Estimation of Natural Radionuclides and Rare Earth Elements Concentration of the Rocks of Abu Khuruq Ring Complex, Egypt
Abstract
1. Introduction
2. Materials and Methods
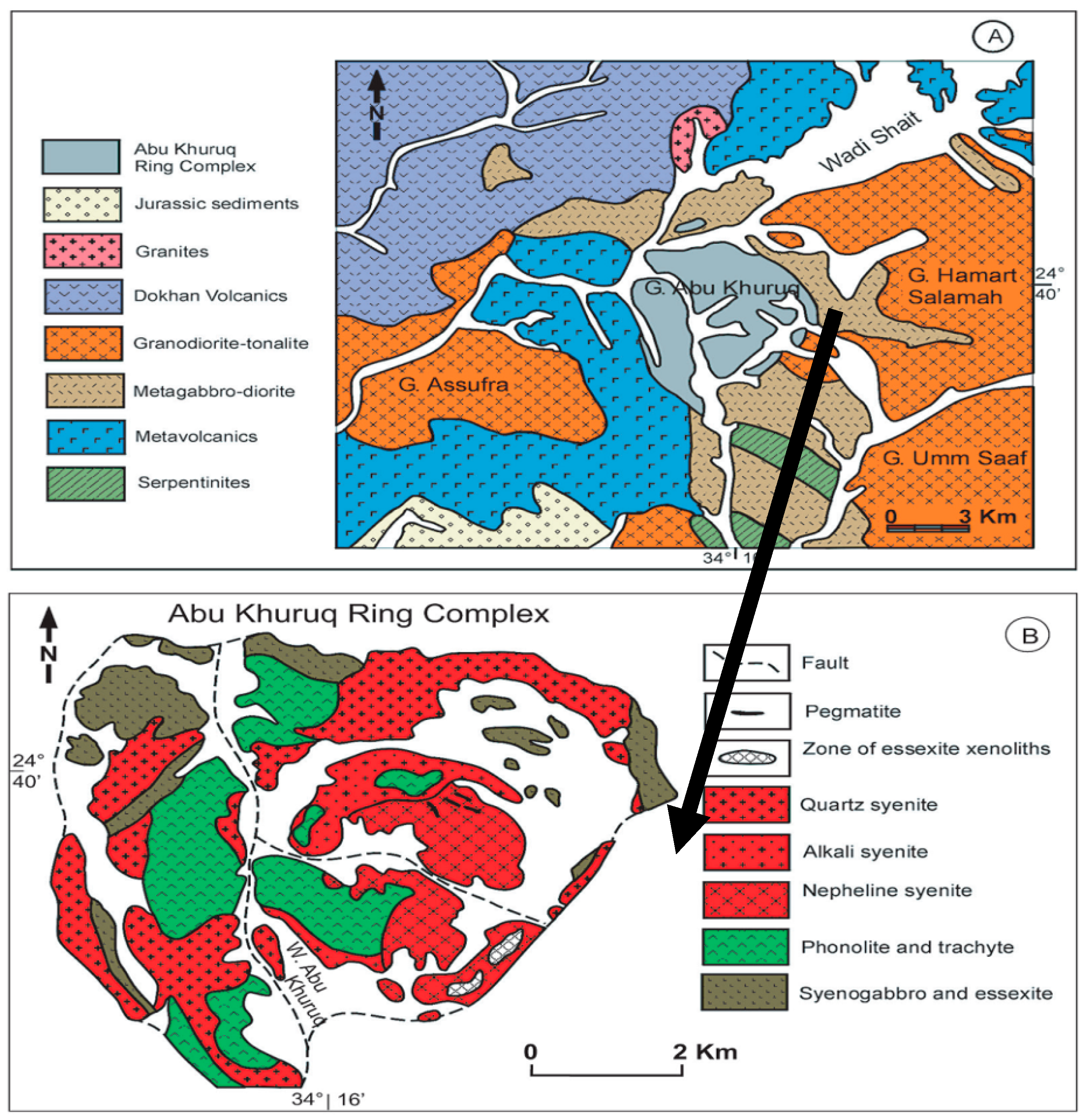
2.1. General Description of the Geology of Abu Khuruq Area
2.2. Sample Preparation
2.3. The Gamma Radiation Measurement
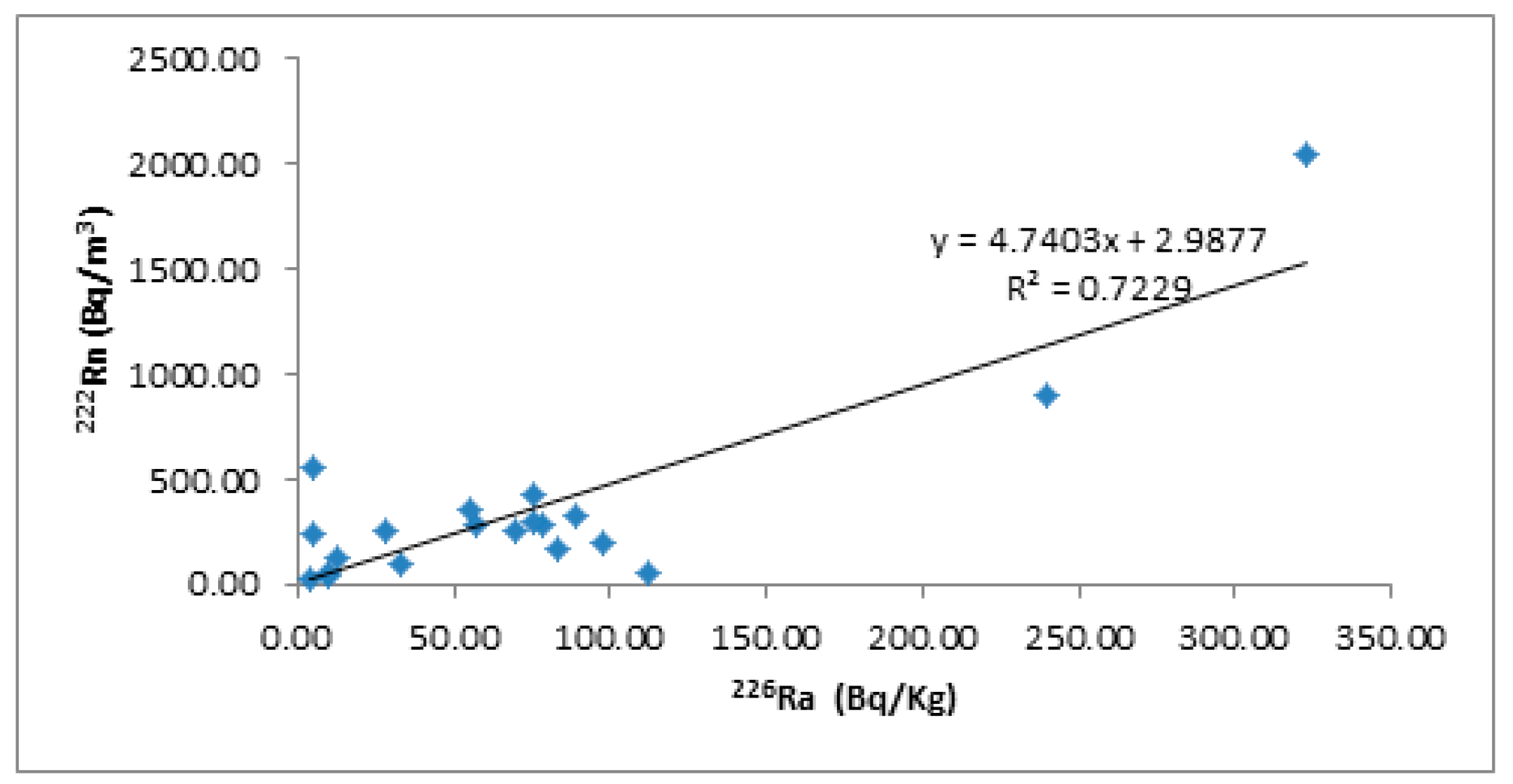
2.4. Radon Measurement
2.5. Rare Earth Elements Concentration
3. Results
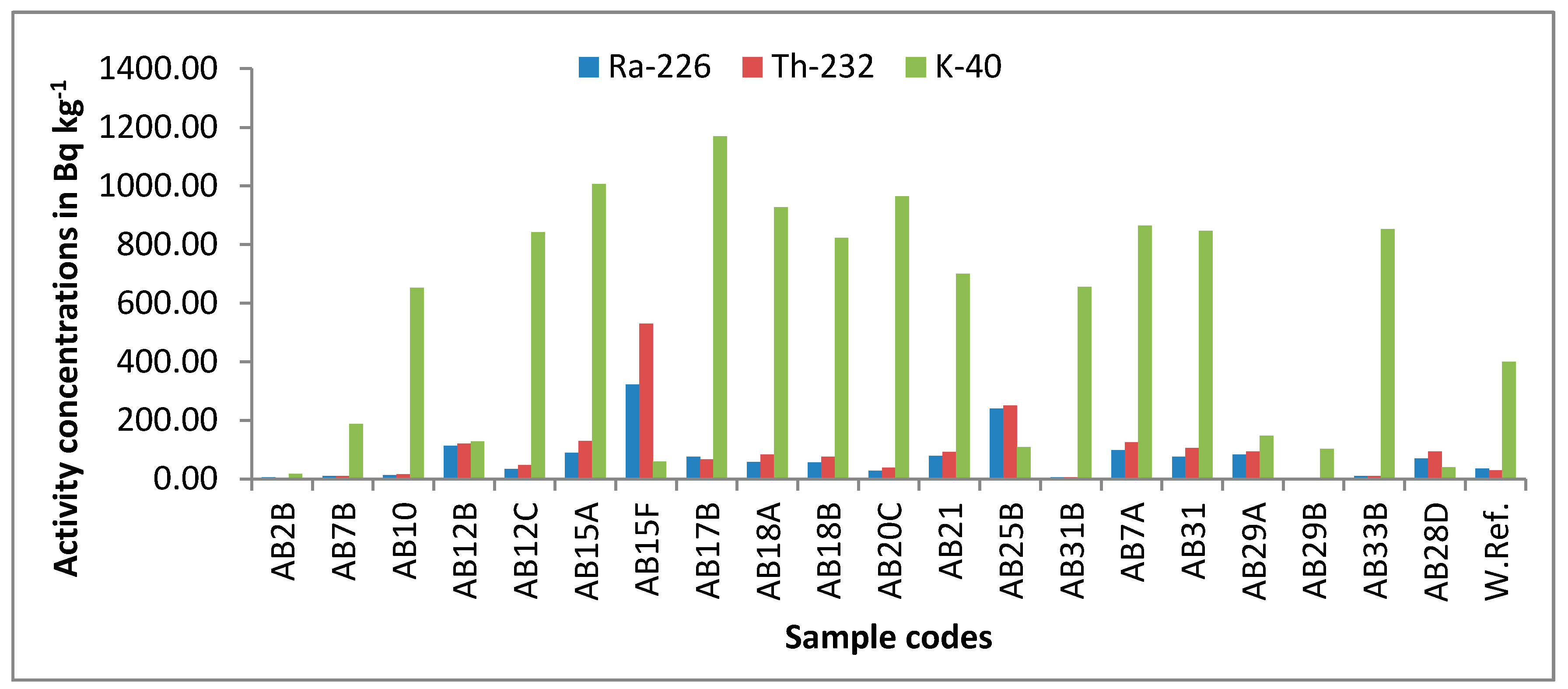
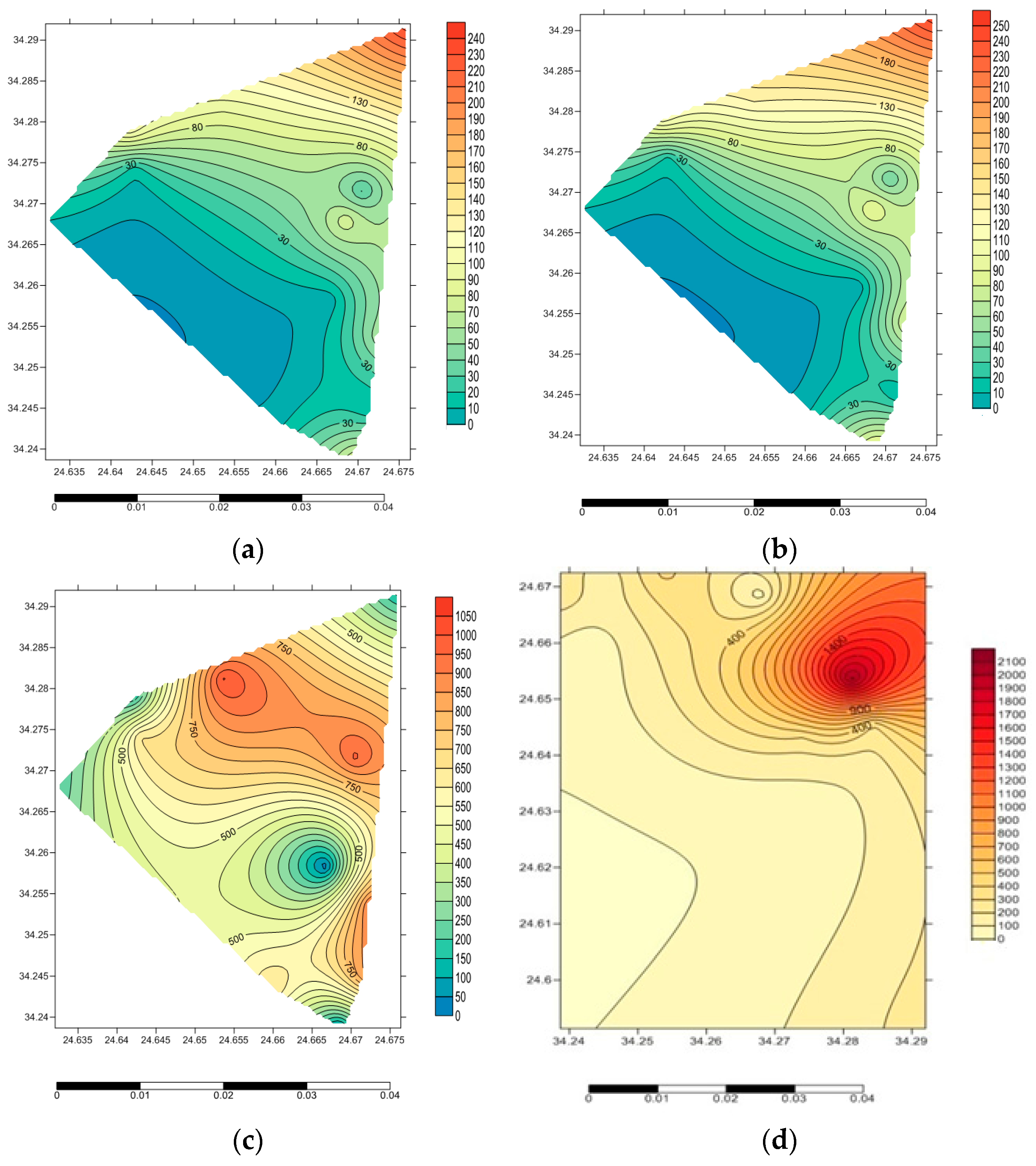
3.1. Activity Concentrations of Radioisotopes in the Investigated Samples
3.2. Radiological Hazard Parameters
3.2.1. Radium Equivalent Activity Raeq
3.2.2. Absorbed Dose Rates (DR) and Annual Effective Dose Rates (AEDR)
3.2.3. External Hazard Index (Hex)
3.2.4. Internal Hazard Index (Hin)
3.2.5. Alpha and Gamma Indices (Iα & Iγ)
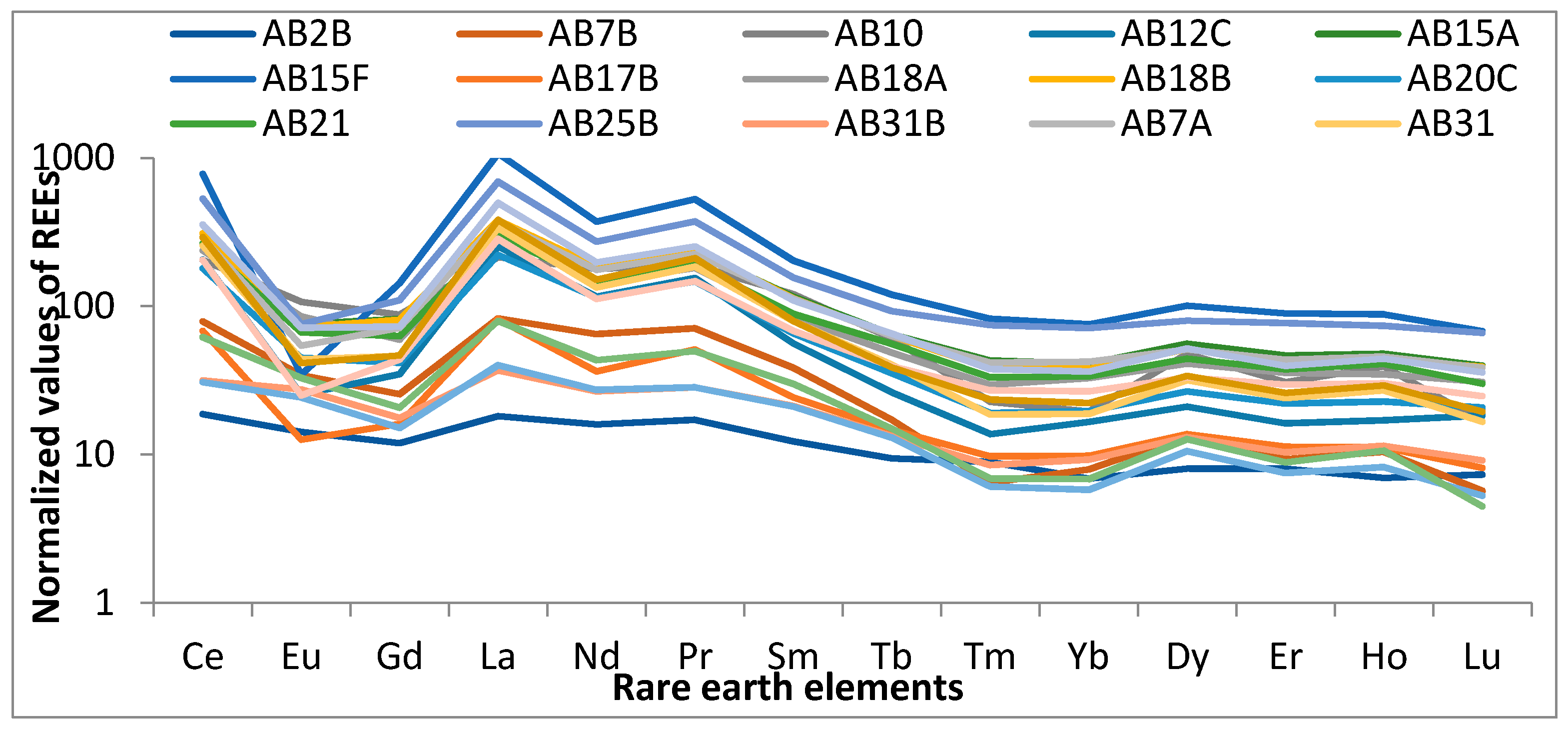
3.3. The Rare Earth Element Concentrations in the Alkaline Rocks of Abu Khuruq
3.3.1. Major Oxides Contents of Rocks
3.3.2. Pearson’s Correlation Coefficient
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNSCEAR. Sources and Effects of Ionizing Radiation. Report to General Assembly, with Scientific; UNSCEAR: New York, NY, USA, 2000. [Google Scholar]
- UNSCEAR. United Nations Scientific Committee on the Effect of Atomic Radiation. Sources and Effects of Ionizing Radiation; UNSCEAR: New York, NY, USA, 1993. [Google Scholar]
- Bollhoefer, A.; Storm, J.; Martin, P.; Tims, S. Geographic variability in radon exhalation at a rehabilitated uranium mine in the northern territory, Australia. Environ. Monit. Assess. 2006, 114, 313–330. [Google Scholar] [CrossRef] [PubMed]
- El-Bahi, S.M. Assessment of radioactivity and radon exhalation rate in Egyptian cement. Health Phys. 2004, 86, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Omeje, M.; Adewoyin, O.O.; Emmanuel, S.J.; Ehi-Eromosele, C.O.; Emenike, C.P.; Usikalu, M.R.; Akinwumi, S.A.; Zaidi, E.; Mohammad, A.S. Natural radioactivity concentrations of 226Ra, 232Th and 40K in commercial building materials and their lifetime cancer risk assessment in dwellers. Hum. Ecol. Risk Assess. Int. J. 2018. [Google Scholar] [CrossRef]
- Mason, B.; Moore, C. Principles of Geochemistry; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Rudnick, R.L.; Gao, S. The Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier-Pergamon: Oxford, UK, 2003; Volume 3. [Google Scholar]
- Me’nager, M.; Heath, M.; Ivanovich, M.; Montjotin, C.; Barillon, C.; Camp, J.; Hasler, S.E. Migration of uranium from uranium mineralised fractures into the rock matrix in granite: Implications for radionuclide transport around a radioactive waste repository. In Proceedings of the 4th International Conference of Chemistry and Migration Behaviour of Actinides and Fission Products in the Geosphere (Migration, 1993), Charleston, SC, USA, 12–17 December 1993; pp. 47–83. [Google Scholar]
- Tzortzis, M.; Tsertos, H.; Christofides, S.; Christodoulides, G. Gamma-ray measurements of naturally occurring radioactive samples from Cyprus characteristic geological rocks. Radiat. Meas. 2003, 37, 221–229. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Zhao, Y.; Zhang, C.; Wei, Y. Rare Earth Elements Supply vs. Clean Energy Technologies: New Problems to Solve. Gospod. Surowcami Min. 2016, 32, 29–44. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- U.S. Geological Survey. The Rare-Earth Elements—Vital to Modern Technologies and Lifestyles. Available online: https://pubs.usgs.gov/fs/2014/3078/pdf/fs2014-3078.pdf (accessed on 9 June 2017).
- Landoll, J.D.; Foland, K.A.; Henderson, C.M.B. Nd isotopes demonstrate the role of contamination in the formation of coexisting quartz and nephelinesyenites at the Abu Khruq complex, Egypt. Contrib. Miner. Petrol. 1994, 117, 305–329. [Google Scholar] [CrossRef]
- El Ramly, M.F.; Hussein, A.A.A. The ring complexes of the eastern desert of Egypt. J. Afa. Earth. Sci. 1985, 3, 77–82. [Google Scholar] [CrossRef]
- Obeid, M.A.; Lalonde, A.E. The Geochemistry and Petrogenesis of the Late Cretaceous Abu Khuruq Alkaline Complex, Eastern Desert Egypt. Can. Miner. 2013, 51, 537–558. [Google Scholar] [CrossRef]
- Canberra. Germanium Detectors User’s Manual; Canberra Industries, Inc.: Meriden, CT, USA, 2013. [Google Scholar]
- Zunic, Z.S.; Kobal, I.; Vaupotic, J.; Kozak, K.; Mazur, J.; Birovl-jev, A.; Janik, M.; Čelikovic, I.; Ujic, P.; Demajo, A.; et al. High natural radia- tion exposure in radon spa areas: A detailed field investigation in NiˇskaBanja (Balkan region). J. Environ. Radioactiv. 2006, 89, 249–260. [Google Scholar] [CrossRef]
- El-Gamal, H.; Sidique, E.; El-Haddad, M.; El-Azab Farid, M. Assessment of the natural radioactivity and radiological hazards in granites of Mueilha area (South Eastern Desert, Egypt). Environ. Earth Sci. 2018, 77, 691. [Google Scholar] [CrossRef]
- ICRP (Internal Commission on Radiological Protection). Protection against Rn-222 at home and at work. ICRP publication 65. Ann. ICRP 1994, 23, 1–48. [Google Scholar]
- Xinwei, L.; Lingqing, W.; Xiaodan, J.; Leipeng, Y.; Gelian, D. Specific activity and hazards of Archeozoic-Cambrian rock samples collected from the Weibei area of Shaanxi, China. Radiat. Prot. Dosimetry 2006, 118, 352–359. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Sources, Effects and Risks of Ionizing Radiation; Annex, B., Ed.; UNSCEAR: New York, NY, USA, 1988. [Google Scholar]
- Beretka, J.; Mathew, P. Natural radioactivity of Australian building materials, industrial wastes and by-products. Health Phys. 1985, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- EC (European Commission). Radiological Protection Principles Concerning the Natural Radioactivityof Building Materials. Radiation Protection 112. Directorate General Environment, NuclearSafety and Civil Protection; EC: Geneva, Switzerland, 1999. [Google Scholar]
- Papadopoulos, A.; Christofides, G.; Koroneos, A.; Papadopoulou, L.; Papastefanou, C.; Stoulos, S. Natural radioactivity and radiation index of the major plutonic bodies in Greece. J. Environ. Radioactiv. 2013, 124, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Aykamış, A.Ş.; Turhan, Ş.; Aysun, U.F.; Baykan, U.N.; Kılıç, A.M. Natural radioactivity, radon exhalation rates and indoor radon concentration of some granite samples used as construction material in Turkey. Radiat. Prot. Dosim. 2013, 157, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Righi, S.; Bruzzi, L. Natural radioactivity and radon exhalation in building materials used in Italian dwellings. J. Environ. Radioactiv. 2006, 88, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, R.; Raghu, Y.; Chandrasekaran, A.; Gandhi, M.S.; Vijayagopal, P.; Venkatraman, B. Determination of natural radioactivity and the associated radiation hazards in building materials used in Polur, Tiruvannamalai District, Tamilnadu, India using gamma ray spectrometry with statistical approach. J. Geochem. Explor. 2016, 163, 41–52. [Google Scholar] [CrossRef]
- Nordic. Naturally Occurring Radiation in the Nordic Countries-Recommendations. 2000. Available online: https://www.gr.is/wp-content/media/2013/07/NaturallyOccurringRadioactivity.pdf (accessed on 18 July 2019).
- Richardson, D.G.; Birkett, T.C. Peralkaline rock-associated rare metals. In The Geology of North America; Eckstrand, O.R., Sinclair, W.D., Thorpe, R.I., Eds.; Geological Society of America: Boulder, CO, USA, 1996; pp. 523–540. [Google Scholar]
- Dostal, J. Rare Earth Element Deposits of Alkaline Igneous Rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of rare earth elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Pilet, S.; Baker, M.B.; Stolper, E.M. Metasomatized lithosphere and the origin of alkaline lavas. Science 2008, 320, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Markl, G.; Marks, M.; Schwinn, G.; Sommer, H. Phase equilibrium constraints on intensive crystallization parameters of the Ilmaussaq Complex, South Greenland. J. Petrol. 2001, 42, 2231–2258. [Google Scholar] [CrossRef]
- Halter, W.; Webster, J.D. The magmatic to hydrothermal transition and its bearing on ore-forming systems. Chem. Geol. 2004, 210, 1–6. [Google Scholar] [CrossRef]
- Şükrü, K.; Ali, S.; Okay, Ç. Major and rare earth element contents in sedimentary rocks of the Haymana formation, Ankara, Turkey. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1918–1928. [Google Scholar] [CrossRef]
- Noack, C.W.; Jain, J.C.; Stegmeier, J.; Hakala, J.A.; Karamalidis, A.K. Rare earth element geochemistry of outcrop and core samples from the Marcellus shale. Geochem. Trans. 2015, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.S.M.; da Silva, Y.J.A.B.; Escobar, M.E.O.; do Nascimento, C.W.A. Spatial variability and geochemistry of rare earth elements in soils from the largest uranium–phosphate deposit of Brazil. Environ. Geochem. Health 2018, 40, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
| Sample Code | Raeq (Bq kg−1) | DR (nGy h−1) | AEDR (mSv y−1) | Hex (Bq kg−1) | Hin (Bq kg−1) | Iα | Iγ |
|---|---|---|---|---|---|---|---|
| AB2B | 10.94 | 4.83 | 0.02 | 0.03 | 0.04 | 0.02 | 0.04 |
| AB7B | 37.67 | 17.71 | 0.09 | 0.10 | 0.13 | 0.05 | 0.14 |
| AB10 | 85.17 | 42.03 | 0.21 | 0.23 | 0.26 | 0.06 | 0.34 |
| AB12B | 294.57 | 126.52 | 0.62 | 0.79 | 1.10 | 0.56 | 1.01 |
| AB12C | 165.15 | 77.71 | 0.38 | 0.45 | 0.54 | 0.17 | 0.62 |
| AB15A | 352.92 | 158.88 | 0.78 | 0.95 | 1.19 | 0.45 | 1.28 |
| AB15F | 1085.62 | 460.83 | 2.26 | 2.93 | 3.80 | 1.61 | 3.75 |
| AB17B | 260.59 | 121.51 | 0.59 | 0.70 | 0.91 | 0.38 | 0.97 |
| AB18A | 246.83 | 113.14 | 0.55 | 0.67 | 0.82 | 0.29 | 0.91 |
| AB18B | 226.56 | 103.63 | 0.51 | 0.61 | 0.76 | 0.28 | 0.83 |
| AB20C | 157.88 | 75.72 | 0.37 | 0.43 | 0.50 | 0.14 | 0.61 |
| AB21 | 263.99 | 118.54 | 0.58 | 0.71 | 0.92 | 0.39 | 0.95 |
| AB25B | 606.94 | 259.36 | 1.27 | 1.64 | 2.29 | 1.19 | 2.09 |
| AB31B | 63.50 | 32.88 | 0.16 | 0.17 | 0.18 | 0.03 | 0.26 |
| AB7A | 342.54 | 153.32 | 0.75 | 0.92 | 1.19 | 0.49 | 1.24 |
| AB31 | 291.65 | 131.45 | 0.64 | 0.79 | 0.99 | 0.37 | 1.06 |
| AB29A | 228.59 | 98.58 | 0.48 | 0.62 | 0.84 | 0.42 | 0.79 |
| AB29B | 19.07 | 9.037 | 0.04 | 0.05 | 0.06 | 0.02 | 0.07 |
| AB33B | 90.09 | 45.96 | 0.22 | 0.24 | 0.27 | 0.05 | 0.37 |
| AB28D | 207.63 | 88.54 | 0.43 | 0.56 | 0.75 | 0.35 | 0.72 |
| Min. | 10.94 | 4.83 | 0.02 | 0.03 | 0.04 | 0.02 | 0.04 |
| Max. | 1085.62 | 460.83 | 2.26 | 2.93 | 3.80 | 1.61 | 3.75 |
| Mean | 251.89 | 112.01 | 0.55 | 0.68 | 0.88 | 0.37 | 0.90 |
| Sample No. | Ce | Eu | Gd | La | Nd | Pr | Sm | Tb | Tm | Yb | Dy | Er | Lu | Ho | ∑LREE | ∑HREE | ∑REEs | LREE/HREE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB2B | 11.50 | 0.80 | 2.38 | 4.30 | 7.30 | 1.59 | 1.82 | 0.34 | 0.22 | 1.11 | 1.98 | 1.28 | 0.18 | 0.38 | 29.69 | 5.49 | 35.18 | 5.41 |
| AB7B | 48.40 | 1.93 | 5.09 | 19.60 | 29.70 | 6.59 | 5.68 | 0.62 | 0.16 | 1.28 | 3.32 | 1.49 | 0.14 | 0.57 | 116.99 | 7.58 | 124.57 | 15.43 |
| AB10 | 125.50 | 6.03 | 17.45 | 50.70 | 81.60 | 17.55 | 17.85 | 2.19 | 0.56 | 3.15 | 11.75 | 4.92 | 0.41 | 2.15 | 316.68 | 25.13 | 341.81 | 12.60 |
| AB12B | 179.50 | 2.33 | 9.29 | 90.70 | 69.00 | 19.55 | 11.75 | 1.40 | 0.58 | 3.58 | 8.36 | 4.17 | 0.48 | 1.60 | 382.12 | 20.17 | 402.29 | 18.94 |
| AB12C | 126.50 | 1.43 | 6.95 | 60.00 | 53.00 | 14.40 | 8.33 | 0.95 | 0.34 | 2.68 | 5.17 | 2.60 | 0.45 | 0.93 | 270.61 | 13.12 | 283.73 | 20.62 |
| AB15A | 177.50 | 4.26 | 16.30 | 78.30 | 86.80 | 21.80 | 17.20 | 2.33 | 1.06 | 6.61 | 13.75 | 7.47 | 0.98 | 2.61 | 402.16 | 34.81 | 436.97 | 11.55 |
| AB15F | 478.00 | 1.94 | 28.50 | 254.00 | 170.00 | 48.90 | 30.10 | 4.32 | 2.03 | 12.15 | 24.80 | 14.35 | 1.67 | 4.82 | 1011.44 | 64.14 | 1075.58 | 15.76 |
| AB17B | 41.90 | 0.71 | 3.20 | 19.40 | 16.60 | 4.75 | 3.60 | 0.53 | 0.24 | 1.58 | 3.38 | 1.81 | 0.20 | 0.61 | 90.16 | 8.35 | 98.51 | 10.79 |
| AB18A | 146.00 | 4.82 | 11.85 | 72.70 | 65.90 | 16.85 | 12.55 | 1.76 | 0.73 | 5.28 | 10.10 | 5.69 | 0.76 | 1.90 | 330.67 | 26.22 | 356.89 | 12.61 |
| AB18B | 190.00 | 4.07 | 16.15 | 90.10 | 84.80 | 22.40 | 16.70 | 2.28 | 0.94 | 6.08 | 12.65 | 6.89 | 0.96 | 2.37 | 424.22 | 32.17 | 456.39 | 13.187 |
| AB20C | 110.50 | 2.53 | 8.25 | 52.70 | 52.30 | 13.65 | 9.73 | 1.28 | 0.47 | 3.16 | 6.56 | 3.53 | 0.51 | 1.24 | 249.66 | 16.75 | 266.41 | 14.90 |
| AB21 | 162.50 | 3.75 | 12.50 | 70.40 | 67.80 | 17.70 | 13.15 | 2.01 | 0.82 | 5.43 | 10.90 | 6.00 | 0.74 | 2.23 | 347.80 | 28.13 | 375.93 | 12.36 |
| AB25B | 326.00 | 4.27 | 21.90 | 164.00 | 124.50 | 34.60 | 23.10 | 3.35 | 1.84 | 11.45 | 19.65 | 12.30 | 1.63 | 4.02 | 698.37 | 54.24 | 752.61 | 12.87 |
| AB31B | 19.40 | 1.55 | 3.53 | 8.75 | 12.20 | 2.63 | 3.16 | 0.48 | 0.21 | 1.49 | 3.23 | 1.66 | 0.23 | 0.63 | 51.21 | 7.92 | 59.13 | 6.47 |
| AB7A | 177.50 | 3.06 | 14.20 | 88.40 | 80.50 | 20.90 | 16.65 | 2.28 | 1.03 | 6.80 | 12.60 | 7.00 | 0.95 | 2.50 | 401.21 | 33.16 | 434.37 | 12.09 |
| AB31 | 157.00 | 2.47 | 9.19 | 80.00 | 61.30 | 17.15 | 11.75 | 1.46 | 0.46 | 3.03 | 7.82 | 3.85 | 0.41 | 1.48 | 338.86 | 18.51 | 357.37 | 18.31 |
| AB29A | 18.90 | 1.37 | 3.01 | 9.50 | 12.50 | 2.63 | 3.12 | 0.47 | 0.15 | 0.93 | 2.60 | 1.20 | 0.13 | 0.45 | 51.03 | 5.93 | 56.96 | 8.60 |
| AB29B | 37.90 | 1.85 | 4.13 | 18.90 | 19.80 | 4.62 | 4.44 | 0.54 | 0.17 | 1.10 | 3.13 | 1.42 | 0.11 | 0.58 | 91.64 | 7.05 | 98.69 | 12.99 |
| AB33B | 218.00 | 4.03 | 14.50 | 118.00 | 90.20 | 23.50 | 16.20 | 2.36 | 0.93 | 5.83 | 12.80 | 6.33 | 0.88 | 2.42 | 484.43 | 31.55 | 515.98 | 15.35 |
| AB28D | 126.00 | 1.41 | 8.72 | 67.30 | 51.20 | 13.70 | 10.10 | 1.42 | 0.67 | 4.30 | 8.16 | 4.74 | 0.61 | 1.65 | 278.43 | 21.55 | 299.98 | 12.92 |
| Average | 143.93 | 2.73 | 10.85 | 70.89 | 61.85 | 16.27 | 11.85 | 1.62 | 0.68 | 4.35 | 9.14 | 4.93 | 0.62 | 1.76 | 318.37 | 23.10 | 341.47 | 13.78 |
| Sample Code | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Cr2O3 | TiO2 | MnO | P2O5 | SrO | BaO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB2B | 46.90 | 19.10 | 9.22 | 8.85 | 8.91 | 3.84 | 0.43 | 0.01 | 0.70 | 0.11 | 0.10 | 0.04 | 0.01 |
| AB7B | 49.50 | 13.35 | 10.10 | 9.36 | 8.67 | 3.07 | 0.97 | 0.07 | 1.53 | 0.14 | 0.33 | 0.07 | 0.05 |
| AB10 | 36.00 | 10.52 | 19.25 | 9.88 | 9.30 | 4.47 | 0.41 | 0.01 | 5.23 | 0.30 | 2.76 | 0.06 | 0.02 |
| AB12B | 57.70 | 18.40 | 5.64 | 2.39 | 0.72 | 7.49 | 4.27 | 0.01 | 0.63 | 0.14 | 0.18 | 0.04 | 0.07 |
| AB12C | 58.00 | 19.00 | 5.41 | 1.92 | 0.32 | 8.61 | 4.37 | 0.01 | 0.35 | 0.14 | 0.12 | 0.04 | 0.06 |
| AB15A | 63.80 | 12.10 | 9.90 | 0.93 | 0.12 | 6.78 | 4.19 | 0.01 | 0.62 | 0.30 | 0.06 | 0.01 | 0.03 |
| AB15F | 54.90 | 19.55 | 6.32 | 1.25 | 0.26 | 8.90 | 4.25 | 0.01 | 0.14 | 0.29 | 0.06 | 0.01 | 0.01 |
| AB17B | 77.70 | 13.25 | 0.94 | 0.19 | 0.14 | 3.56 | 3.34 | 0.01 | 0.07 | 0.03 | 0.02 | 0.01 | 0.07 |
| AB18A | 63.50 | 15.90 | 6.06 | 2.11 | 0.46 | 6.08 | 3.81 | 0.01 | 0.62 | 0.16 | 0.14 | 0.02 | 0.14 |
| AB18B | 64.00 | 14.60 | 3.52 | 3.50 | 0.42 | 5.61 | 4.95 | 0.01 | 0.55 | 0.12 | 0.05 | 0.01 | 0.06 |
| AB20C | 61.50 | 13.40 | 10.05 | 1.44 | 1.25 | 6.75 | 4.56 | 0.01 | 0.54 | 0.33 | 0.11 | 0.01 | 0.03 |
| AB21 | 63.70 | 13.85 | 8.86 | 2.08 | 0.18 | 5.38 | 4.02 | 0.01 | 0.63 | 0.18 | 0.11 | 0.01 | 0.07 |
| AB25B | 70.50 | 10.40 | 7.05 | 1.56 | 0.16 | 4.26 | 4.08 | 0.01 | 0.37 | 0.12 | 0.02 | 0.01 | 0.01 |
| AB31B | 48.95 | 19.45 | 8.59 | 9.82 | 5.44 | 3.86 | 0.43 | 0.03 | 1.36 | 0.12 | 0.19 | 0.06 | 0.03 |
| AB7A | 67.90 | 13.45 | 4.44 | 1.95 | 0.01 | 4.79 | 5.02 | 0.01 | 0.41 | 0.07 | 0.02 | 0.01 | 0.01 |
| AB31 | 57.60 | 18.30 | 6.22 | 2.99 | 0.94 | 7.01 | 4.11 | 0.01 | 0.76 | 0.14 | 0.21 | 0.05 | 0.09 |
| AB29A | 45.00 | 17.55 | 12.35 | 11.90 | 4.39 | 3.04 | 0.32 | 0.01 | 4.07 | 0.14 | 0.10 | 0.07 | 0.01 |
| AB29B | 49.00 | 21.20 | 8.99 | 10.30 | 3.29 | 3.95 | 0.55 | 0.01 | 1.82 | 0.10 | 0.45 | 0.09 | 0.03 |
| AB33B | 63.10 | 15.00 | 8.03 | 2.44 | 0.33 | 6.18 | 3.88 | 0.01 | 0.58 | 0.21 | 0.10 | 0.01 | 0.09 |
| AB28D | 42.14 | 13.02 | 20.95 | 15.72 | 4.72 | 0.00 | 0.00 | 0.00 | 2.10 | 0.35 | 0.00 | 0.00 | 0.00 |
| Min | 36.00 | 10.40 | 0.94 | 0.19 | 0.01 | 3.04 | 0.32 | 0.01 | 0.07 | 0.07 | 0.02 | 0.01 | 0.01 |
| Max | 77.70 | 21.20 | 20.95 | 15.72 | 9.30 | 8.90 | 4.95 | 0.07 | 5.32 | 0.35 | 2.76 | 0.09 | 0.14 |
| Average | 57.05 | 15.59 | 8.81 | 5.29 | 2.70 | 5.25 | 2.87 | 0.02 | 1.29 | 0.18 | 0.36 | 0.03 | 0.05 |
| STDEV | 11.82 | 3.51 | 5.51 | 5.07 | 3.47 | 2.23 | 1.93 | 0.02 | 1.56 | 0.09 | 0.78 | 0.03 | 0.04 |
| REE | Ce | Eu | Gd | La | Nd | Pr | Sm | Tb | Tm | Yb | Dy | Er | Lu | Ho |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce | 1 | |||||||||||||
| Eu | 0.37 | 1 | ||||||||||||
| Gd | 0.93 | 0.62 | 1 | |||||||||||
| La | 0.99 | 0.31 | 0.9 | 1 | ||||||||||
| Nd | 0.97 | 0.53 | 0.98 | 0.95 | 1 | |||||||||
| Pr | 0.99 | 0.45 | 0.95 | 0.98 | 0.99 | 1 | ||||||||
| Sm | 0.94 | 0.6 | 0.99 | 0.92 | 0.99 | 0.97 | 1 | |||||||
| Tb | 0.95 | 0.57 | 0.99 | 0.93 | 0.98 | 0.97 | 0.99 | 1 | ||||||
| Tm | 0.95 | 0.43 | 0.94 | 0.93 | 0.94 | 0.95 | 0.93 | 0.96 | 1 | |||||
| Yb | 0.94 | 0.45 | 0.93 | 0.93 | 0.93 | 0.94 | 0.93 | 0.96 | 0.99 | 1 | ||||
| Dy | 0.96 | 0.55 | 0.98 | 0.94 | 0.98 | 0.97 | 0.98 | 0.99 | 0.97 | 0.97 | 1 | |||
| Er | 0.95 | 0.47 | 0.96 | 0.94 | 0.96 | 0.96 | 0.95 | 0.98 | 0.99 | 0.99 | 0.98 | 1 | ||
| Lu | 0.93 | 0.46 | 0.92 | 0.92 | 0.93 | 0.93 | 0.92 | 0.95 | 0.98 | 0.99 | 0.96 | 0.98 | 1 | |
| Ho | 0.95 | 0.53 | 0.98 | 0.94 | 0.97 | 0.97 | 0.98 | 0.99 | 0.98 | 0.97 | 0.99 | 0.99 | 0.96 | 1 |
| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Cr2O3 | TiO2 | MnO | P2O5 | SrO | BaO | LREE | HREE | TREE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1 | |||||||||||||||
| Al2O3 | −0.26 | 1 | ||||||||||||||
| Fe2O3 | −0.78 | −0.27 | 1 | |||||||||||||
| CaO | −0.85 | 0.14 | 0.75 | 1 | ||||||||||||
| MgO | −0.81 | 0.02 | 0.63 | 0.79 | 1 | |||||||||||
| Na2O | 0.35 | 0.29 | −0.52 | −0.75 | −0.55 | 1 | ||||||||||
| K2O | 0.81 | −0.16 | −0.67 | −0.93 | −0.86 | 0.73 | 1 | |||||||||
| Cr2O3 | −0.15 | −0.06 | −0.03 | 0.18 | 0.45 | −0.16 | −0.24 | 1 | ||||||||
| TiO2 | −0.76 | −0.14 | 0.75 | 0.71 | 0.66 | −0.45 | −0.70 | 0.04 | 1 | |||||||
| MnO | −0.36 | −0.30 | 0.68 | 0.13 | 0.09 | 0.09 | −0.05 | −0.19 | 0.26 | 1 | ||||||
| P2O5 | −0.53 | −0.27 | 0.52 | 0.29 | 0.54 | −0.07 | −0.37 | 0.03 | 0.74 | 0.28 | 1 | |||||
| SrO | −0.62 | 0.48 | 0.19 | 0.55 | 0.59 | −0.17 | −0.62 | 0.42 | 0.56 | −0.29 | 0.39 | 1 | ||||
| BaO | 0.41 | 0.09 | −0.45 | −0.46 | −0.39 | 0.38 | 0.41 | 0.05 | −0.33 | −0.23 | −0.11 | −0.08 | 1 | |||
| LREE | 0.31 | −0.16 | −0.16 | −0.51 | −0.51 | 0.52 | 0.59 | −0.24 | −0.33 | 0.35 | −0.07 | −0.54 | −0.02 | 1 | ||
| HREE | 0.34 | −0.31 | −0.12 | −0.48 | −0.49 | 0.40 | 0.55 | −0.27 | −0.29 | 0.34 | −0.05 | −0.60 | −0.10 | 0.96 | 1 | |
| TREE | 0.31 | −0.17 | −0.16 | −0.51 | −0.51 | 0.52 | 0.59 | −0.25 | −0.33 | 0.35 | −0.07 | −0.55 | −0.03 | 0.99 | 0.97 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gamal, H.; El-Haddad, M. Estimation of Natural Radionuclides and Rare Earth Elements Concentration of the Rocks of Abu Khuruq Ring Complex, Egypt. Symmetry 2019, 11, 1041. https://doi.org/10.3390/sym11081041
El-Gamal H, El-Haddad M. Estimation of Natural Radionuclides and Rare Earth Elements Concentration of the Rocks of Abu Khuruq Ring Complex, Egypt. Symmetry. 2019; 11(8):1041. https://doi.org/10.3390/sym11081041
Chicago/Turabian StyleEl-Gamal, Hany, and Mervat El-Haddad. 2019. "Estimation of Natural Radionuclides and Rare Earth Elements Concentration of the Rocks of Abu Khuruq Ring Complex, Egypt" Symmetry 11, no. 8: 1041. https://doi.org/10.3390/sym11081041
APA StyleEl-Gamal, H., & El-Haddad, M. (2019). Estimation of Natural Radionuclides and Rare Earth Elements Concentration of the Rocks of Abu Khuruq Ring Complex, Egypt. Symmetry, 11(8), 1041. https://doi.org/10.3390/sym11081041





