Abstract
A review. The question of homochirality is an intriguing problem in the field of chemistry, and is deeply related to the origin of life. Though amphiphiles and their supramolecular assembly have attracted less attention compared to biomacromolecules such as RNA and proteins, the lipid world hypothesis sheds new light on the origin of life. This review describes how amphiphilic molecules are possibly involved in the scenario of homochirality. Some prebiotic conditions relevant to amphiphilic molecules will also be described. It could be said that the chiral properties of amphiphilic molecules have various interesting features such as compositional information, spontaneous formation, the ability to exchange components, fission and fusion, adsorption, and permeation. This review aims to clarify the roles of amphiphiles regarding homochirality, and to determine what kinds of physical properties of amphiphilic molecules could have played a role in the scenario of homochirality.
1. Introduction
The question of homochirality arises from the simple fact that living organisms are composed of chiral molecules. Functional biomolecules, such as nucleic acids, proteins, and lipids, are made of enantiomerically-pure chiral building blocks (e.g., sugars, amino acids, and glycerolphosphates). Typical questions regarding homochirality are “How did life choose chirality?”, “Why does the enantiopurity of chiral molecules need to be high in living organisms?”, “How and when did the homochirality occur?”. One specific question about lipids is “Why is the chiral moiety of archaea (sn-glycerol-1-phosphate) the mirror image of that of bacteria and eukaryotes (sn-glycerol-3-phosphate) [1,2,3]?”. One reason that makes it difficult to answer these questions is that we do not know how life appeared, nor when it emerged. Some researchers assume that life emerged when functional biopolymers, such as RNA or proteins, emerged from a soup of their monomers and oligomers, represented by the RNA world [4,5,6], protenoid microsphere [7,8], and the protein world [9]. Others postulate that it was not these polymers, but the formation of a supramolecular assembly that came first (e.g., micelle, vesicles, coacervate, oil droplets, etc.), represented by a lipid world hypothesis [10,11,12]. The latter view is held by relatively few scientists, but it is gaining attention and could provide new insight into the problem of homochirality.
To understand how amphiphilic molecules may have affected homochirality, approaches from a wide range of sciences are required. The importance of amphiphilic molecules, from the view point of the origin of life, has been largely recognized by researchers in system chemistry [13]. This field provides a bird’s eye view of the origin of life, including the roles of lipids, as well as RNA and proteins. Computational methods in this field do not require chemicals and can explore the key features of a supramolecular assembly, including compositional information, reproduction, and the evolution of life [14]. Supramolecular chemistry is key in understanding the roles of cell membranes in a prebiotic system [15], and also provides profound insight into homochirality [16]. The field may be able to answer the basic questions of homochirality: “How are chiral properties connected with the various features of a supramolecular assembly, such as critical aggregate concentration, chemical reaction, and the permeation of chemicals through the boundary of the assembly, etc.?”. The amplification of the chirality is an important phenomena, and a mechanistic study could provide a general framework with which to understand the origin of homochirality [17]. Organic chemistry provides knowledge of the synthetic routes of prebiotic chemicals taking prebiotic reaction conditions into account. In addition to inorganic catalysts, the growing field of organocatalysis may drastically change the view of stereoselective chemical reactions under prebiotic conditions [18]. Analytical chemistry provides basic information about lipids under prebiotic conditions by establishing analytical methods to separate and identify lipids. One surprising fact is that vesicles can be formed from lipids extracted from meteorites [19], and identifying the chemical structures of the components by analytical methods may uncover possible prebiotic molecules, since meteorites are recognized as carbon sources on earth.
This review focuses on amphiphilic molecules in the context of homochirality and aims to (1) show how could amphiphiles be emerged prior to biopolymers, (2) review prebiotic conditions and candidates of prebiotic molecules, and (3) clarify the connection between the features of amphiphilic molecules and homochirality. The main interest lies in (3), but before going into depth, it is worth considering (1), and understand why amphiphilic molecules, specifically lipids, are attracting attention from scientists who are studying the origin of life. By knowing what kinds of prebiotic conditions and prebiotic molecules are probable with the aim of (2), researchers will be able to construct plausible experimental systems or devise theories to solve the problems of homochirality.
2. Amphiphilic Molecules and the Origin of Life
In the following sections, the authors attempt to briefly summarize the relevance of amphiphilic molecules in the context of the origin of life. The emphasis is placed on amphiphiles rather than metabolism in this review (see ref. [13] for a broader view encompassing the metabolic system).
2.1. Theories of Origin of Life
It could be said that the importance of amphiphiles was gradually admitted after Dyson [20,21,22] revisited Oparin’s droplet theory (coacervate theory), though his emphasis was on a metabolic system rather than the compartment of the system. In his lecture in 1984 [22], Dyson categorizes theories about the origin of life into three groups, i.e., droplet theories by Oparin [23], genetic theories by Eigen [24], and clay theories by Cairns-Smith [25]. The criteria to classify the theories are in the order of events (Figure 1). Cells are assumed to form a boundary to confine the content (enzymes and genes), enzymes to form a self-sustaining metabolic cycle, and genes to form biopolymers containing genetic information (e.g., RNA and DNA). The droplet theories assume that the formation of the cell comes first, enzymes come second, and genes last. Genetic theories are represented by the RNA world, which arose from the striking fact that RNA can replicate itself if the oligomer is provided for ligation. The theories place genes first, enzymes second, and cells last. The last theory assumes that inorganic crystals contributed to the emergence of life before nucleic acids.
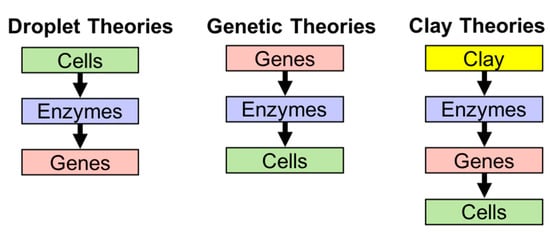
Figure 1.
The three theories described by Dyson [21].
Based on the droplet theory, Dyson describes how the first living organism proliferated as follows [22]: “I propose that the original living creatures were cells with a metabolic apparatus directed by protein enzymes but with no genetic apparatus. Such cells would lack the capacity for exact replication but could grow and divide and reproduce themselves in an approximate statistical fashion.” He carefully distinguished replication and reproduction in the text. The former indicates that it is only possible for a molecule to construct an exact copy of itself, and the latter that cells are able to divide and inherit approximately the same composition (see review [26] for more details about replication and reproduction). The mechanism of replication requires a very precise copy of a molecule; otherwise, that molecule loses the ability to copy itself.
Interestingly, he also suggested that RNA emerges as a parasite of the prebiotic system that utilizes adenosine triphosphate (ATP) as an energy source. ATP has high energy phosphate bonds, and ATP is used as an energy carrier by the present living cells. The prebiotic system possibly discovered a way to synthesize ATP and other nucleoside triphosphates. RNA could have emerged as a parasite of the prebiotic system, since RNA is synthesized from nucleoside triphosphates (ATP, GTP, CTP, and UTP). This idea was inspired by Lynn Margulis, who proposed that mitochondria and the photosynthetic plastids in eukaryotic cells were symbiotically acquired [27]. Her idea is that the evolution of cellular complexity was often caused by parasitism and symbiosis.
Dyson raises three other notions in support of droplet theories. The first is based on von Neumann’s idea of hardware (process information) and software (embodies information) [22] (p. 7) [28]. They have an exact analogue in living cells, mainly proteins (component of metabolism) and nucleic acids (component of replication), respectively. Hardware comes prior to software, and can process information without the software. On the other hand, software has a parasitic character, and it needs a host to make a copy of itself. Therefore, the hardware characteristics of proteins is thought to come prior to nucleic acids with software characteristics. The second reason is that amino-acid synthesis is simpler than nucleotide synthesis. Dyson dealt with Miller’s experiment that shows how an electric discharge in reducing gas mixtures can generate amino acids [29,30]. Whether the atmosphere was reductive or neutral has been discussed after the original report by Miller. We recognize that Miller himself admitted that the atmosphere is more likely to be neutral than reductive in his posthumous work in 2008, yet the fact that an amino acid can be formed relatively easily is unchanged [31]. Nucleotides have far more complex structures compared to amino acids, and it is normally thought that the spontaneous formation of RNA under prebiotic conditions is difficult [13] (p. 286) [32,33]. The possible synthetic pathway of RNA under prebiotic conditions is still debated and has not been settled [34]. It is likely that the synthetic route of ATP was gradually developed prior to the appearance of RNA in the metabolic system of the prebiotic cell. The last reason is that the hypothesis may be testable. He says that a geochemical approach may determine whether or not a primitive cell in a microfossil contains a clue of RNA (e.g., high content of phosphorus). Geochemical research on the origin of life is on its way; yet, the scarcity and poor preservation conditions of Archean rocks on Earth hampers the investigation [35] (p. 9). Other problems specific to the RNA world are described in the literature [32,36,37].
In addition to these reasonings, it is reasonable to put the emergence of cells before enzymes and genes when one considers the problem of the concentration threshold and permeation of the cell membrane [38,39,40]. First, since the replication or enzymatic reaction takes places via an intermolecular interaction, the reaction takes a very long time in very diluted conditions. Considering that the destruction and deactivation of the biopolymers take place at the same time, there should be a certain concentration threshold for the biopolymers to increase. Secondly, the permeability of a biopolymer is relatively low compared to that of small molecules, and if one assumes that those biopolymers obtained a cell membrane in the later stage than the emergence of the biopolymers, then it will be difficult to permeate through the membrane. Even if one assumes that biopolymers get into the cell, they can also be permeated through the membrane to exit from the membrane using the same mechanism by which they entered the cell.
Regarding the problem of the concentration threshold and permeation, however, a clue to overcoming this difficulty was found by Luisi and coworkers. They found that ferritin and a green fluorescent protein in a solution are overcrowded in the cell compartment when liposomes are formed in the solution [40,41]. The concentration within a compartment is high, contrary to the expectation of Poisson statistics (they call it an all-or-nothing situation). The mechanism is under investigation, but this fact is interesting, since it may make it possible to cross the concentration threshold of metabolism or replication. However, some difficulties still remain regarding the assumption that biopolymers emerged prior to the cell membrane.
2.2. Lipid World Hypothesis
Lancet and coworkers proposed the concept of the “lipid world” in 1999 [10], and emphasized the importance of the amphiphilic molecules in the context of the origin of life (see ref. [14] for a review written in 2018). It seems that Dyson considers the role of the cell as an inert compartment of the metabolic system, and he was focused more on the cell content that dominates metabolism and reproduction (i.e., protein enzymes), rather than the cell boundary itself (i.e., assembly of lipid and amphiphilic molecules). However, Lancet proposed that the lipid and amphiphilic molecules could also play an important role in information carriage, reproduction, catalysis, selection, and evolution.
Lancet developed a graded autocatalysis replication domain (GARD) model (Figure 2) to investigate the possibility of the lipid world hypothesis. The model assumes that amphiphilic molecules are formed from high energy monomers, while some amphiphiles show weak catalytic activity. Examples of the catalytic reaction with a supramolecular assembly are reported with the help of metal ions [42,43]. The catalytic activity is described by parameter β derived from a receptor affinity distribution (RAD) model [10] (p. 132) [44,45]. The simulation can describe the growth and splitting of the amphiphilic assembly and monitor the compositional changes during growth and fission. Fission generates progeny assemblies, and it may be problematic if one follows all of the generated assemblies, since the population exponentially increases. For the GARD simulation, one of the two progeny assemblies is discarded in the simulation [10] (p. 4), so the model basically focuses on one assembly. This simulation enables one to investigate the relationship among the compositional information, reproduction, catalysis, selection, and evolution. Unlike the experimental approach, the model is not necessarily restricted by the availability of molecules, but it can introduce kinetic constants derived from experiments which may be more plausible [46,47]. The model is based on the idea of the autocatalytic set by Kauffman, and he defines the term as follows: “By autocatalytic set we mean that each member is the product of at least one reaction catalyzed by at least one other member” [48] (p. 50). The meaning of the autocatalytic set, and the similar terms, autocatalytic reaction and autocatalytic cycle, are sometimes confusing; the difference was recently clarified by Hordijk [49]. A comparison among the models proposed by Lancet, Dyson, and Kauffman is reported in reference [50]. Other cell replication models are also proposed by Solé based on a dissipative particle dynamics approach [51].
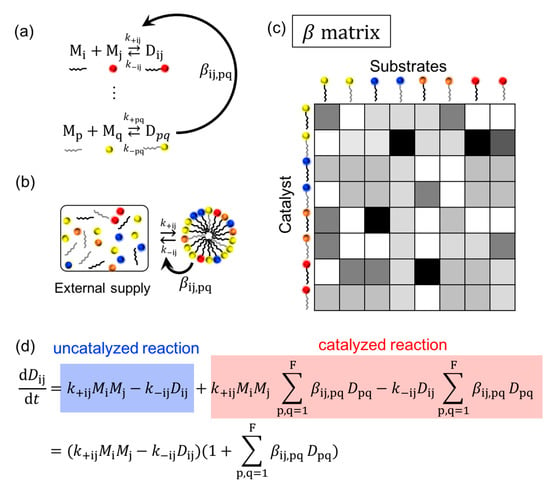
Figure 2.
Description of GARD model based on ref [50,54]. (a) Chemical reaction of amphiphilic dimer, D, from monomers, M. Constants k+ij and k−ij indicates association and dissociation rate constants for a reaction forming a dimer (Dij) from monomers (Mi and Mj), respectively. The semicircle arrow indicates the catalysis of the formation and degradation reactions of Dij by Dpq with the factor βij,pq. (b) Assumed condition of GARD model. Monomers are supplied from the outside and the components of assembly catalyze the reaction. (c) β matrix of the reaction. The gray scaled color indicates the value of β. (d) Rate equation of the system.
The stated ideas of Dyson and Lancet are examples of the recently described metabolism first claim, and are often contrasted with the genetic first claim represented by the RNA world. Both approval [14,52] and disapproval [53] can be found for the metabolism first claim, but it is recognized that there is no decisive evidence to choose between the two, since each has its own shortcomings [13] (p. 287).
2.3. Amphiphiles and Definition of Life
Another piece of key research seeking to unvover the relation between the origin of life and amphiphiles was carried out by Luisi and coworkers (see reviews [15,39,55,56,57]). In the context of the origin of life, he points out that the criterion of life and criterion of evolution are often confused [39]. Having the question “What is life?” in mind, he introduced the word “autopoiesis” (i.e., self-reproduction) to supramolecular chemistry [15]. The autopoietic system is defined as “a system which continuously produces the components that specify it, while at the same time realizing it (the system) as a concrete unity in space and time, which makes the network of production of components possible” [58]. The definition can differentiate the criteria of evolution from those of the living or dead. In addition, it helps to think of a minimal living unit based on the supramolecular assembly. Figure 3 describes this situation. A unit of assembly S (surfactant) is formed from a precursor A. S forms the decay product P, which is eliminated from the system. The kinetics of formation and destruction of S determines whether the assembly grows, maintains (being the state of homeostasis), or decays.
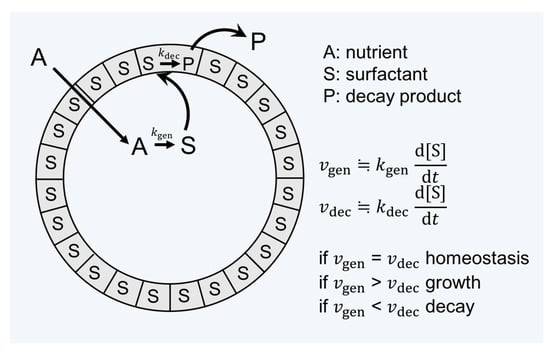
Figure 3.
The concept of autopoiesis applied to the supramolecular assembly from reference [55].
The actual self-reproducible supramolecular system of reversed micelles, micelles, and vesicles are reported [15] (p. 3642). The process of self-reproduction is often referred to as autocatalytic, meaning “the catalysis of a reaction by the products” in this context. For example, in the case of autocatalytic micelles [59], the system is composed of two phases (water and oil), and micelles of sodium caprylates are formed in the aqueous phase by hydrolysis from ethyl caprylate (insoluble in water and forms an oil phase). The rate of the reaction is initially slow, but it exponentially increases after the formation of a certain number of micelles.
It could be interesting to ask whether the finding of the autocatalytic micelle means the discovery of the model system in which the surfactants show a catalytic activity as described in the GARD model. However, the mechanism of the autocatalytic micelle was attributed to a transport phenomenon rather than micellar catalysis [60,61,62]. This indicated that the catalytic property of the autocatalytic micelle is not the identical definition of catalytic activity assumed in the previously described GARD model. Even if this is true, finding a surfactant with catalytic activity or establishing a method to measure the low catalytic activity in the experimental system would be a big challenge, and it enables a dialog between the experiment and theory. It will benefit experimentalists to have guidance by a theoretical model, and it also benefits theoreticians to use the significant phenomena observed by experiment in the model.
3. Prebiotic Condition and Amphiphiles
3.1. Sources of Amphiphiles in Prebiotic Condition
Is it plausible to think that amphiphiles existed under prebiotic conditions? Among the sources of organic compounds (Table 1), the two main sources of amphiphiles are suggested to be extraterrestrial comets and thermal vents. The first source is a meteorite represented by carbonaceous chondrite (Table 1). Carbonaceous chondrite is a type of meteorite mainly composed of silicates, but containing 1.5–4% of carbons in organic forms [63]. Deamer and coworkers reported the striking fact that non-polar molecules extracted from the Murchison carbonaceous chondrite form a vesicle structure [19]. The components of the vesicles are thought to be mainly monocarboxylic acids. Later, analysis of the carbonaceous chondrite revealed that various kinds of monocarboxylic acids are present in meteorites [64,65] (Table 2). These findings led to the idea that a membrane-like structure was formed from lipids such as monocarboxylic acids [11]. The second source of organic compounds is synthesized via the Fischer-Tropsch-type reaction in thermal vents [66,67,68]. For instance, in aqueous solutions of formic acid or oxalic acid, various compounds, such as n-alkanols, n-alkanoic acids, n-alkenes, n-alkanes and alkanones ranging from C2 to over C35 are synthesized [67]. In addition to the Fischer-Tropsch-type reaction, the production of organic molecules catalyzed by various kinds of minerals was also reported [67,68]. It is notable that acyl glycerols can be formed under hydrothermal conditions, since acyl glycerols are a substructure of phospholipids at present, and important candidates for prebiotic supramolecular assembly [69]. The hydrothermal condition is not only preferable for abiotic organic synthesis; it is also proposed as a good condition for the selection and accumulation of amphiphilic molecules [70]. The lifetime of each hydrothermal vent is typically less than 100 years, but ranges from 1–10,000 years [1,71]. Therefore, some questions like “Is the time span enough for prebiotic cells to become independent from the hydrothermal condition?”, and “Could a prebiotic cell increase the region of habitat before the living chimney becomes inactive?” will be important in evaluating whether or not prebiotic cells could have been produced in hydrothermal vents.

Table 1.
Major sources (kg yr−1) of prebiotic organic compounds in the early earth (from ref. [72] (p. 1459)).

Table 2.
Molecular abundances of main organic compounds found in the Murchison, Bells, and Ivuna meteorites from ref [77].
The amphiphiles described above are mainly lipids, but peptides could also have been components of the cell membrane [82]. Szostak and coworkers proposed a system of vesicle membranes encapsulating a dipeptide catalyst [83]. This dipeptide catalyzes the reaction that forms new dipeptides, and the product takes part in the vesicle membranes because of an enhanced affinity for fatty acids, thus promoting vesicle growth. Another peptide/lipid system was reported by Pascal and Ruiz-Mirazo [84]. They used the reaction of a 5(4H)-oxazolone with leucinamide to form a dipeptide product. The presence of a lipid vesicle increases the yield of the peptide product, leading to the stereoselective reversal of the dipeptide product above the critical aggregation concentration. They also demonstrated that the produced dipeptide showed an affinity for the lipid phase. Since amino acids are synthesized by Miller’s experiment [31] and are found in meteorites [85], it is natural to think that a certain amount of peptides may be involved in the formation of the prebiotic cell membrane.
N-carboxyanhydride (NCA)-amino acids are a possible precursor of peptides, and could have been prebiotic compounds involved in the molecular evolution on the early Earth [86,87]. The present protein is synthesized by reactions with the help of the ribosome (composed of rRNA and proteins), mRNA, and tRNA. Under prebiotic conditions, a more primitive synthesis of proteins without such an elaborate reaction should take place. In the case of NCA, it undergoes a simple chemical reaction to form peptides activated by certain chemical species (e.g., CO2 and carbonyl diimidazole). A particularly important finding is the case of activation by carbonyl diimidazole. It was found that α-amino acids can be efficiently oligomerized from NCA using carbonyl diimidazole, while β-amino acids do not oligomerize [88]. Proteins at the present time are composed from only α-amino acids, but as described below, the source of organic compounds (e.g., meteorites) contains not only α-amino acids, but also β-amino acids. If the polymerization of NCA took place with the help of carbonyl diimidazole, the selectivity would naturally form proteins made of α-amino acids.
Many studies of the origin of life utilize phospholipids to form vesicles, since they are the essential building blocks of current cell membranes. Their chiral configuration shows an interesting chiral recognition of amino-acid-related compounds. However, it should be noted that the formation of complex phospholipids (e.g., phosphatidylcholine) from fatty acids, glycerol, and phosphate would have been difficult under prebiotic conditions [89,90,91]. Phospholipids that can be found in the present cell membranes are products of highly-evolved metabolic pathways incorporated by multiple enzymes. However, the complexity of phospholipids does not mean that the study of the origin of life using phospholipids is wrong, but that the synthetic pathway of phospholipids was gradually formed in the prebiotic system [89]. Interplay between the phospholipids and other biomolecules (e.g., amino acids or nucleic acids) will provide a possible pathway to form more complex biopolymers, and to the interaction between lipids and other biomolecules and polymers [33,83,92,93,94,95,96,97].
The critical aggregation concentration is usually high for short-chain lipids or peptides, and doubts could be cast over whether or not a boundary structure can be formed with such short-chain lipids under prebiotic conditions. The pseudo-phase separation approach has been reported for micelles [98,99], and this model predicts the critical aggregation concentration of mixed micelles based on the parameter βij = N(Wii + Wjj − 2Wij)/RT. Wii, Wjj, and Wij are the pair-wise interaction of the surfactants i and j, N is Avogadro’s number, R is the gas constant and T is the temperature. When βij is close to 0, it will behave as an ideal system, but when the value is negative (e.g., in the case of a mixture of cationic and anionic surfactants), the critical aggregation concentration becomes lower than that of each component. Szostak and coworkers revealed that a similar model works not only for micelles, but also for vesicles composed of prebiotically-relevant lipid mixtures [100]. This result supports the fact that the boundary structure can be formed even if short chain amphiphiles take part in the vesicle structure.
3.2. Chiral Amphiphiles in Prebiotic Condition
It has been reported that carbonaceous chondrites show an enantiomeric excess of organic compounds. Amino acid and sugar are thought to be important chiral organic molecules, since they are chiral building blocks of proteins and nucleic acids. It is interesting that many of the amino acids and sugars found in meteorites have excess L and D configurations, respectively [101,102]. The chirality coincides with the handedness of the building blocks of biopolymers of present life and the fact supports that the source of the organic molecules are extraterrestrial.
It is also interesting that some of the amphiphiles extracted from a carbonaceous chondrite have an asymmetric carbon in their structures (e.g., branched monocarboxylic acids in the Murchison meteorite [64], and hydroxy acids found in the Murchison, GRA 95229 and LAP 02342 meteorites [81]). Most of these chemicals may have a very high solubility in water to form a membrane structure, but some could be seen as model chiral amphiphiles to investigate how the chiral structure affects the properties of a self-assembled entity.
4. Amphiphilic Molecule and Homochirality
The question of “How amphiphiles and homochirality are related?” is less understood compared to the relationship between polymers and homochirality. One reason for this is that it has been thought that the first biological entities were biopolymers, such as proteins and RNA, rather than lipids; thus, the discussion of homochirality has been mainly limited to biopolymers. The question was examined relatively recently [13,103] (p. 303). The relation between a supramolecular assembly and chirality is complicated compared to biopolymers, and it is necessary to clarify the connection between the features of an amphiphilic molecule and homochirality. Lancet and coworkers listed the advantages of lipid assemblies compared to biopolymers in the context of the origin of life [14]. In Figure 4, the physical properties in the list and two other physical properties (adsorption and permeation) are shown. Each physical property could be influenced by the chiral properties. The following sections are devoted to review studies related to chirality, and to some of the physical properties of the amphiphilic molecules.
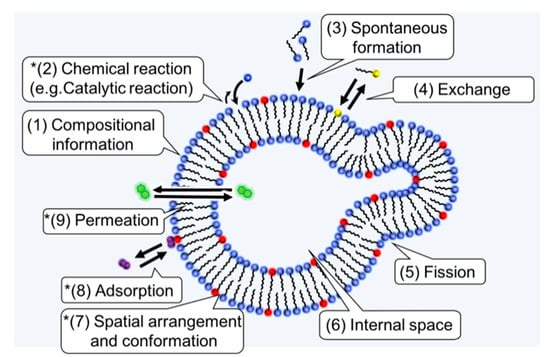
Figure 4.
Functions of amphiphiles that could be important for the origin of life and homochirality. The properties (1)–(6) were taken from a table in reference [14] (p. 19) and slightly modified. (7)–(9) were added in this review to discuss the possible roles of chiral amphiphiles. In this article, only the properties with an asterisk are reviewed in detail.
4.1. Chemical Reactions
4.1.1. Advantage Factors
The developing process of homochirality can be described as shown in Figure 5 [104,105]. Without any asymmetric perturbation of chirality (i.e., the source of chirality), constituents of the pre-biotic system can be racemic on average (1). However, perturbation of the chiral purity (2a) or statistical fluctuation of the enantiopurity of chiral molecules (2b) can influence the asymmetrical initial conditions (3). Examples of the chiral perturbation are listed in Table 3. The enantiomeric excess of organic molecules in meteorites could have affected the initial conditions of the racemic soup on earth. The process of (2a) and (2b) also affects the enhancement of asymmetry (4). The examples of the enhancement of asymmetry and the effect of the advantage factor in chemical reactions are described in the following section. Lastly, the system reaches chiral purity of the biological entity at the present time (5). The description of the advantage factors and chemical reactions described below was originally considered for biopolymers, but is general enough to also be applied to a supramolecular assembly.
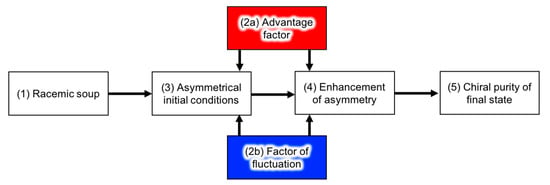
Figure 5.
Process of developing homochirality.

Table 3.
Physical advantage factors [105,106].
Various types of physical advantage factors to induce chiral asymmetry of the system was summarized by Goldanskii and coworkers [105,106]. It was stated that the combinations of the static field are not a “true” advantage factor, and do not lead to inducing asymmetry. Quantified values of the asymmetry factor g are defined as follows.
where kL and kD are the rate constants for the mirror-image reactions. This value quantitatively evaluates how much each advantage factor can contribute to the chiral asymmetry. Table 3 lists this value for each reaction.
4.1.2. Reaction Building Blocks
Considering that the living organism at the present time consists of chirally-pure biopolymers, such as proteins, RNA, and DNA, it is natural to think that the g values of these advantage factors could be too weak, and that another mechanism to enhance or amplify the chirality of the system is necessary. One model that describes the spontaneous mirror-symmetry breaking was proposed by F. Frank in 1953 [107]. He proposed a model reaction of autocatalysis that results in products with a high enantiopurity, even if the starting chemicals are racemic. This approach was generalized by Morozov by describing the dynamic reaction of chiral polarization (η) [104,108].
where xL and xD are the concentrations of the L and D chiral molecules. |η| = 1 corresponds to the chirally-pure state while |η| = 0 corresponds to a racemic state. Based on the approach by Morozov, a broad class of kinetic diagrams and their dynamic equations are described in detail by Goldanskii and Kuzmin (Table 4) [105].
where θ is the concentration of antipodes in the system. The parameters and can be replaced by and . The table has several important features:

Table 4.
Basic type of process in chiral system from reference [105].
- (1)
- The table provides “reaction building blocks” which can construct complex models for the reaction process in an extremely simple way.
- (2)
- The classification enables one to identify the type of process that efficiently leads to breaking the mirror symmetry.
- (3)
- The table takes g into account to evaluate the contribution of the advantage factors to the process.
Figure 6 qualitatively describes the time dependency of the chiral polarization for each type of process. The racemization process decreases |η| with time. The neutral process does not change |η|. The deracemization process increases the value of |η| with time. The deracemization process is the key to increasing the chiral asymmetry of the system.
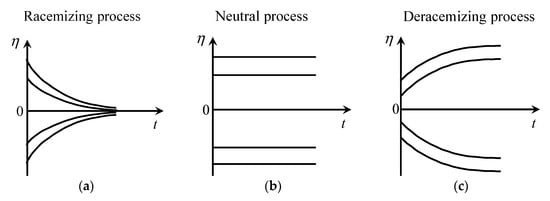
Figure 6.
Function of from reference [105]. (a) Racemizing process. (b) Neutral process. (c) Deracemizing process.
Lancet and coworkers investigated the homochirality based on the GARD model [103] (see Figure 2). Some models for the mirror symmetry breaking designed a reaction network based on the reaction building blocks shown in Table 4. However, for the GARD model, it randomly generated a large variety of chemical species including amphiphiles and catalytic networks without designing the reaction system. The model assumed that the NG types of the chiral molecules form a catalytic network with equal amounts of the D and L optical isomers. The parity-violating energy difference between enantiomers is excluded and pairs of enantiomers having the same properties. A key chiral property is the ability of the chiral compound Li to distinguish an enantiomer of other chiral compounds, Lj and Dj, described by the enantio-discrimination factor αij = βLi,Lj/βLi,Dj, where βij is the catalytic intensity. It was observed that some chemical species in the stationary compositional states were enriched, with one of the two enantiomers indicating spontaneous chiral symmetry breaking. A considerable degree of chiral selection was observed when the values of αij are highly relative to the typical values. This indicates that assembly-based enantioselection could not have occurred during the early stage of the origin of life, since such a high discrimination factor is expected only for large molecules. Lancet and coworkers suggested that the homochirality could be a consequence of catalytic networks, rather than a prerequisite for the initiation of primeval life processes.
4.2. Spatial Arrangement and Conformation
When a chiral unit and racemic or achiral unit are confined in a polymer or supramolecular assembly, these units act cooperatively, which can result in non-linear relationships between the mole fraction of a chiral unit and chiral properties (e.g., optical activity and enantioselective catalytic activity). Such phenomena were first found for helical polymers, and similar phenomena were also found later for supramolecular assemblies, mainly in the form of one-dimensional helical fibers. The Italian researcher Pino and coworkers studied stereo-regular vinyl polymers and determined the cooperative phenomena in the 1960s [109,110,111]. However, understanding the phenomena was hindered by a weak cooperativity and the absence of a chromophore to observe the chiroptical properties that reflect the chiral conformational properties. Green and coworkers studied poly(isocyanates) (Nylon-1), which showed evident non-linear relationships between the mole fraction of the chiral unit and optical activity [112,113]. The mechanism behind the phenomena was described by one-dimensional Ising models utilizing four main parameters, i.e., temperature, energy of a helix sense reversal, the energy difference between the units of opposite helical senses, and the degree of polymerization [114,115,116,117,118]. The “optical activity” is often measured as a physical property that responds non-linearly to the mole fraction of the chiral unit, because the optical activity indicates the helical conformation of the polymer. However, this does not mean that those amplifications are limited to the chiroptical properties. Suginome and coworkers have shown that for the poly(quinoxaline-2,3-diyl)s, the majority rule [119,120] and sergeants-and-soldiers principal [121] can be coupled with asymmetric catalysis. The reported catalytic reaction of poly(quinoxaline-2,3-diyl)s may only have a slight relation with the prebiotic catalytic reaction, but implies possible roles of those phenomena in the homochirality if such a catalytic activity is observed in the supramolecular assembly. Many reviews are available for the amplification of chirality in helical polymers [113,122,123,124,125,126,127].
Cooperative phenomena are not limited to polymers whose units are covalently connected, but also for supramolecular assemblies whose units are non-covalently connected. A wide range of helical supramolecular assemblies are reviewed by Yashima and coworkers [128] and Meijer and coworkers [17,129,130,131]. Figure 7 depicts a model of cooperative supramolecular copolymerization of a disk-like monomer unit [17], a case of majority rule (Figure 7a), and a sergeants-and-soldiers reaction (Figure 7b). For the majority rules, a mismatch between the handedness of the helical structure (ΔH0MMP) and chirality of the monomer and nucleation (ΔH0NP) are assumed to reduce the enthalpy of the elongation process (ΔH0ELO). For the sergeants-and-soldiers principal, the possible reactions are similar as those in the majority-rules model, but the principal distinguishes the enthalpy of chiral units from achiral units, since they have different achiral physical properties. The model is limited to a supramolecular assembly that undergoes one-dimensional growth, but it implies the possibility of amplification in the other chiral supramolecular system. It is an open question whether or not majority rules and sergeants-and-soldiers principal are involved in the emergence of homochirality in the context of the lipid world.
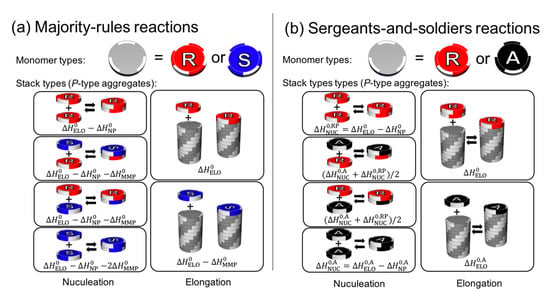
Figure 7.
Theoretical models of cooperative supramolecular copolymerization from reference [17]. Formation of M- and P-type helical aggregates are possible but only the P-type aggregates are shown in this figure for the sake of simplicity. The M-type helical aggregate can be described in a similar manner by switching the helicity of the aggregates from P to M and switching the (S) and (R)-isomers. (a) Majority-rules reactions. Two types of monomers, (R)-isomer (red) and (S)-isomer (blue), forming P-type helical aggregate. The model is described by ΔH0ELO, ΔH0NP, ΔH0MMP, and ΔS0, where ΔH0ELO is the elongation enthalpy, ΔH0NP is the nucleation penalty, ΔH0MMP is the mismatch penalty, and ΔS0 is the entropy. The (R)-isomer prefer P-helicity while (S)-isomer prefer M-helicity. This preference is taken in account by ΔH0MMP. (b) Sergeants-and-soldiers reactions. Mismatch does not happen for this case but additional parameters, ΔH0,AELO, ΔH0,ANP, and ΔS0,A, are introduced to consider the dependence of the energy on the helicity for achiral monomers.
4.3. Adsorption
It was reported that the membrane of liposomes assists in the formation of the amino acid sequence of the peptide chain by the adsorption of monomers. For instance, Luisi and coworkers reported that the 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane facilitates the NCA polycondensation of hydrophobic tryptophan-containing peptides up to 29 mer [132]. They also reported that 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) assisted the NCA-D/L-Tryptophan (L-Trp) condensation [97]. They concluded that the chiral structure of the lipid and the corresponding liposome phase transition temperature do not significantly affect the distribution of the enantiomeric composition of the resulting Trp oligomers.
Umakoshi and coworkers, however, reported that the liposome of DPPC enantioselectively adsorbs L-amino acids after 48 h of coincubation [133]. They tested 10 different amino acids (histidine, serine, aspartic acid, tyrosine, proline, cysteine, tryptophan, phenylalanine, leucine, and valine) and found that the L-stereoisomer of all the amino acids (except for serine) were preferentially adsorbed on the DPPC surface. The extreme cases were L-tryptophan and L-histidine, and their SL/D values (adsorbed concentration ratio of L-amino acid to D-amino acid) exceeded 1000. The adsorption isotherms were attributed to the Langmuir type. This finding implies the possibility of the condensation of the L-amino acid on the surface of the DPPC liposome. They also reported the enantioselective adsorption of ibuprofen [134].
Taking advantage of the enantioselective adsorption, the group showed that the chiral liposome can support homochiral oligomerization of an amino acid [135]. They first allowed L- or D-Histidine to be adsorbed on L-POPC for 72 h, then performed the oligomerization at 25 °C for 48 h by adding 1-hydroxybenzotriazole and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride to a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (L-POPC) suspension. They concluded that the homochiral oligomerization of L-amino acids was efficiently achieved compared to the D-amino acids. This result shows that the chirality of the lipid helps to induce the homochirality of peptides. Questions as to whether or not the opposite chiral transfer (peptides to phospholipid) happens, and whether such a reaction occurs with prebiotic molecules, such as NCA, may be interesting, and further investigation is anticipated.
4.4. Permeation
Though there are almost no studies on the enantioselective permeation from the point of view of homochirality, some studies from a pharmaceutical point of view have been reported. The blood-brain barrier protects our central nervous system against external aggressions, but it also prevents therapeutics from reaching targets in the brain. Teixido and Giralt developed a phenylproline tetrapeptide that can cross the blood-brain barrier by paracellular hydrophilic diffusion [136]. They synthesized a library of the 16 phenylproline tetrapeptides stereoisomers, and revealed the relationship among their stereochemistry, transport properties, and permeability. They categorized the tetrapeptides into two groups depending on the stereochemistry; (Group 1) peptides with a higher symmetry (i.e., containing an even number of L or D phenylproline units) and (Group 2) peptides with less symmetric peptides (i.e., containing an odd number of L or D phenylproline units). The enantiomers of the former group did not show similar transport and permeation properties. The latter showed a significant difference between the enantiomers, and basically, the L-rich enantiomer showed higher transport and permeation properties compared to the D-rich enantiomers. Other studies of stereochemical effects on the permeability of human and rat skin are reported based on transdermal drug delivery research [137,138].
5. Conclusions
Though it may not be a common view compared to the RNA world, the lipid world hypothesis provides new insight into the scenario of the origin of life. It emphasizes that an amphiphilic molecule could have played a key role in the origin of life prior to the emergence of the biopolymers. This area of research provides new knowledge about the supramolecular assembly and its interaction with other biomolecules and biopolymers. Far less is known about the relationship between the lipid world and homochirality, and it seems that there are various undiscovered features that chiral amphiphiles have when one considers the physical properties that are unique to supramolecular assemblies (compositional information, spontaneous formation, fission, permeation, etc.). Considering the scenario of homochirality, it is of great interest to determine how those properties could have connected with mirror symmetry breaking, and to elucidate the cooperative phenomena of chiral molecules, such as the sergeants-and-soldiers principal and majority rules.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/11/8/966/s1: derivation of dynamic equations (S1–S54).
Author Contributions
Conceptualization, N.S. and Y.I.; Writing-Original Draft Preparation, N.S. and Y.I.; Writing—Review and Editing, N.S. and Y.I., Supervision, N.S. and Y.I.
Funding
This study was supported by JSPS KAKENHI Grant Number JP17K14083.
Acknowledgments
N.S. thanks E. Yashima, T. Ikai, and D. Taura, and the group members for the fruitful discussion on the amplification of chirality in helical polymers and supramolecular assembly.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y. Early evolution of membrane lipids: How did the lipid divide occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.; Siliakus, M.F.; Exterkate, M.; Jain, S.; Jumde, V.R.; Andringa, R.L.H.; Kengen, S.W.M.; Minnaard, A.J.; Driessen, A.J.M.; van der Oost, J. Converting escherichia coli into an archaebacterium with a hybrid heterochiral membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Forterre, P. The two ages of the RNA world, and the transition to the DNA world: A story of viruses and cells. Biochimie 2005, 87, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Molecular replication. Nature 1992, 358, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.O. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2010, 39, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Self-sequencing of amino acids and origins of polyfunctional protocells. Orig. Life 1984, 14, 485–488. [Google Scholar] [CrossRef]
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- Ikehara, K. Possible steps to the emergence of life: The [gadv]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Orig. Life Evol. Biospheres 2001, 31, 119–145. [Google Scholar] [CrossRef]
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Walde, P. Surfactant assemblies and their various possible roles for the origin(s) of life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Luisi, P.L. Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem. Commun. 2010, 46, 3639–3653. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Dallavalle, S.; Walde, P.; Colonna, S.; Luisi, P.L. Autopoietic self-reproduction of chiral fatty acid vesicles. J. Am. Chem. Soc. 1997, 119, 292–301. [Google Scholar] [CrossRef]
- Markvoort, A.J.; ten Eikelder, H.M.M.; Hilbers, P.A.J.; de Greef, T.F.A.; Meijer, E.W. Theoretical models of nonlinear effects in two-component cooperative supramolecular copolymerizations. Nat. Commun. 2011, 2, 509. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Deamer, D.W. Boundary structures are formed by organic components of the murchison carbonaceous chondrite. Nature 1985, 317, 792–794. [Google Scholar] [CrossRef]
- Dyson, F.J. A model for the origin of life. J. Mol. Evol. 1982, 18, 344–350. [Google Scholar] [CrossRef]
- Dyson, F. Origins of Life; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Dyson, F. Origins of life. In Nishina Memorial Lectures: Creators of Modern Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 746, pp. 71–97. [Google Scholar]
- Oparin, A.I. Biochemical processes in the simplest structures. In The Origin of Life on the Earth; Elsevier: Amsterdam, The Netherlands, 1959; pp. 428–436. [Google Scholar]
- Eigen, M.; Gardiner, W.; Schuster, P.; Winkler-Oswatitsch, R. The origin of genetic information. Sci. Am. 1981, 244, 88–118. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. The origin of life and the nature of the primitive gene. J. Theor. Biol. 1965, 10, 53–88. [Google Scholar] [CrossRef]
- Szathmáry, E. The origin of replicators and reproducers. Philos. Trans. Royal. Soc. B 2006, 361, 1761–1776. [Google Scholar] [CrossRef]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef]
- Von Neumann, J. The general and logical theory of automata. In Cerebral Mechanisms in Behavior; the Hixon Symposium; Wiley: Oxford, UK, 1951; pp. 1–41. [Google Scholar]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others)a. Biol. Direct 2012, 7, 23. [Google Scholar] [CrossRef]
- Mallik, S.; Kundu, S. The lipid-RNA world. arXiv 2012, arXiv:1211.0413. [Google Scholar]
- Biscans, A. Exploring the emergence of RNA nucleosides and nucleotides on the early earth. Life 2018, 8, 57. [Google Scholar] [CrossRef]
- Nakashima, S.; Kebukawa, Y.; Kitadai, N.; Igisu, M.; Matsuoka, N. Geochemistry and the origin of life: From extraterrestrial processes, chemical evolution on earth, fossilized life’s records, to natures of the extant life. Life 2018, 8, 39. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2010, 4, a003608. [Google Scholar] [CrossRef]
- Hud, N.V.; Cafferty, B.J.; Krishnamurthy, R.; Williams, L.D. The origin of RNA and “my grandfather’s axe”. Chem. Biol. 2013, 20, 466–474. [Google Scholar] [CrossRef]
- D‘Aguanno, E.; Altamura, E.; Mavelli, F.; Fahr, A.; Stano, P.; Luisi, P. Physical routes to primitive cells: An experimental model based on the spontaneous entrapment of enzymes inside micrometer-sized liposomes. Life 2015, 5, 969–996. [Google Scholar] [CrossRef]
- Luisi, P.L. Chemistry constraints on the origin of life. Isr. J. Chem. 2015, 55, 906–918. [Google Scholar] [CrossRef]
- Luisi, P.L.; Stano, P.; de Souza, T. Spontaneous overcrowding in liposomes as possible origin of metabolism. Orig. Life Evol. Biosph. 2015, 44, 313–317. [Google Scholar] [CrossRef]
- Luisi, P.L.; Allegretti, M.; Pereira de Souza, T.; Steiniger, F.; Fahr, A.; Stano, P. Spontaneous protein crowding in liposomes: A new vista for the origin of cellular metabolism. ChemBioChem 2010, 11, 1989–1992. [Google Scholar] [CrossRef]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in micellar systems. Angew. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef]
- Fendler, J.H. Atomic and molecular clusters in membrane mimetic chemistry. Chem. Rev. 1987, 87, 877–899. [Google Scholar] [CrossRef]
- Lancet, D.; Sadovsky, E.; Seidemann, E. Probability model for molecular recognition in biological receptor repertoires: Significance to the olfactory system. Proc. Natl. Acad. Sci. USA 1993, 90, 3715–3719. [Google Scholar] [CrossRef]
- Rosenwald, S.; Kafri, R.A.N.; Lancet, D. Test of a statistical model for molecular recognition in biological repertoires. J. Theor. Biol. 2002, 216, 327–336. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Markovitch, O.; Zidovetzki, R.; Lancet, D. Replication of simulated prebiotic amphiphile vesicles controlled by experimental lipid physicochemical properties. Phys. Biol. 2011, 8, 066001. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lancet, D.; Zidovetzki, R. Replication of simulated prebiotic amphiphilic vesicles in a finite environment exhibits complex behavior that includes high progeny variability and competition. Astrobiology 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Farmer, J.D.; Kauffman, S.A.; Packard, N.H. Autocatalytic replication of polymers. Physica D 1986, 22, 50–67. [Google Scholar] [CrossRef]
- Hordijk, W. Autocatalytic confusion clarified. J. Theor. Biol. 2017, 435, 22–28. [Google Scholar] [CrossRef]
- Segré, D. A statistical chemistry approach to the origin of life. Chemtracts–Biochem. Mol. Biol. 1999, 12, 382–397. [Google Scholar]
- Solé, R.V. Evolution and self-assembly of protocells. Int. J. Biochem. Cell Biol. 2009, 41, 274–284. [Google Scholar] [CrossRef]
- Shapiro, R. A replicator was not involved in the origin of life. IUBMB Life 2000, 49, 173–176. [Google Scholar] [CrossRef]
- Anet, F.A.L. The place of metabolism in the origin of life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Segré, D.; Lancet, D.; Kedem, O.; Pilpel, Y. Graded autocatalysis replication domain (gard): Kinetic analysis of self-replication in mutually catalytic sets. Orig. Life Evol. Biospheres 1998, 28, 501–514. [Google Scholar] [CrossRef]
- Luisi, P.L. The minimal autopoietic unit. Orig. Life Evol. Biospheres 2015, 44, 335–338. [Google Scholar] [CrossRef]
- Stano, P.; Luisi, P.L. Semi-synthetic minimal cells: Origin and recent developments. Curr. Opin. Biotechnol. 2013, 24, 633–638. [Google Scholar] [CrossRef]
- Stano, P.; Luigi Luisi, P. Self-reproduction of micelles, reverse micelles, and vesicles: Compartments disclose a general transformation pattern. In Advances in Planar Lipid Bilayers and Liposomes; LiuLiu, A.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2008; Volume 7, pp. 221–263. [Google Scholar]
- Luisi, P.L.; Varela, F.J. Self-replicating micelles—A chemical version of a minimal autopoietic system. Orig. Life Evol. Biospheres 1989, 19, 633–643. [Google Scholar] [CrossRef]
- Bachmann, P.A.; Luisi, P.L.; Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 1992, 357, 57–59. [Google Scholar] [CrossRef]
- Buhse, T.; Nagarajan, R.; Lavabre, D.; Micheau, J.C. Phase-transfer model for the dynamics of “micellar autocatalysis”. J. Phys. Chem. A 1997, 101, 3910–3917. [Google Scholar] [CrossRef]
- Buhse, T.; Pimienta, V.; Lavabre, D.; Micheau, J.C. Experimental evidence of kinetic bistability in a biphasic surfactant system. J. Phys. Chem. A 1997, 101, 5215–5217. [Google Scholar] [CrossRef]
- Buhse, T.; Lavabre, D.; Nagarajan, R.; Micheau, J.C. Origin of autocatalysis in the biphasic alkaline hydrolysis of c-4 to c-8 ethyl alkanoates. J. Phys. Chem. A 1998, 102, 10552–10559. [Google Scholar] [CrossRef]
- Pizzarello, S.; Shock, E. The organic composition of carbonaceous meteorites: The evolutionary story ahead of biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Olah, G.A.; Mathew, T.; Prakash, G.K.S. Chemical formation of methanol and hydrocarbon (“organic”) derivatives from co2 and h2—carbon sources for subsequent biological cell evolution and life’s origin. J. Am. Chem. Soc. 2016, 139, 566–570. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid formation by aqueous fischer-tropsch-type synthesis over a temperature range of 100 to 400 degrees c. Orig. Life Evol. Biospheres 2001, 31, 103–118. [Google Scholar] [CrossRef]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by fischer- tropsch-type reactions. Orig. Life Evol. Biospheres 1999, 29, 153–166. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig. Life Evol. Biospheres 2006, 36, 93–108. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M. Selection of prebiotic molecules in amphiphilic environments. Life 2017, 7, 3. [Google Scholar] [CrossRef]
- Lowell, R.P.; Rona, P.A.; Von Herzen, R.P. Seafloor hydrothermal systems. J. Geophys. Res. Solid Earth 1995, 100, 327–352. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Irvine, W.; Becker, L.; Blank, J.; Brucato, J.R.; Colangeli, L.; Derenne, S.; Despois, D.; Dutrey, A.; Fraaije, H.; et al. Astrophysical and astrochemical insights into the origin of life. Rep. Prog. Phys. 2002, 65, 1427–1487. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef]
- Kadko, D.; Baker, E.; Alt, J.; Baross, J. Global impact of submarine hydrothermal processes: Ridge. In Vents Workshop, NSF RIDGE Initiative and NOAA Vents Program; Durham Ridge Office: Durham, NH, USA, 1995. [Google Scholar]
- Elderfield, H.; Schultz, A. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 1996, 24, 191–224. [Google Scholar] [CrossRef]
- Delsemme, A.H. Cometary origin of carbon, nitrogen and water on the earth. Orig. Life Evol. Biospheres 1991, 21, 279–298. [Google Scholar] [CrossRef]
- Monroe, A.A.; Pizzarello, S. The soluble organic compounds of the bells meteorite: Not a unique or unusual composition. Geochim. Cosmochim. Acta 2011, 75, 7585–7595. [Google Scholar] [CrossRef]
- Pizzarello, S. The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc. Chem. Res. 2006, 39, 231–237. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in orgueil and ivuna: Tracing the parent body of ci type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Pizzarello, S.; Wang, Y.; Chaban, G.M. A comparative study of the hydroxy acids from the murchison, gra 95229 and lap 02342 meteorites. Geochim. Cosmochim. Acta 2010, 74, 6206–6217. [Google Scholar] [CrossRef]
- Fishkis, M. Steps towards the formation of a protocell: The possible role of short peptides. Orig. Life Evol. Biosph. 2007, 37, 537–553. [Google Scholar] [CrossRef]
- Adamala, K.; Szostak, J.W. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef]
- Murillo-Sánchez, S.; Beaufils, D.; González Mañas, J.M.; Pascal, R.; Ruiz-Mirazo, K. Fatty acids‘ double role in the prebiotic formation of a hydrophobic dipeptide. Chem. Sci. 2016, 7, 3406–3413. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Polypeptides and 100 years of chemistry of α-amino acidn-carboxyanhydrides. Angew. Chem. Int. Ed. 2006, 45, 5752–5784. [Google Scholar] [CrossRef]
- Ehler, K.W.; Orgel, L.E. N, N‘-carbonyldiimidazole-induced peptide formation in aqueous solution. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1976, 434, 233–243. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Polymerization of β-amino acids in aqueous solution. Orig. Life Evol. Biospheres 1998, 28, 47–60. [Google Scholar] [CrossRef]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Namani, T.; Deamer, D.W. Stability of model membranes in extreme environments. Orig. Life Evol. Biospheres 2008, 38, 329–341. [Google Scholar] [CrossRef]
- Budin, I.; Szostak, J.W. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 5249–5254. [Google Scholar] [CrossRef]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Anella, F.; Danelon, C. Reconciling ligase ribozyme activity with fatty acid vesicle stability. Life 2014, 4, 929–943. [Google Scholar] [CrossRef]
- Anella, F.; Danelon, C. Prebiotic factors influencing the activity of a ligase ribozyme. Life 2017, 7, 17. [Google Scholar] [CrossRef]
- Olasagasti, F.; Kim, H.J.; Pourmand, N.; Deamer, D.W. Non-enzymatic transfer of sequence information under plausible prebiotic conditions. Biochimie 2011, 93, 556–561. [Google Scholar] [CrossRef]
- Wilson, M.A.; Wei, C.; Pohorille, A. Towards co-evolution of membrane proteins and metabolism. Orig. Life Evol. Biospheres 2014, 44, 357–361. [Google Scholar] [CrossRef]
- Hitz, T.; Blocher, M.; Walde, P.; Luisi, P.L. Stereoselectivity aspects in the condensation of racemic nca−amino acids in the presence and absence of liposomes. Macromolecules 2001, 34, 2443–2449. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Vora, S.; George, A.; Desai, H.; Bahadur, P. Mixed micelles of some anionic-anionic, cationic-cationic, and ionic-nonionic surfactants in aqueous media. J. Surfactants Deterg. 1999, 2, 213–221. [Google Scholar] [CrossRef]
- Budin, I.; Prywes, N.; Zhang, N.; Szostak, J.W. Chain-length heterogeneity allows for the assembly of fatty acid vesicles in dilute solutions. Biophys. J. 2014, 107, 1582–1590. [Google Scholar] [CrossRef]
- Burton, A.; Berger, E. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef]
- Cooper, G.; Rios, A.; Nuevo, M. Monosaccharides and their derivatives in carbonaceous meteorites: A scenario for their synthesis and onset of enantiomeric excesses. Life 2018, 8, 36. [Google Scholar] [CrossRef]
- Kafri, R.; Markovitch, O.; Lancet, D. Spontaneous chiral symmetry breaking in early molecular networks. Biol. Direct 2010, 5, 38. [Google Scholar] [CrossRef]
- Morozov, L.L.; Kuz Min, V.V.; Goldanskii, V.I. Comparative analysis of the role of statistical fluctuations and factor of advantage (parity nonconservation) in the origins of optical activity. Orig. Life 1983, 13, 119–138. [Google Scholar] [CrossRef]
- Gol’danskiĭ, V.I.; Kuz’min, V.V. Spontaneous breaking of mirror symmetry in nature and the origin of life. Sov. Phys. Usp. 1989, 32, 1–29. [Google Scholar] [CrossRef]
- Avetisov, V.A.; Kuz’min, V.V.; Goldanskii, V.I. Handedness, origin of life and evolution. Phys. Today 1991, 44, 33–41. [Google Scholar] [CrossRef]
- Frank, F.C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Morozov, L. Mirror symmetry breaking in biochemical evolution. Orig. Life 1979, 9, 187–217. [Google Scholar] [CrossRef]
- Pino, P.; Lorenzi, G.P. Optically active vinyl polymers. Ii. The optical activity of isotactic and block polymers of optically active α-olefins in dilute hydrocarbon solution. J. Am. Chem. Soc. 1960, 82, 4745–4747. [Google Scholar] [CrossRef]
- Pino, P.; Ciardelli, F.; Lorenzi, G.P.; Montagnoli, G. Optically active vinyl polymers. Ix. Optical activity and conformation in dilute solution of isotactic poly-α-olefins. Makromol. Chem. 1963, 61, 207–224. [Google Scholar] [CrossRef]
- Luisi, P.L.; Pino, P. Conformational properties of optically active poly-Alpha-olefins in solution. J. Phys. Chem. 1968, 72, 2400–2405. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A helical polymer with a cooperative response to chiral information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef]
- Green, M.M.; Park, J.W.; Sato, T.; Teramoto, A.; Lifson, S.; Selinger, R.L.B.; Selinger, J.V. The macromolecular route to chiral amplification. Angew. Chem. Int. Ed. 1999, 38, 3138–3154. [Google Scholar] [CrossRef]
- Lifson, S.; Green, M.M.; Andreola, C.; Peterson, N.C. Macromolecular stereochemistry: Helical sense preference in optically active polyisocyanates. Amplification of a conformational equilibrium deuterium isotope effect. J. Am. Chem. Soc. 1989, 111, 8850–8858. [Google Scholar] [CrossRef]
- Selinger, J.V.; Selinger, R.L.B. Theory of chiral order in random copolymers. Phys. Rev. Lett. 1996, 76, 58–61. [Google Scholar] [CrossRef]
- Selinger, J.V.; Selinger, R.L.B. Cooperative chiral order in copolymers of chiral and achiral units. Phys. Rev. E 1997, 55, 1728–1731. [Google Scholar] [CrossRef]
- Selinger, J.V.; Selinger, R.L.B. Cooperative chiral order in polyisocyanates: New statistical problems. Macromolecules 1998, 31, 2488–2492. [Google Scholar] [CrossRef]
- Gu, H.; Sato, T.; Teramoto, A.; Varichon, L.; Green, M.M. Molecular mechanisms for the optical activities of polyisocyanates induced by intramolecular chiral perturbations. Polym. J. 1997, 29, 77–84. [Google Scholar] [CrossRef][Green Version]
- Ke, Y.Z.; Nagata, Y.; Yamada, T.; Suginome, M. Majority-rules-type helical poly(quinoxaline-2,3-diyl)s as highly efficient chirality-amplification systems for asymmetric catalysis. Angew. Chem. Int. Ed. 2015, 54, 9333–9337. [Google Scholar] [CrossRef]
- Yamamoto, T.; Murakami, R.; Komatsu, S.; Suginome, M. Chirality-amplifying, dynamic induction of single-handed helix by chiral guests to macromolecular chiral catalysts bearing boronyl pendants as receptor sites. J. Am. Chem. Soc. 2018, 140, 3867–3870. [Google Scholar] [CrossRef]
- Nagata, Y.; Nishikawa, T.; Suginome, M. Exerting control over the helical chirality in the main chain of sergeants-and-soldiers-type poly(quinoxaline-2,3-diyl)s by changing from random to block copolymerization protocols. J. Am. Chem. Soc. 2015, 137, 4070–4073. [Google Scholar] [CrossRef]
- Green, M.M.; Cheon, K.S.; Yang, S.Y.; Park, J.W.; Swansburg, S.; Liu, W. Chiral studies across the spectrum of polymer science. Acc. Chem. Res. 2001, 34, 672–680. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and amplification of chirality by helical polymers. Chem. Eur. J. 2004, 10, 42–51. [Google Scholar] [CrossRef]
- Fujiki, M. Helix generation, amplification, switching, and memory of chromophoric polymers. Top. Curr. Chem. 2008, 284, 119–186. [Google Scholar]
- Yashima, E.; Maeda, K.; Furusho, Y. Single and double-stranded helical polymers: Synthesis, structures, and functions. Acc. Chem. Res. 2008, 41, 1166–1180. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef]
- Maeda, K.; Yashima, E. Helical polyacetylenes induced via noncovalent chiral interactions and their applications as chiral materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef]
- Palmans, A.R.A.; Meijer, E.W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef]
- Blocher, M.; Liu, D.; Walde, P.; Luisi, P.L. Liposome-assisted selective polycondensation of α-amino acids and peptides. Macromolecules 1999, 32, 7332–7334. [Google Scholar] [CrossRef]
- Ishigami, T.; Suga, K.; Umakoshi, H. Chiral recognition of l-amino acids on liposomes prepared with l-phospholipid. ACS Appl. Mater. Interfaces 2015, 7, 21065–21072. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kishi, Y.; Ishigami, T.; Suga, K.; Umakoshi, H. Chiral selective adsorption of ibuprofen on a liposome membrane. J. Phys. Chem. B 2016, 120, 2790–2795. [Google Scholar] [CrossRef]
- Ishigami, T.; Kaneko, Y.; Suga, K.; Okamoto, Y.; Umakoshi, H. Homochiral oligomerization of l-histidine in the presence of liposome membranes. Colloid. Polym. Sci. 2015, 293, 3649–3653. [Google Scholar] [CrossRef]
- Arranz-Gibert, P.; Guixer, B.; Malakoutikhah, M.; Muttenthaler, M.; Guzmán, F.; Teixidó, M.; Giralt, E. Lipid bilayer crossing—The gate of symmetry. Water-soluble phenylproline-based blood-brain barrier shuttles. J. Am. Chem. Soc. 2015, 137, 7357–7364. [Google Scholar] [CrossRef]
- Valentová, J.; Bauerová, K.; Farah, L.; Devínsky, F. Does stereochemistry influence transdermal permeation of flurbiprofen through the rat skin? Arch. Dermatol. Res. 2010, 302, 635–638. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B.; Kommuru, T.; Afouna, M.; Reddy, I. Transport of chiral molecules across the skin. In Chirality in Drug Design and Development; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).