Effects of Salt Stress on the Leaf Shape and Scaling of Pyrus betulifolia Bunge
Abstract
1. Introduction
2. Experimental Process and Data Analysis
2.1. Experimental Materials
2.2. Leaf Image Acquisition and Leaf Edge Extraction
2.3. Data Acquisition of Leaf-Shape Indices
2.4. Data Analysis
2.4.1. Leaf Water Content
2.4.2. The Relationship between Leaf Area and Dry Weight
2.4.3. The Relationship between Leaf Area and LMA
2.4.4. The Relationship between Leaf Area and QPA
2.4.5. The Relationship between Leaf Area and QWL
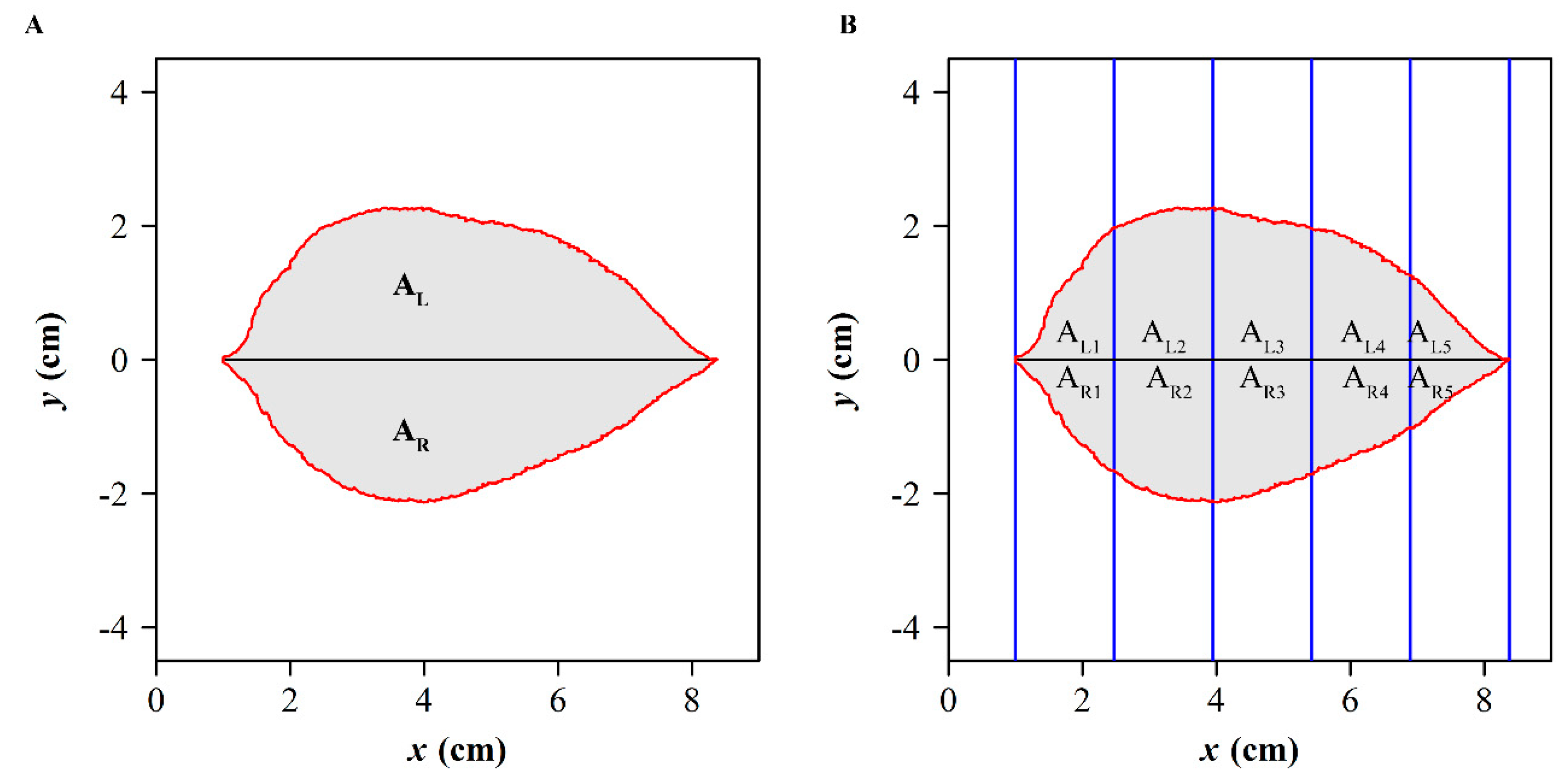
2.4.6. The Areal Quotient of the Left Side and Right Side
2.4.7. Standardized Index for Bilateral Symmetry
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; University of New South Wales Press Ltd.: Canberra, Australia, 1995. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Responses to Abiotic Stresses. In Biochemistry and Molecular Biology of Plants; Gruissem, W., Buchannan, B., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1249. [Google Scholar]
- Zhao, K.F.; Li, F.C.; Zhang, F.S. Halophytes in China; Science Press: China, Beijing, 2013. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.F.; Fan, H. Saline Plants and Their Adaptive Physiology to Saline Habitats; Science Press: Beijing, China, 2005. [Google Scholar]
- Essa, T.A. Effect of salinity stress on growth and nutrient composition of three soybean (Glycine max L. Merrill) cultivars. J. Agron. Crop Sci. 2002, 188, 86–93. [Google Scholar] [CrossRef]
- Gulen, H.; Turhan, E.; Eris, A. Changes in peroxidase activities and soluble proteins in strawberry varieties under salt-stress. Acta Physiol. Plant 2006, 28, 109–116. [Google Scholar] [CrossRef]
- Davenport, S.B.; Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuus L. cells. Plant Growth Regul. 2003, 40, 81–88. [Google Scholar] [CrossRef]
- Kim, J.K.; Bamba, T.; Harada, K.; Fukusaki, E.; Kobayashi, A. Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J. Exp. Bot. 2007, 58, 415–424. [Google Scholar] [CrossRef]
- Morabito, D.; Jolivet, Y.; Prat, D.; Dizengremel, P. Differences in the physiological responses of two clones of Eucalyptus microtheca selected for their salt tolerance. Plant Sci. 1996, 114, 129–139. [Google Scholar] [CrossRef]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, R.S.; Wilson, B.; Hughes, J.D.; McCulloch, C. The upscaling of transpiration from individual trees to areal transpiration in tree belts. Plant Soil 2007, 297, 223–232. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and energy exchange. Q. Rev. Biol. 1966, 41, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Christianson, M.L. Differences in the scaling of area and mass of Ginkgo biloba (Ginkgoaceae) leaves and their relevance to the study of specific leaf area. Am. J. Bot. 2011, 98, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S. Effect of biochar on growth and ion contents of bean plant under saline condition. Environ. Sci. Pollut. Res. 2018, 25, 11556–11564. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Rozendaal, D.M.A. Leaf size and leaf display of thirty-eight tropical tree species. Oecologia 2008, 158, 35–46. [Google Scholar] [CrossRef]
- Jucsor, N.; Ciulca, S.; Sumalan, R. Estimation of leaf area in cup plant (Silphium perfoliatum) using different leaf traits. J. Horticul. For. Biotechnol. 2018, 22, 40–44. [Google Scholar]
- Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Navas-Ureña, J.; Pinto Gomes, C.J.; Quinto-Canas, R.; Cano, E.; Spampinato, G. Similarity analysis between species of the genus Quercus L. (Fagaceae) in southern Italy based on the fractal dimension. PhytoKeys 2018, 113, 79–95. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Luo, H.; Chen, L.; Peng, J.; Fan, J. Plant recognition via leaf shape and margin features. Multimed. Tools Appl. 2019, in press. [Google Scholar] [CrossRef]
- Sinclair, C.; Hoffman, A.A. Monitoring salt stress in grapevines: Are measures of plant trait variability useful? J. Appl. Ecol. 2003, 40, 928–937. [Google Scholar] [CrossRef]
- Baneh, H.D.; Hassani, A.; Shaieste, F.G. Effects of salinity on leaf mineral composition and salt injury symptoms of some Iranian wild grapevine (Vitis vinifera L. ssp. sylvestris) genotypes. OENO One 2014, 48, 231–235. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B. The scaling of leaf area and mass: The cost of light interception increases with leaf size. Proc. R. Soc. B 2007, 274, 2109–2114. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D.; Niinemets, Ü.; Reich, P.B.; Sellin, A.; Shipley, B.; Wright, I.J. ‘‘Diminishing returns’’ in the scaling of functional leaf traits across and within species groups. Proc. Natl. Acad. Sci. USA 2007, 104, 8891–8896. [Google Scholar] [CrossRef]
- De Carvalho, J.O.; Toebe, M.; Tartaglia, F.L.; Bandeira, C.T.; Tambara, A.L. Leaf area estimation from linear measurements in different ages of Crotalaria juncea plants. An. Acad. Bras. Ciênc. 2017, 89, 1851–1868. [Google Scholar] [CrossRef]
- Montgomery, E.G. Correlation studies in corn. In Annual Report No. 24; Agricultural Experimental Station: Lincoln, NB, USA, 1911. [Google Scholar]
- Shi, P.J.; Liu, M.D.; Yu, X.J.; Gielis, J.; Ratkowsky, D.A. Proportional relationship between leaf area and the product of leaf length and width of four types of special leaf shapes. Forests 2019, 10, 178. [Google Scholar] [CrossRef]
- Palaniswamy, K.M.; Gomez, K.A. Length-width method for estimating leaf area of rice. Agron. J. 1974, 66, 430. [Google Scholar] [CrossRef]
- Wang, P.; Ratkowsky, D.A.; Xiao, X.; Yu, X.J.; Su, J.L.; Zhang, L.F.; Shi, P.J. Taylor’s power law for leaf bilateral symmetry. Forests 2018, 9, 500. [Google Scholar] [CrossRef]
- Leigh, A.; Zwieniecki, M.A.; Rockwell, F.E.; Boyce, C.K.; Nicotra, A.B.; Holbrook, N.M. Structural and hydraulic correlates of heterophylly in Ginkgo biloba. New Phytol. 2011, 189, 459–470. [Google Scholar] [CrossRef]
- Bailey, L.H. Pyrus; Standard Cyclopedia of Horticulture: Macmillan, NY, USA, 1917. [Google Scholar]
- Rubtsov, G.A. Geographical distribution of the genus Pyrus and trends and factors in its evolution. Am. Nat. 1944, 78, 358–366. [Google Scholar] [CrossRef]
- Okubo, M.; Sakuratani, T. Effects of sodium chloride on survival and stem elongation of two Asian pear rootstock seedlings. Sci. Hortic. 2000, 85, 85–90. [Google Scholar] [CrossRef]
- Robbani, M.; Banno, K.; Yamaguchi, K.; Fujisawa, N.; Liu, J.Y.; Kakegawa, M. Selection of dwarfing pear rootstock clones from Pyrus betulaefolia and P. calleryana seedlings. J. Jpn. Soc. Hortic. Sci. 2006, 75, 1–10. [Google Scholar] [CrossRef]
- Okubo, M.; Furukawa, Y.; Sakuratani, T. Growth, flowering and leaf properties of pear cultivars grafted on two Asian pear rootstock seedlings under NaCl irrigation. Sci. Hortic. 2000, 85, 91–101. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tamura, F.; Chun, J.P.; Ikeda, T.; Imanishi, K.; Tanabe, K. Enhancement in salt tolerance of Japanese pear by using Pyrus betulaefolia rootstock. Hortic. Res. (Jpn.) 2007, 6, 47–52. [Google Scholar] [CrossRef]
- Tamura, F. Recent advances in research on Japanese pear rootstocks. J. Jpn. Soc. Hortic. Sci. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Shi, P.J.; Huang, J.G.; Hui, C.; Grissino-Mayer, H.D.; Tardif, J.C.; Zhai, L.H.; Wang, F.S.; Li, B.L. Capturing spiral radial growth of conifers using the superellipse to model tree-ring geometric shape. Front. Plant Sci. 2015, 6, 856. [Google Scholar] [CrossRef]
- Shi, P.J.; Ratkowsky, D.A.; Li, Y.; Zhang, L.F.; Lin, S.Y.; Gielis, J. A general leaf area geometric formula exists for plants: Evidence from the simplified Gielis equation. Forestry 2018, 9, 714. [Google Scholar] [CrossRef]
- Su, J.L.; Niklas, K.J.; Huang, W.W.; Yu, X.J.; Yang, Y.Y.; Shi, P.J. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 17 April 2018).
- Shi, P.J.; Zheng, X.; Ratkowsky, D.A.; Li, Y.; Wang, P.; Cheng, L. A simple method for measuring the bilateral symmetry of leaves. Symmetry 2018, 10, 118. [Google Scholar] [CrossRef]
- Zhou, H.H.; Li, W.H. Responses and adaptation of xylem hydraulic conductivity to salt stress in Populus euphratica. Chin. J. Plant Ecol. 2015, 31, 81–91. [Google Scholar]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Aust. J. Plant Physiol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Bernstein, N.; Silk, W.K.; Läuchli, A. Growth and development of sorghum leaves under conditions of NaCl stress. Planta 1993, 191, 433–439. [Google Scholar] [CrossRef]
- Sumida, A.; Komiyama, A. Crown spread patterns for five deciduous broad-leaved woody species ecological significance of the retention patterns of larger branches. Ann. Bot. 1997, 80, 759–766. [Google Scholar] [CrossRef]
- Sumida, A.; Terazawa, I.; Togashi, A.; Komiyama, A. Spatial arrangement of branches in relation to slope and neighbourhood competition. Ann. Bot. 2002, 89, 301–310. [Google Scholar] [CrossRef]
- King, D.A. Allometry of saplings and understorey trees of a Panamanian forest. Funct. Ecol. 1990, 4, 27–32. [Google Scholar] [CrossRef]
- Küppers, M. Ecological significance of above-ground architectural patterns in woody plants: A question of cost-benefit relationships. Trends Ecol. Evol. 1989, 4, 375–379. [Google Scholar] [CrossRef]
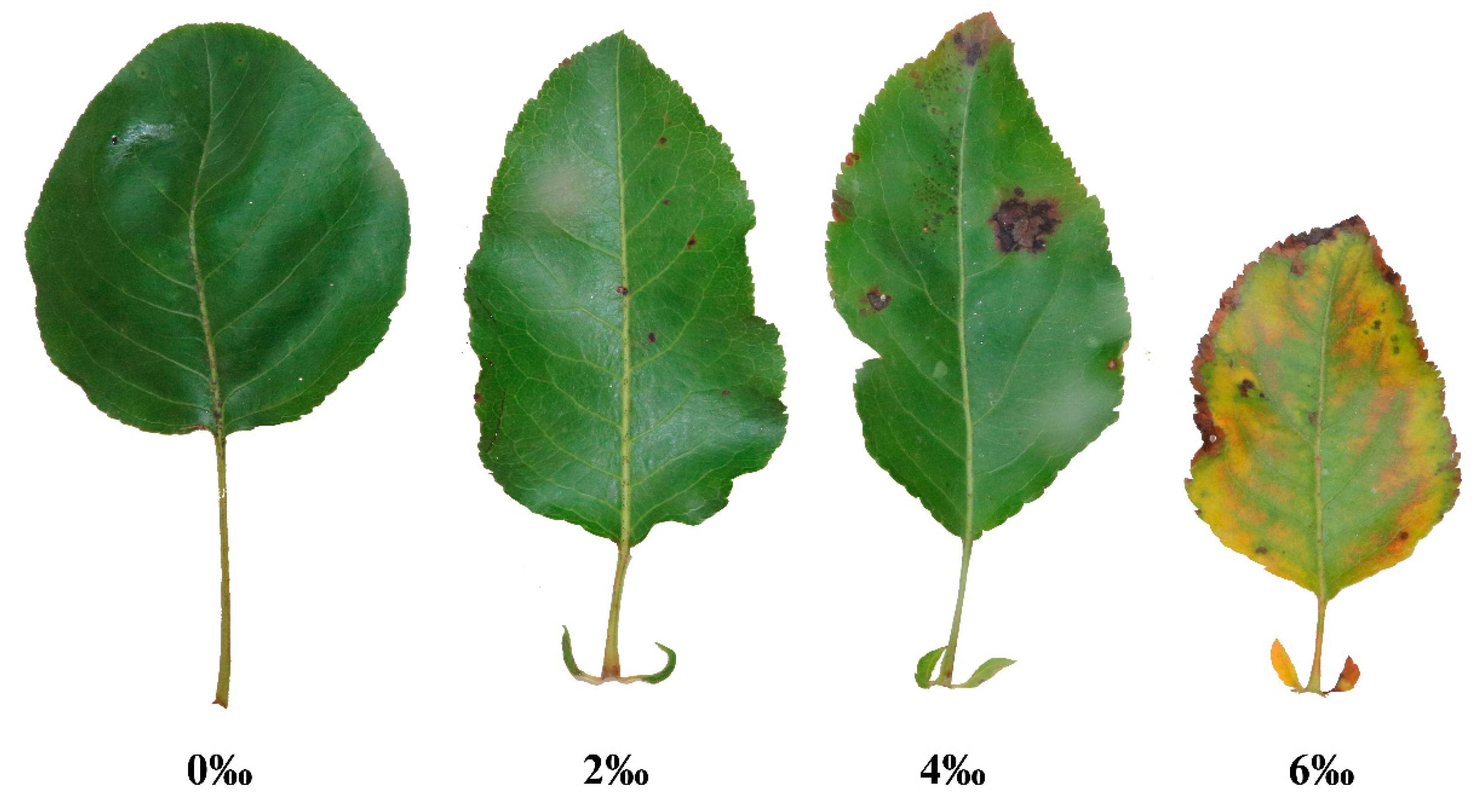
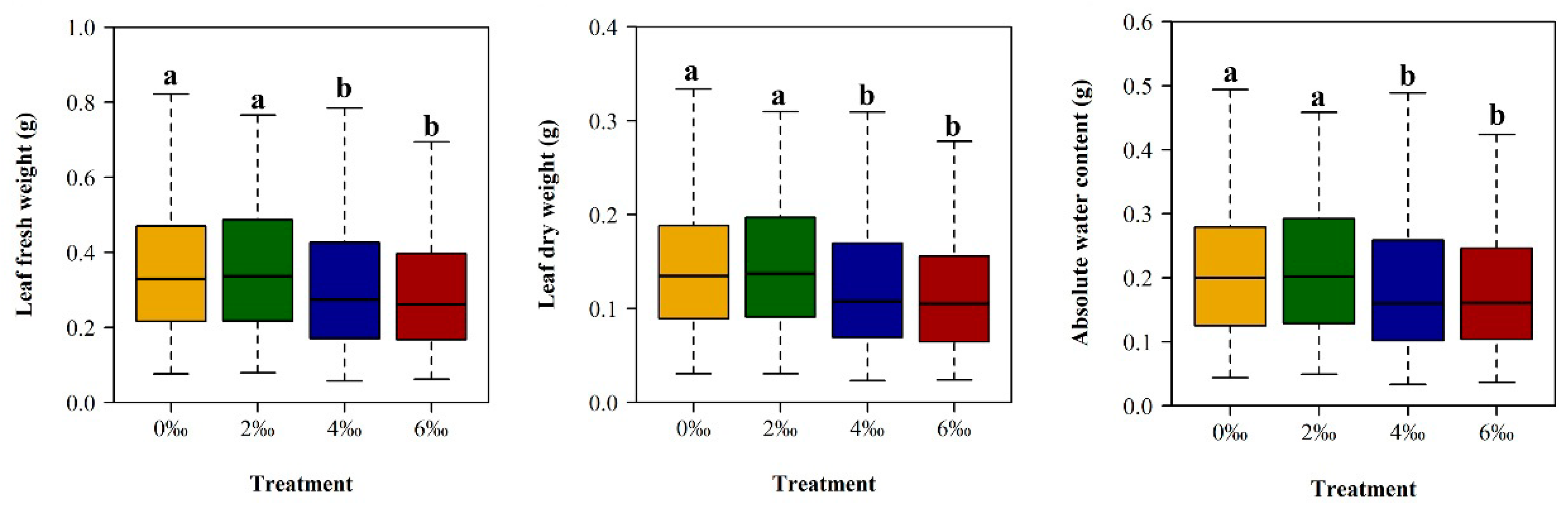
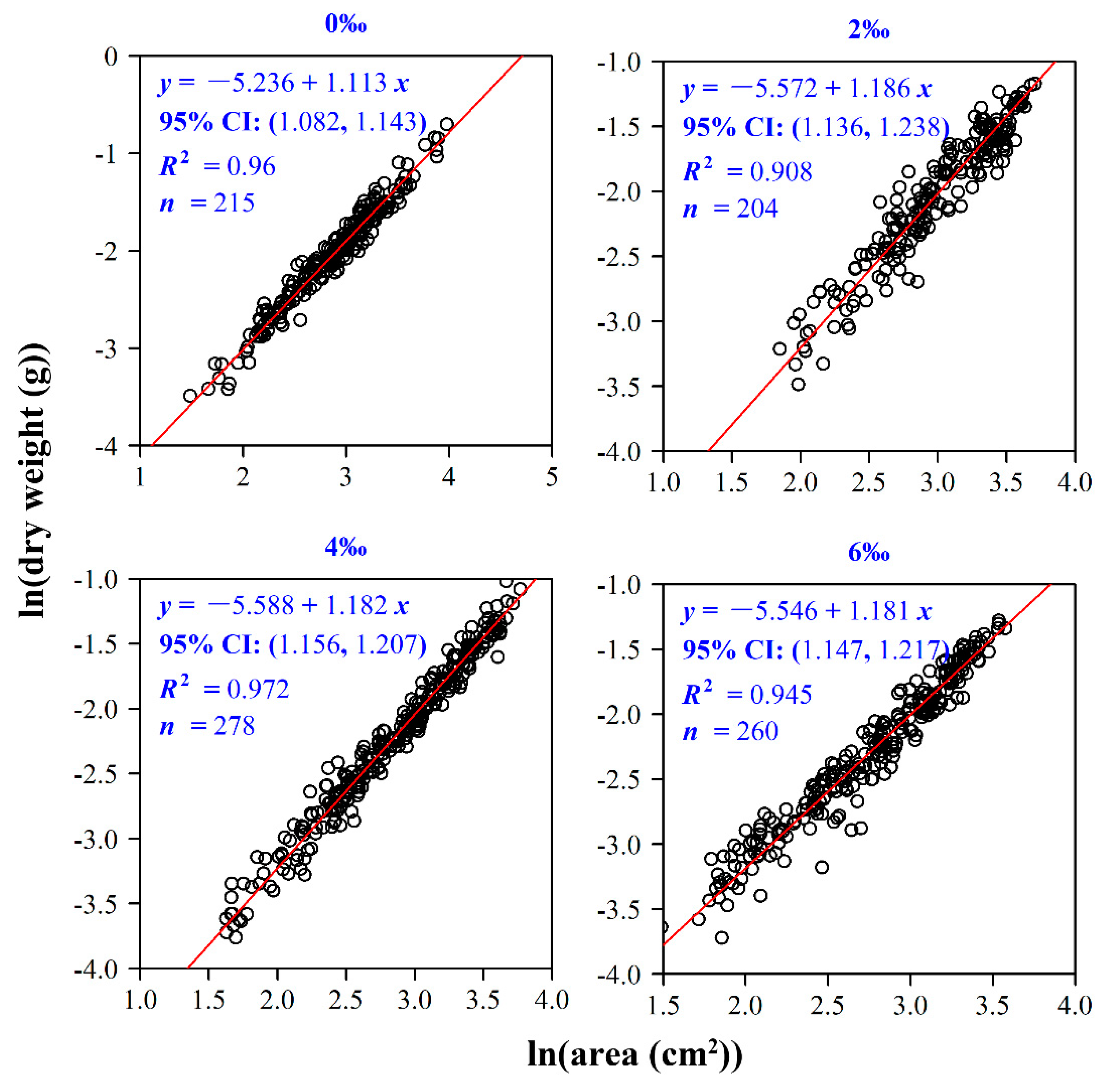
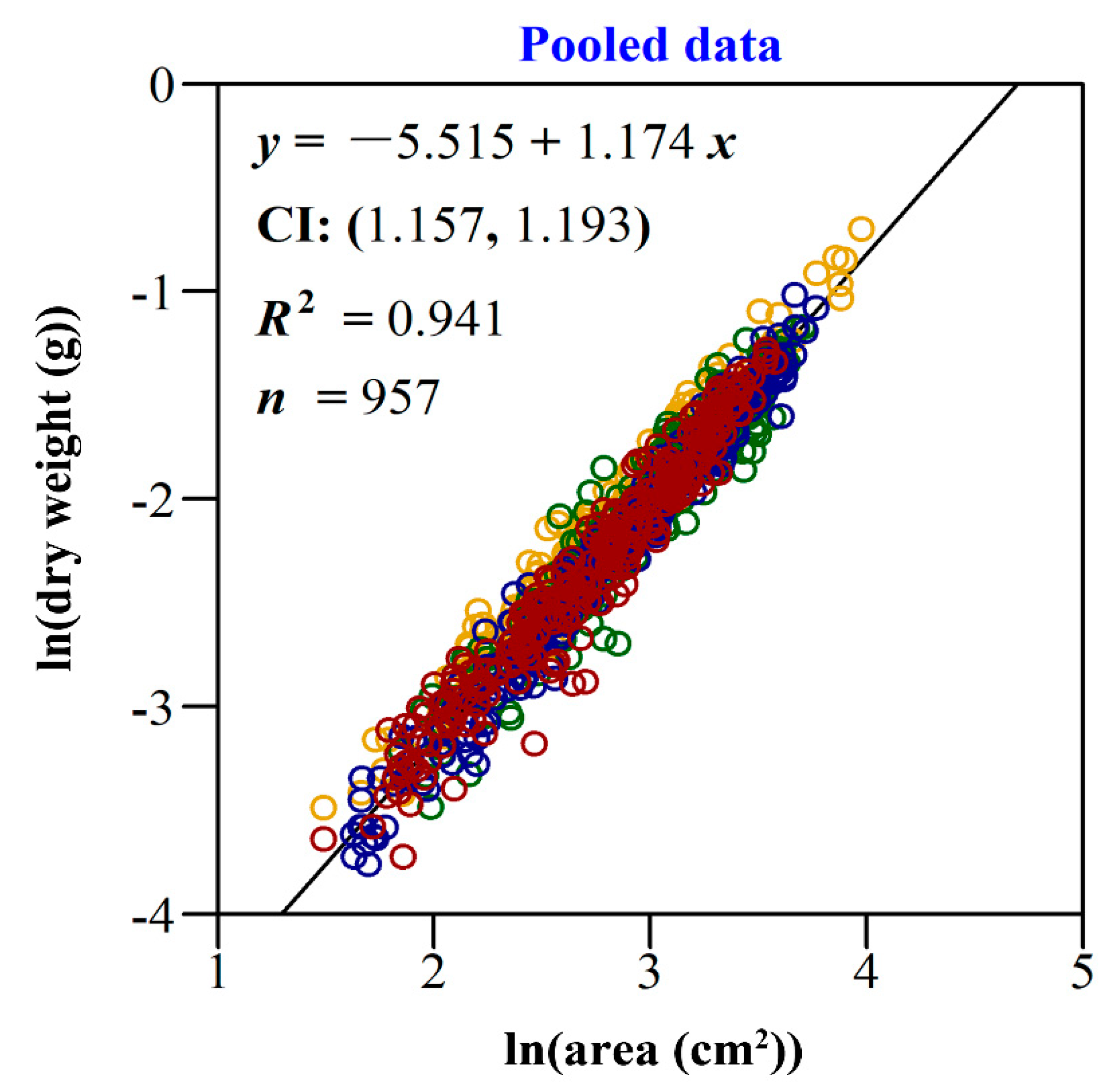
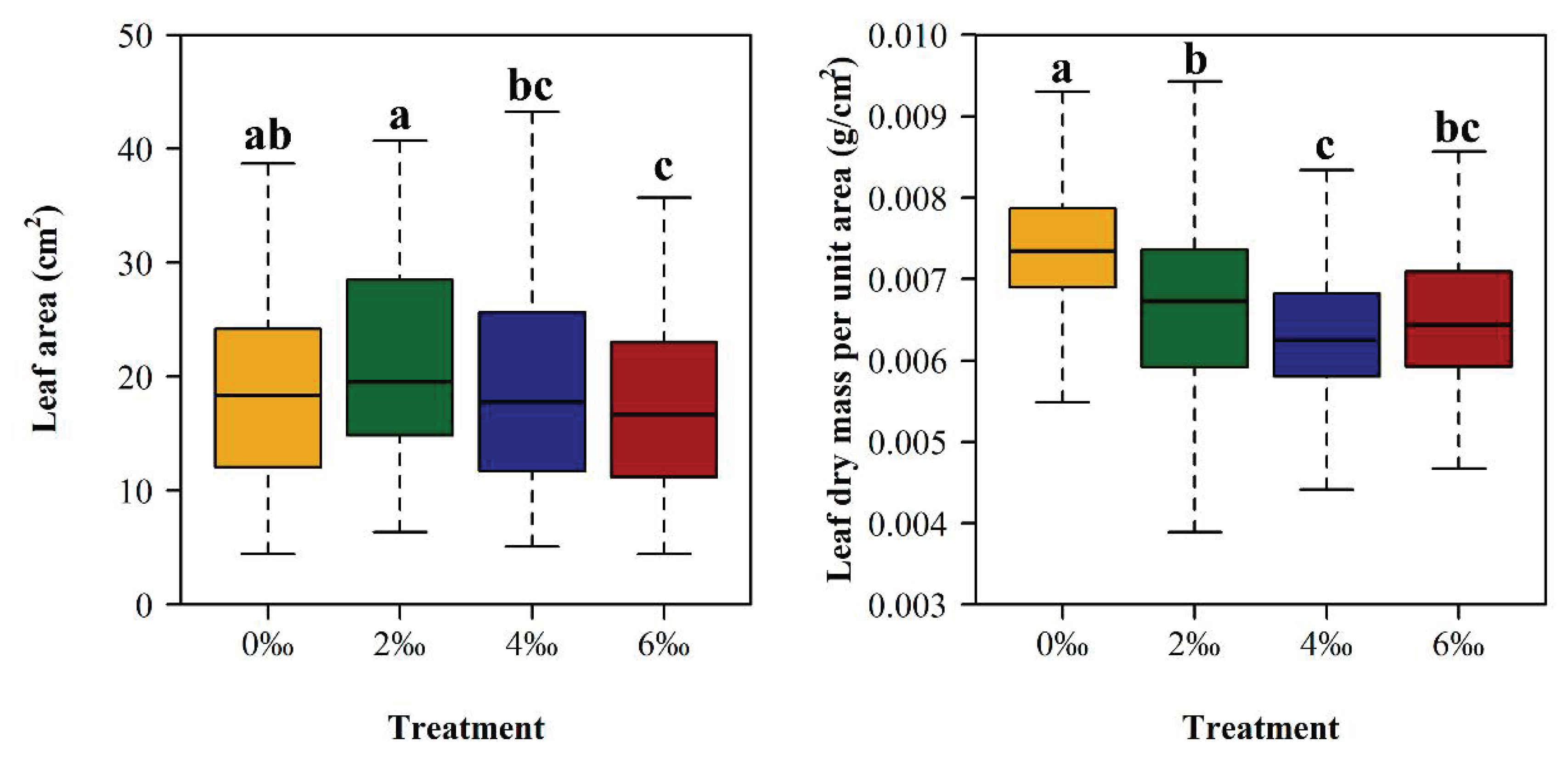
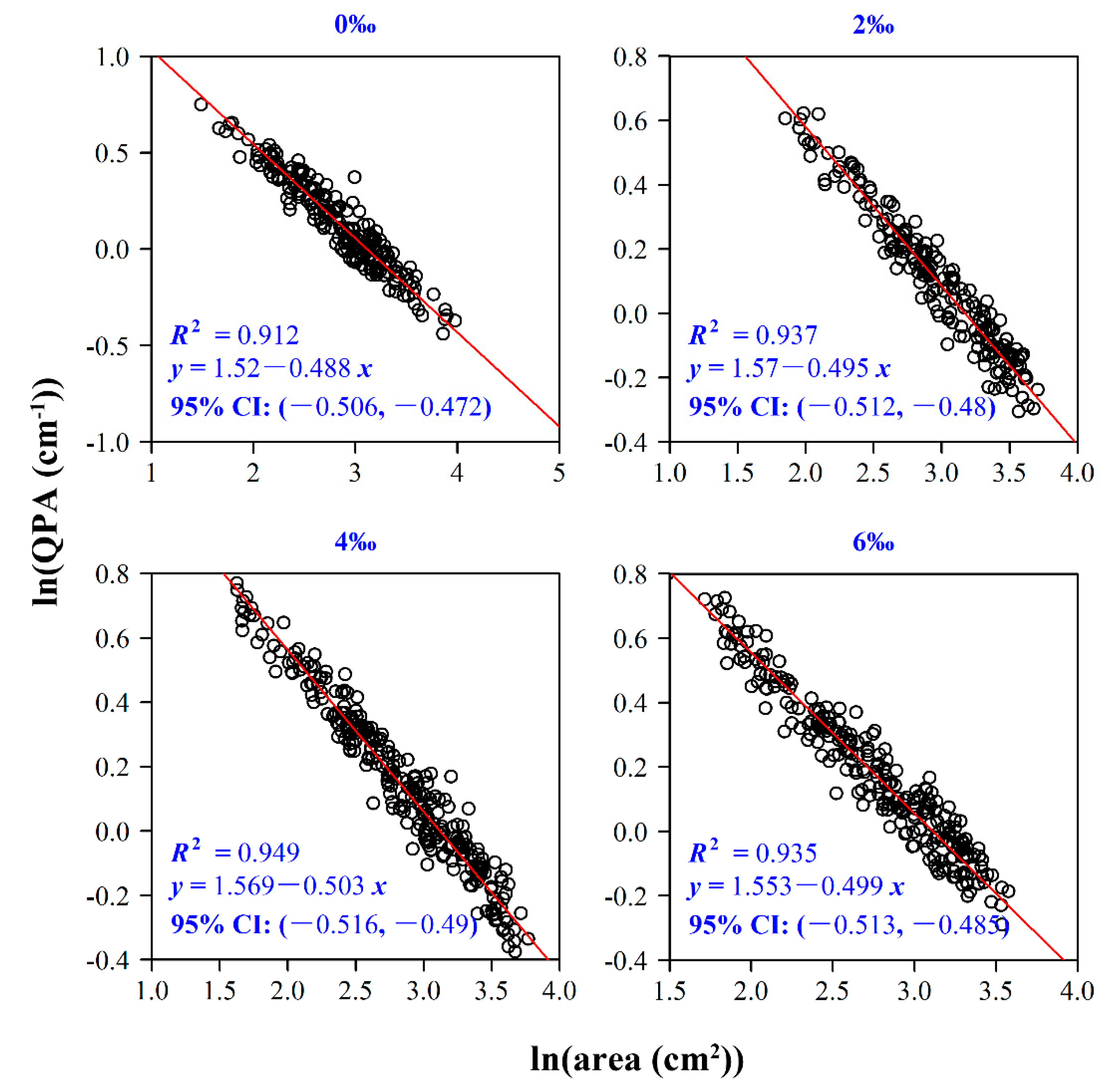
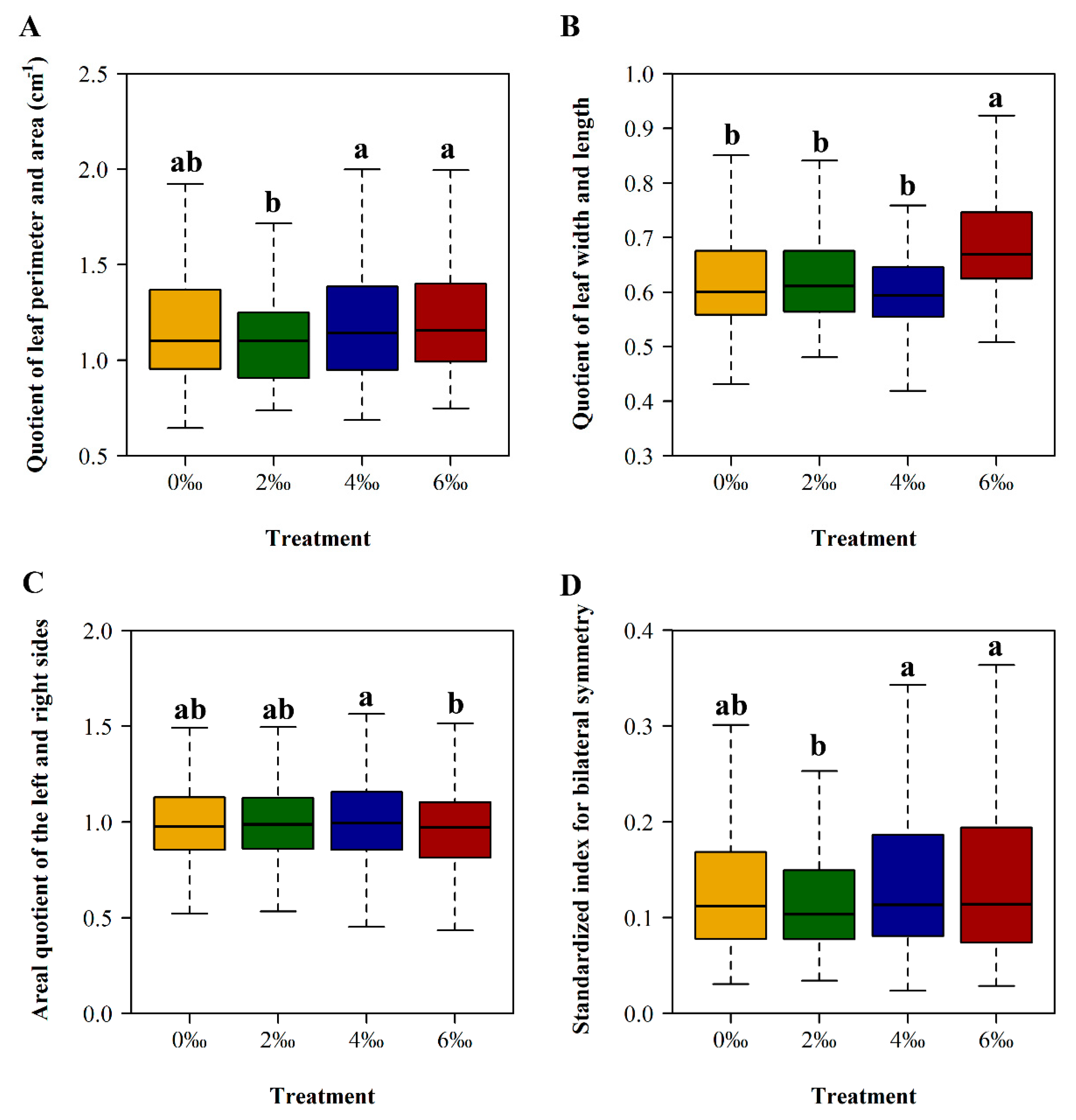
| Treatment | A (cm2) | LMA (g/cm2) |
|---|---|---|
| 0‰ | 19.36 ± 9.14 ab | 0.0074 ± 0.0009 a |
| 2‰ | 21.43 ± 8.55 a | 0.0067 ± 0.0011 b |
| 4‰ | 19.03 ± 9.02 bc | 0.0063 ± 0.0009 c |
| 6‰ | 17.26 ± 7.39 c | 0.0065 ± 0.0008 bc |
| Treatment | QPA-A | P | QWL-A | P |
|---|---|---|---|---|
| 0‰ | −0.8739 | <0.01 | −0.0204 | 0.7664 |
| 2‰ | −0.9168 | <0.01 | −0.1022 | 0.1459 |
| 4‰ | −0.9094 | <0.01 | −0.0001 | 0.9984 |
| 6‰ | −0.9081 | <0.01 | −0.1808 | <0.01 |
| Treatment | Estimate | SD | R2 |
|---|---|---|---|
| 0‰ | 0.6048 | 0.0059 | 0.9799 |
| 2‰ | 0.6167 | 0.0062 | 0.9801 |
| 4‰ | 0.6002 | 0.0053 | 0.9792 |
| 6‰ | 0.6707 | 0.0052 | 0.9845 |
| Treatment | AQ | SI |
|---|---|---|
| 0‰ | 1.0101 ± 0.2506 ab | 0.1337 ± 0.0783 ab |
| 2‰ | 1.0202 ± 0.2676 ab | 0.1265 ± 0.0740 b |
| 4‰ | 1.0789 ± 0.4167 a | 0.1517 ± 0.1075 a |
| 6‰ | 0.9785 ± 0.2986 b | 0.1510 ± 0.1079 a |
| Treatment Code | AQ-A | P | SI-A | P |
|---|---|---|---|---|
| 0‰ | 0.0169 | 0.8054 | −0.1449 | <0.05 |
| 2‰ | −0.0791 | 0.2607 | −0.2468 | <0.01 |
| 4‰ | −0.2669 | <0.01 | −0.4138 | <0.01 |
| 6‰ | 0.1075 | 0.0837 | −0.4400 | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Shi, P.; Hui, C.; Miao, L.; Liu, C.; Zhang, Q.; Feng, C. Effects of Salt Stress on the Leaf Shape and Scaling of Pyrus betulifolia Bunge. Symmetry 2019, 11, 991. https://doi.org/10.3390/sym11080991
Yu X, Shi P, Hui C, Miao L, Liu C, Zhang Q, Feng C. Effects of Salt Stress on the Leaf Shape and Scaling of Pyrus betulifolia Bunge. Symmetry. 2019; 11(8):991. https://doi.org/10.3390/sym11080991
Chicago/Turabian StyleYu, Xiaojing, Peijian Shi, Cang Hui, Lifei Miao, Changlai Liu, Qiuyue Zhang, and Chaonian Feng. 2019. "Effects of Salt Stress on the Leaf Shape and Scaling of Pyrus betulifolia Bunge" Symmetry 11, no. 8: 991. https://doi.org/10.3390/sym11080991
APA StyleYu, X., Shi, P., Hui, C., Miao, L., Liu, C., Zhang, Q., & Feng, C. (2019). Effects of Salt Stress on the Leaf Shape and Scaling of Pyrus betulifolia Bunge. Symmetry, 11(8), 991. https://doi.org/10.3390/sym11080991







