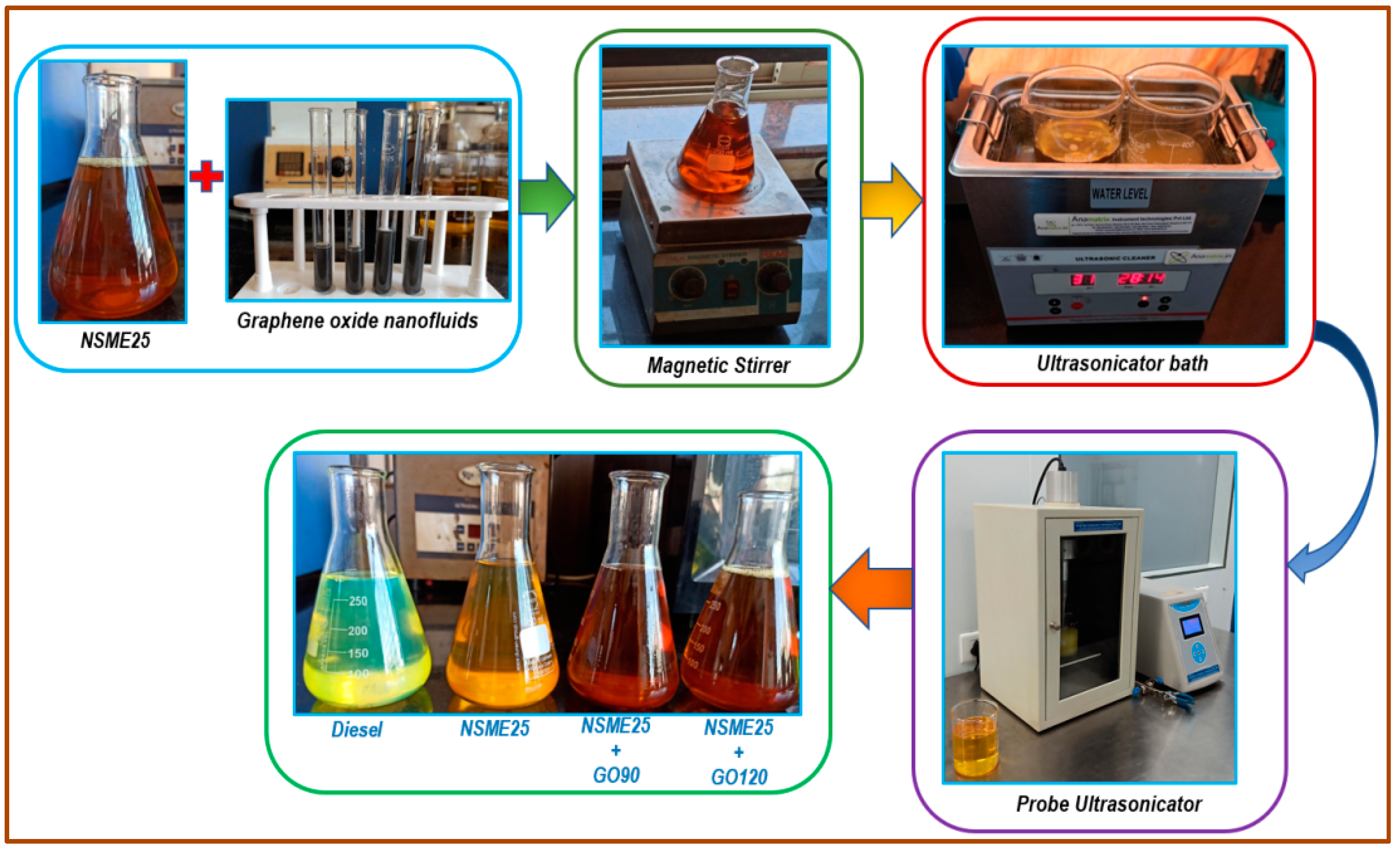
2.4. Preparation of Fuel Blend
2.5. Physicochemical Properties of Fuel Blends
3. Uncertainty Analysis of Expected Errors
4. Results
4.1. The Effect of Graphene Oxide Nanoparticles and n-Butanol in Biodiesel Nigella sativa on the Combustion Characteristics of CRDI Engines
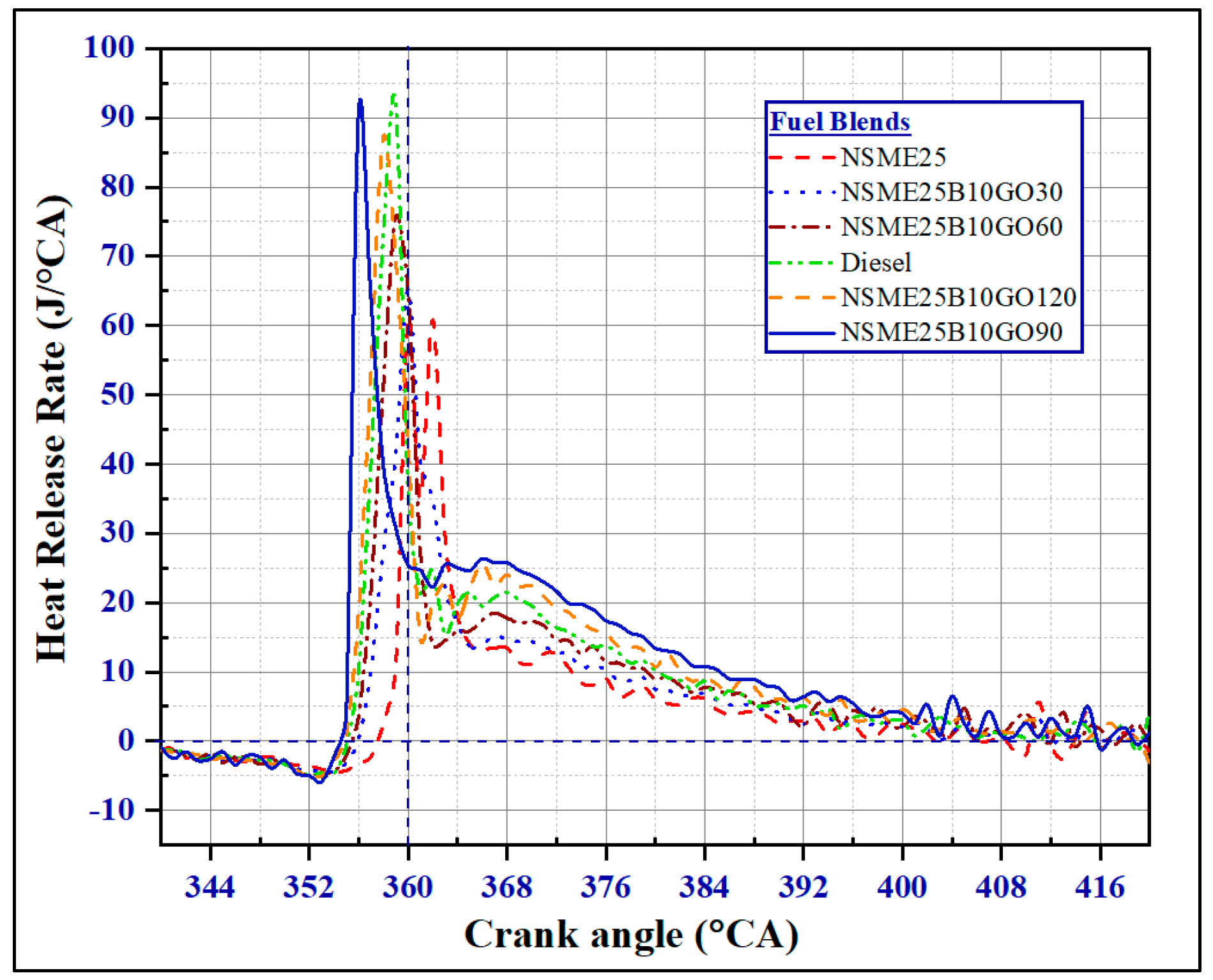
4.1.1. The Effect of Fuel Additives on Heat Release Rate
4.1.2. Effect of Fuel Additives on the In-Cylinder Temperature of the Engine
4.1.3. The Effect of Fuel Additives on Exhaust Gas Temperature (EGT)
4.1.4. The Effect of Fuel Additives on Cylinder Pressure (CP)
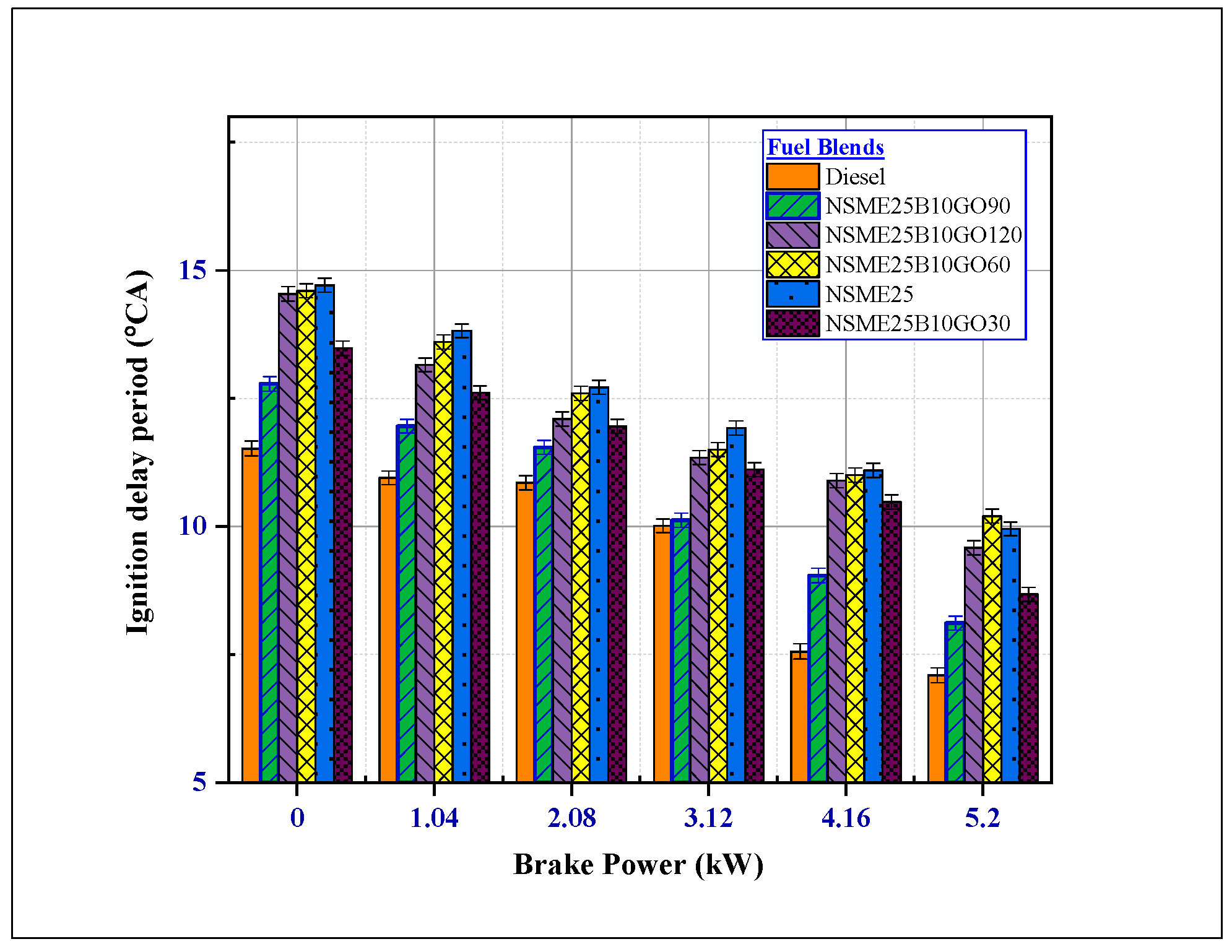
4.1.5. The Effect of Fuel Additives on Ignition Delay (ID) Period
4.2. The Effect of Graphene Oxide Nanoparticle and n-Butanol in Nigella sativa Biodiesel on the CRDI Engine Performance Characteristics
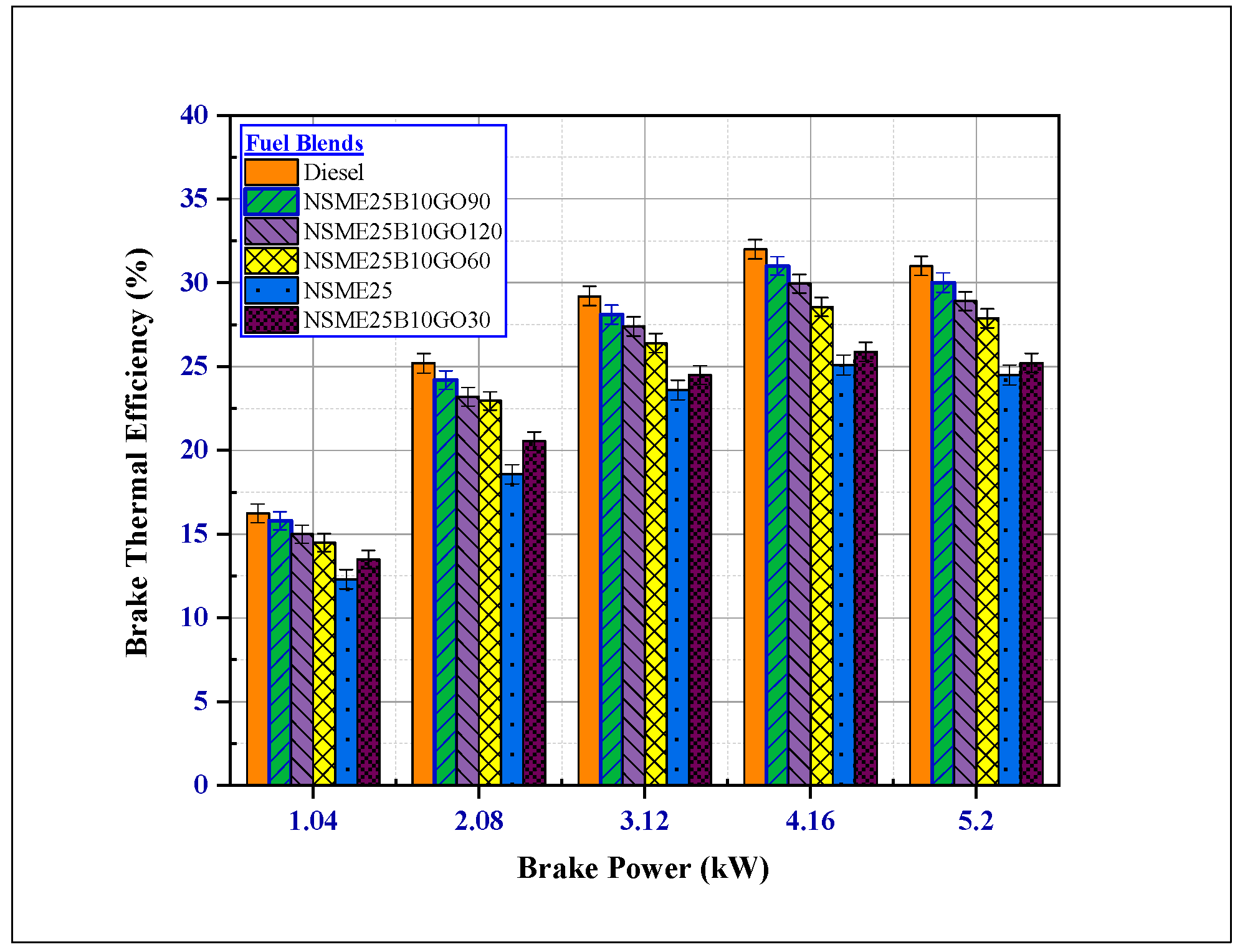
4.2.1. The Effect of Fuel Additives on Brake Thermal Efficiency (BTE)
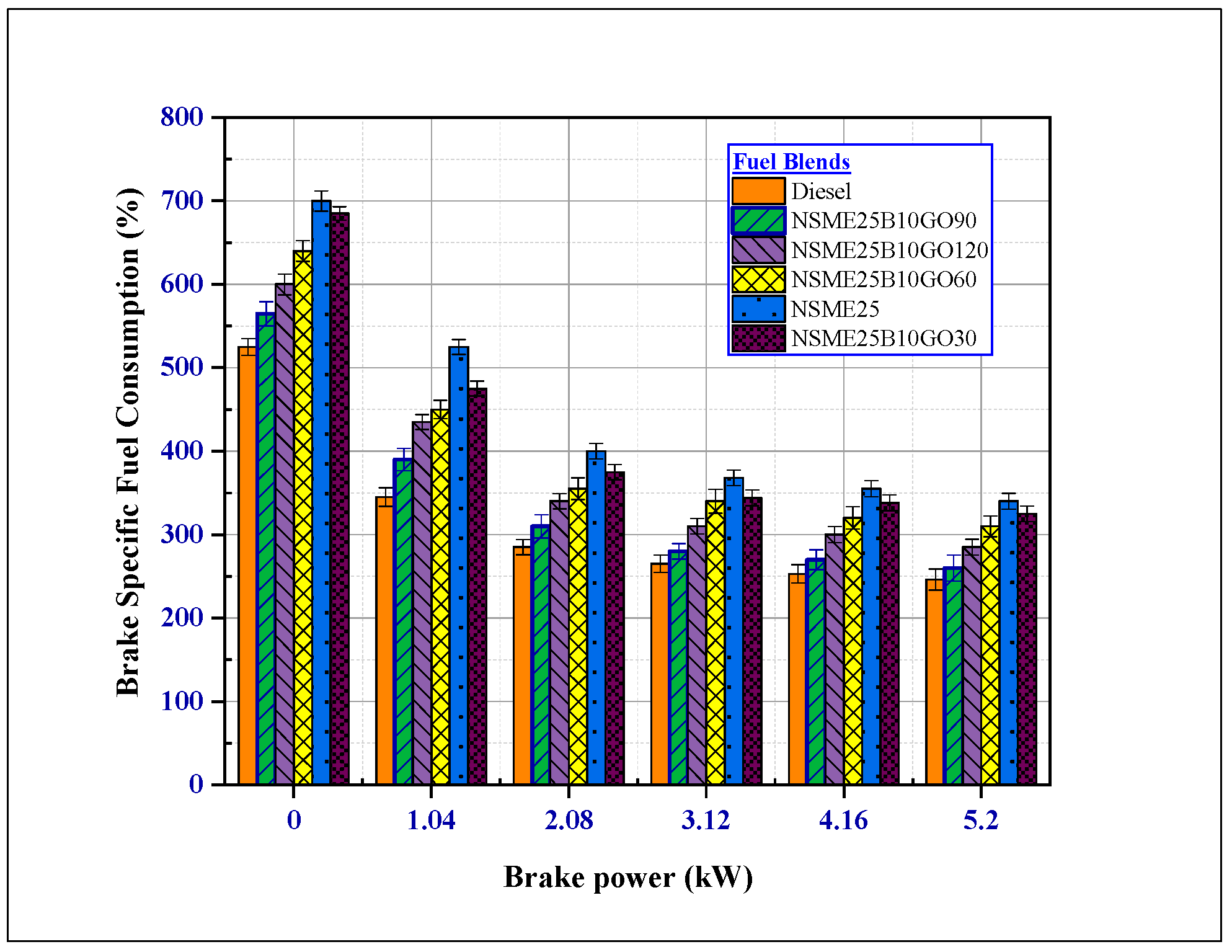
4.2.2. The Effect of Additives on Brake-Specific Fuel Consumption (BSFC)
4.3. The Effect of Graphene Oxide Nanoparticle and n-Butanol in Nigella sativa Biodiesel on the Emissions of the CRDI Engine
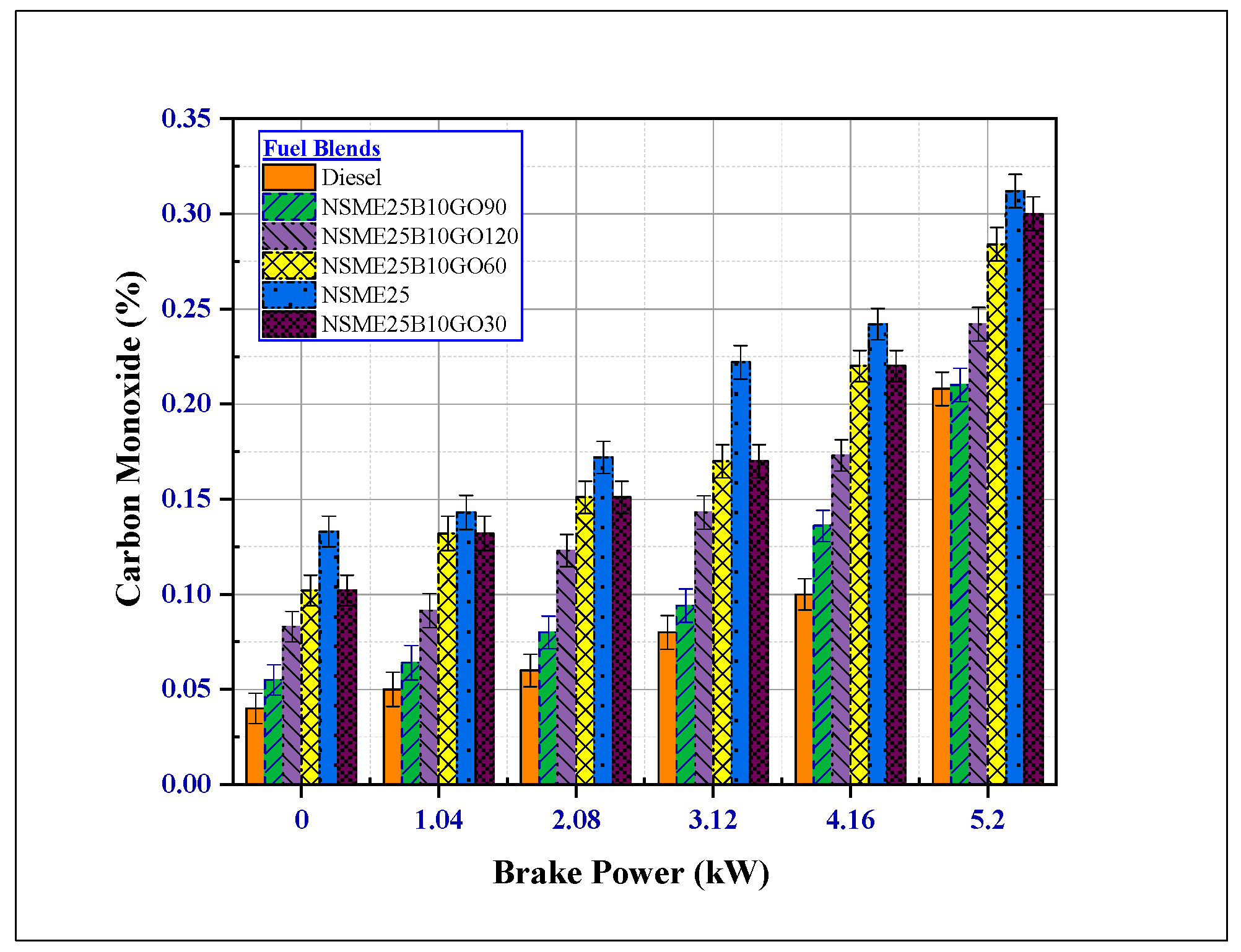
4.3.1. The Effect of Fuel Additives on Carbon Monoxide (CO) Emissions
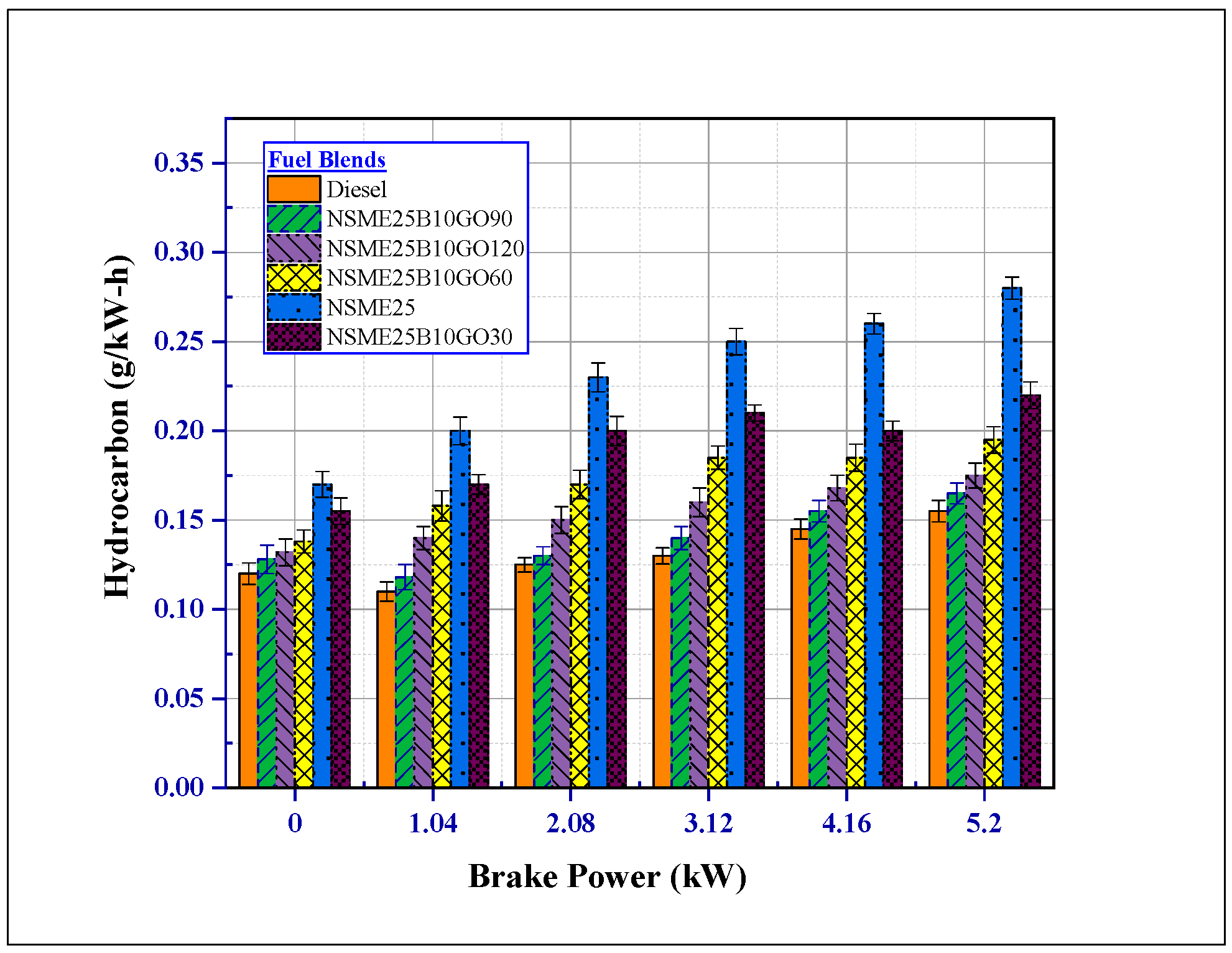
4.3.2. The Effect of Fuel Additives on Hydrocarbon (HC) Emissions
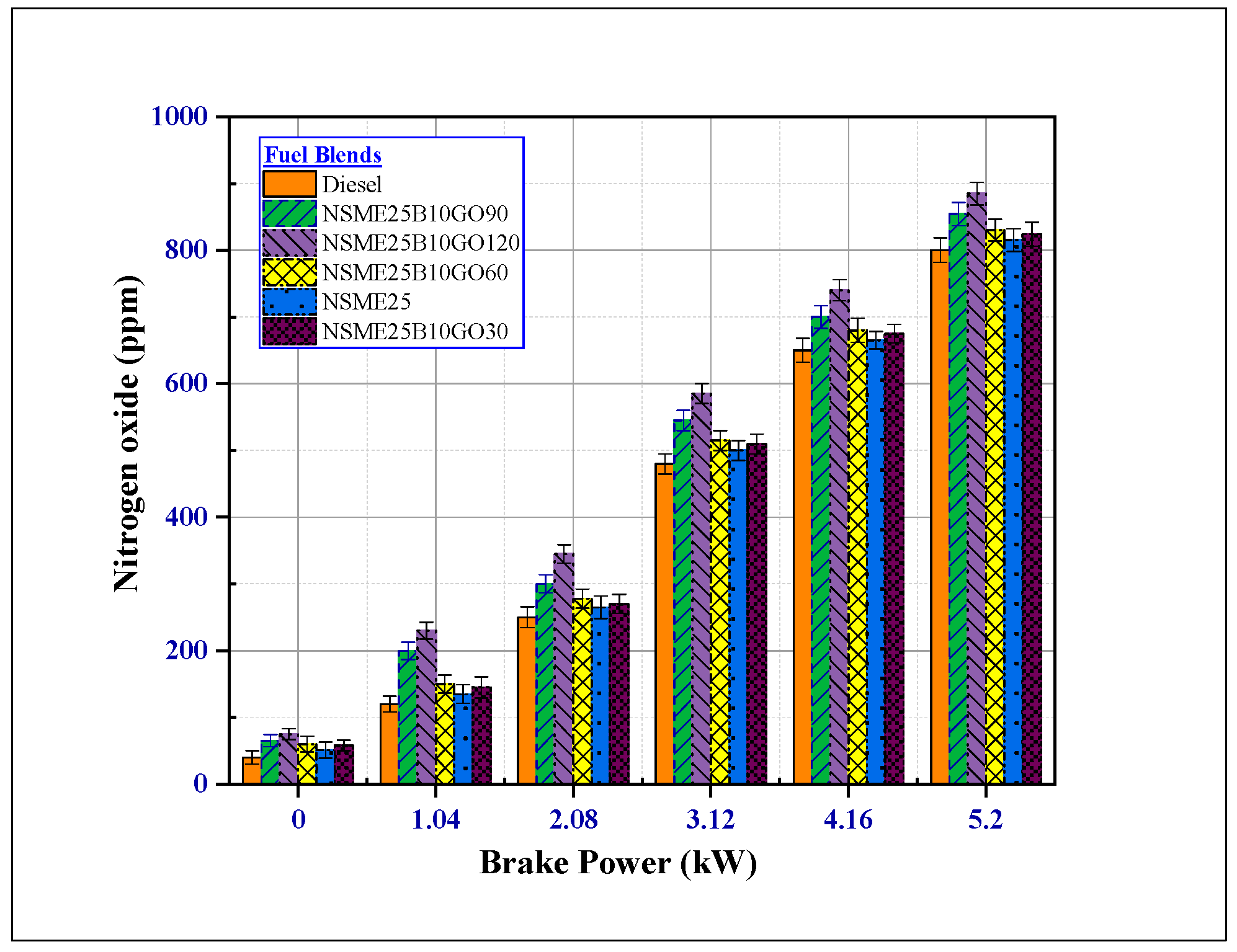
4.3.3. The Effect of Fuel Additives on Nitrogen Oxide (NOx) Emissions
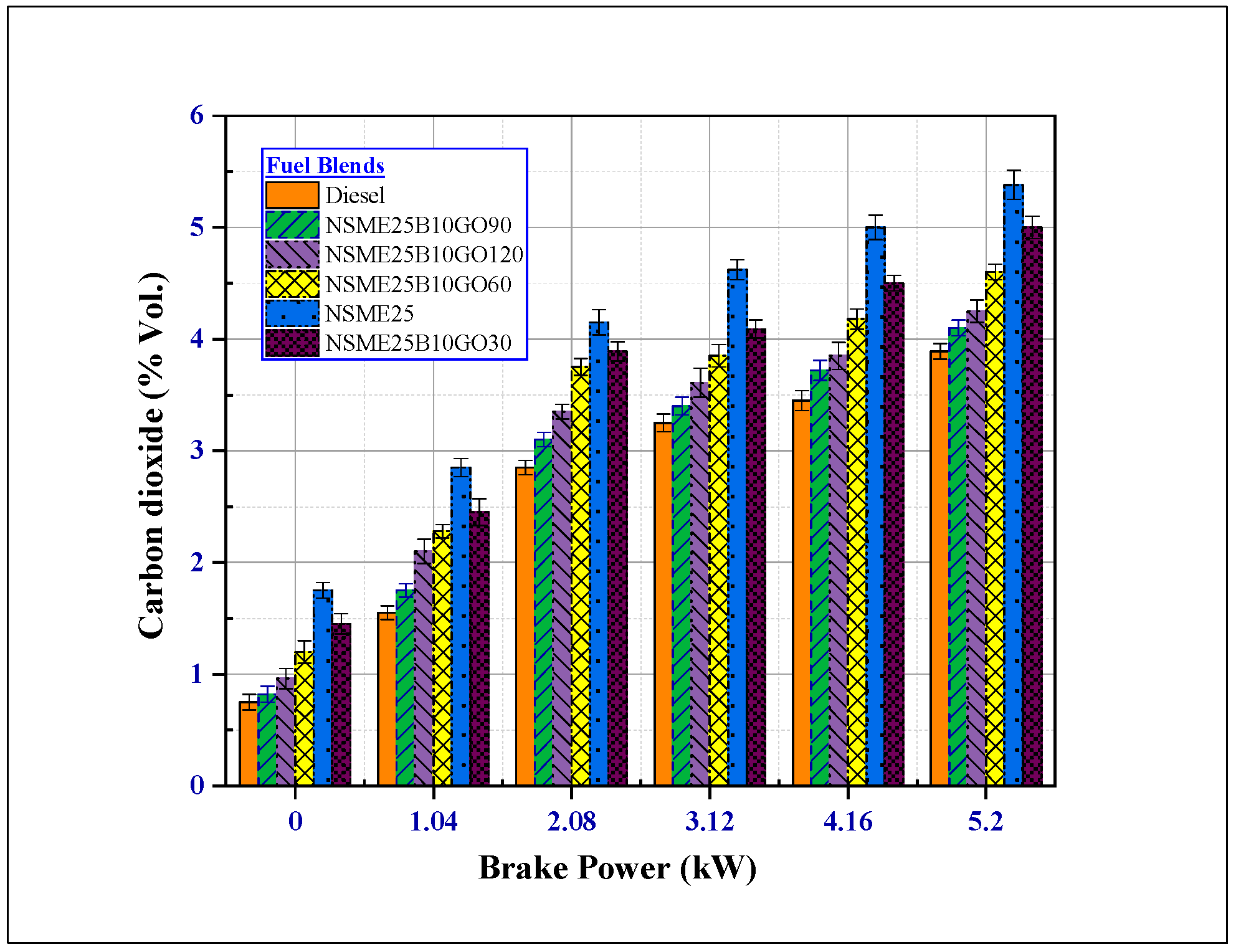
4.3.4. Effects of Fuel Additives on Carbon Dioxide (CO2) Emissions
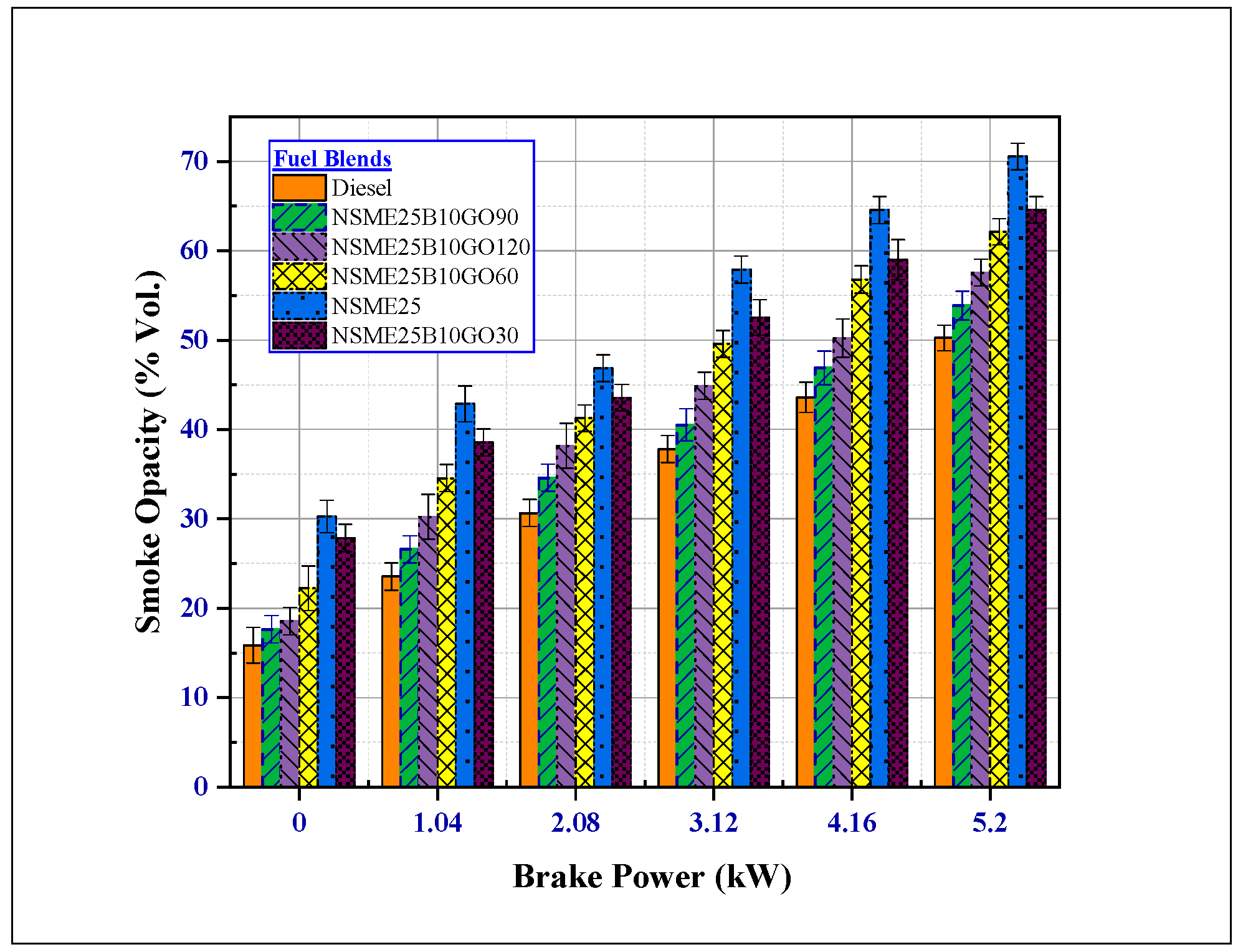
4.3.5. Effect of Fuel Additives on Smoke Emissions
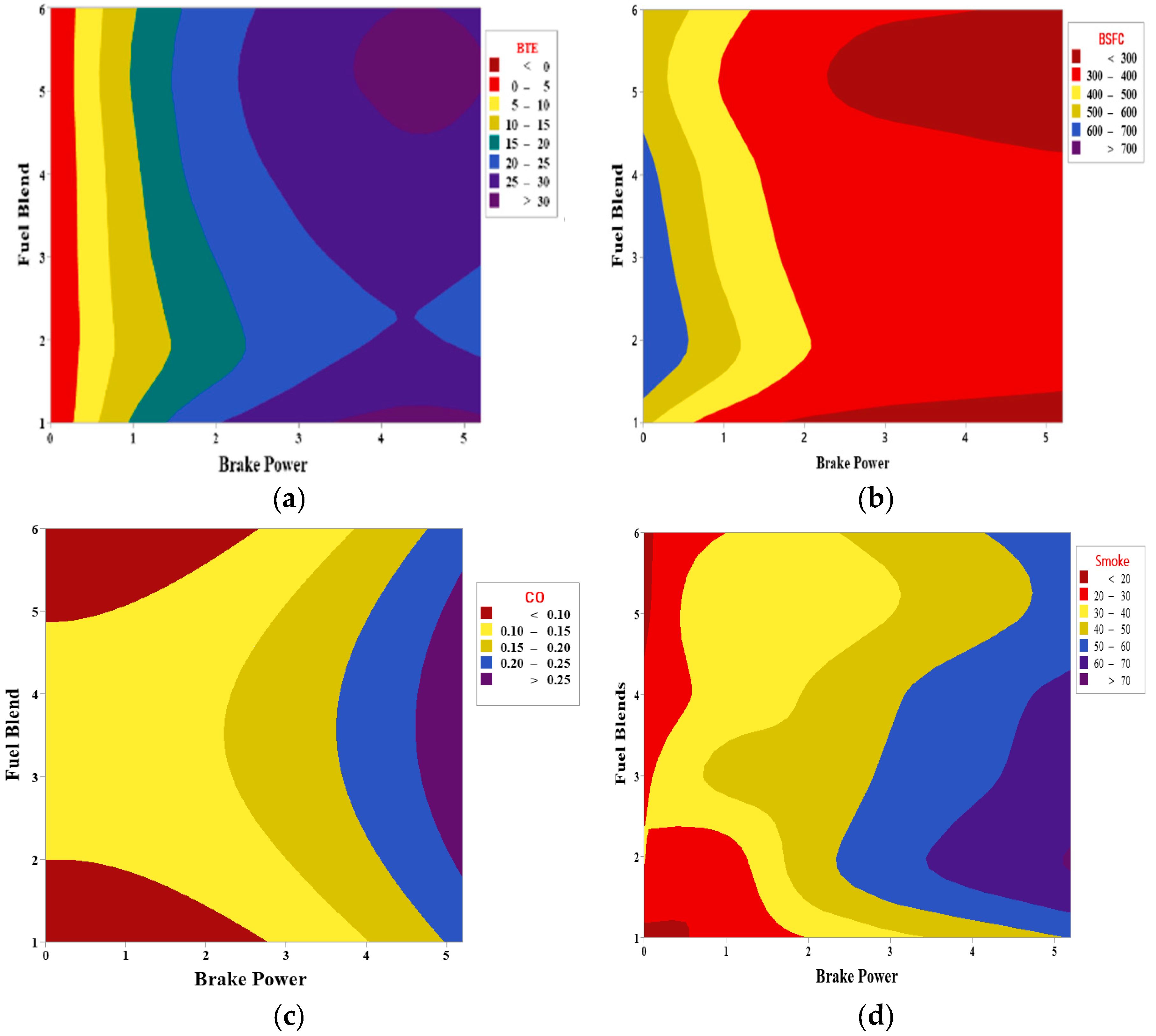
5. Response Surface Methodology of Nanofuel Blends
6. Conclusions
- The NSME25, n-butanol and graphene oxide nanoparticle emulsion fuel illustrated an enhanced BTE value compared to the NSME (B25) fuel blend; the BTE of NSME25B10GO90 was nearly equivalent to diesel fuel. For NSME25B10GO90 fuel, the BTE increased by 18.37% compared to the NSME25. The enhancement in the BTE for all nanofuel blends is due to the catalytic effect of GO NPs.
- In addition, the BSFC values for all nanofuel blends with n-butanol decreased due to complete fuel combustion and an improved A: F mixing ratio; this is due to excess oxygen atoms in the combustion chamber and higher in-cylinder temperature.
- The toroidal combustion chamber demonstrated better A:F mixing due to an enhanced swirling motion and six-hole fuel injector at an injection pressure of 900 bar; this led to an instantaneous combustion, which improved the heat release rate and cylinder pressure.
- The NOx emissions for the NSME25, n-butanol and GO NPs fuel blend increased due to surplus oxygen molecules present in the combustion chamber and high combustion chamber temperature; lower NOx emissions were observed for NSME25 and diesel fuel. Meanwhile, the NSME25B10GO120 emitted maximum NOx emissions at all loads.
- The addition of GO NPs to the Nigella sativa reduced all the other noxious emissions (CO2, HC, smoke, and CO). At maximum load, NSME25B10GO90 fuel blend smoke, HC and CO emissions decreased by 31.68%, 48.571% and 50.15% compared with NSME25 fuel blend.
- The enhancement of the BTE increased the EGT for diesel and nanofuel blend, leading to rapid combustion of fuel blends, and lowered the ignition delay at maximum load shortly after the SOC period.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| NPs | Nanoparticles | GO | Graphene oxide |
| CRDI | Common rail direct injection | SDBS | Sodium dodecyl benzene sulphonate |
| CI | Compression ignition | IC | Internal combustion |
| nm | Nanometer | A: F | Air-to-fuel ratio |
| g/kWh | Grams per kilowatt hour | ppm | Parts per million |
| CC | Combustion chamber | m | Meter |
| ATDC | After top dead centre | TCC | Toroidal combustion chamber |
| FFA | Free fatty acid | BTDC | Before top dead centre |
| A:F | Air Fuel mixture | CR | Compression ratio |
| ID | Injection delay | PP | Peak cylinder pressure |
| CO2 | Carbon dioxide | HC | Hydrocarbon |
| NOX | Oxides of nitrogen | CO | Carbon monoxide |
| BTE | Brake thermal efficiency | PM | Particulate matter |
| SFC | Specific fuel consumption | BSFC | Brake-specific fuel consumption |
| max. | Maximum | vol | Volume |
| T | Temperature | P | Pressure |
| ASTM | American Society for Testing and Materials | Tw | Wall temperature |
| CN | Cetane number | ηth | Thermal efficiency |
| IP | Injection pressure | IT | Injection timing |
| EGT | Exhaust gas temperature | HRR | Heat release rate |
| CD | Combustion duration | CP | Cylinder pressure |
| °CA | Crank angle (degrees) | D100 | 100% diesel |
| NSME | Nigella sativa methyl ester (Nigella biodiesel) | NSME25 | 25% Nigella sativa methyl ester blended with diesel |
| NSME25 B10GO30 | NSME25 with 10% n-butanol and 30 ppm GO NPs | NSME25 B10GO60 | NSME25 with 10% n-butanol and 60 ppm GO NPs |
| NSME25 B10GO90 | NSME25 with 10% n-butanol and 90 ppm GO NPs | NSME25 B10GO120 | NSME25 with 10% n-butanol and 120 ppm GO NPs |
References
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Ali, M.A.B.; Kamangar, S.; Cho, H.M.; Akram, N. An investigation on the influence of aluminium oxide nano-additive and honge oil methyl ester on engine performance, combustion and emission characteristics. Renew. Energy 2020, 146, 2291–2307. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Khan, T.Y.; Bashir, M.N.; Akram, N.; Farade, R.; Afzal, A. The effects of graphene oxide nanoparticle additive stably dispersed in dairy scum oil biodiesel-diesel fuel blend on CI engine: Performance, emission and combustion characteristics. Fuel 2019, 257, 116015. [Google Scholar] [CrossRef]
- Aghabarari, B.; Dorostkar, N.; Martinez-Huerta, M. Synthesis of biodiesel from Nigella sativa seed oil using surfactant-Brønsted acidic-combined ionic liquid as catalyst. Fuel Process. Technol. 2014, 118, 296–301. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Kittur, P.; Parmar, F.; Batakatti, S.; Kulkarni, P.; Kallannavar, V. Production of jath Oil Ethyl Ester (MOEE) and its Performance test on four stroke single cylinder VCR engine. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Philadelphia, PA, USA, 2017; Volume 225, p. 012029. [Google Scholar]
- Azam, M.M.; Waris, A.; Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar]
- Delvi, M.K.; Soudagar, M.E.M.; Khan, H.; Ahmed, Z.; Shariff, I.M. Biodiesel Production Utilizing Diverse Sources, Classification of Oils and Their Esters, Performance and Emission Characteristics: A Research. IJRTE 2020. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Badruddin, I.A.; Kalam, M.A.; Kittur, M.I.; Akram, N.; Ullah, M.A.; Khan, T.Y.; Mokashi, I. Production of honge oil methyl ester (HOME) and its performance test on four stroke single cylinder VCR engine. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2019; Volume 2142, p. 200006. [Google Scholar]
- Sheriff, S.A.; Kumar, I.K.; Mandhatha, P.S.; Jambal, S.S.; Sellappan, R.; Ashok, B.; Nanthagopal, K. Emission reduction in CI engine using biofuel reformulation strategies through nano additives for atmospheric air quality improvement. Renew. Energy 2020, 147, 2295–2308. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Cho, H.M.; Masjuki, H.H.; Kalam, M.A.; Ong, H.C.; Gul, M.; Harith, M.H.; Yusoff, M.N.A.M. Critical review on sesame seed oil and its methyl ester on cold flow and oxidation stability. Energy Rep. 2020, 6, 40–54. [Google Scholar] [CrossRef]
- Achten, W.M.; Mathijs, E.; Verchot, L.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha biodiesel fueling sustainability? Biofuels Bioprod. Biorefin. Innov. Sustain. Econ. 2007, 1, 283–291. [Google Scholar] [CrossRef]
- Heidari-Maleni, A.; Gundoshmian, T.M.; Jahanbakhshi, A.; Ghobadian, B. Performance improvement and exhaust emissions reduction in diesel engine through the use of graphene quantum dot (GQD) nanoparticles and ethanol-biodiesel blends. Fuel 2020, 267, 117116. [Google Scholar] [CrossRef]
- Basha, J.S. Impact of Carbon Nanotubes and Di-Ethyl Ether as additives with biodiesel emulsion fuels in a diesel engine—An experimental investigation. J. Energy Inst. 2018, 91, 289–303. [Google Scholar] [CrossRef]
- Akram, N.; Sadri, R.; Kazi, S.N.; Zubir, M.N.M.; Ridha, M.; Ahmed, W.; Soudagar, M.E.M.; Arzpeyma, M. A comprehensive review on nanofluid operated solar flat plate collectors. J. Therm. Anal. Calorim. 2020, 139, 1309–1343. [Google Scholar] [CrossRef]
- Ooi, J.B.; Ismail, H.M.; Swamy, V.; Wang, X.; Swain, A.K.; Rajanren, J.R. Graphite oxide nanoparticle as a diesel fuel additive for cleaner emissions and lower fuel consumption. Energy Fuels 2016, 30, 1341–1353. [Google Scholar] [CrossRef]
- Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Sohn, K.; Huang, J. Self-propagating domino-like reactions in oxidized graphite. Adv. Funct. Mater. 2010, 20, 2867–2873. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Taghizadeh-Alisaraei, A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Performance and emission characteristics of a CI engine fuelled with carbon nanotubes and diesel-biodiesel blends. Renew. Energy 2017, 111, 201–213. [Google Scholar] [CrossRef]
- Venkateswaran, S.P.; Nagarajan, G. Effects of the re-entrant bowl geometry on a DI turbocharged diesel engine performance and emissions—A CFD approach. J. Eng. Gas Turbines Power 2010, 132. [Google Scholar] [CrossRef]
- Bapu, B.R.; Saravanakumar, L.; Prasad, B.D. Effects of combustion chamber geometry on combustion characteristics of a DI diesel engine fueled with calophyllum inophyllum methyl ester. J. Energy Inst. 2017, 90, 82–100. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Novel environmentally friendly fuel: The effects of nanographene oxide additives on the performance and emission characteristics of diesel engines fuelled with Ailanthus altissima biodiesel. Renew. Energy 2018, 125, 283–294. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; Hassan, H.; Ookawara, S. Performance, combustion, and emission characteristics of a diesel engine fueled with Jatropha methyl ester and graphene oxide additives. Energy Convers. Manag. 2018, 166, 674–686. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Ebadi, M.T.; Mamat, R.; Yusaf, T. Biodiesels from three feedstock: The effect of graphene oxide (GO) nanoparticles diesel engine parameters fuelled with biodiesel. Renew. Energy 2020, 145, 190–201. [Google Scholar] [CrossRef]
- Ooi, J.B.; Ismail, H.M.; Tan, B.T.; Wang, X. Effects of graphite oxide and single-walled carbon nanotubes as diesel additives on the performance, combustion, and emission characteristics of a light-duty diesel engine. Energy 2018, 161, 70–80. [Google Scholar] [CrossRef]
- Debbarma, S.; Misra, R.D.; Das, B. Performance of graphene-added palm biodiesel in a diesel engine. Clean Technol. Environ. Policy 2020, 1–12. [Google Scholar] [CrossRef]
- Paramashivaiah, B.M.; Banapurmath, N.R.; Rajashekhar, C.R.; Khandal, S.V. Studies on effect of graphene nanoparticles addition in different levels with simarouba biodiesel and diesel blends on performance, combustion and emission characteristics of CI engine. Arab. J. Sci. Eng. 2018, 43, 4793–4801. [Google Scholar] [CrossRef]
- Khan, T.Y.; Atabani, A.E.; Badruddin, I.A.; Ankalgi, R.F.; Khan, T.M.; Badarudin, A. Ceiba pentandra, Nigella sativa and their blend as prospective feedstocks for biodiesel. Ind. Crops Prod. 2015, 65, 367–373. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Goreja, W. Black Seed: Nature’s Miracle Remedy; Karger Publishers: Basel, Switzerland, 2003. [Google Scholar]
- Encyclopaedia Britannica. Black cumin. In Black Cumin—PLANT AND SEED; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2018; Available online: https://www.britannica.com/plant/black-cumin (accessed on 6 May 2020).
- Ain, Q.T.; Haq, S.H.; Alshammari, A.; Al-Mutlaq, M.A.; Anjum, M.N. The systemic effect of PEG-nGO-induced oxidative stress in vivo in a rodent model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef]
- Farade, A.R.; Wahab, N.I.B.A.; Mansour, D.E.A.; Azis, N.B.; Banapurmath, N.R.; Soudagar, M.E.M. Investigation of the dielectric and thermal properties of non-edible cottonseed oil by infusing h-BN nanoparticles. IEEE Access 2020. [Google Scholar] [CrossRef]
- Wilson, N.R.; Pandey, P.A.; Beanland, R.; Young, R.J.; Kinloch, I.A.; Gong, L.; Liu, Z.; Suenaga, K.; Rourke, J.P.; York, S.J.; et al. Graphene oxide: Structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 2009, 3, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Rakopoulos, D.; Rakopoulos, C.D.; Giakoumis, G.; Dimaratos, M.; Kyritsis, C. Effects of butanol—Diesel fuel blends on the performance and emissions of a high-speed DI diesel engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Han, Z.; Du, B.; Liu, Y.; Lee, C. Study on performance and emissions of a passenger-car diesel engine fueled with butanol—Diesel blends. Energy 2013, 55, 638–646. [Google Scholar] [CrossRef]
- Ibrahim, A. Performance and combustion characteristics of a diesel engine fuelled by butanol—Biodiesel—diesel blends. Appl. Therm. Eng. 2016, 103, 651–659. [Google Scholar] [CrossRef]
- Al-Samaraae, R.; Atabani, A.E.; Uguz, G.; Kumar, G.; Arpa, O.; Ayanoglu, A.; Mohammed, M.N.; Farouk, H. Perspective of safflower (Carthamus tinctorius) as a potential biodiesel feedstock in Turkey: Characterization, engine performance and emissions analyses of butanol—Biodiesel—diesel blends. Biofuels 2017, 1–17. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Saravanan, B.; Somasundaram, P.; Jegadheesan, C.; Chaturvedi, B.; Sharma, S.; Patni, G. A novel study on the effect lemon peel oil as a fuel in CRDI engine at various injection strategies. Energy Convers. Manag. 2018, 172, 517–528. [Google Scholar] [CrossRef]
- Örs, I.; Sarıkoç, S.; Atabani, A.E.; Ünalan, S.; Akansu, S.O. The effects on performance, combustion and emission characteristics of DICI engine fuelled with TiO2 nanoparticles addition in diesel/biodiesel/n-butanol blends. Fuel 2018, 234, 177–188. [Google Scholar]
- Hawi, M.; Elwardany, A.; Ismail, M.; Ahmed, M. Experimental investigation on performance of a compression ignition engine fueled with waste cooking oil biodiesel–diesel blend enhanced with iron-doped cerium oxide nanoparticles. Energies 2019, 12, 798. [Google Scholar] [CrossRef]
- Banapurmath, N.R.; Sankaran, R.; Tumbal, A.V.; TN, N.; Hunashyal, A.M.; Ayachit, N.H. Experimental investigation on direct injection diesel engine fuelled with graphene, silver and multiwalled carbon nanotubes-biodiesel blended fuels. Int. J. Automot. Eng. Technol. 2014, 3, 129–138. [Google Scholar] [CrossRef]
- Javed, S.; Murthy, Y.S.; Satyanarayana, M.R.S.; Reddy, R.R.; Rajagopal, K. Effect of a zinc oxide nanoparticle fuel additive on the emission reduction of a hydrogen dual-fuelled engine with jatropha methyl ester biodiesel blends. J. Clean. Prod. 2016, 137, 490–506. [Google Scholar] [CrossRef]
- Ahmed, A.; Shah, A.N.; Azam, A.; Uddin, G.M.; Ali, M.S.; Hassan, S.; Ahmed, H.; Aslam, T. Environment-friendly novel fuel additives: Investigation of the effects of graphite nanoparticles on performance and regulated gaseous emissions of CI engine. Energy Convers. Manag. 2020, 211, 112748. [Google Scholar] [CrossRef]
- Fatah, M.A.; Farag, H.; Ossman, M. Production of biodiesel from non-edible oil and effect of blending with diesel on fuel properties. Eng. Sci. Technol. Int. J. 2012, 2, 583–591. [Google Scholar]
- Nanthagopal, K.; Ashok, B.; Tamilarasu, A.; Johny, A.; Mohan, A. Influence on the effect of zinc oxide and titanium dioxide nanoparticles as an additive with Calophyllum inophyllum methyl ester in a CI engine. Energy Convers. Manag. 2017, 146, 8–19. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Hassan, H. Investigation of the effect of adding graphene oxide, graphene nanoplatelet, and multiwalled carbon nanotube additives with n-butanol-Jatropha methyl ester on a diesel engine performance. Renew. Energy 2019, 132, 558–574. [Google Scholar] [CrossRef]
- Gupta, H.N. Fundamentals of Internal Combustion Engines; PHI Learning Pvt. Ltd.: New Delhi, India, 2012. [Google Scholar]
- El-Seesy, A.I.; Abdel-Rahman, A.K.; Bady, M.; Ookawara, S.J.E.C. Performance, combustion, and emission characteristics of a diesel engine fueled by biodiesel-diesel mixtures with multi-walled carbon nanotubes additives. Energy Convers. Manag. 2017, 135, 373–393. [Google Scholar] [CrossRef]
- Annamalai, M.; Dhinesh, B.; Nanthagopal, K.; Sivarama Krishnan, P.; Lalvani, J.I.J.; Parthasarathy, M.; Annamalai, K. An assessment on performance, combustion and emission behavior of a diesel engine powered by ceria nanoparticle blended emulsified biofuel. Energy Convers. Manag. 2016, 123, 372–380. [Google Scholar] [CrossRef]
- Aalam, C.S.; Saravanan, C.; Kannan, M. Experimental investigations on a CRDI system assisted diesel engine fuelled with aluminium oxide nanoparticles blended biodiesel. Alex. Eng. J. 2015, 54, 351–358. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Kamruzzaman, M.; Shahin, M.M.; Rashed, M.M. Evaluation of oxygenated n-butanol-biodiesel blends along with ethyl hexyl nitrate as cetane improver on diesel engine attributes. J. Clean. Prod. 2017, 141, 928–939. [Google Scholar] [CrossRef]
- Altun, S.; Oner, C.; Yasar, F.; Adin, H. Effect of n-butanol blending with a blend of diesel and biodiesel on performance and exhaust emissions of a diesel engine. Ind. Eng. Chem. Res. 2011, 50, 9425–9430. [Google Scholar] [CrossRef]
- Mirzajanzadeh, M.; Tabatabaei, M.; Ardjmand, M.; Rashidi, A.; Ghobadian, B.; Barkhi, M.; Pazouki, M. A novel soluble nano-catalysts in diesel–biodiesel fuel blends to improve diesel engines performance and reduce exhaust emissions. Fuel 2015, 139, 374–382. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Aydin, M. Experimental investigation on the performance, combustion and exhaust emission characteristics of a compression-ignition engine fueled with cottonseed oil biodiesel/diethyl ether/diesel fuel blends. Energy Convers. Manag. 2020, 205, 112355. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Kishna, R.S.; Atabani, A.E.; Ala’a, H.; Kumar, G.; Ashok, B. A compressive review on the effects of alcohols and nanoparticles as an oxygenated enhancer in compression ignition engine. Energy Convers. Manag. 2020, 203, 112244. [Google Scholar] [CrossRef]
- Watson, A.Y.; Richard, R.B.; Kennedy, D. (Eds.) Air Pollution, the Automobile, and Public Health; National Academies: Washington, DC, USA, 1988.
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Ebadi, M.T.; Mamat, R.; Yusaf, T. Performance and emission characteristics of a CI engine using graphene oxide (GO) nano-particles additives in biodiesel-diesel blends. Renew. Energy 2020, 145, 458–465. [Google Scholar] [CrossRef]
- Vairamuthu, G.; Sundarapandian, S.; Kailasanathan, C.; Thangagiri, B. Experimental investigation on the effects of cerium oxide nanoparticle on Calophyllum inophyllum (Punnai) biodiesel blended with diesel fuel in DI diesel engine modified by nozzle geometry. J. Energy Inst. 2016, 89, 668–682. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Najafi, B.; Mirsalim, S.M.; Gharehghani, A.; Mohammadi, P.; Aghbashlo, M.; Ghaffari, A.; Khounani, Z.; Shojaei, T.R.; et al. A novel emulsion fuel containing aqueous nano cerium oxide additive in diesel–biodiesel blends to improve diesel engines performance and reduce exhaust emissions: Part I–Experimental analysis. Fuel 2017, 207, 741–750. [Google Scholar] [CrossRef]
- Zeldovich, Y.; Frank-Kamenetskii, D.; Sadovnikov, P. Oxidation of Nitrogen in Combustion; Publishing House of the Acad of Sciences of USSR: Moscow, Russia, 1947.
- Szwaja, S.; Naber, J.D. Combustion of n-butanol in a spark-ignition IC engine. Fuel 2010, 89, 1573–1582. [Google Scholar] [CrossRef]
- Soudagar, M.; Afzal, A.; Kareemullah, M. Waste coconut oil methyl ester with and without additives as an alternative fuel in diesel engine at two different injection pressures. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]






| Biofuel Blends | Biodiesel and GO NPs Size | Dosage of Graphene | Engine Type | Application Output | Ref. |
|---|---|---|---|---|---|
| D, B10, B20 and B20 nano-additive blends | Ailanthus altissima; 150 nm | 30, 60, and 90 ppm | 4-S Lombardini3LD510, SC, DI, CI engine, 1500 rpm, 18:1 CR. |
| [23] |
| D, B20 and B20 nano-additive blends | Jatropha; 8 nm thick, 5 µm wide | 50 ppm 75 ppm | 4-S HATZ-1B30-2, SC, DI, CI engine, 1500 rpm 23°BDTC, AC, 21.5: 1 CR, 373 cm3 displacement |
| [24] |
| D, B20 and B20 nano-additive blends | Camelina oil, Tree of heaven, Evening primrose; 150 nm | 60 ppm | 4-S Lombardini- 3LD510, SC, Non-TC, DI, CI 1500 rpm, engine, 18:1 CR |
| [25] |
| D and D nano-additive blends | GDD; 200 nm | 25 ppm | 4-S HATZ 1B30 light-duty, SC, DI, CI engine 3500 rpm, displacement 347 cm3, NA, 21.5: 1 CR |
| [26] |
| D, B20 and B20 nano-additive blends | Dairy scum oil; 23–27 nm | 20, 40 and 60 ppm | 4-S Kirloskar (TV1), SC, DI, CI engine, 2400 rpm, 23°BDTC, WC, 17.5: 1 CR, 3 FI nozzles |
| [3] |
| D, B30 and B30 nano-additive blends | Palm; 23–26 nm | 50, 75 and 100 ppm | 4-S Kirloskar (TV1), SC, DI, CI engine, 2400 rpm, 0–25°BDTC, WC, 17.5: 1 CR, HCC |
| [27] |
| D, B20, B20 nano-additive blends | Simarouba, 22.5–26 nm | 20, 40 and 60 ppm | 4-S Kirloskar (TV1), SC, DI, CI engine, 1500 rpm, 23°BDTC, WC, 17.5: 1 CR, 3 FI nozzles |
| [28] |
| Fatty Acid | IUPAC Systematic Name | Carbon Content (C) | Nigella Sativa Biodiesel % Weight |
|---|---|---|---|
| Arachidic acid | Eicosanoic | 20:0 | 0.75 |
| Behenic acid | docosanoic acid | 22:0 | 1.8 |
| Erucic acid | cis-13-Docosenoic acid | 22:1 | 6.8 |
| Linoleic acid | cis-9-cis-12 Octadecadienoic | 18:2 | 45.66 |
| Linolenic acid | cis-9-cis-12 | 18:3 | 0.35 |
| Myristic acid | Tetradecanoic | 14:0 | 0.2 |
| Oleic acid | cis-9-Octadecenoic | 18:1 | 21.56 |
| Palmitic acid | Hexadecanoic | 16:0 | 15.65 |
| Stearic acid | Octadecanoic | 18:0 | 3.85 |
| Fuel Blend | Diesel | Diesel qty. | Biodiesel | Biodiesel Qty. | n-butanol | n-butanol Dosage | GO NPs Dosage | SDBS Surfactant |
|---|---|---|---|---|---|---|---|---|
| Diesel | 100% | 1000 mL | - | - | - | - | - | - |
| NSME25 | 75% | 750 mL | 25% | 250 mL | - | - | - | - |
| NSME25B10GO30 | 65% | 650 mL | 25% | 250 mL | 10% | 100 mL | 30 ppm | 15 mg |
| NSME25B10GO60 | 65% | 650 mL | 25% | 250 mL | 10% | 100 mL | 60 ppm | 25 mg |
| NSME25B10GO90 | 65% | 650 mL | 25% | 250 mL | 10% | 100 mL | 90 ppm | 35 mg |
| NSME25B10GO120 | 65% | 650 mL | 25% | 250 mL | 10% | 100 mL | 120 ppm | 45 mg |
| Properties | Unit | ASTM Standards (D6751-15c) | Test Limit | Diesel a | NSME25 a | NSME25 B10GO30 a | NSME25 B10GO60 a | NSME25 B10GO90 a | NSME25 B10GO120 a |
|---|---|---|---|---|---|---|---|---|---|
| Kinematic Viscosity | cSt at 40 °C | ASTMD445 | 1.9–4.1 | 2.35 | 4.812 | 3.256 | 3.395 | 3.412 | 3.564 |
| Calorific value | MJ/kg | ASTM D5865 | Min. 35,000 | 45.456 | 41.25 | 42.105 | 42.759 | 43.58 | 43.017 |
| Density | kg/m3 at 15 °C | ASTM D4052 | 820–840 | 822.68 | 877.61 | 860.5 | 856.45 | 850.41 | 852.6 |
| Specific Gravity | gm/cc | ASTM D891 | 0.87–0.90 | 0.8 | 0.862 | 0.856 | 0.855 | 0.854 | 0.8548 |
| Flash Point | °C | ASTMD93 | Min. 93 | 78 | 130.55 | 120.86 | 118.45 | 110.68 | 112.81 |
| Pour Point | °C | ASTM D97-12 | −15 to 16 | −4 | 3.2 | 2.52 | 2.18 | 2.02 | 2.1 |
| Cloud point | °C | ASTM D2500-11 | −3 to 12 | −1 | 5 | 5.47 | 5.43 | 5.42 | 5.423 |
| Cetane Number | - | ASTMD613 | Min. 40 | 49.52 | 45.68 | 50.63 | 50.85 | 51.27 | 51.91 |
| Parameters | Accuracy (±) | Uncertainty (%) |
|---|---|---|
| CO emission (%) | ±0.01% | ±0.35 |
| NOx emission (ppm) | ±10 ppm | ±0.4 |
| HC emission (ppm) | ±10 ppm | ±0.4 |
| Exhaust gas temperature (°C) | ±1 | ±0.5 |
| Smoke meter (HSU) | ±1 | ±0.35 |
| Brake Thermal Efficiency (%) | - | ±0.5 |
| Brake Specific Fuel Consumption (%) | - | ±0.4 |
| Heat Release Rate (J/°CA) | - | ±0.18 |
| BTE = 4.35 + 13.519 BP − 2.790 Blend − 1.645 BP*BP + 0.410 Blend*Blend + 0.043 BP*Blend |
| BSFC = 503.6 − 156.8 BP + 75.2 Blend + 19.43 BP*BP − 10.85 Blend*Blend − 0.10 BP*Blend |
| CO = −0.0009 − 0.0040 BP + 0.0713 Blend + 0.00630 BP*BP − 0.01041 Blend*Blend + 0.00083 BP*Blend |
| Smoke = 6.36 + 9.57 BP + 11.73 Blend − 0.368 BP*BP − 1.637 Blend*Blend − 0.156 BP*Blend |
| S | R-sq | R-sq(adj) | R-sq(pred) | |
|---|---|---|---|---|
| Smoke | 5.46660 | 87.71% | 85.66% | 82.51% |
| CO | 0.0390499 | 76.49% | 72.57% | 65.10% |
| BTE | 1.97657 | 96.95% | 96.44% | 95.37% |
| BSFC | 45.0148 | 88.82% | 86.96% | 83.43% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.; Soudagar, M.E.M.; Kumar, R.H.; Safaei, M.R.; Farooq, M.; Khidmatgar, A.; Banapurmath, N.R.; Farade, R.A.; Abbas, M.M.; Afzal, A.; et al. Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry 2020, 12, 961. https://doi.org/10.3390/sym12060961
Khan H, Soudagar MEM, Kumar RH, Safaei MR, Farooq M, Khidmatgar A, Banapurmath NR, Farade RA, Abbas MM, Afzal A, et al. Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry. 2020; 12(6):961. https://doi.org/10.3390/sym12060961
Chicago/Turabian StyleKhan, Hurmathulla, Manzoore Elahi M. Soudagar, Rajagopal Harish Kumar, Mohammad Reza Safaei, Muhammad Farooq, Abdulqhadar Khidmatgar, Nagaraj R Banapurmath, Rizwan A. Farade, Muhammad Mujtaba Abbas, Asif Afzal, and et al. 2020. "Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics" Symmetry 12, no. 6: 961. https://doi.org/10.3390/sym12060961
APA StyleKhan, H., Soudagar, M. E. M., Kumar, R. H., Safaei, M. R., Farooq, M., Khidmatgar, A., Banapurmath, N. R., Farade, R. A., Abbas, M. M., Afzal, A., Ahmed, W., Goodarzi, M., & Taqui, S. N. (2020). Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry, 12(6), 961. https://doi.org/10.3390/sym12060961








